Introduction
Diabetic retinopathy (DR) and retinopathy of
prematurity (ROP) are types of retinal microangiopathy, which
involve blood-retina barrier (BRB) injury and the occurrence of
inflammation. In addition to the BRB injury and inflammatory
cytokine upregulation, retinal neovascularization (RNV) appears,
resulting in bleeding, seepage and hyperplasia, and causing severe
vision loss. According to previous reports, RNV-associated diseases
are one of the most serious irreversible diseases, which can cause
blindness, worldwide (1,2). Anti-vascular endothelial growth
factor (VEGF) drugs, including ranibizumab, are the most effective
therapeutic drugs at present. However, their clinical application
is restricted as patients require multiple injections, which may
increase the risk of infections and increases economic burden
(3). Therefore, details
investigations of the pathogenesis of RNV, and the identification
of more effective and economic therapeutic strategies are urgently
required to overcome these problems.
Previous studies have shown that chronic hypoxia of
the retina is a basic histopathological change in retinal
microangiopathies. VEGF, released by the hypoxic retina, is the key
trigger factor in neovascularization and inflammation. Calcineurin
is distributed on endothelial cells, which can be activated by
VEGF. The activation of calcineurin can further initiate the
dephosphorylation of NFATc1, which transfers into the nucleus and
upregulates the expression of certain inflammatory cytokines,
including interleukin (IL)-8, IL-2 and cyclooxygenase-2 (COX-2). As
the inflammatory cytokines are upregulated, the vascular walls are
altered. The typical histopathological changes include loss of
endothelial cells and thickening of the basilar membrane (4), which forms tight junctions. The tight
junctions between endothelial cells are important in the retinal
barrier, which are predominantly destroyed in retinal
microangiopathy. The destruction to tight junctions renders the
barriers of the retina, particularly the inner BRB, seriously
damaged.
FK506, an immunosuppressive drug, is used for as a
treatment following transplantation, for atopic dermatitis and
rheumatoid arthritis (5–7). FK506 binds to FK506-binding proteins
and forms complexes. These complexes then bind to calcineurin,
leading to inhibition of the dephosphorylation of NFATc1, which
suppresses the expression of inflammatory cytokines and T cell
activation (8). FK506 can
effectively downregulate the infiltration of inflammatory cells and
the expression of E-selectin, intercellular cell adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
in inflamed tissues in vivo (9,10).
FK506 has been shown to be closely associated with thrombotic
microangiopathy (11,12), however, the direct effects of FK506
on retinal microvascular endothelia cells (RMECs) remain to be
fully elucidated. In order to determine an effective treatment for
RNV diseases, the present study investigated whether FK506 can
suppress hypoxia-induced inflammation and protect tight junction
function via the calcineurin-nuclear factor of activated T-cells 1
(CaN-NFATc1) signaling pathway in RMECs.
Materials and methods
Mouse (m)RMEC culture
The mRMECs (cat. no. CD-1065) were purchased from
Cell Biologics, Inc. (Chicago, IL, USA). The mRMECs were cultured
in a 100 mm collagen-coated culture dish containing 10 ml
supplemented medium (RPMI-1640; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) with 20% fetal bovine serum (16000044;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
maintained at 37°C in a humidified atmosphere of 5% CO2
and 95% air. The culture medium was replaced every 2 days. The
cells used in the present study were between passages 3 and 4. When
the cells had grown to 70% confluence, they were cultured under
hypoxic conditions (93% N2, 5% CO2, 2%
O2) at 37°C for 24 h. Then cells were then washed twice
with phosphate-buffered saline (PBS) and then exposed to FK506
(F4679; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at different
concentrations (0.1, 1 and 10 µM) for 24 and 48 h, respectively.
FK506 was dissolved in dimethyl sulfoxide (<0.1%), which caused
no deleterious effect on the viability of the mRMECs in preliminary
experiments.
Measurement of trans-epithelial
electrical resistance (TEER)
The permeability of the mRMEC monolayers was
assessed via TEER measurement. The TEER across the endothelial cell
layer was calculated using a Millicell-ERS Voltohmmeter (EMD
Millipore, Bedford, MA, USA). Briefly, the mRMECs were seeded onto
a membrane at a density of 3×105 cells/cm2.
The TEER measurements were implemented following treatment of the
cells with FK506 at different concentrations (0.1, 1 and 10 µM).
The TEER value of the blank Transwell membrane was used as a blank
value, and was subtracted from the sample value. The TEER values
were calculated as Ω × cm2 and presented as the mean ±
standard error of mean of three independent experiments. Each cell
monolayer with a TEER value >100 Ω × cm2 was
considered to contain tight junctions.
Measurement of cell permeability using
fluorescein isothiocyanate (FITC)-dextran
The mRMECs were plated in 12 mm diameter Transwell
polyethylene terephthalate membrane inserts (0.4 µm pore size) at a
density of 2×105 per insert. The permeability was
measured using FITC-dextran, with 1 mg/ml FITC-dextran added to the
apical compartment 4 h prior to the assessment. The samples were
collected (25 µl) from the lower compartment for assessment, and
PBS was added to 250 µl. The fluorescence was measured on a
fluorescence luminometer at wavelengths of 492 nm (excitation) and
520 nm (emission).
Western blot analysis
The cells were lysed on ice for 20 min using RIPA
lysis buffer with protease inhibitor, and were centrifuged at
13,400 × g at 4°C for 10 min. The supernatant was collected and
total protein concentration was measured by BCA protein assay
(Beyotime Institute of Biotechnology, Shanghai, China). The
proteins were then boiled in sodium dodecyl sulfate (SDS) sample
buffer for 10 min and then equal amounts of lysate protein (10
µl/lane) were added to 10% SDS-polyacrylamide gel electrophoresis
gels. Following electrophoresis, the proteins were transferred onto
a polyvinylidene fluoride membrane and the membranes were blocked
in 5% skim milk in Tris-HCl buffer salt solution-Tween (TBST) for 2
h at room temperature. The membranes were then incubated with the
following primary antibodies overnight at 4°C: Anti-zonula
occludens-1 (ZO-1; 61-7300; 1:500; Thermo Fisher Scientific, Inc.),
anti-p-NFATc1 (SC32979; 1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-t-NFATc1 (SC13033; 1:500; Santa Cruz
Biotechnology, Inc.). β-actin (3700S; 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) was used as a loading control.
The membranes were then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G secondary
antibody (A21010; 1:5,000) and HRP-conjugated goat anti-rabbit IgG
secondary antibody (A21020; 1:5,000; both from Abbkine Scientific
Co., Ltd., Wuhan, China) for 2 h at room temperature. The
immunoreactive bands were visualized with enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Immunofluorescent microscopy
The mRMECs were plated on glass slides in a 12-well
plate at a density of 1×105 per well and pretreated with
hypoxia and FK506 (10 µM). Following washing with PBS, the cells
were fixed with 4% paraformaldehyde for 30 min at room temperature.
The cells were then incubated with polyclonal rabbit primary
antibodies against ZO-1 (1:500; Cell Signaling Technology, Inc.) in
5% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) at 4°C
overnight, followed by blocking with 5% BSA for 2 h at room
temperature. The next day, following washing in PBS, the slides
were incubated with Alexa Fluor 555 conjugated anti-rabbit IgG
secondary antibody (4413S; 1:1,000; Cell Signaling Technology,
Inc.) for 2 h at room temperature, and the nuclei were labeled with
4′, 6-diamidino-2-phenylindole for 10 min. Images were captured
under a confocal microscope (Zeiss 510; Carl Zeiss AG, Oberkochen,
Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA were extracted from the mRMECs using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
purity and concentration of RNA samples were confirmed by the ratio
of optical densities at 260 and 280 nm. Then, 1 µl RNA was
reverse-transcribed in a reaction mixture containing 1 U RNase
inhibitor, 500 ng random primers, 3 mM MgCl2, 0.5 mM
dNTP, 1X RT buffer and 10 U reverse transcriptase (Promega,
Madison, WI, USA). Ths synthesized cDNA was used as a template for
PCR reaction using GoTaq polymerase (Promega Corporation, Madison,
WI, USA). The RT-qPCR analysis was performed using SYBR-Green
qRT-PCR Master mix, according to the manufacturer's protocol
(Biotool, Houston, TX, USA). qRT-PCR was performed for 40 cycles of
denaturation at 94°C for 50 sec, annealing at 55°C for 50 sec and
extension at 72°C for 50 sec. The quantification cycle values were
normalized against β-actin and the 2−∆∆Cq method
(13) was used to calculate target
gene expression. The results determined as the relative value
compared with that of the control. The primers for target genes
were obtained from the NCBI GeneBank database (http://www.ncbi.nlm.nih.gov/genbank/).
The primers for COX-2, inducible nitric oxide synthase (iNOS),
monocyte chemotactic protein-1 (MCP-1) and β-actin were as follows:
COX-2 forward, 5′-CCAGATGATATCTTTGGGGAGAC-3′ and reverse,
5′-CTTGCATTGATGGTGGCTG-3′; iNOS forward,
5′-ACAACAGGAACCTACCAGCTCA-3′ and reverse,
5′-GATGTTGTAGCGCTGTGTGTCA-3′; MCP-1 forward,
5′-ACTGAAGCCAGCTCTCTCTTCCTC-3′ and reverse,
5′-TTCCTTCTTGGGGTCAGCACAGAC-3′; β-actin forward,
5′-GGCGGACTATGACTTAGTTG-3′ and reverse, 5′-AAACAACAATGTGCAATCAA-3′.
The samples were examined in triplicate and the experiment was
repeated twice.
Enzyme-linked immunosorbent assay
(ELISA)
The supernatants of the mRMEC culture media were
harvested 48 h following FK506 treatment and stored at −80°C until
assayed. The levels of IL-6, ICAM-1 and VCAM-1 in the supernatants
were measured using an ELISA kit. A 96-well plate containing 100 µl
of captured antibody per well was incubated overnight at room
temperature. Following incubation, the plate was blocked with 1%
BSA in PBS for 1 h at room temperature. The samples were added (100
µl) and incubated for 2 h at room temperature. Following three
washes with 0.05% Tween-20 in PBS, 100 µl of monoclonal antibodies
were added to each well for 2 h at room temperature.
Streptavidin-HRP was added to each well and incubated for 20 min at
room temperature. The color reagent A (H2O2)
and color reagent B (tetramethylbenzidine) substrate solution
reactions were terminated at 20 min with 2
NH2SO4. Each well was immediately read on a
microplate reader at 450 nm. The measurements were performed in
triplicate.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean of three independent experiments. Differences in mean
values among groups were subjected to one-way factorial analysis of
variance followed by the Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 20.0 software (IBM
SPSS, Armonk, NY, USA).
Results
FK506 treatment attenuates decreased
TEER values in hypoxia-induced mRMECs
At 24 and 48 h, the TEER values of the hypoxia
groups were markedly lower, compared with the TEER in the control
group. Compared with the hypoxia group, the TEER values of the
FK506 groups (1 and 10 µM) were significantly higher, with 10 µM
exhibiting a more marked effective, compared with 1 µM (Fig. 1).
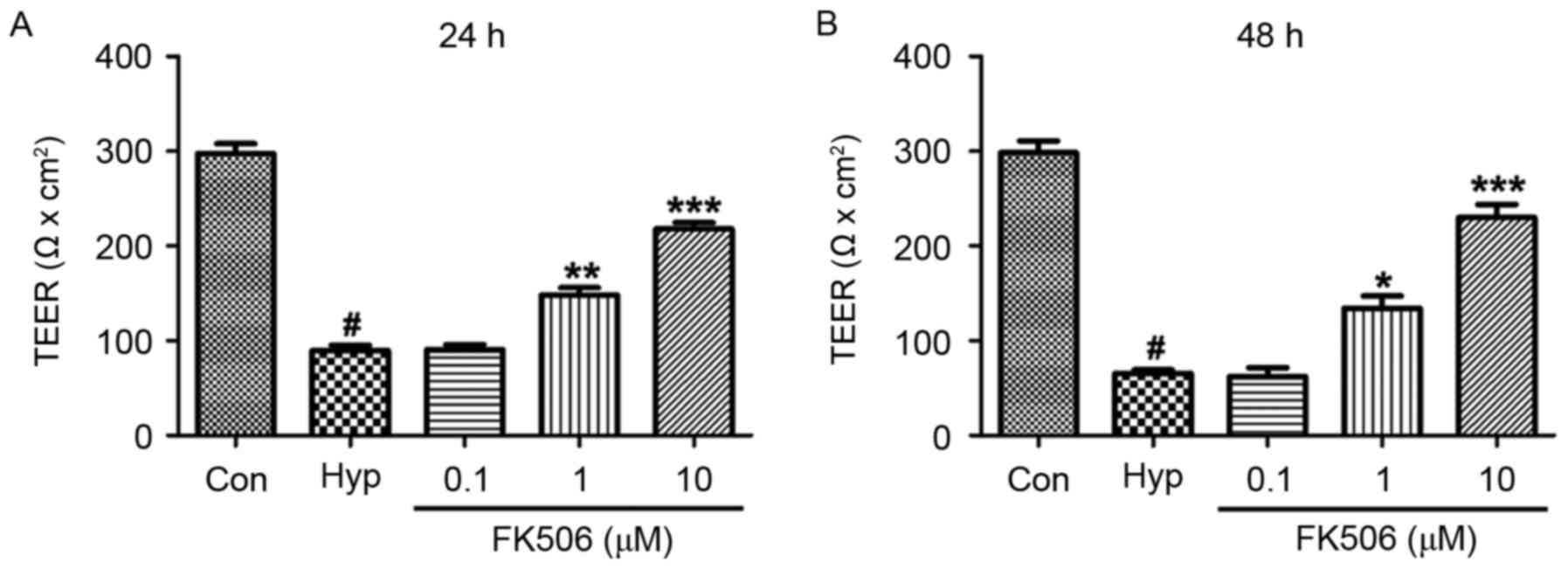 | Figure 1.Effect of FK506 on changes of TEER in
hypoxia-induced mRMECs. Following culture under hypoxic conditions
(93% N2, 5% CO2, 2% O2) at 37°C
for 24 h, the mRMECs were treated with FK506 at concentrations of
0.1, 1 and 10 µM for (A) 24 h and (B) 48 h. The results are
representative of three independent experiments and data are
expressed as the mean ± standard error of the mean.
#P<0.05, vs. Con; *P<0.05, **P<0.01 and
***P<0.001, vs. Hyp. TEER, trans-epithelial electrical
resistance; mRMECs, mouse retinal microvascular endothelial cells;
Con, control; Hyp, hypoxia. |
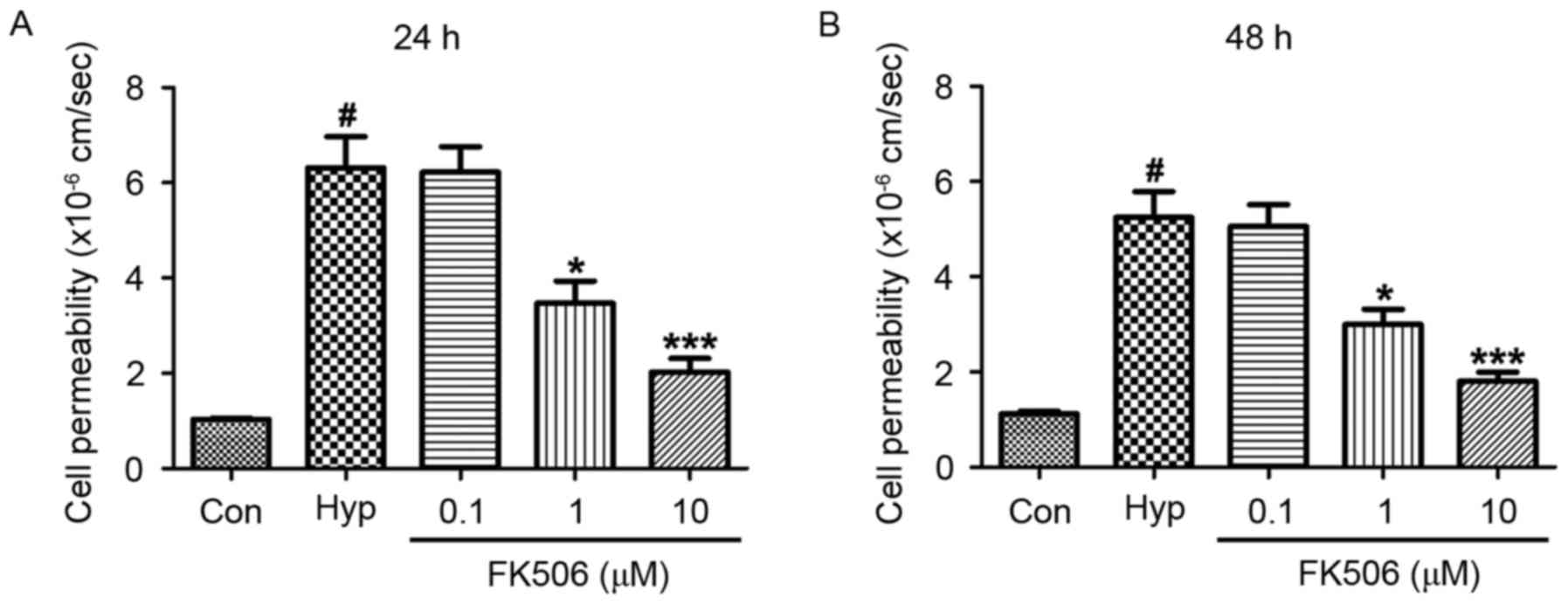
FK506 treatment inhibits increased
cell permeability in hypoxia-induced mRMECs
Compared with the control group, the cell
permeability of the hypoxia group was increased at 24 and 48 h.
However, compared with the hypoxia group, the permeability of cells
in the groups treated with FK506 at concentrations of 1 and 10 µM
were significantly decreased. The reduction in cell permeability in
the 10 µM group was more marked, compared with that in the 1 µM
group (Fig. 2).
FK506 treatment attenuates the
decreased protein expression of ZO-1 in hypoxia-induced mRMECs
ZO-1 is a protein associated with tight junctions,
which is involved in the epithelial function and barrier system.
The results of the western blot analysis showed that the expression
of ZO-1 was decreased in the mRMECs exposed to hypoxia. However,
the protein expression levels of ZO-1 in the 1 and 10 µM FK506
groups were increased significantly, with higher expression in the
10 µM FK506 group (Fig. 3A and
B).
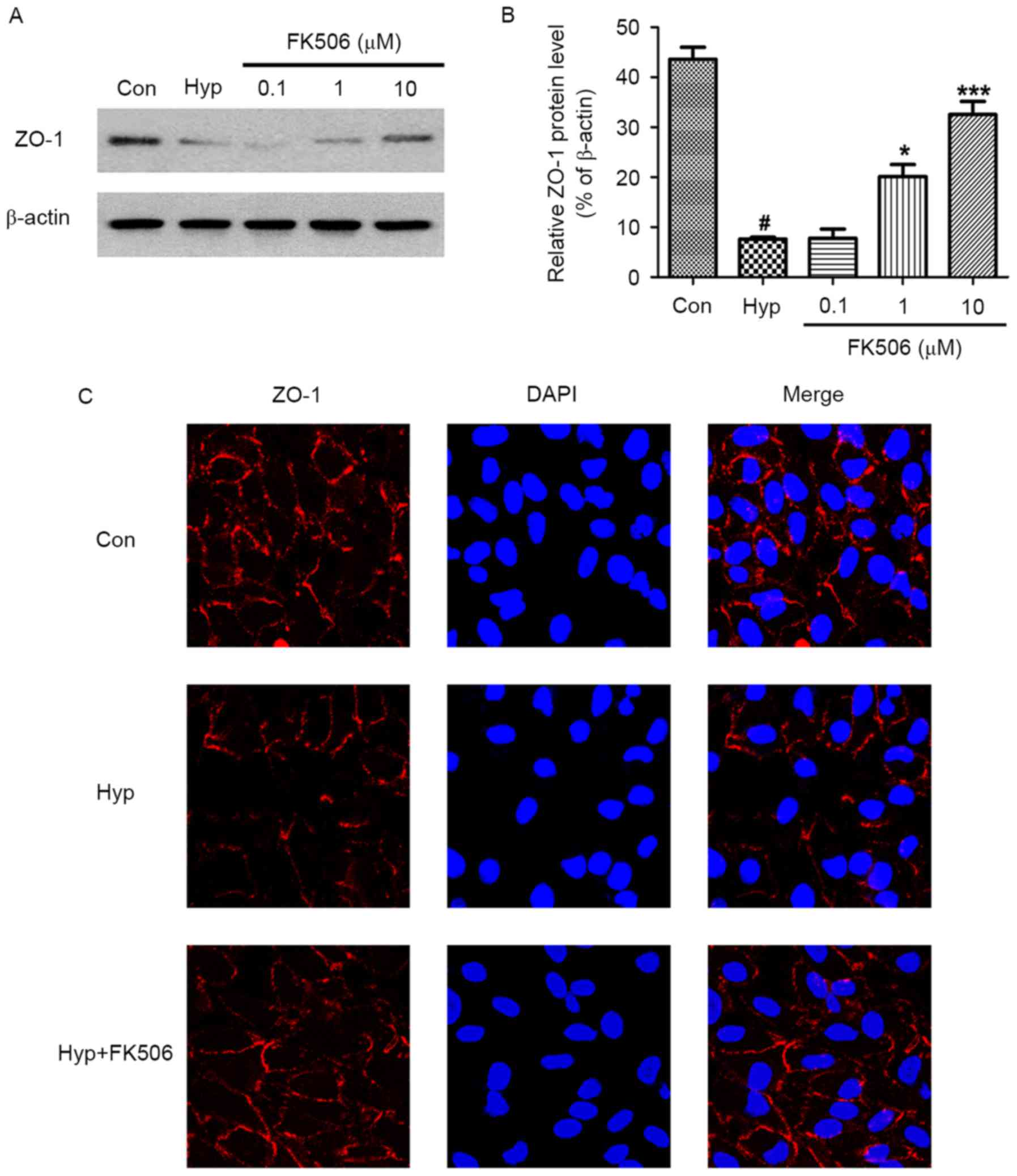 | Figure 3.Effect of FK506 on changes of the
protein expression of ZO-1 in hypoxia-induced mRMECs. Following
culture under hypoxia conditions (93% N2, 5%
CO2, 2% O2) at 37°C for 24 h, the mRMECs were
treated with FK506 at concentrations of 0.1, 1 and 10 µM for 24 h.
(A) Protein expression levels of ZO-1 in each group were determined
using western blot analysis. β-actin was used as an internal
standard. (B) Quantification of the expression levels of ZO-1
against β-actin in each group. (C) Protein expression of ZO-1 was
also detected using immunofluorescent microscopy (magnification,
×40). mRMECs in the FK506 group were treated at 10 µM, and all
groups were stained with ZO-1 (red) and DAPI (blue). The results
are representative of three independent experiments and data are
expressed as the mean ± standard error of the mean.
#P<0.05, vs. Con; *P<0.05 and ***P<0.001, vs.
Hyp. mRMECs, mouse retinal microvascular endothelial cells; ZO-1,
zonula occludens-1; DAPI, 4′, 6-diamidino-2-phenylindole; Con,
control; Hyp, hypoxia. |
The results of the immunofluorescence analysis were
similar. Compared with the control group, the expression of ZO-1 in
the hypoxia group was lower. Following treatment with FK506 at a
concentration of 10 µM, the protein expression of ZO-1 was elevated
(Fig. 3C). These results showed
that FK506 suppressed the damage to tight junctions in
hypoxia-induced mRMECs.
FK506 treatment inhibits the increased
mRNA expression of COX-2, iNOS, MCP-1 in hypoxia-induced
mRMECs
RT-qPCR analysis was performed to examine the mRNA
expression levels of COX-2, iNOS and MCP-1 in the control, hypoxia
and FK506 groups. As shown in Fig.
4A-C, hypoxia significantly increased the mRNA expression
levels of COX-2, iNOS and MCP-1. No significant differences were
found between the hypoxia group and the 0.1 µM FK506 group.
However, compared with the hypoxia group, the mRNA expression
levels were significantly decreased in the 1 and 10 µM FK506 group,
and was more marked in the 10 µM group.
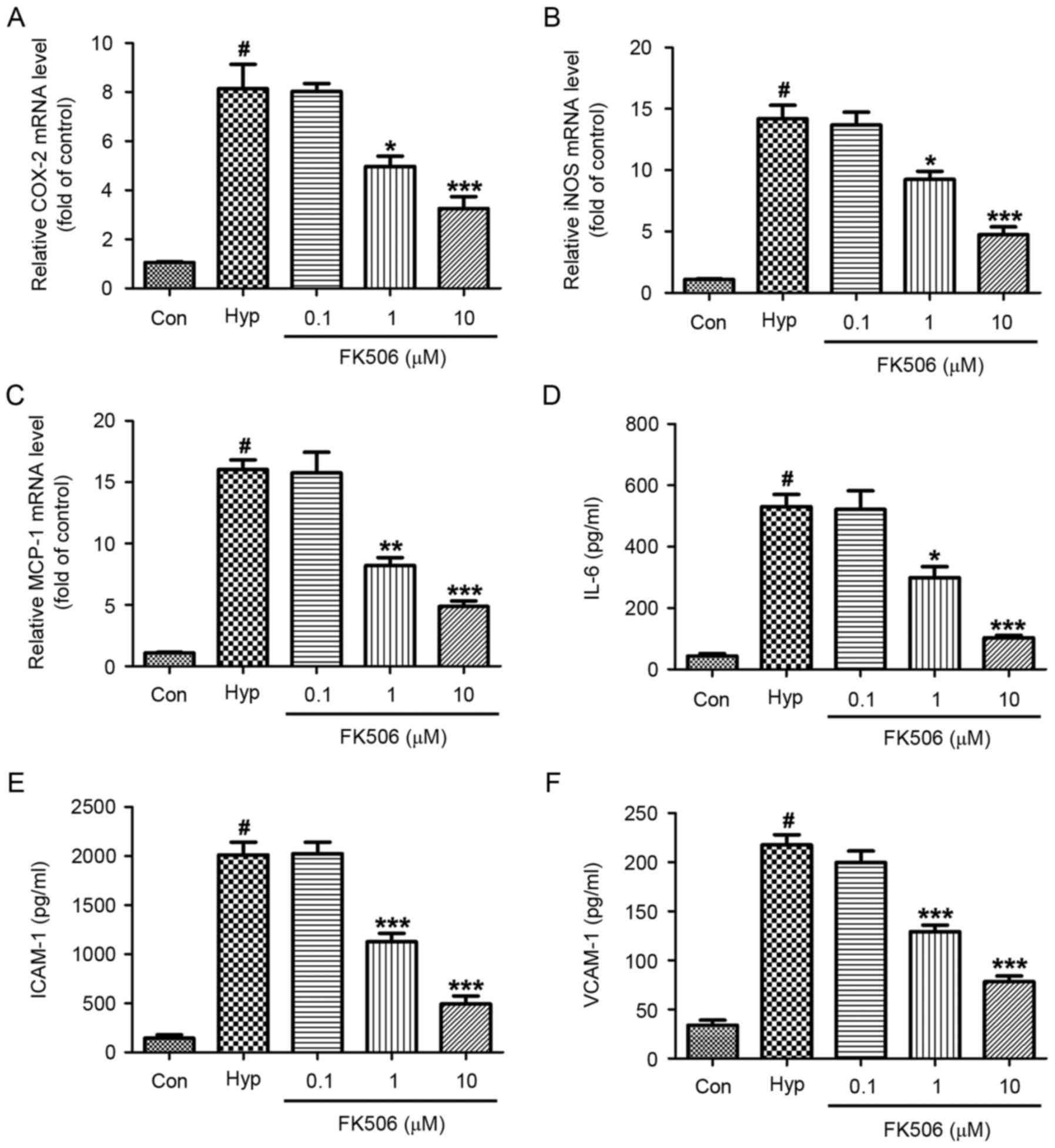 | Figure 4.Effect of FK506 on changes of the
expression levels of COX-2, iNOS, MCP-1, IL-6, ICAM-1 and VCAM-1 in
hypoxia-induced mRMECs. Following culture under hypoxic conditions
(93% N2, 5% CO2, 2% O2) at 37°C
for 24 h, the mRMECs were treated with FK506 at concentrations of
0.1, 1 and 10 µM for 24 h. The mRNA levels of (A) COX-2, (B) iNOS
and (C) MCP-1 were determined using reverse
transcription-quantitative polymerase chain reaction analysis
against the control group. The expression levels of (D) IL-6, (E)
ICAM-1 and (F) VCAM-1 in supernatants were determined using an
enzyme-linked immunosorbent assay. The results are representative
of three independent experiments and data are expressed as the mean
± standard error of the mean. #P<0.05, vs. Con;
*P<0.05, **P<0.01 and ***P<0.001, vs. Hyp. mRMECs, mouse
retinal microvascular endothelial cells; COX-2, cyclooxygenase-2;
iNOS, inducible nitric oxide synthase; MCP-1, monocyte chemotactic
protein-1; IL-6, interleukin-6; ICAM-1, intercellular cell adhesion
molecule-1; VCAM-1, vascular cell adhesion molecule-1; Con,
control; Hyp, hypoxia. |
FK506 treatment inhibits the increased
expression levels of IL-6, ICAM-1 and VCAM-1 in hypoxia-induced
mRMECs
ELISA was performed to determine the concentrations
of IL-6, ICAM-1 and VCAM-1 in the supernatants. It was found that
the concentrations of IL-6, ICAM-1 and VCAM-1 in the hypoxia groups
were significantly higher, compared with those in the control
groups. The concentrations were decreased following treatment with
FK506 (1 and 10 µM), which was more marked in the 10 µM group
(Fig. 4D-F).
FK506 treatment attenuates the
decreased expression of p-NFATc1 and the increased expression of
t-NFATc1 in hypoxia-induced mRMECs
Western blot analysis was performed to examine the
protein expression of NFATc1. As shown in Fig. 5, hypoxia significantly increased
the protein expression of total (t-)NFATc1, whereas the expression
of phosphorylated (p-)NFATc1 was downregulated, compared with the
control group. Following treatment with FK506 at concentrations of
1 and 10 µM, the expression of t-NFATc1 was significantly
decreased, whereas the expression of p-NFATc1 was significantly
increased, compared with that in the hypoxia group. These changes
in protein expression were more marked in the 10 µM group.
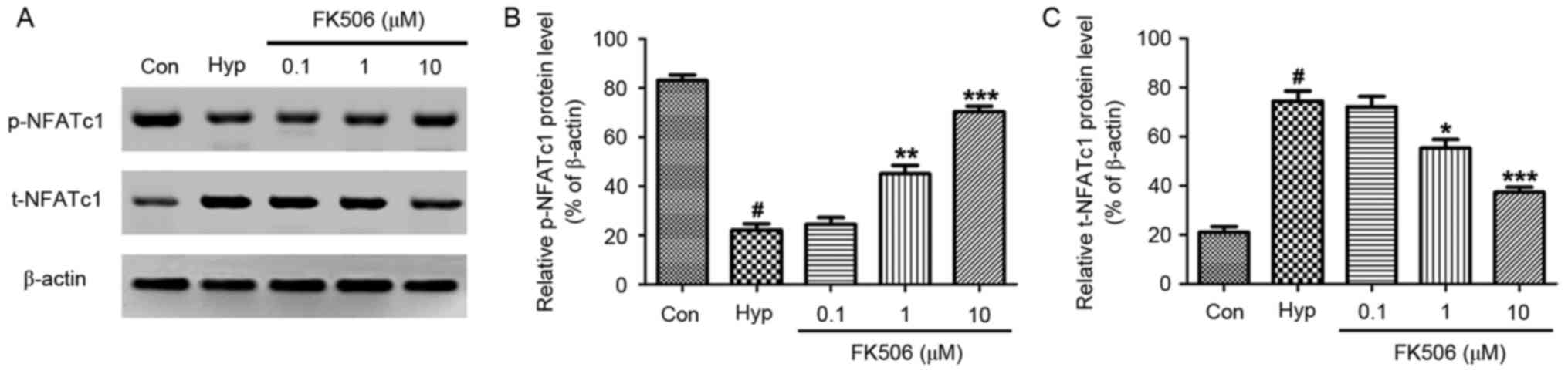 | Figure 5.Effect of FK506 on changes of the
expression of NFATc1 in hypoxia-induced mRMECs. Following culture
under hypoxic conditions (93% N2, 5% CO2, 2%
O2) at 37°C for 24 h, the mRMECs were treated with FK506
at concentrations of 0.1, 1 and 10 µM for 24 h. (A) Protein
expression levels of p-NFATc1 and t-NFATc1 in each group were
determined using western blot analysis. β-actin was used as
internal standard. (B) Quantification of the expression levels of
p-NFATc1 against β-actin in each group. (C) Quantification of the
expression levels of t-NFATc1 against β-actin in each group. The
results are representative of three independent experiments and
data are expressed as the mean ± standard error of the mean.
#P<0.05, vs. Con; *P<0.05, **P<0.01 and
***P<0.001, vs. Hyp. mRMECs, mouse retinal microvascular
endothelial cells; NFATc1, nuclear factor of activated T-cells 1;
p-NFATc1, phosphorylated-NFATc1; t-NFATc1, total NFATc1; Con,
control; Hyp, hypoxia. |
Discussion
RMECs, a specialized cell type of retinal vessels,
are involved in the physiological and pathological processes of
RNV. It has been reported that RMECs are sensitive to hypoxia and
that the tight junctions of RMECs are destroyed following exposure
to hypoxia. Subsequently, neovascularization appears and several
severe symptoms occur, including bleeding, exudation and edema.
Angiogenesis is recognized as a crucial factor in RNV diseases,
including DR and ROP, which lead to impaired vision. Anti-VEGF is
the most effective therapy at present. However, it has restrictions
in clinical applications (3).
Therefore, it is important and urgent to identify a more effective
and economic therapy.
FK506 is a potent immunosuppressive agent with
clinical applications, including transplantation, atopic dermatitis
and rheumatoid arthritis (5–7).
Investigations on inflammation have shown that FK506 can suppress T
cell activation and inflammatory cytokine expression, including
IL-2 (8). It can also effectively
downregulate the nuclear factor-κB pathway in various cell types
(14–17). Studies on microangiopathy have
suggested that FK506 can reduce the high glucose-induced
upregulated expression of VEGF in Müller cells (18). In addition, FK506 can inhibit
hyperplasia of the vascular wall in the injured arteria carotis
communis (19). Evidence has
indicated that FK506 can suppress the progress of microangiopathy
(11,12), however, the precise effects of
FK506 on RMECs remain to be fully elucidated. The results of the
present study revealed for the first time, to the best of our
knowledge, several important findings concerning the role of FK506
in suppressing hypoxia-induced inflammation and damage to tight
junctions in mRMECs.
Previous studies have shown that TEER values
decrease and permeability increases following endothelial barrier
injury by hypoxia. In the present study, it was found that TEER
values were significantly increased and permeability values were
significantly decreased following treatment with FK506 (1 and 10
µM), with the dose of 10 µM being more effective. Therefore, it was
confirmed that FK506 protected against damage to tight junctions in
hypoxia-induced mRMECs. In order to confirm this, western blot
analysis and immunofluorescent staining were performed to measure
the protein expression levels of ZO-1. ZO-1 is a type of
cytoskeletal protein, which is involved in maintaining the stable
structure of tight junctions. The present study found that the
protein expression of ZO-1 was lower following exposure to hypoxia,
however, it was expressed at high levels following treatment with
FK506 (1 and 10 µM). Although 1 and 10 µM FK506 were effective, the
protein level of ZO-1 was higher in the 10 µM group. Consequently,
these data suggested that FK506 inhibited hypoxia-induced tight
junction damage in the mRMECs.
Previous studies have shown that tight junctions in
inflammation are severely damaged. Corneal inflammation has been
shown to downregulate tight junction expression and endothelial
cell integrity in a mouse model (20). Commonly used anti-inflammatory
treatment for patients with inflammatory disease reduces
inflammation and maintains normal barrier integrity (21). To determine whether FK506 protects
tight junctions in hypoxia-induced mRMECs by suppressing
inflammation, the present study performed RT-qPCR analysis and
ELISA to analyze the changes in the expression of of COX-2, iNOS,
MCP-1, IL-6, ICAM-1 and VCAM-1, all of which are known to be key
factors responsible for hypoxia-induced retinal inflammation and
neovascularization. Following exposure to hypoxia, the
concentrations of these cytokines increased markedly. However,
treatment with FK506 (1 and 10 µM) significantly reduced the levels
of all these cytokines. These findings indicated that FK506
protected tight junctions by suppressing hypoxia-induced
inflammation in mRMECs.
NFATc1 proteins are calcineurin-dependent, rapidly
inducible transcription factors. Calcium mobilization and the
activation of calcineurin leads to NFATc1 dephosphorylation,
followed by its translocation into the nucleus where it acts as a
transcription factor (22). NFATc1
proteins are present predominantly in lymphoid cells and are
involved in different cellular functions, including the immune
response, cell proliferation, and development and differentiation,
in addition to pathological events, including cancer (23,24).
In previous years, several studies have shown that the CaN-NFATc1
signaling pathway not only regulates the growth of the cardiac
system (25) and peripheral
vasculature (26), but is also
involved in the pathological neovascularization of proliferative
vitreoretinopathy (27) and tumors
(28). In 2013, Bretz et al
demonstrated that VEGF induces NFATc1 to transfer into the nucleus
in RMECs. Inhibitors of NFATc1 and CaN inhibited RMEC proliferation
and vessel formation and, in a model of oxygen-induced retinopathy,
the area of retinal neovasculature reduced following intravitreal
injection of CaN-NFATc1 interaction inhibitor-6 and FK506 (27). Therefore, the CaN-NFATc1 signaling
pathway is crucial in RNV. Based on these findings, the present
study hypothesized that FK506 suppresses hypoxia-induced
inflammation and protects tight junction function in mRMECs via the
CaN-NFATc1 signaling pathway. The results of the present study
revealed that the levels of t-NFATc1 were increased in hypoxia,
whereas the levels of p-NFATc1 were decreased. Following treatment
with FK506, the levels of t-NFATc1 were decreased and levels of
p-NFATc1 were increased.
In conclusion, the results of the present study
showed that FK506 suppressed hypoxia-induced inflammation and
protected tight junction function via the CaN-NFATc1 signaling
pathway in mRMECs. FK506 may be a potential effective therapy for
hypoxia-induced microangiopathy.
Acknowledgements
This study was financially supported by the Chinese
government in the form of the National Natural Science Foundation
of China (grant no. 81670873).
References
|
1
|
Gliem M, Finger RP, Fimmers R, Brinkmann
CK, Holz FG and Issa P Charbel: Treatment of choroidal
neovascularization due to angioid streaks: A comprehensive review.
Retina. 33:1300–1314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Lookeren Campagne M, LeCouter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mintz-Hittner HA, Kennedy KA and Chuang
AZ: BEAT-ROP Cooperative Group: Efficacy of intravitreal
bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med.
364:603–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kollias AN and Ulbig MW: Diabetic
retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl
Int. 107:75–83. 2010.PubMed/NCBI
|
|
5
|
Todo S, Fung JJ, Starzl TE, Tzakis A,
Demetris AJ, Kormos R, Jain A, Alessiani M, Takaya S and Shapiro R:
Liver, kidney and thoracic organ transplantation under FK 506. Ann
Surg. 212:295–307. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baumgart DC, Pintoffl JP, Sturm A,
Wiedenmann B and Dignass AU: Tacrolimus is safe and effective in
patients with severe steroid-refractory or steroid-dependent
inflammatory bowel disease-a long-term follow-up. Am J
Gastroenterol. 101:1048–1056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thestrup-Pedersen K: Tacrolimus treatment
of atopic eczema/dermatitis syndrome. Curr Opin Allergy Clin
Immunol. 3:359–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weiwad M, Edlich F, Kilka S, Erdmann F,
Jarczowski F, Dorn M, Moutty MC and Fischer G: Comparative analysis
of calcineurin inhibition by complexes of immunosuppressive drugs
with human FK506 binding proteins. Biochemistry. 45:15776–15784.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haydon GH and Hayes PC: New
immunosuppressive treatment in transplantation medicine. Baillieres
Clin Gastroenterol. 8:455–464. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuzuki S, Toyama-Sorimachi N, Kitamura F,
Tobita Y and Miyasaka M: FK506 (tacrolimus) inhibits extravasation
of lymphoid cells by abrogating VLA-4/VCAM-1 mediated
transendothelial migration. FEBS Lett. 430:414–418. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kahan BD: Cyclosporine. N Engl J Med.
321:1725–1738. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobson P, Uberti J, Davis W and
Ratanatharathorn V: Tacrolimus: A new agent for the prevention of
graft-versus-host disease in hematopoietic stem cell
transplantation. Bone Marrow Transplant. 22:217–225. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeyda M, Geyeregger R, Poglitsch M,
Weichhart T, Zlabinger GJ, Koyasu S, Hörl WH, Stulnig TM,
Watschinger B and Saemann MD: Impairment of T cell interactions
with antigen-presenting cells by immunosuppressive drugs reveals
involvement of calcineurin and NF-kappaB in immunological synapse
formation. J Leukoc Biol. 81:319–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du S, Hiramatsu N, Hayakawa K, Kasai A,
Okamura M, Huang T, Yao J, Takeda M, Araki I, Sawada N, et al:
Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506)
via induction of the C/EBP family: Implication for unfolded protein
response. J Immunol. 182:7201–7211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino T, Nakase H, Honzawa Y, Matsumura
K, Yamamoto S, Takeda Y, Ueno S, Uza N, Masuda S, Inui K and Chiba
T: Immunosuppressive effects of tacrolimus on macrophages
ameliorate experimental colitis. Inflamm Bowel Dis. 16:2022–2033.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura-Yanagidaira T, Takahashi Y, Sano
K, Murata T and Hayashi T: Development of spontaneous neuropathy in
NF-κBp50-deficient mice by calcineurin-signal involving impaired
NF-κB activation. Mol Vis. 17:2157–2170. 2011.PubMed/NCBI
|
|
18
|
Xia W, Xia J, Zhang XF, Zhong L, Sun ZT
and Wang YM: Inhibition of FK506 on the expression of vascular
endothelial growth factor in retinal Müller cells cultured by high
concentration glucose. Chin J Exp Ophthalmol. 32:998–1003.
2014.
|
|
19
|
Xiao ZX, Xie YY and Zhen RD: Effect of
FK506 on injured vascular wall. Hainan Med J. 4:1–3. 2009.(In
Chinese).
|
|
20
|
Larkin DF: Longitudinal changes to tight
junction expression and endothelial cell integrity in a mouse model
of sterile corneal inflammation. Invest Ophthalmol Vis Sci.
57:34852016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cannon AR, Akhtar S, Hammer AM, Morris NL,
Javorski MJ, Li X, Kennedy RH, Gamelli RL and Choudhry MA: Effects
of mesalamine treatment on gut barrier integrity after burn injury.
J Burn Care Res. 37:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rao A, Luo C and Hogan PG: Transcription
factors of the NFAT family: Regulation and function. Annu Rev
Immunol. 15:707–747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hogan PG, Chen L, Nardone J and Rao A:
Transcriptional regulation by calcium, calcineurin, and NFAT. Genes
Dev. 17:2205–2232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mancini M and Toker A: NFAT proteins:
Emerging roles in cancer progression. Nat Rev Cancer. 9:810–820.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang CP, Neilson JR, Bayle JH, Gestwicki
JE, Kuo A, Stankunas K, Graef IA and Crabtree GR: A field of
myocardial-endocardial NFAT signaling underlies heart valve
morphogenesis. Cell. 118:649–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Graef IA, Chen F, Chen L, Kuo A and
Crabtree GR: Signals transduced by Ca(2+)/calcineurin and NFATc3/c4
pattern the developing vasculature. Cell. 105:863–875. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bretz CA, Savage S, Capozzi M and Penn JS:
The role of the NFAT signaling pathway in retinal
neovascularization. Invest Ophthalmol Vis Sci. 54:7020–7027. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minami T, Jiang S, Schadler K, Suehiro J,
Osawa T, Oike Y, Miura M, Naito M, Kodama T and Ryeom S: The
calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium
is critical for the establishment of lung metastases. Cell Rep.
4:709–723. 2013. View Article : Google Scholar : PubMed/NCBI
|