Introduction
Gastric cancer (GC) is a heterogeneous disease that
evolves from various genetic and epigenetic alterations (1). According to global cancer statistics
(2012), GC is the 5th most common cancer and is the 3rd most common
cause of cancer-associated death. In 2012, an estimated 951,600 new
stomach cancer cases and 723,100 mortalities occurred worldwide,
and the incidence rates were highest in Eastern Asia (particularly
in Korea, Mongolia, Japan and China) (2). The majority of patients are treated
with surgical resection and chemotherapy at the time of diagnosis;
however, the overall 5-year survival rate of GC patients remains
unsatisfactory (3–5). Therefore, investigating the
underlying mechanisms of effector molecules and signaling pathways
that promote the initiation and progression of GC require further
investigation.
Previous studies reported that angiogenesis serves
an important role in the pathogenesis of certain cancers, including
GC (6,7). Vascular endothelial growth factor
(VEGF) is as an angiogenic cytokine that specifically binds to
receptor tyrosine kinases (RTKs), including VEGF receptor (R)1
(encoded by the FLT1 gene), VEGFR2 (encoded by the
KDR gene) and VEGFR3 (encoded by the FLT4 gene)
(8,9). Reduced paracrine secretion of VEGF
from tumor cells may suppress angiogenic activity (10). An additional angiogenic cytokine,
transforming growth factor-β1 (TGF-β1) is an important
transcriptional regulator of the extracellular matrix, and high
expression of TGF-β1 is associated with significantly poor overall
disease-free survival (11). This
suggests that angiogenesis is tightly controlled by angiogenic
cytokines and inflammatory factors in GC.
Serum soluble interleukin-2 receptor (sIL-2R) is an
important monitoring index which reflects cellular immunity
function in the body (12). It has
been reported that sIL-2R levels may be used as an independent
prognostic index in follicular lymphoma patients (13). High sIL-2R levels in patients with
tumors may inhibit the proliferation of T cells (14). However, whether sIL-2R is involved
in progression of GC remains to be determined. The aim of the
present study was to detect the serum levels of sIL-2R in patients
with GC and to determine whether they are associated with VEGF and
TGF-β1, which may provide an insight into whether sIL-2R may be
used as a clinical biomarker for GC.
Materials and methods
Patients
The original research was approved by the Medical
Ethics Committee of Henan Provincial People's Hospital, People's
Hospital of Zhengzhou University (approval no. KY2012-005; Henan,
China) and written informed consent was provided by the patients. A
total of 35 newly diagnosed GC patients were enrolled between
January 2013 and April 2015. Individuals with hypertension and
diabetes and those who had received surgery were excluded. A total
of 32 age-and sex-matched healthy individuals also enrolled at
Henan Provincial People's Hospital were used as controls. The
characteristics of subjects enrolled in the study are presented in
Table I.
 | Table I.Clinical characteristics of gastric
cancer patients and healthy controls. |
Table I.
Clinical characteristics of gastric
cancer patients and healthy controls.
| Parameter | Healthy controls | Gastric cancer
patients |
|---|
| Total | 32 | 35 |
| Age (years) | 57±7.54 | 62±17.21 |
| Sex
(male/female) | 17/15 | 19/16 |
| Tumor location in the
stomach |
|
|
|
Upper | – | 11 |
|
Middle | – | 15 |
|
Lower | – | 9 |
| Stage |
|
|
| II
A-B | – | 2 |
| III
A-C | – | 24 |
| IV | – | 9 |
Serum cytokine measurements
All blood samples were collected, mixed with EDTA
and centrifuged at 1,000 × g for 15 min at room temperature, and
the supernatants (serum) were stored at −80°C until analysis.
Soluble IL-2R (CSB-E04629H, CUSABIO; Flarebio Biotech LLC, Wuhan,
China), VEGF and TGF-β1 in the serum samples were determined using
ELISA kits (VEGF, DVE00; TGF-β1, DB100B; R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's instructions.
The final concentration in each sample was calculated by
interpolation of the standard curve.
Angiogenic capacity
The angiogenic capacity of vascular-like structures
was determined using human umbilical vein endothelial cells
(HUVECs) that were purchased from Cell Resource Center, China
infrastructure of cell line resources (Beijing, China). HUVECs
(2×105/well) were pretreated with recombinant human
(rh)sIL-2R (200 ng/ml; PromoCell GmbH, Heidelberg, Germany), VEGF
(20 ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), TGF-β1
(10 ng/ml; ProSpec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA)
or culture medium respectively for at 37°C and 5% CO2
for 24 h, then cells were collected and seeded into 96-well plates
coated with 50 µl Matrigel® (15) (BD Biosciences, Franklin Lakes, NJ,
USA). The plates were maintained at 37°C and 5% CO2 for
6 h. Tubes were defined as straight cellular extensions forming a
closed loop and digital images (at ×200 magnification) of each well
were taken by an inverted microscope (Leica QUIPS; Leica
Microsystems, Ltd., Milton Keynes, UK) and three random digital
images in each well were counted.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Student's t-test was used to determine differences in
serum cytokines between GC patients and healthy individuals.
Associations between parameters were calculated by the Spearman's
rank or Pearson correlation coefficient. P<0.05 was considered
to indicate a statistically significant difference.
Results
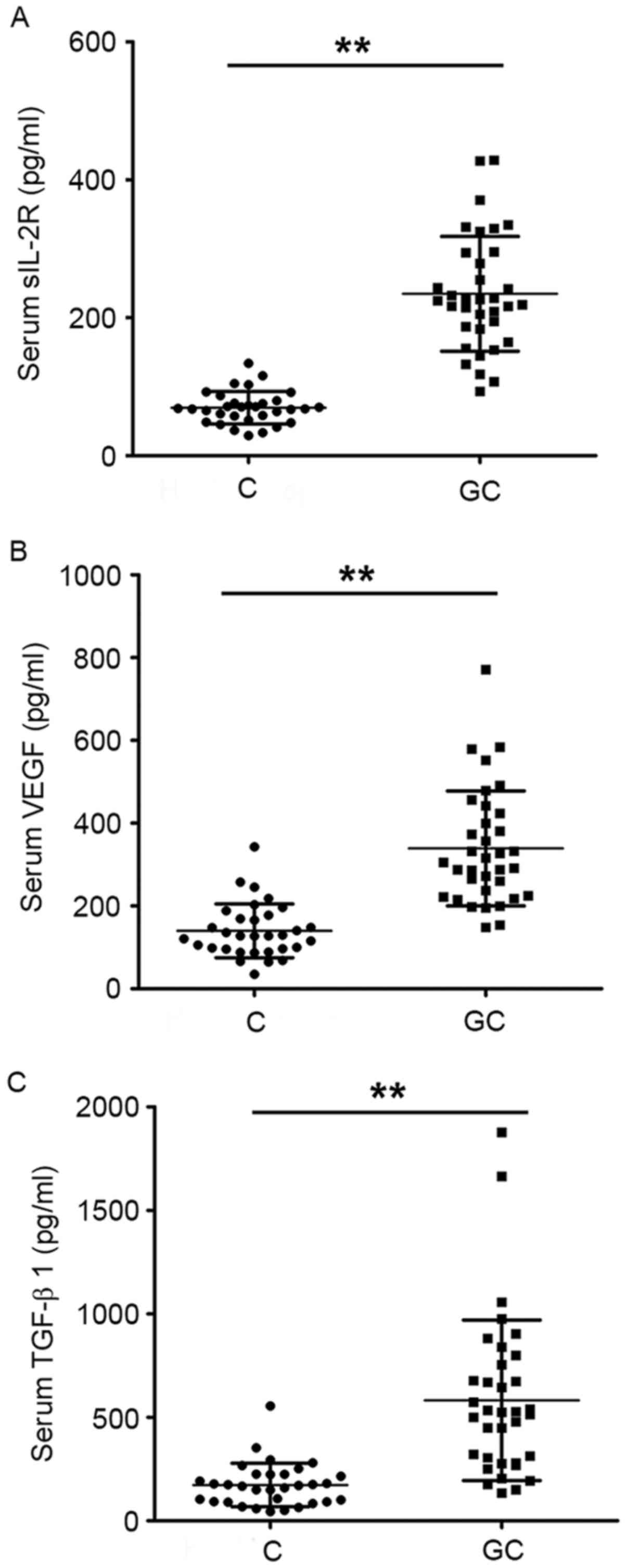
Serum levels of sIL-2R, VEGF and TGF-β1 were
significantly increased in GC patients compared with healthy
individuals (P<0.01; Fig.
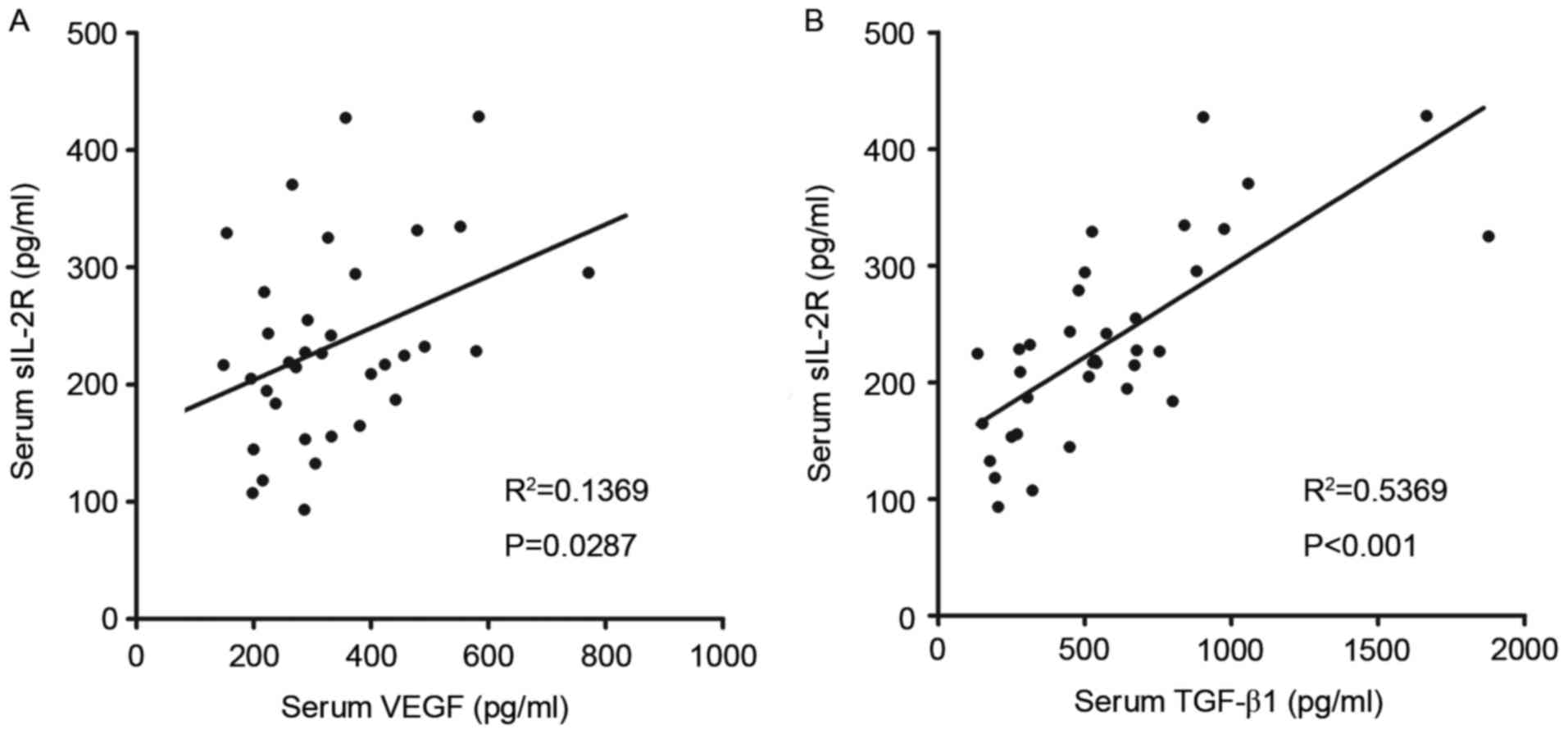
1A-C). It was also revealed that there were significant
positive associations between serum levels of sIL-2R and VEGF
(R2=0.1369, P=0.0287; Fig.
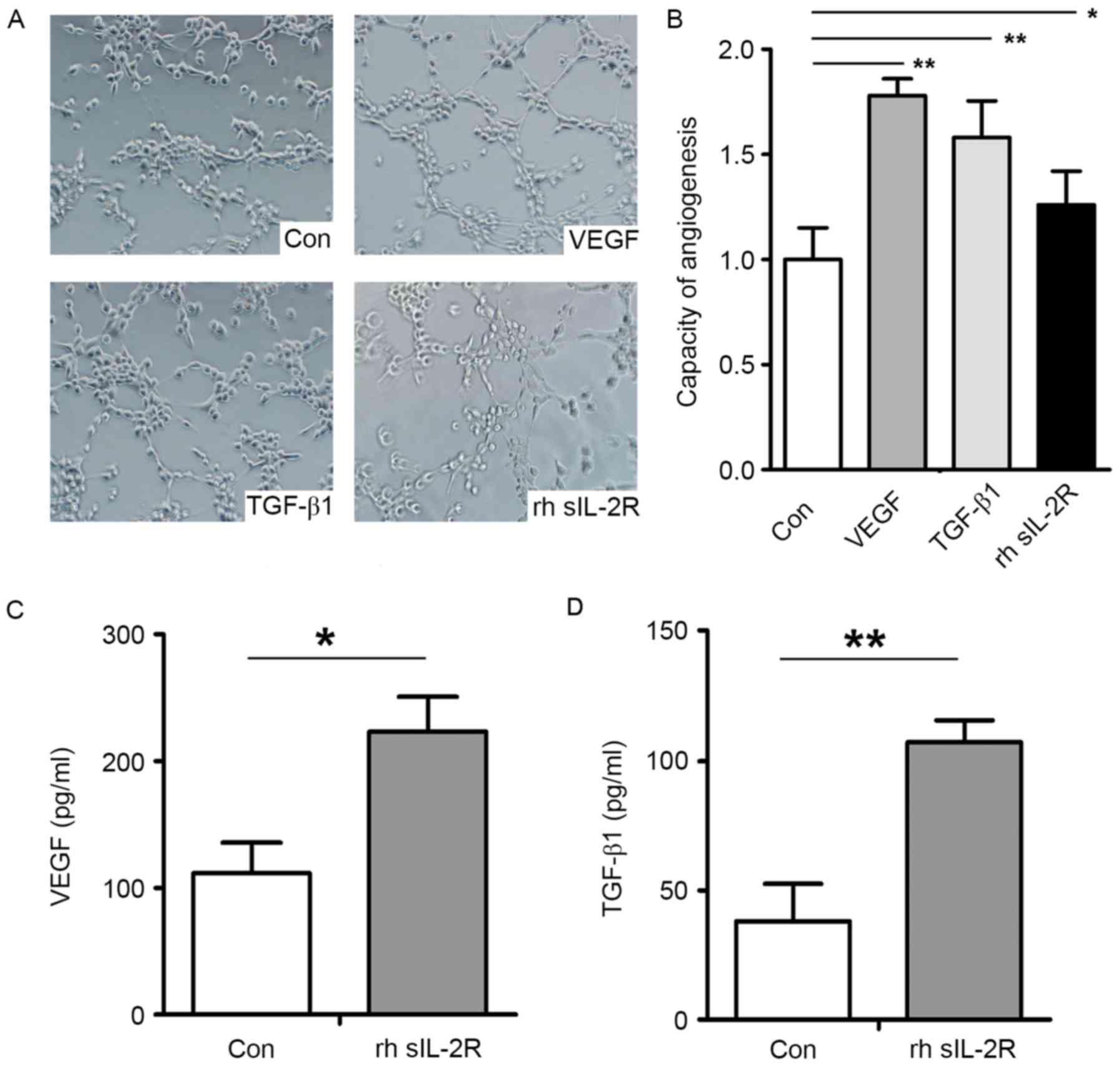
2A) and TGF-β1 (R2=0.5369, P<0.001; Fig. 2B). The angiogenic capacity of
HUVECs was enhanced after pretreatment with rhsIL-2R (P<0.05),
VEGF (P<0.01) and TGF-β1 (P<0.01) compared with the control
group (Fig. 3A and B). In
addition, rhsIL-2R pretreatment significantly increased the
secretion of VEGF (P<0.05; Fig.
3C) and TGF-β1 (P<0.01; Fig.
3D) in HUVECs.
Discussion
It is now accepted that angiogenesis is a crucial a
step involved in tumor pathogenesis, and angiogenic and
anti-angiogenic signaling pathways provide a switch for tumor
progression (16,17). Investigating the specific
contribution of pro-angiogenic factors in tumors may lead to the
identification of therapeutic targets and reliable biomarkers in
the clinic. In the present study, serum sIL-2R, VEGF and TGF-β1
levels were measured in GC patients and healthy controls. It was
revealed that serum levels of sIL-2R, VEGF and TGF-β1 were
significantly increased in GC patients compared with healthy
individuals. In addition, sIL-2R was significantly associated with
VEGF and TGF-β1, which are important angiogenetic factors. This
data suggested that sIL-2R may be an angiogenesis marker in GC.
sIL-2R is a glycoprotein which is derived from the α
chain of IL-2 receptors of the mononuclear and T-cell membranes,
and its molecular weight is 45 kDa (18). The secretion of sIL-2R serves a
significant role in regulating cell immune function. When IL-2
binds to sIL-2R, differentiation of memory T cells into effector T
cells is promoted to help fight infection. Previous studies
demonstrated that the expression levels of sIL-2R were increased in
the serum of patients with metastatic melanoma, and appeared to aid
the prediction of patient outcome (19). Increased serum sIL-2R expression
levels have an effect on a hamster cheek pouch carcinoma model
subsequent to heavy-ion beam irradiation (20). This suggests that there may be an
essential role for sIL-2R in the tumor microenvironment. In the
present study, there were significantly increased serum sIL-2R
levels in the serum of GC patients than healthy controls. This
suggests that the secretion of sIL-2R serves an important role in
GC progression.
TGF-β1 is a prototypical member of the TGF protein
superfamily, is involved in cell growth, differentiation, motility
and angiogenesis, and is associated with a negative prognosis
(21–23). In addition, TGF-β1 induces VEGF
expression in vascular endothelial cells (24), suggesting that TGF-β1 is an
important component of angiogenic activity. In previous studies,
VEGF has been reported to be expressed in response to immunity and
inflammation and also exerts a systemic influence on immune cell
development and function in tumors (25,26).
Incremental levels of circulating VEGF inhibit T cell immune
responses in colorectal cancer. In the present study, there was a
significant positive association between serum levels of sIL-2R and
VEGF and TGF-β1. Therefore, the association between sIL-2R and
angiogenesis was investigated in vitro. Pretreatment with
rhsIL-2R significantly increased the secretion of VEGF and TGF-β1,
and the angiogenic capacity of HUVECs was enhanced after rhsIL-2R
pretreatment. These results suggested that sIL-2R may serve a role
in angiogenesis during GC progression. However, further study is
required to establish if sIL2R is a factor that promotes the
angiogenic capacity of HUVECs directly. The underlying mechanism of
how sIL2R induces VEGF expression in the tumor microenvironment
requires further investigation.
In conclusion, the results of the present study
suggested that GC patients exhibit enhanced serum levels of sIL-2R,
VEGF and TGF-β1. sIL-2R was positively associated with VEGF and
TGF-β1. The angiogenic capacity of HUVECs was enhanced by
pretreatment with sIL-2R. These results suggested that serum levels
of sIL-2R serve a role in GC progression, and may be a marker of
angiogenesis in GC.
References
|
1
|
Baniak N, Senger JL, Ahmed S, Kanthan SC
and Kanthan R: Gastric biomarkers: A global review. World J Surg
Oncol. 14:2122016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HS, Lee H, Jeung HC, Noh SH, Chung HC,
Roh JK, Nam CM and Rha SY: Advanced detection of recent changing
trends in gastric cancer survival: Up-to-date comparison by period
analysis. Jpn J Clin Oncol. 41:1344–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohtsu A: Chemotherapy for metastatic
gastric cancer: Past, present, and future. J Gastroenterol.
43:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Zhou R, Zhao Y, Dong S, Zhang J,
Luo Y, Huang N, Shi M, Bin J, Liao Y and Liao W: MACC-1 promotes
endothelium-dependent angiogenesis in gastric cancer by activating
TWIST1/VEGF-A signal pathway. PLoS One. 11:e01571372016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh M: FGFR inhibitors: Effects on
cancer cells, tumor microenvironment and whole-body homeostasis
(Review). Int J Mol Med. 38:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang X, Xu F, Li X, Ma C, Zhang Y and Xu
W: VEGF signal system: The application of antiangiogenesis. Curr
Med Chem. 21:894–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q,
Jiao H and Chen J: Salinomycin exerts anti-angiogenic and
anti-tumorigenic activities by inhibiting vascular endothelial
growth factor receptor 2-mediated angiogenesis. Oncotarget.
7:26580–26592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H and Zhou S: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanazawa S, Yamaguchi K, Kinoshita Y,
Komiyama Y, Muramatsu M and Nomura S: Elevation of soluble
interleukin-2 receptor in patients with non-small cell lung cancer
treated with gefitinib. J Cancer Res Clin Oncol. 132:719–725. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwamuro M, Shinagawa K, Okada H, Takata K,
Yoshino T and Yamamoto K: Elevated soluble IL-2 receptor levels
correlate with tumor bulk of follicular lymphomas with intestinal
involvement. Clin Biochem. 47:191–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eller T, Aluoja A, Maron E and Vasar V:
Soluble interleukin-2 receptor and tumor necrosis factor levels in
depressed patients in Estonia. Medicina (Kaunas). 45:971–977.
2009.PubMed/NCBI
|
|
15
|
Ni M, Yang ZW, Li DJ, Li Q, Zhang SH, Su
DF, Xie HH and Shen FM: A potential role of alpha-7 nicotinic
acetylcholine receptor in cardiac angiogenesis in a
pressure-overload rat model. J Pharmacol Sci. 114:311–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu
Q and Li Q: Helicobacter pylori promotes angiogenesis depending on
Wnt/beta-catenin-mediated vascular endothelial growth factor via
the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer.
16:3212016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Wang H, Ni Y, Yao Z, Ye L and Tian
J: DCT015, a new sorafenib derivate, inhibits tumor growth and
angiogenesis in gastric cancer models. Tumour Biol. 37:9221–9232.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubin LA, Galli F, Greene WC, Nelson DL
and Jay G: The molecular basis for the generation of the human
soluble interleukin 2 receptor. Cytokine. 2:330–336. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vuoristo MS, Laine S, Huhtala H, Parvinen
LM, Hahka-Kemppinen M, Korpela M, Kumpulainen E and
Kellokumpu-Lehtinen P: Serum adhesion molecules and interleukin-2
receptor as markers of tumour load and prognosis in advanced
cutaneous melanoma. Eur J Cancer. 37:1629–1634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An X, Li M, Li N, Liu B, Zhang H and Wang
J: Effect of heavy-ion beam irradiation on the level of serum
soluble interleukin-2 receptors in hamster cheek pouch carcinoma
model. Biomed Rep. 2:408–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KS, Park JM, Kong T, Kim C, Bae SH,
Kim HW and Moon J: Retinal angiogenesis effects of TGF-β1 and
paracrine factors secreted from human placental stem cells in
response to a pathological environment. Cell Transplant.
25:1145–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller-Pillasch F, Menke A, Yamaguchi H,
Elsasser HP, Bachem M, Adler G and Gress TM: TGFbeta and the
extracellular matrix in pancreatitis. Hepatogastroenterology.
46:2751–2756. 1999.PubMed/NCBI
|
|
23
|
Yang XJ, Chen GL, Yu SC, Xu C, Xin YH, Li
TT, Shi Y, Gu A, Duan JJ, Qian C, et al: TGF-β1 enhances
tumor-induced angiogenesis via JNK pathway and macrophage
infiltration in an improved zebrafish embryo/xenograft glioma
model. Int Immunopharmacol. 15:191–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari G, Pintucci G, Seghezzi G, Hyman
K, Galloway AC and Mignatti P: VEGF, a prosurvival factor, acts in
concert with TGF-beta1 to induce endothelial cell apoptosis. Proc
Natl Acad Sci USA. 103:17260–17265. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terme M, Pernot S, Marcheteau E, Sandoval
F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E
and Taieb J: VEGFA-VEGFR pathway blockade inhibits tumor-induced
regulatory T-cell proliferation in colorectal cancer. Cancer Res.
73:539–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamzah J, Jugold M, Kiessling F, Rigby P,
Manzur M, Marti HH, Rabie T, Kaden S, Gröne HJ, Hämmerling GJ, et
al: Vascular normalization in Rgs5-deficient tumours promotes
immune destruction. Nature. 453:410–414. 2008. View Article : Google Scholar : PubMed/NCBI
|