Introduction
Bone mesenchymal stromal cells (BMSCs) are plastic
adherent cells and are characterized by their multi-potency, and
the ability to give rise to colony forming unit-fibroblasts (CFU-F)
(1). These types of cells can
differentiate into osteogenic, adipogenic and chondrogenic
lineages. Therefore, BMSCs are commonly used to promote tissue
regeneration.
Bone regeneration is a complicated and highly
regulated process. Bones are regenerated in two distinct ways:
Intramembranous ossification and endochondral ossification
(2). Flat bones arise from
intramembranous ossification, and long bones arise from
endochondral ossification, and the mesenchyme condensation marks
the beginning of both bone regeneration processes (3). In intramembranous ossification, bones
are directly formed by osteoblastic differentiation of BMSCs,
whereas endochondral ossification involves the cooperation of
multiple cell types, during which chondrocytes mediate the growth
and formation of the skeleton (4).
In endochondral ossification, chondrocytes in the cartilage stop
proliferating, undergo hypertrophy, and secrete type X collagen
(5). Hypertrophic chondrocytes
guide vessel penetration, osteoblastic differentiation of BMSCs and
osteoblast migration. Finally, hypertrophic chondrocytes are
programmed to undergo apoptosis, and blood vessels and osteoblasts
infiltrate the cartilage matrix and subsequently achieve bone
growth and regeneration. Therefore, stimulation of vessel formation
and osteoblastic differentiation greatly promote bone regeneration
(6).
Bone tissue engineering is a promising therapy to
increase vessel formation and bone regeneration (6). An active blood vessel network is a
precondition for implants to integrate with the local tissue
(7). Endothelial progenitor cells
(EPCs) are hematologic precursor cells, defined by Asahara et
al (8), that are known for
their pro-angiogenic ability (9).
The high mobility of EPCs enables the cells to migrate to trauma
sites and stimulate neovascularization (10). Therefore, EPCs are often attached
to implants to increase vessel penetration. However, the effects of
EPCs remain controversial. Duttenhoefer et al (11) identified that EPCs may negatively
regulate osteoblastic differentiation of BMSCs in vitro. In
contrast, Goerke et al (12) have argued that EPCs support bone
regeneration by stimulating vessel formation. Therefore,
understanding the communication between EPCs and BMSCs will aid the
development of future tissue engineering treatments.
Extracellular vesicles (EVs) are a group of vesicles
that include apoptotic bodies, exosomes and microvesicles (13), which are released by almost all
cells in the body, including reticulocytes (14), dendritic cells (15), B cells (16), tumor cells (17), mast cells (18), T cells (19), epithelial cells (20) and endothelial cells (21). EVs are widely distributed
throughout the body (22) and
serve crucial roles in cell-to-cell communication. These vesicles
can be internalized by the recipient cells through endocytosis
(23), and then release functional
proteins, DNA and RNA (24). Via
this method, EVs can effectively transport and deliver content
between cells and regulate homeostasis.
In the present study, the role of EPC-derived EVs in
the regulation of osteoblastic differentiation and proliferation of
BMSCs was examined. The results demonstrated that EPC-derived EVs
can enter BMSCs through endocytosis and release their cargo in the
Golgi apparatus, thereby modulating differentiation and
proliferation of BMSCs in vitro.
Materials and methods
Cell culture
Mouse BMSCs were isolated from 7–8-week-old male
C57BL/6 mice (n=3, weight: 20 g, 12-h light/dark cycle, free access
to food and water) supplied by the Shanghai Jiaotong University
Affiliated Sixth People's Hospital and housed at 22°C in 50%
humidity according to established protocols. The use of all samples
was approved by and was conducted in accordance with the Ethical
Committee of Shanghai Jiaotong University Affiliated Sixth People's
Hospital (Shanghai, China). Briefly, BMSCs were collected according
to current protocols (25). Cells
from passages 3–5 were used in the experiments. BMSCs were cultured
with Dulbecco's modified Eagle's medium (DMEM; cat no. 12571071)
and 10% foetal bovine serum (cat no. 10099141) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified environment with 5% CO2.
EPCs isolated from the bone marrow of 7–8-week-old
C57BL/6 mice were obtained from BioChain (cat no. 7030031). These
cells express various endothelial markers, including CD31, CD105,
vascular endothelial growth factor receptor 1 and neuropilin-1, and
have the spindle morphology common to EPCs. These cells are
considered to be late-stage EPCs due to the fact that they are
negative for CD133, an early stage endothelial progenitor marker.
All EPCs were cultured with DMEM and 10% foetal bovine serum at
37°C in a humidified environment with 5% CO2. The
conditioned medium (EPC-CM) was collected following incubation for
24 h.
For the CFU-F assay, BMSCs were continuously treated
with EPC-CM, EPC-derived extracellular vesicles (5 µg/ml in all
experiments, EPC-EV), EV-depleted EPC-CM (noEV) and BMSC culture
medium (BMSC-group).
For the osteogenic differentiation assay, the
osteogenic medium (OM) contained 0.05 mM ascorbate-2 phosphate,
10−8 dexamethasone, and 10 mM β-glycerophosphate. BMSCs
were divided into the following 4 groups: EPC-CM + OM, EPC-EV + OM
group, noEV + OM and the OM group.
Extracellular vesicle isolation
EVs were isolated from the supernatant of EPC-CM
following culturing for 24 h according to current
ultracentrifugation protocols (26). Briefly, the EPC culture medium was
collected, centrifuged at 500 × g for 30 min at room temperature to
remove dead cells and then at 16,500 × g for 20 min at 4°C; this
was followed by filtration through a 0.22 µm filter to eliminate
cell debris. Then, EVs underwent ultracentrifugation (Beckman Ti70
rotor; Beckman Coulter, Inc., Brea, CA, USA) at 120,000 × g for 2 h
at 4°C. The protein content of EVs was measured using a BCA Protein
Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Electron microscopy
Collected EVs were fixed in 4% paraformaldehyde
(PFA) in PBS for 30 min at room temperature. Fixed EVs were placed
onto a formvar carbon-coated grid and dried at room temperature for
20 min. Subsequent to being washed with PBS, the EVs were fixed in
1% glutaraldehyde for 5 min, washed with water and stained with
saturated aqueous uranyl oxalate for 5 min at room temperature. EVs
were then embedded in 0.4% uranyl acetate and 1.8% methylcellulose
and incubated on ice for 10 min. The excess liquid was then
removed. The grid was dried at room temperature for 10 min and
viewed at a magnification of ×20,000 using an electron microscope
(Philips CM 120; Medical Systems B.V., Eindhoven, The
Netherlands).
Confocal microscopy
Harvested EVs were labelled with PKH67 Green
Fluorescent Cell Linker (cat no. PKH67GL-1KT; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) according to the manufacturer's protocols
and were then added to BMSCs in the culture medium. A total of
1×105 BMSCs were seeded in each well of a 6-well plate.
Subsequently, BMSCs were treated with EPC-derived EVs (5 µg/ml)
when the cells reached 70–80% cell confluency. Following 4 h
culture, the cells were washed three times with PBS and then fixed
with 4% PFA. The endoplasmic reticulum (ER-Tracker Red; cat no.
E34250), Golgi apparatus (Golgi-RFP; cat no. C10593) and lysosomes
(Lyso-Tracker Red; cat no. L12492) (all from Life Technologies;
Thermo Fisher Scientific, Inc.) were stained according to the
manufacturer's protocols. The cell nuclei were stained with DAPI
according to manufacturer's protocol (cat no. BD5010; Bioworld
Technology, Inc., St. Louis Park, MN, USA).
CFU-F assay
BMSCs were diluted in DMEM with 10% FBS and seeded
into a 6-well plate at 100 cells/well and treated with EPC-CM
(EPC-CM group) and BMSC culture medium (BMSC). Following 14 days of
culture at 37°C in a humidified environment with 5% CO2,
the cells were washed three times with 1X PBS and fixed by addition
of ice-cold 100% ethanol. The cells were stained with 0.1% crystal
violet solution for 10 min at room temperature. Images were
captured by light microscopy.
Alizarin Red staining and MTT
Cells were fixed in 70% ethanol for 30 min and
rinsed with double-distilled H2O and then stained with
40 mM Alizarin Red S (cat no. 130223; Sigma-Aldrich; Merck KGaA),
pH 4.0, for 15 min with gentle agitation. Cells were rinsed 3 times
with double-distilled H2O and then rinsed for 15 min
with 1X PBS with gentle agitation. Images were captured using a
light microscope. The MTT (Molecular Probes Life Technologies;
Thermo Fisher Scientific, Inc.) assay was performed according to
the manufacturer's protocols, and the absorbance was measured at
450 nm with a microplate reader (MK3; Thermo Fisher Scientific,
Inc.).
Western blotting
The cells were washed three times with PBS and lysed
in ice-cold lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1% (w/v)
Nonidet P-40, 0.1% (w/v) SDS, and 1% (w/v) sodium deoxycholate]
supplemented with phenylmethylsulfonyl fluoride (Shen Neng Bo Cai
Corporation, Shanghai, China). The lysates were incubated on ice
for 30 min and then centrifuged at 9,000 × g for 10 min at 4°C to
precipitate the debris. The protein concentrations were determined
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Then, 30 µg of the protein lysates were separated using 10 and 18%
(w/v) SDS-polyacrylamide gel electrophoresis (PAGE) and
electroblotted onto PVDF membranes (Roche Diagnostics, Basel,
Switzerland). Membranes were blocked with 5% non-fat dry milk in
Tris-buffer saline with Tween-20 (TBST) for 2 h and probed with
primary antibodies diluted in TBST containing 5% milk at 4°C
overnight. Primary antibodies used included rabbit anti-mouse
alkaline phosphatase (ALP) antibodies (cat no. ab108337; 1:1,000),
rabbit anti-mouse osteocalcin (OCN) antibodies (cat no. ab93876;
1:1,000), rabbit anti-mouse osteopontin (OPN) antibodies (cat no.
ab8448; 1:500), rabbit anti-mouse runt-related transcription
factor-2 (RUNX-2) antibodies (cat no. ab23981; 1:1,000) and rabbit
anti-mouse GAPDH (cat no. ab9485; 1:1,000) (all from Abcam,
Cambridge, MA, USA). Membranes were washed and incubated with
horseradish peroxidase conjugated goat anti-rabbit secondary
antibody (cat no. A0208; 1:1,000; Beyotime Institute of
Biotechnology, Haimen, China) for 1 h at room temperature. Targeted
proteins were visualized using an enhanced chemiluminescence (ECL)
detection system (ChemiDoc™ XRS+ imaging system; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Osteogenic gene expression in BMSCs at day 14 was
measured using RT-qPCR for the following marker genes: ALP, RUNX2,
OPN and OCN in BMSCs. Total RNA was extracted using the TRIzol
reagent (Thermo Fisher Scientific, Inc.) and qualified by
absorbance at 260 nm. cDNA was synthesized from 1 µg of RNA by
Takara RNA PCR kit (Takara Bio, Inc., Otsu, Japan) following the
manufacturer's protocols. Real-time PCR was performed using a SYBR
Green Master Mix (Takara, Bio, Inc.) on ABI StepOnePlus system
(Applied Biosystems; Thermo Fisher Scientific, Inc.): SYBR Premix
Ex Taq, 12.5 µl; PCR forward primer, 1 µl; PCR reverse primer 1 µl;
diethylpyrocarbonate water, 8.5 µl; DNA, 2 µl. The cycling
conditions were: 95°C for 30 sec (1 cycle); 95°C for 5 sec, then
60°C for 30 sec (40 cycles). Primer sequences used were: ALP
forward, GGC AGC TTG ACC TCC TCG GAA GAC A and reverse, AGC ATG GGG
GCC AGA CCA AAG ATA G; RUN X2 forward, CCC CTC CTA CCT GAG CCA GAT
GAC G and reverse, AAG GGC CCA GTT CTG AAG CAC CTG A; OPN forward,
ACA GCA TCG TCG GGA CCA GAC TCG T and reverse, GGT AGT GAG TTT TCC
TTG GTC GGC G; OCN forward, GCC CTC ACA CTC CTC GCC CTA TT and
reverse, GGG TCT CTT CAC TAC CTC GCT GCC; β-actin forward, CGG GAA
ATC GTG CGT GAC AT and reverse, GGA CTC GTC ATA CTC CTG CTT GC. The
relative expression level of genes was normalized to the value of
GAPDH by the 2−ΔΔCq method (26), allowing the calculation of
differences in gene expression using the ABI software.
Statistical analysis
CFU-F analysis, Alizarin staining, MTT and RT-qPCR
analyses were repeated three times, and data are presented as the
mean ± standard deviation. Differences among the results were
assessed using Bonferroni's multiple comparison tests, and
statistical significance was analysed using SPSS, version 20.0
software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
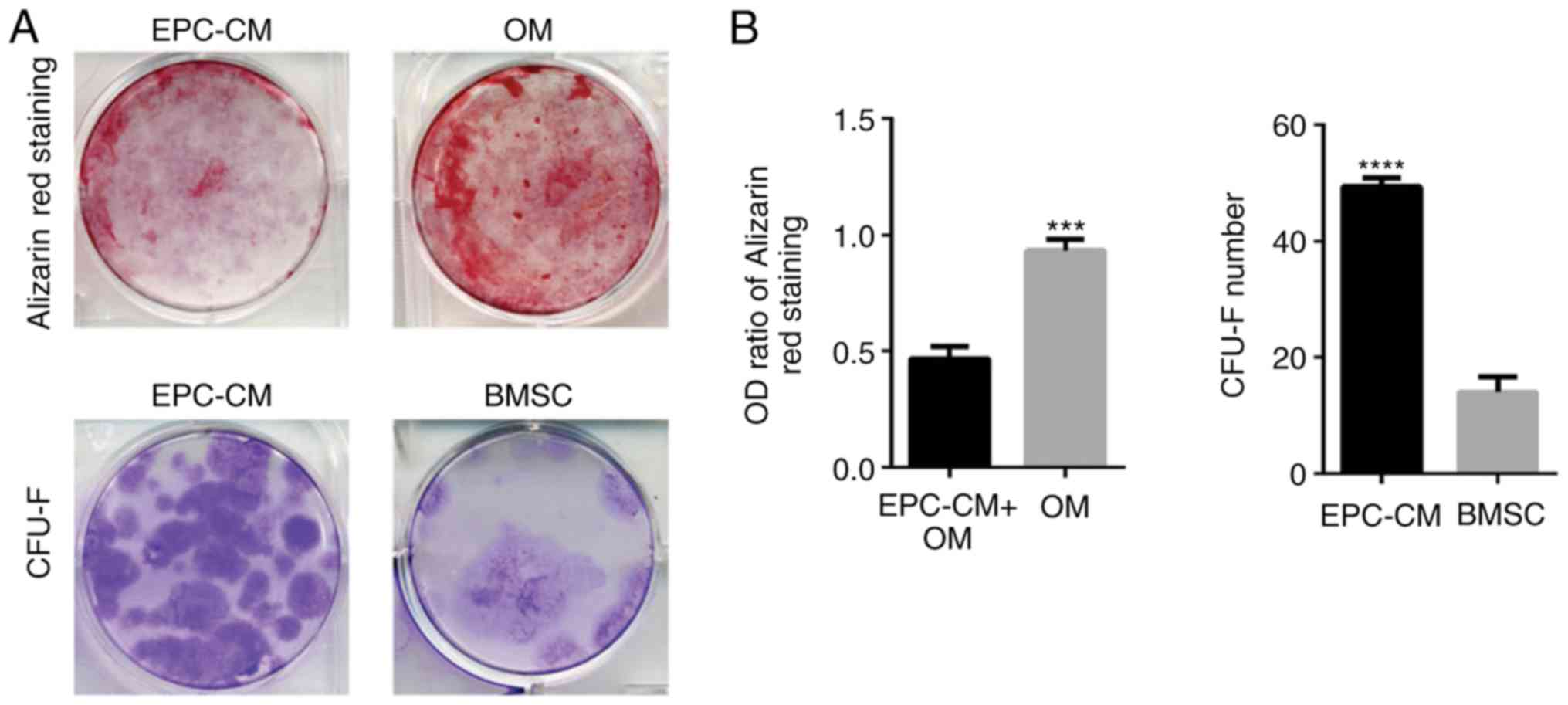
EPC-CM inhibits osteogenic
differentiation and promotes proliferation of BMSCs
To examine the potential influence of EPC-derived
EVs on BMSCs, whether EPC-CM could regulate the osteoblastic
differentiation and proliferation of BMSCs was measured. The
results indicated that the OM group had an increased number of
calcium deposits compared with that of the EPCs-CM + OM group
(Fig. 1A). The OD ratio of both
groups at day 14 also confirmed the results (Fig. 1B; P=0.003). Furthermore, it was
identified that the BMSCs in the EPCs-CM group formed an increased
number of CFU-Fs compared with the BMSCs in culture medium
(Fig. 1; P=0.0038). Altogether,
these results demonstrate that EPC-CM inhibits osteoblastic
differentiation and stimulates cell proliferation of BMSCs. These
results confirm the results of an earlier report (11).
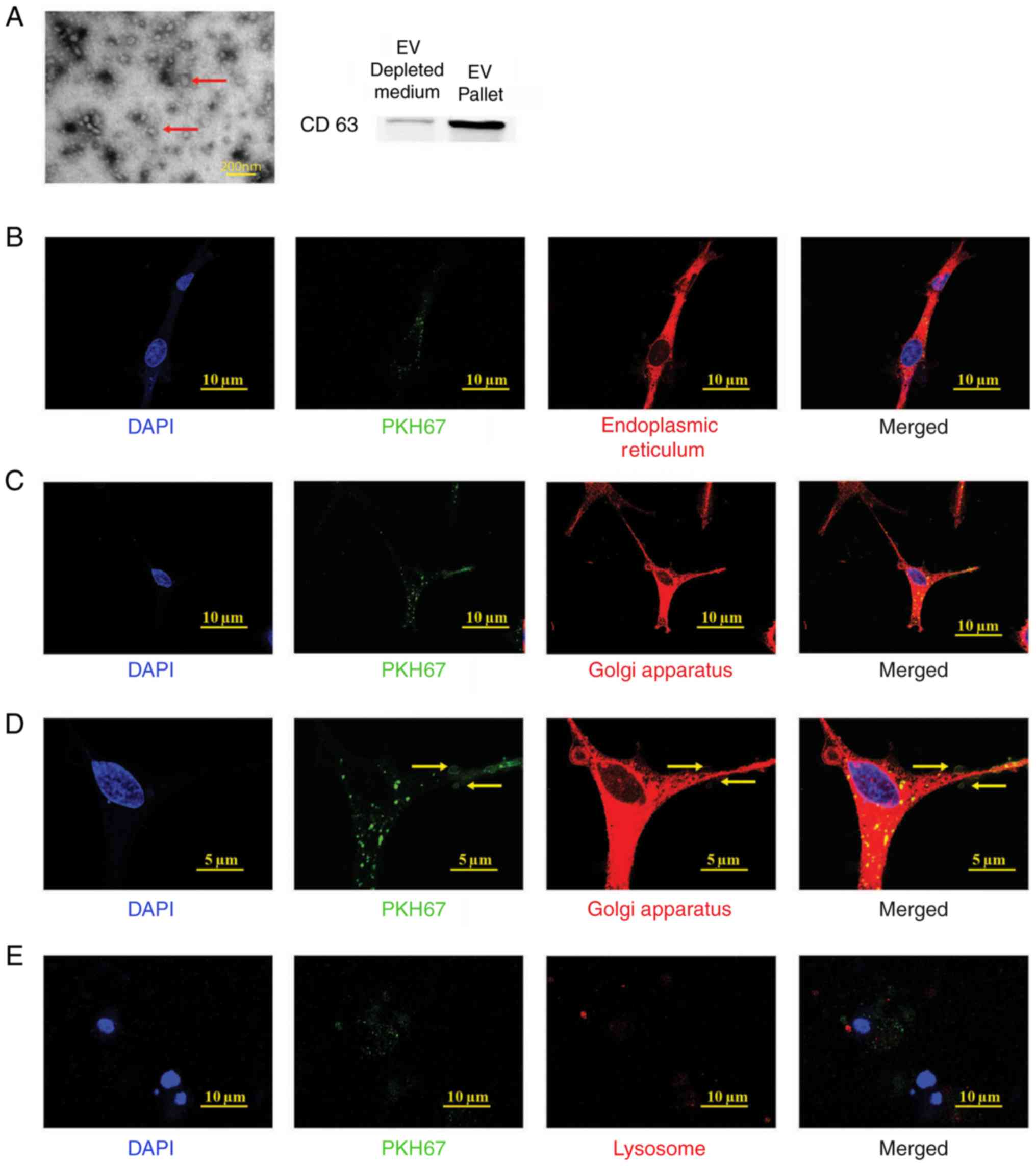
EPC-derived extracellular vesicle
isolation
Prior to examining the function of EPC-derived
extracellular vesicles (EPC-EVs), it was determined whether EPC-EVs
are isolated and can enter BMSCs. The EPC-EVs were isolated
according to a commonly used ultracentrifugation protocol and
analysed using electron microscopy (Fig. 2A, red arrows) (27). In addition, CD63, a common marker
of EVs, was used in western blot analysis to further confirm that
EVs are purified from EPC-CM (Fig.
2A) (28). Subsequently, it
was examined whether the EPC-EVs were absorbed by the BMSCs.
Therefore, the collected EVs were labelled with PKH67 (green), a
stable green fluorescent cell membrane linker. Subsequently, the
PKH67-stained vesicles were added to the BMSC culture medium.
Following a 4 h incubation, the cells were fixed and labelled with
diverse organelle-specific dyes (red) and were imaged and analysed
with confocal microscopy. As presented in Fig. 2B, the PKH67-labelled EVs (green
dots) were absorbed by the BMSCs and presented in the endoplasmic
reticulum (ER-Tracker Red), Golgi apparatus (Golgi-RFP) and
lysosomes (Lyso-Tracker Red). These results indicated that EPC-EVs
fused with the cells and were degraded by the Golgi apparatus,
releasing their cargo along the way. However, it was not possible
to demonstrate an association between EPC-EVs and lysosomes, which
have been suggested to digest EVs (29).
EPC-derived extracellular vesicles
inhibit osteogenic differentiation of BMSCs
Because EPC-EVs are absorbed by BMSCs, the function
of EPC-EVs was investigated using osteoblastic differentiation
assays. Alizarin Red staining demonstrated that there was a marked
decrease in calcium deposits in the EPC-CM + OM group and EPC-EV +
OM group compared with the noEV + OM group and OM group on days 7
and 14 (Fig. 3A). Western blot
analyses further confirmed that osteoblastic differentiated-related
proteins were downregulated in the EPC-CM + OM group and EPC-EV +
OM group (Fig. 3B). Accordingly,
the expression of osteoblastic markers and osteogenic genes,
including ALP, RUNX2, OPN and OCN, were additionally decreased in
the EPC-CM + OM and EPC-EV + OM groups. Compared with the EV-group,
the expression levels of ALP, OCN, OPN and RUNX2 were decreased by
2.4-, 2.0-, 3.1- and 1.6-fold, respectively, in the OM group
(Fig. 3C). Together, these results
suggested that EPC-EVs inhibited osteoblastic differentiation,
presumably by modulating the expression of osteogenic genes.
However, the expression of osteogenic genes in the noEV + OM group
remained reduced compared with that of the OM group, thus
indicating that other factors in the EPC-CM may also serve a role
in regulating the osteoblastic differentiation of BMSCs.
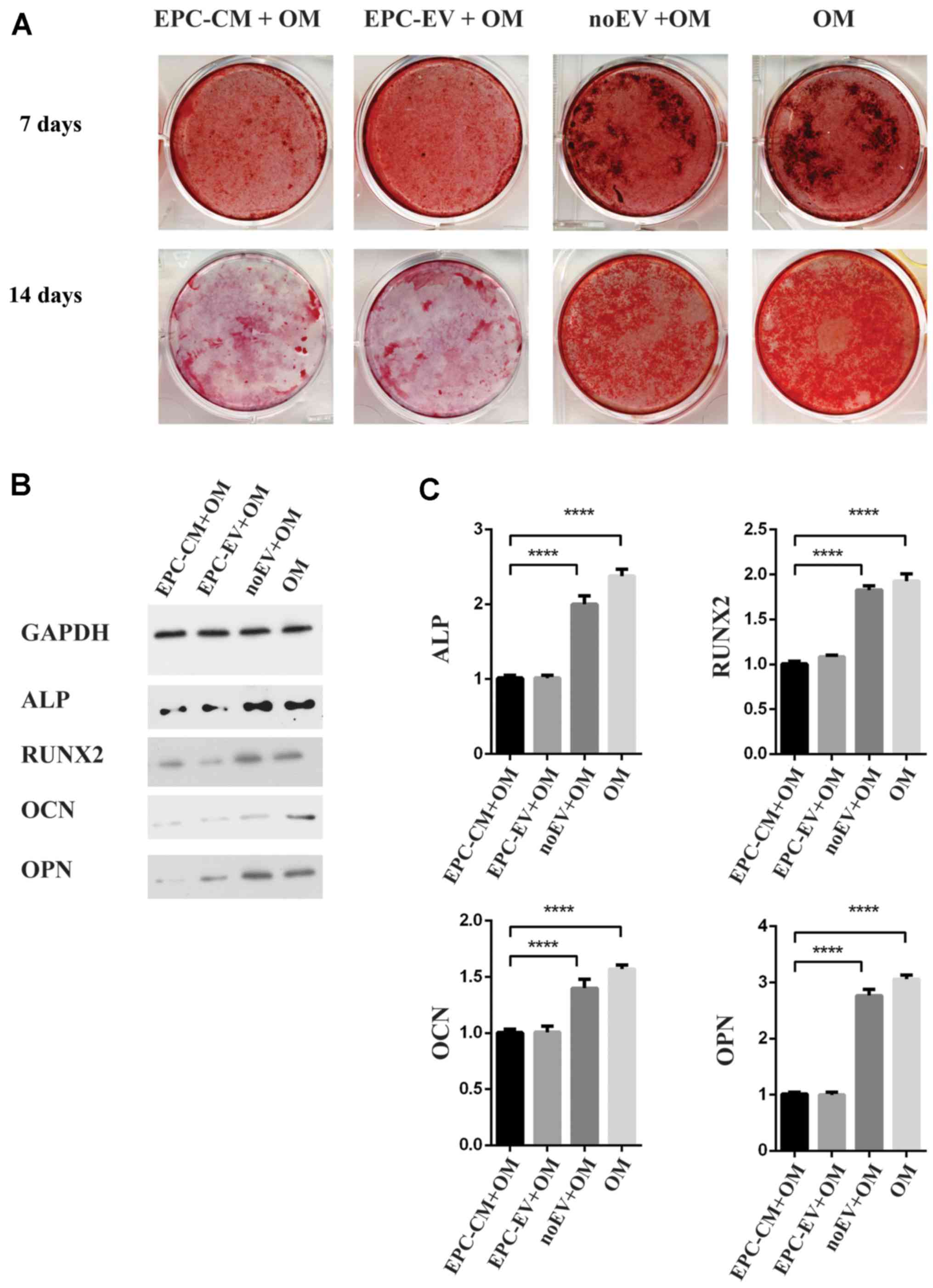 | Figure 3.(A) The macroscopic Alizarin Red
staining for the OM-group, the EPC-CM + OM group, EPC-EV + OM
group, noEV + OM group and OM group at day 1 and day 14. (B)
Western blot analysis of ALP, OCN, OPN and RUNX2 at day 14. (C) The
expression of ALP, OCN, OPN and RUNX2 increased by 1.9-, 3.8-, 3.2-
and 2.4-fold, respectively, in the OM group vs. the EPC-EV + OM
group; ****P<0.0001. OM, osteogenic medium; ALP, alkaline
phosphatase; OCN, osteocalcin; OPN, osteopontin; RUNX2,
runt-related transcription factor-2; EPC, endothelial progenitor
cell; CM, conditioned medium; EV, extracellular vesicle; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
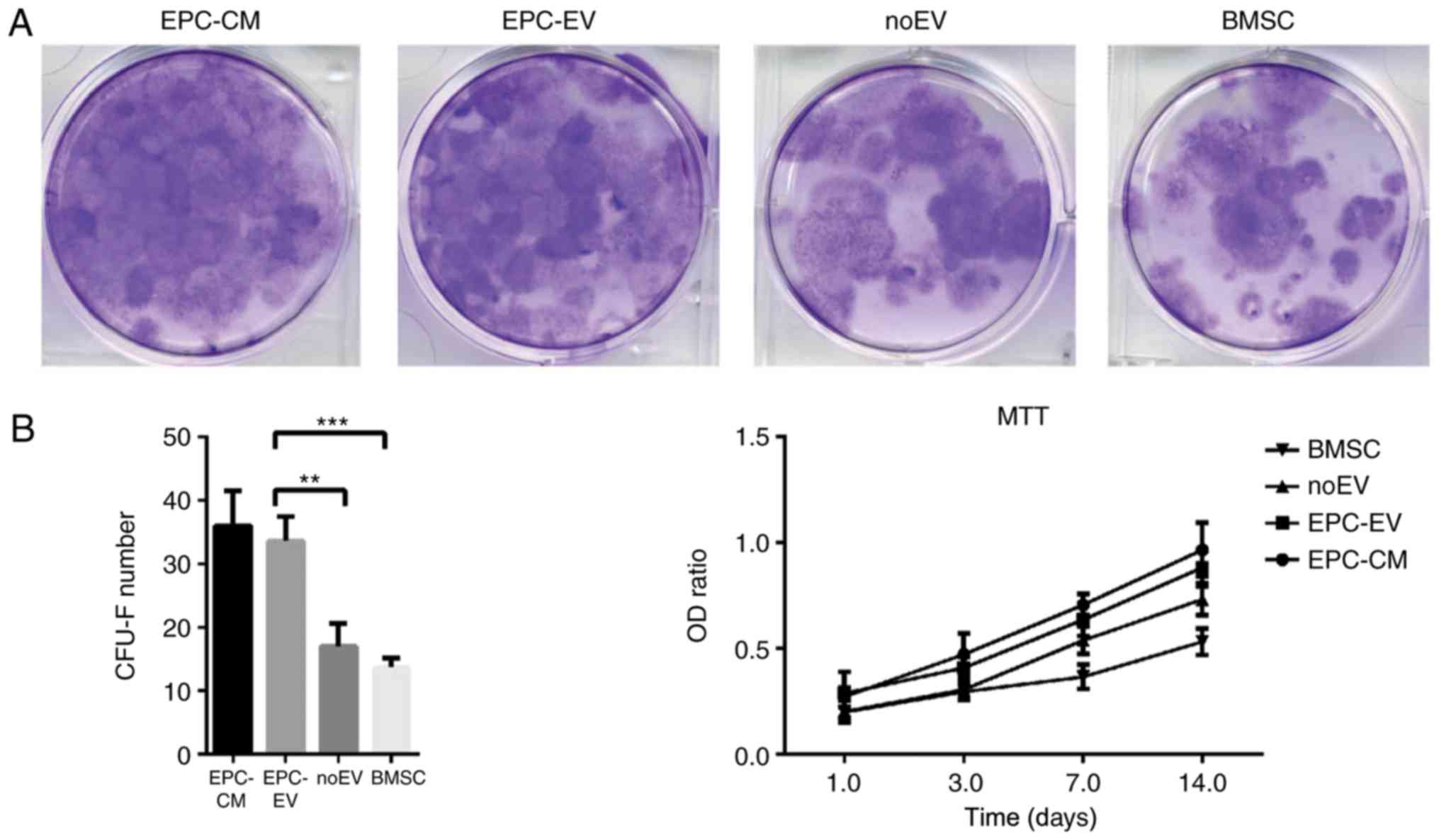
EPC-derived extracellular vesicles
regulate colony formation and proliferation of BMSCs
Given that EPC-EVs negatively regulate osteoblastic
differentiation of BMSCs, it was determined whether EPC-EVs may
additionally serve a role in regulating BMSCs CFU-F. Therefore, a
CFU-F assay was performed and cell proliferation was analysed using
MTT assays. Following 14 days of culture, CFU-Fs were increased in
the EPC-CM and EPC-EV groups compared with the noEV and OM groups.
Similar results were identified in the MTT assays (Fig.4). Altogether, these results
suggested that EPC-derived EVs have a positive influence on the
proliferation of BMSCs.
Discussion
EPCs have been demonstrated to induce vessel
formation in vivo and in vitro and are commonly used
in tissue engineering to promote bone regeneration (30). EPCs exhibit good vessel formation
when coated with matrigel (31)
and attenuate limb ischaemic mouse models (32). However, EPCs have a controversial
influence on the proliferation and osteoblastic differentiation of
BMSCs. Duttenhoefer et al (11) demonstrated that BMSCs co-cultured
with EPCs exhibit a significantly reduced level of osteogenic gene
expression and less calcium deposition. Furthermore, Wen et
al (33) established an
EPC/BMSC indirect Transwell culture system, and identified that
co-cultured BMCs proliferate more than mono-cultured MSCs. In the
present study, the osteoblastic differentiation of BMSCs treated
with EPC-derived EVs was inhibited, whereas the number of CFU-Fs
was increased.
Although the osteoblastic differentiation of BMSCs
is inhibited by EPC-EVs, the exact mechanism remains unclear. EVs
deliver a large variety of cargo, including proteins, DNA and RNA.
The proteins and RNA in EVs serve critical roles in regulating the
differentiation and function of recipient cells (34). Spectrometry data of EVs have
identified over 4,000 different proteins in the exosomes (35). Although the proteins differ
substantially according to their origin and have different
functions, some proteins are shared by all types of EVs (36). These shared proteins are associated
with cell-to-cell communication. Trafficking-associated proteins,
including heat shock protein (HSP) 70 and HSP90, and cytoskeletal
proteins, including myosin, actin and tubulin, are widely
distributed in EVs (37).
Additionally, studies have demonstrated that small RNAs in EVs also
have an influence on target cells (38,39).
Koppers-Lalic et al (34)
reviewed the known small RNAs in EVs and noted that the functional
RNAs are critical in the regulation of cell commitment,
differentiation and activity. Therefore, profiling of proteins,
DNAs and RNAs in EPC-derived EVs will aid in the exploration of
communication between EPCs and BMSCs.
The confocal microscopy data indicated that the
green round-like fluorescence (red arrow) in the EVs was
internalized, transported to and degraded by the Golgi apparatus,
the lumen of which was stained red (white arrow). These results
suggested that the cargo in the EVs was released in the Golgi
apparatus rather than in the lysosomes. However, the exact
mechanism of EV degradation remains controversial. Baixauli et
al (40) observed that EVs
that are internalized by cells may fuse with either the lysosomes
or the multivesicular bodies. Alvarez-Erviti et al (41) proposed that lysosomal dysfunction
in SH-SY5Y cells is associated with increased EV release, thus
indicating a critical role of lysosomes in EV degradation. Previous
studies have demonstrated that other cell organelles may also serve
a prominent role in EV degradation. Campanella et al
(42) identified that EVs are
transported to the Golgi apparatus by HSP60 located in tumor cells.
A previous study additionally demonstrated that the Golgi apparatus
is key to EV degradation (43).
Therefore, how the cells degrade the EVs requires further
investigation.
In conclusion, EPC-CM inhibits osteogenic gene
expression and calcium deposition in BMSCs in vitro.
EPC-derived EVs are one of the major regulators in EPC-CM.
Osteoblastic differentiation of BMSCs was inhibited by EPC-derived
EVs, whereas BMSC proliferation was increased. BMSCs treated with
EPC-derived EVs exhibited fewer calcium deposits however increased
CFU-Fs. Western blot analysis and RT-qPCR results also demonstrated
that osteoblastic differentiation was inhibited. In addition, it
was observed that EPC-EVs were delivered to and degraded by the
Golgi apparatus rather than by the lysosomes in BMSCs. The present
study demonstrated that EPCs communicate and regulate BMSCs through
EPC-derived EVs and provides a foundation for further exploration
of the communication between EPCs and BMSCs.
Acknowledgements
The current study was funded by the National Natural
Science Foundation of China (grant no. 81272003 to Dr Changqing
Zhang).
References
|
1
|
Dhahri D, Sato-Kusubata K, Ohki-Koizumi M,
Nishida C, Tashiro Y, Munakata S, Shimazu H, Salama Y, Eiamboonsert
S, Nakauchi H, et al: Fibrinolytic crosstalk with endothelial cells
expands murine mesenchymal stromal cells. Blood. 128:1063–1075.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradley EW, Carpio LR, van Wijnen AJ,
McGee-Lawrence ME and Westendorf JJ: Histone deacetylases in bone
development and skeletal disorders. Physiol Rev. 95:1359–1381.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seeman E: Bone modeling and remodeling.
Crit Rev Eukaryot Gene Expr. 19:219–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ornitz DM and Marie PJ: FGF signaling
pathways in endochondral and intramembranous bone development and
human genetic disease. Genes Dev. 16:1446–1465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kronenberg HM: Developmental regulation of
the growth plate. Nature. 423:332–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanczler JM and Oreffo RO: Osteogenesis
and angiogenesis: The potential for engineering bone. Eur Cell
Mater. 15:100–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almubarak S, Nethercott H, Freeberg M,
Beaudon C, Jha A, Jackson W, Marcucio R, Miclau T, Healy K and
Bahney C: Tissue engineering strategies for promoting vascularized
bone regeneration. Bone. 83:197–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Murohara T, Sullivan A, Silver
M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Palma M, Murdoch C, Venneri MA, Naldini
L and Lewis CE: Tie2-expressing monocytes: Regulation of tumor
angiogenesis and therapeutic implications. Trends Immunol.
28:519–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van der Pouw Kraan TC, Van Der Laan AM,
Piek JJ and Horrevoets AJ: Surfing the data tsunami, a
bioinformatic dissection of the proangiogenic monocyte. Vascul
Pharmacol. 56:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duttenhoefer F, de Freitas RL, Loibl M,
Bittermann G, Richards RG, Alini M and Verrier S: Endothelial
progenitor cell fraction contained in bone marrow-derived
mesenchymal stem cell populations impairs osteogenic
differentiation. Biomed Res Int. 2015:6595422015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goerke SM, Obermeyer J, Plaha J, Stark GB
and Finkenzeller G: Endothelial progenitor cells from peripheral
blood support bone regeneration by provoking an angiogenic
response. Microvasc Res. 98:40–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan BT and Johnstone RM: Fate of the
transferrin receptor during maturation of sheep reticulocytes in
vitro: Selective externalization of the receptor. Cell. 33:967–978.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thery C, Regnault A, Garin J, Wolfers J,
Zitvogel L, Ricciardi-Castagnoli P, Raposo G and Amigorena S:
Molecular characterization of dendritic cell-derived exosomes.
Selective accumulation of the heat shock protein hsc73. J Cell
Biol. 147:599–610. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mears R, Craven RA, Hanrahan S, Totty N,
Upton C, Young SL, Patel P, Selby PJ and Banks RE: Proteomic
analysis of melanoma-derived exosomes by two-dimensional
polyacrylamide gel electrophoresis and mass spectrometry.
Proteomics. 4:4019–4031. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raposo G, Tenza D, Mecheri S, Peronet R,
Bonnerot C and Desaymard C: Accumulation of major
histocompatibility complex class II molecules in mast cell
secretory granules and their release upon degranulation. Mol Biol
Cell. 8:2631–2645. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blanchard N, Lankar D, Faure F, Regnault
A, Dumont C, Raposo G and Hivroz C: TCR activation of human T cells
induces the production of exosomes bearing the TCR/CD3/zeta
complex. J Immunol. 168:3235–3241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Niel G, Raposo G, Candalh C, Boussac
M, Hershberg R, Cerf-Bensussan N and Heyman M: Intestinal
epithelial cells secrete exosome-like vesicles. Gastroenterology.
121:337–349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burger D, Viñas JL, Akbari S, Dehak H,
Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS and Burns KD: Human
endothelial colony-forming cells protect against acute kidney
injury: Role of exosomes. Am J Pathol. 185:2309–2323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denzer K, van Eijk M, Kleijmeer MJ,
Jakobson E, de Groot C and Geuze HJ: Follicular dendritic cells
carry MHC class II-expressing microvesicles at their surface. J
Immunol. 165:1259–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morelli AE, Larregina AT, Shufesky WJ,
Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z,
Watkins SC, et al: Endocytosis, intracellular sorting, and
processing of exosomes by dendritic cells. Blood. 104:3257–3266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clayton A, Turkes A, Dewitt S, Steadman R,
Mason MD and Hallett MB: Adhesion and signaling by B cell-derived
exosomes: The role of integrins. FASEB J. 18:977–979.
2004.PubMed/NCBI
|
|
25
|
Meirelles Lda S and Nardi NB: Murine
marrow-derived mesenchymal stem cell: Isolation, in vitro
expansion, and characterization. Br J Haematol. 123:702–711. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Narayanan R, Huang CC and Ravindran S:
Hijacking the Cellular Mail: Exosome mediated differentiation of
mesenchymal stem cells. Stem Cells Int. 2016:38086742016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fleury A, Martinez MC and Le Lay S:
Extracellular vesicles as therapeutic tools in cardiovascular
diseases. Front Immunol. 5:3702014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sivaraj KK and Adams RH: Blood vessel
formation and function in bone. Development. 143:2706–2715. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gulati R, Jevremovic D, Peterson TE,
Chatterjee S, Shah V, Vile RG and Simari RD: Diverse origin and
function of cells with endothelial phenotype obtained from adult
human blood. Circ Res. 93:1023–1025. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi J, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM and Asahara T: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen L, Wang Y, Wen N, Yuan G, Wen M, Zhang
L, Liu Q, Liang Y, Cai C, Chen X and Ding Y: Role of endothelial
progenitor cells in maintaining stemness and enhancing
differentiation of mesenchymal stem cells by indirect cell-cell
interaction. Stem Cells Dev. 25:123–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koppers-Lalic D, Hogenboom MM, Middeldorp
JM and Pegtel DM: Virus-modified exosomes for targeted RNA
delivery; a new approach in nanomedicine. Adv Drug Deliv Rev.
65:348–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simpson RJ, Jensen SS and Lim JW:
Proteomic profiling of exosomes: Current perspectives. Proteomics.
8:4083–4099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Dommelen SM, Vader P, Lakhal S,
Kooijmans SA, van Solinge WW, Wood MJ and Schiffelers RM:
Microvesicles and exosomes: Opportunities for cell-derived membrane
vesicles in drug delivery. J Control Release. 161:635–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gibbings D and Voinnet O: Control of RNA
silencing and localization by endolysosomes. Trends Cell Biol.
20:491–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pegtel DM, Cosmopoulos K, Thorley-Lawson
DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD,
Würdinger T and Middeldorp JM: Functional delivery of viral miRNAs
via exosomes. Proc Natl Acad Sci USA. 107:6328–6333. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baixauli F, Lopez-Otin C and Mittelbrunn
M: Exosomes and autophagy: Coordinated mechanisms for the
maintenance of cellular fitness. Front Immunol. 5:4032014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alvarez-Erviti L, Seow Y, Schapira AH,
Gardiner C, Sargent IL, Wood MJ and Cooper JM: Lysosomal
dysfunction increases exosome-mediated alpha-synuclein release and
transmission. Neurobiol Dis. 42:360–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Campanella C, Bucchieri F, Merendino AM,
Fucarino A, Burgio G, Corona DF, Barbieri G, David S, Farina F,
Zummo G, et al: The odyssey of Hsp60 from tumor cells to other
destinations includes plasma membrane-associated stages and Golgi
and exosomal protein-trafficking modalities. PLoS One.
7:e420082012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin Y, Wang L, Gao Z, Chen G and Zhang C:
Bone marrow stromal/stem cell-derived extracellular vesicles
regulate osteoblast activity and differentiation in vitro and
promote bone regeneration in vivo. Sci Rep. 6:219612016. View Article : Google Scholar : PubMed/NCBI
|