Introduction
Staphylococcus aureus is an important type of
bacteria, which has high colonization rate on the lesions of normal
skin and in inflammatory skin diseases (1,2).
Infection and colonization with S. aureus triggers the onset
and aggravates the course of various inflammatory diseases,
including atopic dermatitis (1,3) and
psoriasis (4). The secretion of
S. aureus enterotoxin B (SEB), one type of staphylococcal
superantigens, is a vital mechanism of how S. aureus acts.
Keratinocytes, which account for 90% of epidermis, are also the
main cells that SEB directly contact. Keratinocytes function as the
barrier of the skin, and have an important role in skin immunity
(5). For example, it has been
proved that human keratinocytes were able to process endogenous and
exogenous antigen and induced rapid effector function in
antigen-specific memory CD4+ and CD8+ T cells (6). Previous researches also suggested
that keratinocytes support superantigens-driven proliferation in
resting T cells via cell-cell contacts in vitro (7,8). The
present study considered whether the direct contact is a
prerequisite and foundation for human keratinocytes to activate T
cells and whether there is a non-contact way to allow keratinocyte
function in situ with T cells.
It is widely accepted that exosomes facilitate the
direct extracellular transfer of proteins, lipids and RNA, in
vitro and in vivo, which enables them to participate in
intercellular communication without direct contact (9,10).
Exosomes are nano-size membrane vesicles (diameter, 30–100 nm) that
form within late endocytic compartments, which are called
multivesicular bodies, and are released to the extracellular medium
by fusion of the plasma membrane (11). They contain a distinct set of
proteins defined as exosomal markers, including tetraspanin family
molecules (CD9, CD63, CD81), heat shock proteins (HSP) 70, HSP90,
and components of the endosomal sorting complex required for
transport machinery [e.g., programmed cell death 6 interacting
protein and tumour susceptibility 101 (TSG101)] (12,13).
Furthermore, exosomes also typically carry a specific set of
proteins associated with the cell type they originated from. For
example, platelet-derived exosomes express the von Willebrand
factor and CD41a, the exosomes from dendritic cells (DCs) express
DC-specific maker CD11c, major histocompatibility complex (MHC) I
and II, and costimulatory molecules [CD86 and intercellular
adhesion molecule 1 (ICAM-1)] (14,15).
Besides the protein, exosomes function similarly to original cells,
such as, one proposed role of exosomes released by DCs is to spread
antigens or MHC-peptide complexes to T cells or DCs causing immune
reactions (16–18).
Therefore, due to the fact that keratinocytes
directly presented SEB to resting CD3+ T cells in
vitro, it was assumed that keratinocyte-derived exosomes, which
could act as a ‘truck’ between keratinocytes and T cells, may also
have the ability to function in superantigen-associated immunity.
To address this question, HaCaT cells (keratinocyte cell line) were
used to analyze the immune characteristics of exosomes derived from
HaCaT cells. Experimental evidence was thus provided that HaCaT
cells pretreated with SEB could induce the proliferation of T cells
in a Transwell co-culture system, and the results demonstrated that
HaCaT cells secrete exosomes and these exosomes carried MHC I and
MHC II molecules when derived from HaCaT cells pretreated with
interferon γ (IFNγ). Furthermore, HaCaT-exosomes could interact
with T cells, resulting in superantigen-induced proliferation of
CD4+ and CD8+ T cells in vitro.
Materials and methods
Reagents
SEB (Academy of Military Medical Science, Beijing,
China), Leaf™ purified anti-human CD3, CD28 antibody (cat. nos.
300313 and 302913; BioLegend, Inc., San Diego, CA, USA), IFNγ and
IL-2 (PeproTech, Inc., Rocky Hill, NJ, USA) were used for cell
stimulation. The following mouse antibodies were used for western
blotting (all from Abcam, Cambridge, UK): Anti-human CD63,
anti-human TSG101, anti-human calnexin, anti-human major
histocompatibility complex, class I, A (HLA-A), anti-human major
histocompatibility complex, class II, DR (HLA-DR), anti-human
ICAM-1 and anti-human β-actin. Antibodies used for flow cytometry
analysis were as follows (all from BioLegend, Inc.): Phycoerythrin
(PE)-anti-human CD4, PE-cyanine7 anti-human CD8, allophycocyanin
anti-human CD3 and PE-Cy7 anti-human CD69.
Cell sorting
Peripheral blood mononuclear cells (PBMCs) were
isolated by means of Ficoll-Hypaque (Dakewei Biotech Co., Ltd,
Shenzhen, China) density gradient centrifugation from heparinized
venous blood obtained from healthy donors, as previously described
(19). Human cells were obtained
under protocols approved by the Medical Ethics Committee of Second
Affiliated Hospital of Third Military Medical University, PLA
(Chongqing, China). CD3+ T cells were sorted by positive
selection with the IMagnet™ (BD IMag™; BD Biosciences, Franklin
Lakes, NJ, USA) as described by the manufacturer. The purity of
isolated CD3+CD69− T cells was >95%,
detected by flow cytometer (Beckman Coulter, Inc., Brea, CA, USA)
and analyzed by FlowJo software (version 7.6.1; FlowJo LLC,
Ashland, OR, USA)
Cell culture and preconditioning
The HaCaT cells (Institute of Biochemistry and Cell
Biology, CAS, Shanghai, China), and the sorted CD3+ T
cells were cultured in RPMI medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
100 U/ml penicillin-streptomycin at 37°C in 5% CO2. In
the co-culture assay, HaCaT cells (2×104 cells/well)
were incubated with or without IFNγ overnight (300 U/ml) and were
then treated for another 4 h with or without the superantigens SEB
(100 ng/ml). Finally, these cells were washed for three times to
remove the solute SEB and IFNγ. For exosome collection, the treated
HaCaT cells were resuspended in fresh culture medium with 10%
exosomes-free FBS (prepared by overnight ultracentrifugation at
100,000 × g). After 48 h, cell culture supernatant was collected
for exosome purification.
Cell proliferation assay
In the cell to cell experiment, Transwell chambers
(8-µm pore membrane; EMD Millipore, Billerica, MA, USA) were used
to determine the capacity of SEB-loaded HaCaT cells to promote T
cell proliferation under non-contact conditions. Purified
CD3+ T cells were labeled with 5 µM carboxyfluorescein
succinimidyl ester (CFSE; Invitrogen; Thermo Fisher Scientific,
Inc.) and then were seeded on 24-well cell culture cluster plates
at a concentration of 4×105 cells/well in volumes of 500
µl. SEB-loaded HaCaT cells (+/-IFNγ) were seeded on the Transwell
chambers at a concentration of 2×104 cells/well in
volumes of 200 µl. In the cell proliferation experiment of
exosomes, the assays were performed in 96-well round-bottom plates
in complete RPMI modified medium. The CFSE-labeled T cells
(2×105/well) in a total volume of 200 µl were stimulated
with HaCaT-exosomes (+/-SEB, +/-IFNγ). As a positive control to
detect whether the CFSE-labeled T cells were able to proliferate,
CD3+ T cells were cultured in plates precoated with 4
µg/ml anti-CD3 and 2 µg/ml anti-CD28 antibodies and stimulated with
IL-2 (30 IU/ml), as previously described (20). After 5 days, T cells were staining
with 5 µg/ml anti-human CD3, CD4 and CD8 antibody. Finally, the
stained cells were analyzed using a flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA).
Exosomes purification
The cell culture supernatants were centrifuged at
300 × g for 10 min, 2,000 × g for 10 min at 4°C, and filtered
through a 0.22 µm sterilizing filter (Corning Incorporated,
Corning, NY, USA). Exosomes were pelleted by ultracentrifugation at
100,000 × g for 70 min at 4°C and then were resuspended in PBS.
Protein level was quantified by bicinchoninic acid protein assay
(Beyotime Institute of Biotechnology, Haimen, China).
Transmission electron microscopy
To analyze the structure of exosomes derived from
HaCaT cells (+/-IFNγ), a 10 µl suspension of exosomes (+/-IFNγ) was
loaded onto Formvar carbon-coated 200 mesh copper grids for 10 min
at room temperature. Excessive fluid was slightly drained with
filter paper and then the adsorbed exosomes were negatively stained
with 1% phosphotungstic acid for 5 min. Finally, the air-dried
grids were observed using a transmission electron microscope
(JEM-1400 PLUS, JEOL, Ltd., Tokyo, Japan) operating at 100 kV.
Fluorescence confocal microscopy
The PKH67 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used to label exosomes according to the manufacturer's
protocols. Briefly, exosomes (~100 µg in 100 µl) were resuspended
in 0.5 ml of diluent C (Sigma-Aldrich; Merck KGaA) and then mixed
with PKH67 diluted in diluent C for a final concentration of
2×10−6 M PKH67. The exosomes dye suspension was
incubated for 5 min at room temperature. Excessive dye from the
labeled exosomes was neutralized with 1 ml 5% bovine serum albumin.
Finally, the supernatant were removed by centrifugation (100,000 ×
g for 70 min at 4°C) and PKH67-labeled exosomes were resuspended in
50 µl PBS.
PKH67-labeled exosomes derived from HaCaT cells were
co-incubated with CD3+ T cells for 4 h at 37°C. The
slides of cells were then washed in PBS three times followed by
fixation in 4% paraformaldehyde for 15 min at room temperature. The
cellular nuclei were stained with 0.5 µg/ml DAPI. Imaging was
performed using a fluorescence confocal microscope. Images were
analyzed with Leica Application Suite Advanced Fluorescence
software (version 2.3.0, build 5131; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA).
SDS-PAGE and western blot
analysis
HaCaT cells or exosomes (+/-IFNγ) were lysed in
radioimmunoprecipitation lysis buffer (50 mM Tris, pH 7.4, 150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium
orthovanadate, sodium fluoride, EDTA, leupeptin and 1 mM PMSF).
Protein of cells and exosomes was quantified by bicinchoninic acid
protein assay (Beyotime Institute of Biotechnology, Haimen, China).
Equal amounts of protein (~20 µg) were subjected to 10% SDS-PAGE,
transferred to a polyvinylidene difluoride membrane (PVDF; 0.45 µm;
EMD Millipore). PVDF membranes were blocked with 5% non-fat milk
for 2 h at room temperature, and then incubated with the diluted
anti-CD63, anti-TSG101, anti-calnexin antibody, anti-HLA-A,
anti-HLA-DR, anti-ICAM-1, anti-β-actin solution (1:1,000) overnight
at 4°C. Washed membranes were probed with a horseradish
peroxidase-conjugated secondary antibody (cat. no. A0216; Beyotime
Institute of Biotechnology, Haimen, China) at a 1:1,000 dilution
for 2 h. The results were visualized by chemiluminescence substrate
(Pierce; Thermo Fisher Scientific, Inc.). Densitometry was
performed using ImageJ software (version 1.45; National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
When appropriate, data are presented as the mean ±
standard deviation. Multiple comparisons were made using one-way
analysis of variance followed by the Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
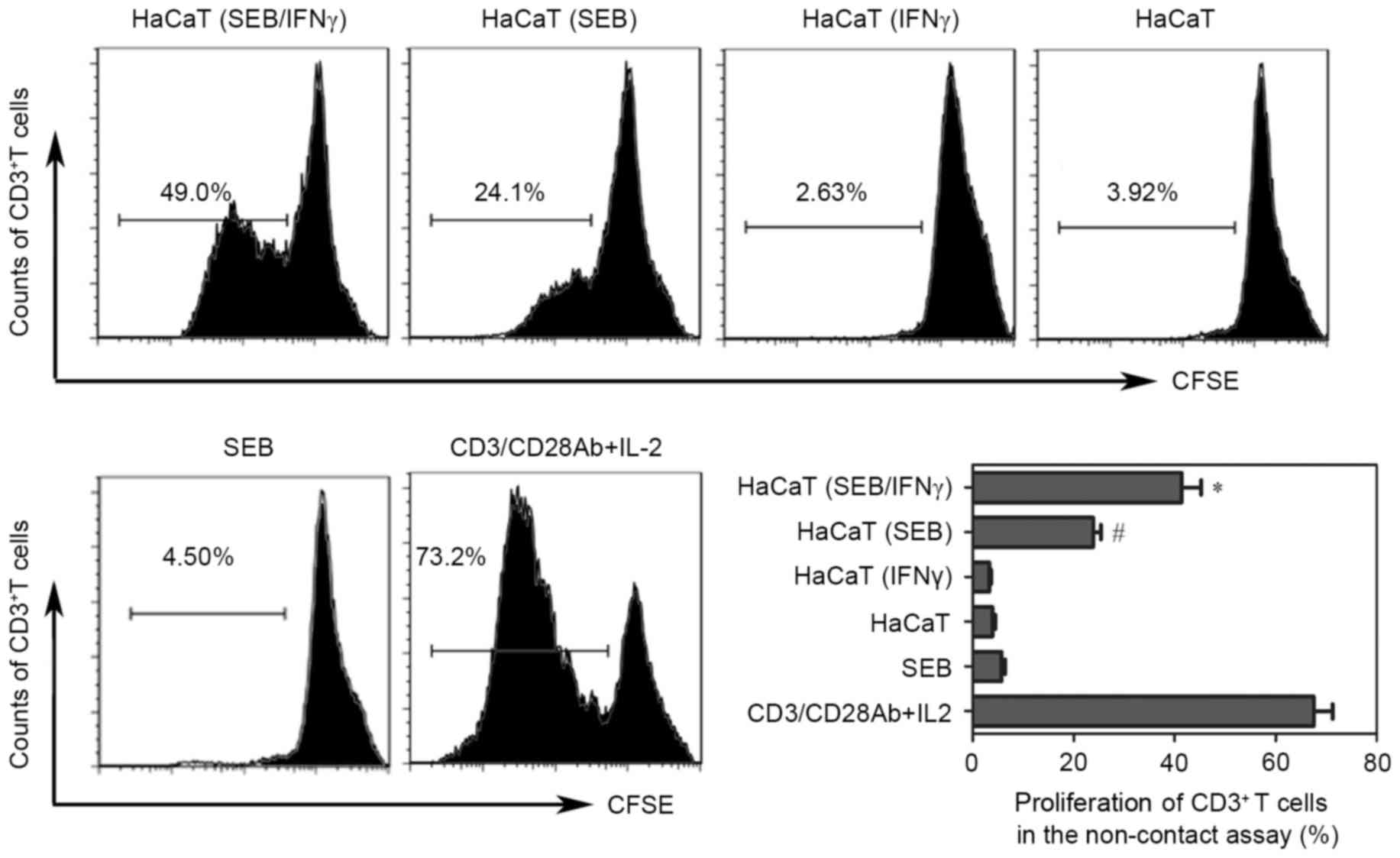
SEB-loaded HaCaT cells (+/-IFNγ)
induce T cell proliferation without direct contact
To detect the ability of HaCaT cells in presenting
SEB to resting T cells under non-contact conditions, indirect
co-culture experiments were performed using the Transwell system.
The results demonstrated that SEB-loaded HaCaT cells induced the
proliferation of resting T cells (Fig.
1). Previous studies have suggested that under inflammatory
conditions, such as when stimulated by IFNγ, keratinocytes express
MHC II, ICAM-1 and increased levels of MHC I to make these cells
compatible with competent presentation of antigens to T cells
(6). Therefore, T cell
proliferation driven by SEB-loaded HaCaT cells pretreated with IFNγ
was also investigated. The data indicated that the proliferation of
T cells was increase compared with the untreated condition in this
indirect co-culture system.
HaCaT cells produce typical exosomes with or without
IFNγ. Exosomes were purified from HaCaT culture supernatants by
ultracentrifugation, and identified with electron microscopy and
western blotting analysis. The data demonstrated that exosomes
derived from the HaCaT cells stimulated with or without IFNγ both
had a typical exosomal round shape with a diameter between 30 and
100 nm (Fig. 2A and B).
Additionally, these exosomes contained exosome marker proteins such
as CD63 and TSG101. The exosomes were negative for calnexin, which
is specifically expressed and located in endoplasmic reticulum
(Fig. 2C and D).
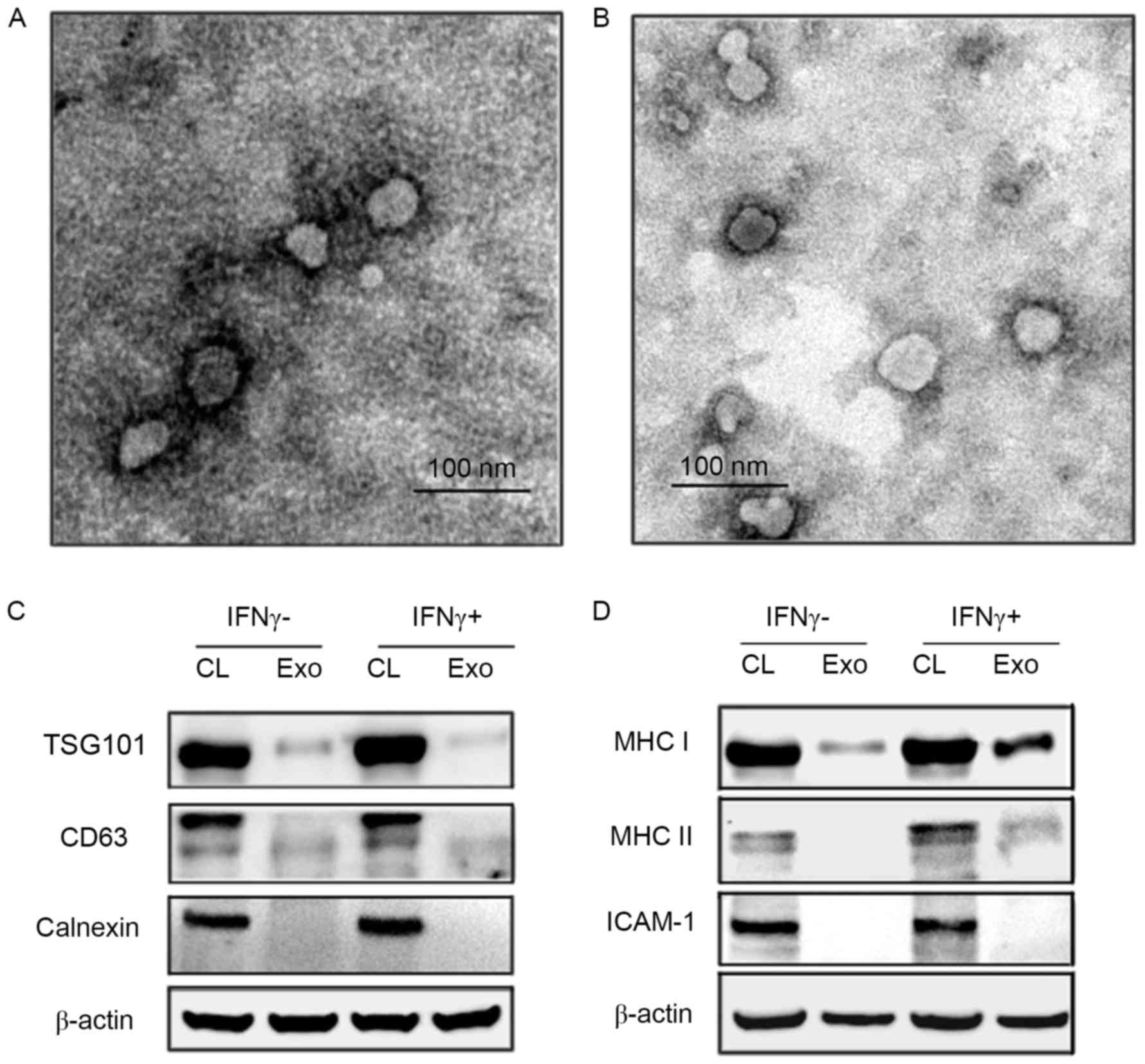 | Figure 2.Analysis of the features of exosomes
secreted by HaCaT cells. (A) Exosomes derived from HaCaT cells
(IFNγ-) and (B) exosomes derived from HaCaT cells (IFNγ+) with
characterizations of HaCaT-released exosomes observed by
transmission electron microscope (×120,000). Two types of exosomes
from HaCaT were roughly identical, ranging from 30 to 100 nm. (C)
Western blotting were performed using vesicles protein to validate
exosomal markers CD63, TSG101 and negative protein calnexin. (D)
Western blotting was performed for immune molecules MHC I, MHC II
molecules and ICAM-1 in the HaCaT-exosomes. CL, cell lysate; Exo,
exosomes. IFNγ, interferon γ; CL, cell lysate; Exo, exosomes;
TSG101, tumour susceptibility 101; MHC, major histocompatibility
complex; ICAM-1, intracellular adhesion molecule-1. |
HaCaT cell exosomes express MHC I and
induced MHC II molecules
To assess the ability of HaCaT-exosomes to present
peptides, whether HaCaT-exosomes contained MHC I and MHC II
molecules with or without stimulation of IFNγ was determined. The
data demonstrated that HaCaT-exosomes expressed low levels of MHC I
but no MHC II molecules (Fig. 2D),
suggesting that HaCaT-exosomes have the potential function of
presenting peptide to CD8+ T cells under resting
condition. When HaCaT cells were pretreated by IFNγ, HaCaT-exosomes
expressed low level of MHC II molecules and the MHC I was further
upregulated (Fig. 2D).
In addition, previous studies have suggested an
important role of ICAM-1 and the leukocyte function-associated
antigen-1 (LFA-1) interaction in presentation function of
keratinocytes for superantigens (7) and in T cell adherence to
keratinocytes (21). The exosomes
from HaCaT cells did not express ICAM-1 molecules under steady
state or inflammatory conditions (+/-IFNγ; Fig. 2D).
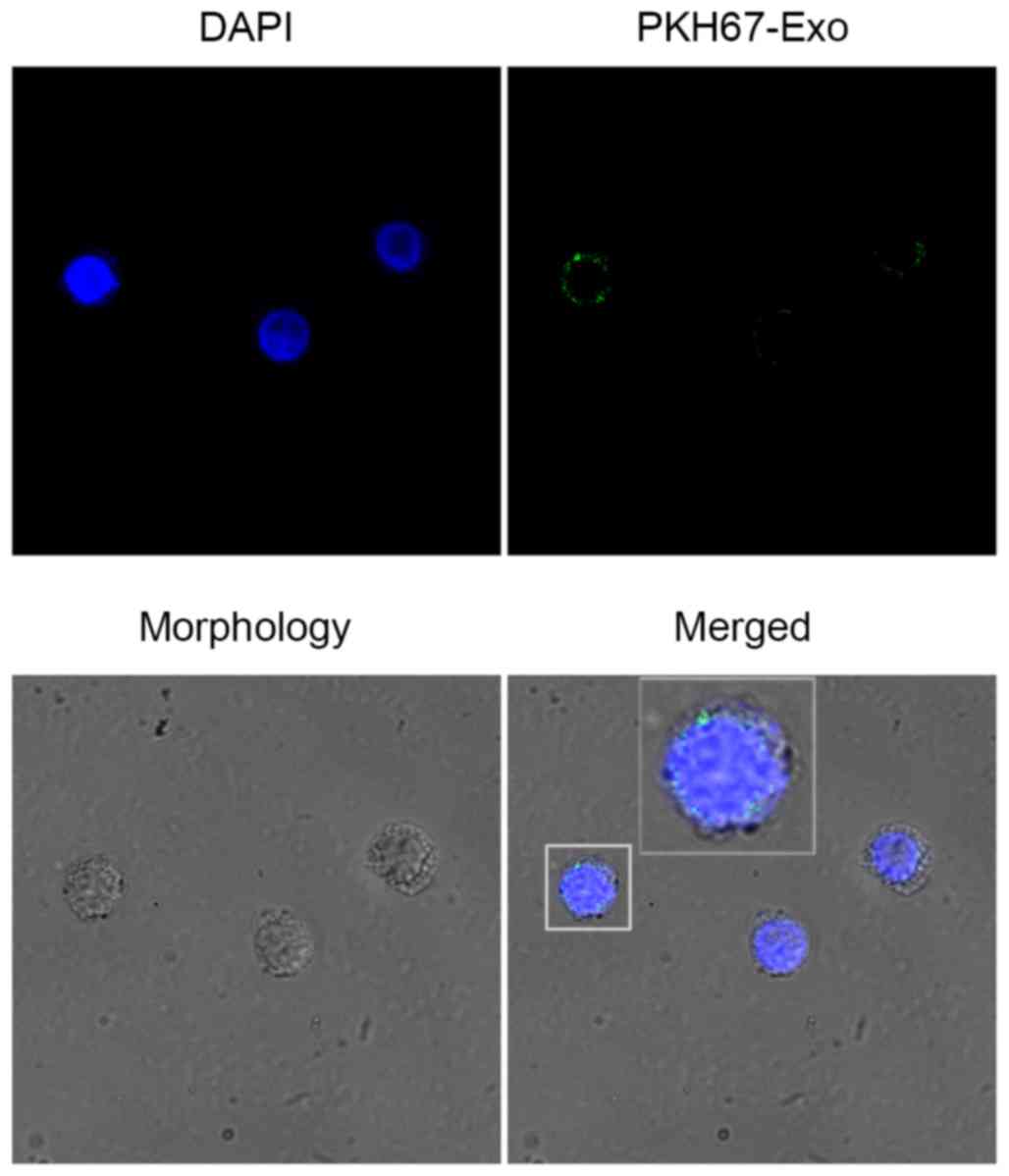
HaCaT-derived exosomes interact with T
cells
To explore whether the HaCaT-exosomes could interact
with T cells, confocal fluorescence microscopy was used to track
the PKH67-labeled HaCaT-exosomes in T cells. After incubation of
PKH67 labeled exosomes with the T cells for 4 h at 37°C, we
observed an obvious green fluorescence in the T cells indicating an
active exosomes interaction with T cells (Fig. 3). This result suggests a potential
role of exosomes in a short-range interaction between HaCaT cells
and T cells.
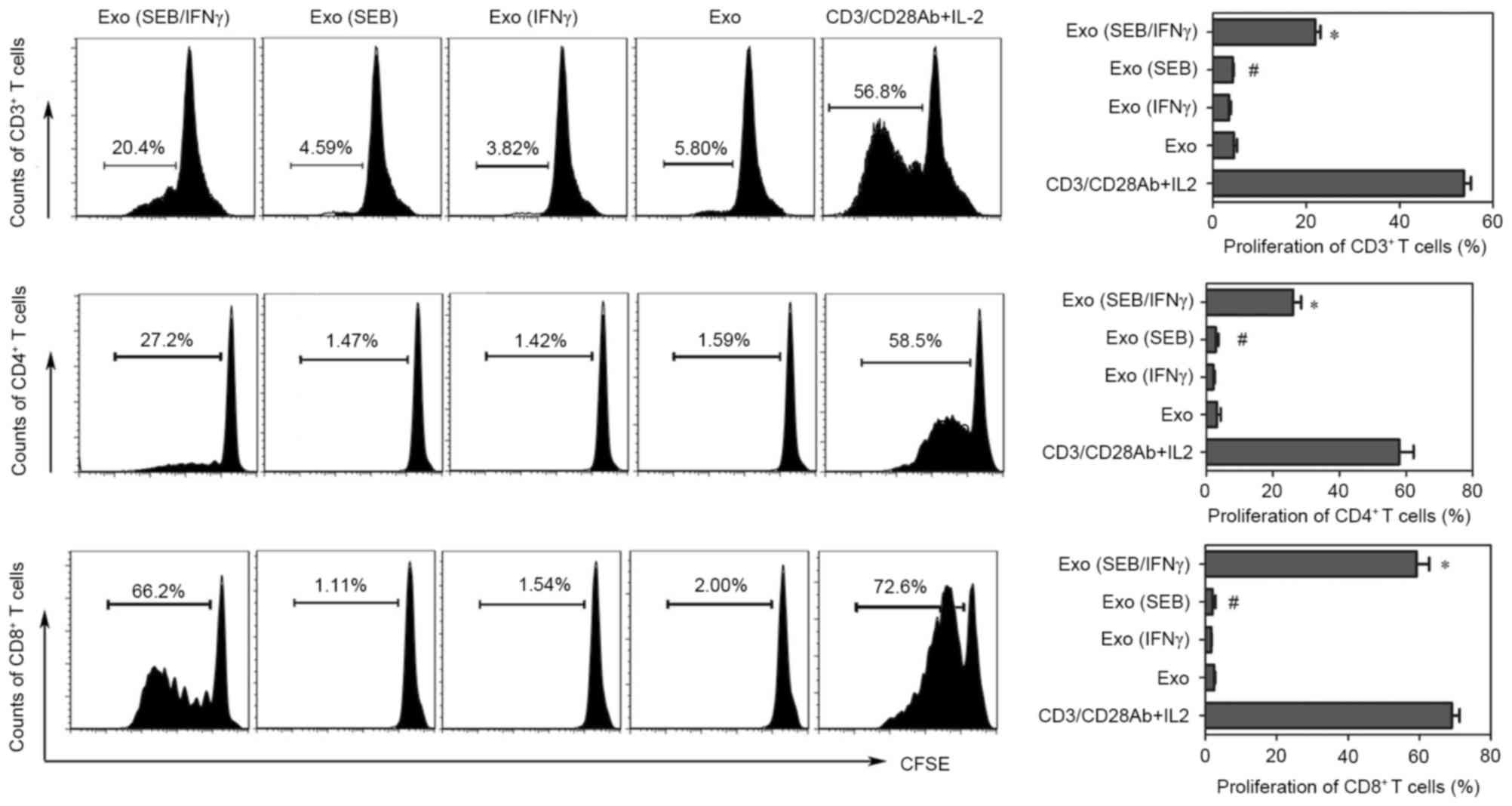
SEB-loaded-exosomes from HaCaT cells
can induce the proliferation of CD4+ and CD8+
T cells
In the proliferation experiments, the results
demonstrated that exosomes from SEB loaded HaCaT cells could induce
the proliferation of CD3+ T cells when the HaCaT cells
were pre-stimulated with IFNγ. It was previously reported that
Staphylococcus aureus could induce notable proliferation of
CD4+ and CD8+ T cells, which were two main
subpopulations of CD3+ T cells (22). Therefore, the CD4+ and
CD8+ T cells in the CD3+ T cells were gated
to detect the proliferations. Correspondingly, the
CD3+CD4+ and CD3+CD8+ T
cells proliferated following induction by exosomes from SEB-HaCaT
cells treated with IFNγ (Fig.
4).
Discussion
Keratinocytes are the first and major point of
contact with many potential antigens; therefore, it is important to
understand how keratinocyte present antigens to T cells both for
disease pathogenesis and also for potential delivery of vaccination
antigens via the skin (6).
Previous studies demonstrated that exosomes have an important role
in modulation of immune responses. However, data about potential
immunomodulatory functions of human keratinocyte exosomes are
scant. In this study, to the best of our knowledge, it was
demonstrated for the first time that HaCaT cells (human
keratinocyte cell line) induce T cell proliferation in a
non-contact manner. Furthermore, it was demonstrated that HaCaT
cells release the nano-size exosomes to react with the T cells.
This novel biological function of exosomes reveals a new
short-range mechanism between keratinocytes and T cells in skin
immunity to superantigens.
Keratinocytes are thought to display features of
antigen-presenting cells (APCs) (5) and IFNγ is reported to upregulate MHC
class II expression on keratinocytes, thus enhancing their antigen
presenting capacities. In the present study, SEB-loaded HaCaT cells
(IFNγ treated and untreated) induced the proliferation of
CD3+ T cells under indirect co-culture conditions.
Keratinocytes are able to transfer immune information to T cells
via soluble factors; for example, keratinocytes produce a large
range of cytokines, including pro-inflammatory cytokines and
chemokines (23). However, none of
these known factors directly induce the proliferation of resting T
cells, thus the role of exosomes in the SEB-associated immunity was
investigated.
In the current study, immune potential of
HaCaT-exosomes was investigated. A previous study have suggested
that keratinocytes constitutively expressed MHC class I molecules
and had induced expression of MHC class II molecules under
inflammatory conditions in vivo (24) or following treatment with IFNγ
in vitro (6), thus we
investigated the level of MHC class I and II in HaCaT-exosomes with
or without IFNγ stimulation. The result indicated that compared
with the HaCaT cells, exosomes contained lower MHC I levels but did
not contain MHC II. The expression of MHC I increased and MHC II
was induced at a low level in the exosomes derived from the HaCaT
cells following IFNγ stimulation. The presence of MHC I and MHC II
molecules implies the potential capacities of HaCaT-exosomes in
antigen-presentation to CD4+ and CD8+ T
cells. Additionally, the expression of MHC I and II in exosomes
derived from HaCaT cells were different from the results detected
in murine keratinocytes, where murine keratinocytes-derived
exosomes did not express MHC II, irrespective of IFNγ stimulation
(25). These divergent results may
be due to the species difference between humans and mice; unlike
human keratinocytes, not all murine keratinocytes express MHC II
in vitro (26).
Keratinocyte-derived exosomes were observed to be
internalized by DCs (25) and bone
marrow-derived DCs (27) in
vitro. In the current study, HaCaT-derived exosomes could
interact with T cells. However, vesicles <200 nm in diameter
cannot be detected by confocal microscopy techniques, and detailed
visualization of exosomes can only be observed by electron
microscopy (28). Thus, how
individual exosomes interact with target cells remains unknown. A
previous study proposed this process to involve binding at the cell
surface via specific receptors, and then internalizing through the
endocytic pathway of the target cell, and/or by direct fusion with
the membrane (28). For example,
previous study indicated that T cells recruited exosomes secreted
by DCs depending on the interaction of ICAM-1/LFA-1 (29). Unfortunately, in the current study,
no ICAM-1 was detected in HaCaT-exosomes, so factors functioning in
the interaction between HaCaT-exosomes and T cells are unknown and
require further research.
HaCaT-exosomes did induce SEB-associated
proliferation of resting CD4+ and CD8+ T
cells in the experiments of the current study, though it is unclear
which factors mediated the interaction between HaCaT-exosomes and T
cells. The present study demonstrated that exosomes induce the
proliferation of CD4+ and CD8+ T cells when
derived from IFNγ-treated HaCaT cells rather than untreated ones.
Superantigens are a type of special antigen. They bind directly to
MHC II molecules on APCs instead of intracellular processing into
the APCs, and simultaneously they bind to the variable region of
the Vβ chain of a T cell receptor (30). Due to the special binding mechanism
and various Vβ isotypes, superantigens bypass the normal mechanisms
of conventional MHC-restricted antigen presentation and the number
of T cells activated by superantigens exceeds that of a
conventional antigen. For example, the S. aureus161:2
(carrying the genes for staphylococcal enterotoxin A and H) could
activate the human mucosal-associated invariant T cells, γδ T
cells, and conventional CD4+ and CD8+ T cells
in vitro (22). Given that
superantigens require the MHC II molecules on antigen-presenting
cells as the binding site, stimulation of IFNγ increased the
expression of MHC II in exosomes, thus potentially accounting for
the increase of T cells proliferation supporting by HaCaT-exosomes.
In addition, as in bone marrow dendritic cells (BM-DCs), exosomes
are more efficient to activate ovalbumin (OVA)-specific MHC class
I-restricted T cell hybridomas, when derived from OVA peptide
loaded mature BM-DCs rather than those from immature BM-DCs
(31). Keratinocytes are thought
to be non-professional APCs, so their exosomes are much more
similar to those of the immature DC than those of mature ones. The
current study and these previous finding suggest an
immunomodulatory role of exosomes depending on the state of the
corresponding origin cell. Furthermore, the presence of
staphylococcal colonization and staphylococcal superantigens is
associated with various skin diseases (30). For example, superantigens-mediated
T cell activation may initiate and propagate psoriasis (32,33).
S. aureus, 60% producing superantigens (most often SEB), is
present in >50% of patients with psoriasis and the severity of
psoriasis is significantly correlated to enterotoxin production of
S. aureus strains (34). As
keratinocytes are the most abundant cell type in the skin, they are
also the main cells with which S. aureus contact. Therefore,
it is reasonable to presume that in skin inflammatory diseases
superantigens are produced by S. aureus and then
keratinocytes release exosomes, which may worsen the inflammation
by inducing superantigen-associated proliferation of T cells. If
the hypothesis is viable that keratinocytes-exosomes are capable of
playing a similar role in the SEB or other superantigen-associated
inflammatory disease in vivo, the exosomes inhibitor could
decrease immune responses, thus offering a potential future
therapeutic approach.
In conclusion, superantigens produced by microbes
may cause a potent T-cell activation and proliferation in their
target tissues, which maintains chronic inflammation if not
appropriately treated. The current study verified a novel
non-contact, but supportive function between keratinocyte and
targeted T cells in the SEB-associated cutaneous immunity.
Furthermore, the results unveil a novel role of
keratinocyte-derived exosomes in superantigen-associated diseases,
and indicates that IFNγ stimulation is an important factor in
influencing the immunity of keratinocyte-exosomes. However, which
factors these effects are mediated by and what the possible
reaction could be in vivo remains unclear.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 81271767).
References
|
1
|
Abeck D and Mempel M: Staphylococcus
aureus colonization in atopic dermatitis and its therapeutic
implications. Br J Dermatol. 139 Suppl 53:S13–S16. 1998. View Article : Google Scholar
|
|
2
|
Miko BA, Uhlemann AC, Gelman A, Lee CJ,
Hafer CA, Sullivan SB, Shi Q, Miller M, Zenilman J and Lowy FD:
High prevalence of colonization with Staphylococcus aureus clone
USA300 at multiple body sites among sexually transmitted disease
clinic patients: An unrecognized reservoir. Microbes Infec.
14:1040–1043. 2012. View Article : Google Scholar
|
|
3
|
Mernelius S, Carlsson E, Henricson J,
Löfgren S, Lindgren PE, Ehricht R, Monecke S, Matussek A and
Anderson CD: Staphylococcus aureus colonization related to severity
of hand eczema. Eur J Clin Microbiol Infect Dis. 35:1355–1361.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elfatoiki FZ, El Azhari M, El Kettani A,
Serhier Z, Othmani MB, Timinouni M, Benchikhi H, Chiheb S and
Fellah H: Psoriasis and Staphylococcus aureus skin colonization in
Moroccan patients. Pan Afr Med J. 23:332016.PubMed/NCBI
|
|
5
|
Nestle FO, Di Meglio P, Qin JZ and
Nickoloff BJ: Skin immune sentinels in health and disease. Nat Rev
Immunol. 9:679–691. 2009.PubMed/NCBI
|
|
6
|
Black AP, Ardern-Jones MR, Kasprowicz V,
Bowness P, Jones L, Bailey AS and Ogg GS: Human keratinocyte
induction of rapid effector function in antigen-specific memory
CD4+ and CD8+ T cells. Eur J Immunol. 37:1485–1493. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nickoloff BJ, Mitra RS, Green J, Zheng XG,
Shimizu Y, Thompson C and Turka LA: Accessory cell function of
keratinocytes for superantigens. Dependence on lymphocyte
function-associated antigen-1/intercellular adhesion molecule-1
interaction. J Immunol. 150:2148–2159. 1993.PubMed/NCBI
|
|
8
|
Li LB, Goleva E, Hall CF, Ou LS and Leung
DY: Superantigen-induced corticosteroid resistance of human T cells
occurs through activation of the mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J
Allergy Clin Immunol. 114:1059–1069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simons M and Raposo G: Exosomes-vesicular
carriers for intercellular communication. Curr Opin Cell Biol.
21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Théry C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol Chapter.
3:Unit 3.22. 2006. View Article : Google Scholar
|
|
12
|
Théry C, Ostrowski M and Segura E:
Membrane vesicles as conveyors of immune responses. Nat Rev
Immunol. 9:581–593. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohno S, Ishikawa A and Kuroda M: Roles of
exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv
Rev. 65:398–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaput N, Flament C, Viaud S, Taieb J,
Roux S, Spatz A, André F, LePecq JB, Boussac M, Garin J, et al:
Dendritic cell derived-exosomes: biology and clinical
implementations. J Leukoc Biol. 80:471–478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson SM, Admyre C, Scheynius A and
Gabrielsson S: Different types of in vitro generated human
monocyte-derived dendritic cells release exosomes with distinct
phenotypes. Immunology. 123:491–499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bobrie A, Colombo M, Raposo G and Théry C:
Exosome secretion: Molecular mechanisms and roles in immune
responses. Traffic. 12:1659–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montecalvo A, Shufesky WJ, Stolz DB,
Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins
PD, Larregina AT and Morelli AE: Exosomes as a short-range
mechanism to spread alloantigen between dendritic cells during T
cell allorecognition. J Immunol. 180:3081–3090. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
André F, Chaput N, Schartz NE, Flament C,
Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, et
al: Exosomes as potent cell-free peptide-based vaccine. I.
Dendritic cell-derived exosomes transfer functional MHC class
I/peptide complexes to dendritic cells. J Immunol. 172:2126–2136.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallone R, Mannering SI, Brooks-Worrell
BM, Durinovic-Belló I, Cilio CM, Wong FS and Schloot NC:
T-CellWorkshop Committee, Immunology of Diabetes Society: Isolation
and preservation of peripheral blood mononuclear cells for analysis
of islet antigen-reactive T cell responses: Position statement of
the T-cell workshop committee of the immunology of diabetes
society. Clin Exp Immunol. 163:33–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang TT, Zhao YL, Peng LS, Chen N, Chen W,
Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al: Tumour-activated
neutrophils in gastric cancer foster immune suppression and disease
progression through GM-CSF-PD-L1 pathway. Gut. Mar 8–2017.(Epub
ahead of print). View Article : Google Scholar :
|
|
21
|
Dustin ML, Singer KH, Tuck DT and Springer
TA: Adhesion of T lymphoblasts to epidermal keratinocytes is
regulated by interferon gamma and is mediated by intercellular
adhesion molecule 1 (ICAM-1). J Exp Med. 167:1323–1340. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johansson MA, Björkander S, Forsberg M
Mata, Qazi KR, Celades M Salvany, Bittmann J, Eberl M and
Sverremark-Ekström E: Probiotic lactobacilli modulate
Staphylococcus aureus-induced activation of conventional and
unconventional T cells and NK cells. Front Immunol. 7:2732016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gröne A: Keratinocytes and cytokines. Vet
Immunol Immunopathol. 88:1–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nickoloff BJ and Turka LA: Immunological
functions of non-professional antigen-presenting cells: New
insights from studies of T-cell interactions with keratinocytes.
Immunol Today. 15:464–469. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotzerke K, Mempel M, Aung T, Wulf GG,
Urlaub H, Wenzel D, Schön MP and Braun A: Immunostimulatory
activity of murine keratinocyte-derived exosomes. Exp Dermatol.
22:650–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaspari AA and Katz SI: Induction and
functional characterization of class II MHC (Ia) antigens on murine
keratinocytes. J Immunol. 140:2956–2963. 1988.PubMed/NCBI
|
|
27
|
Morelli AE, Larregina AT, Shufesky WJ,
Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z,
Watkins SC, et al: Endocytosis, intracellular sorting, and
processing of exosomes by dendritic cells. Blood. 104:3257–3266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Théry C: Exosomes: Secreted vesicles and
intercellular communications. F1000 Biol Rep. 3:152011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nolte-'t Hoen EN, Buschow SI, Anderton SM,
Stoorvogel W and Wauben MH: Activated T cells recruit exosomes
secreted by dendritic cells via LFA-1. Blood. 113:1977–1981. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macias ES, Pereira FA, Rietkerk W and
Safai B: Superantigens in dermatology. J Am Acad Dermatol.
64:455–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Utsugi-Kobukai S, Fujimaki H, Hotta C,
Nakazawa M and Minami M: MHC class I-mediated exogenous antigen
presentation by exosomes secreted from immature and mature bone
marrow derived dendritic cells. Immunol Lett. 89:125–131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao G, Feng X, Na A, Yongqiang J, Cai Q,
Kong J and Ma H: Acute guttate psoriasis patients have positive
streptococcus hemolyticus throat cultures and elevated
antistreptococcal M6 protein titers. J Dermatol. 32:91–96. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leung DY, Travers JB, Giorno R, Norris DA,
Skinner R, Aelion J, Kazemi LV, Kim MH, Trumble AE, Kotb M, et al:
Evidence for a streptococcal superantigen-driven process in acute
guttate psoriasis. J Clin Invest. 96:2106–2112. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomi NS, Kränke B and Aberer E:
Staphylococcal toxins in patients with psoriasis, atopic
dermatitis, and erythroderma, and in healthy control subjects. J Am
Acad Dermatol. 53:67–72. 2005. View Article : Google Scholar : PubMed/NCBI
|