Introduction
Chronic myeloid leukemia (CML) is a malignant
myeloproliferative disorder characterized by reciprocal chromosomal
translocation between chromosomes 9 and 22, which leads to the
formation of the breakpoint cluster region-abelson (BCR/ABL)
oncoprotein with constitutively active tyrosine kinase (1). According to its progression, CML can
be divided into three phases: Chronic phase (CP), accelerated phase
(AP) and blastic phase (BP), and the majority of cases are
diagnosed in the CP (2). It has
been demonstrated that, following allogeneic cell transplantation,
the survival rate of patients with CML is significantly prolonged
and may be cured; however, this treatment is only suitable for
young patients with CP CML and fully matched human leukocyte
antigen donors (3), whereas the
mean age of diagnosis of CML is 64 years old (4). Due to the specific characteristic of
CML, tyrosine kinase inhibitors targeting BCR/ABL have been
developed, including imatinib. However, drug resistance has been
found in patients with CML (5). To
date, CML accounts for 15% of all cases of leukemia and affects
1/100,000 individuals per year in Western countries (6). The prevalence of this disease is
likely to increase in the future; therefore, it is essential to
improve current understanding of the pathogenesis of CML.
Previous studies have demonstrated that the
investigation of transcription factors is a useful and crucial
method for understanding the mechanism of diseases. CCAAT/enhancer
binding protein α (C/EBPα) is a prototypical basic region-leucine
zipper transcription factor, which acts as an oncogene in several
types of cancer (7). C/EBPα has
been reported to be involved in acute lymphoblastic leukemia (ALL)
(8). Several studies have
demonstrated that C/EBPα can regulate genes, which are key to cell
differentiation in AML, and can inhibit cell cycle and apoptosis
(9,10). Zhang et al revealed that
SRY-related high mobility group box containing transcription factor
4 (SOX4) is a direct downstream target and important mediator of
C/EBPα in ALL (11), however, its
mechanism in CML remains not to be elucidated. SOX4, which belongs
to the SoxC class of transcription factors, primarily contributes
to regulation of the proliferation and survival of mesenchymal and
neural progenitors (9), B and T
cell maturation (12), cardiac
outflow formation and myeloid differentiation (13,14).
The aberrant expression of SOX4 in adult tissues has been linked to
the occurrence and progression of cancer in humans and mice, and
the overexpression of SOX4 is usually found in the majority of
types of cancer, including bladder, hepatic, lung, gastric,
prostate and hematopoietic cancer (15,16).
Ramezani-Rad et al demonstrated that SOX4 can act as a
central mediator to enable oncogenic survival signals via
phosphoinositide 3-kinase (PI3k)/AKT and mitogen-activated protein
kinase (MAPK) signaling, which results in a poor clinical outcome
in ALL (17).
In the present study, in order to investigate
changes in SOX4 and C/EBPα in CML, 79 patients with CML were
enrolled. The expression of SOX4 and C/EBPα were compared between
patients with CML and healthy controls, and the expression prior to
and following imatinib treatment were compared in patients with
CML. The aims of these investigations were to provide novel insight
into CML targeted therapy.
Materials and methods
Patients and study design
Between January 2014 and October 2015, a total of 79
patients with CML, confirmed by morphology, immunology,
cytogenetics and molecular biology (MICM) were enrolled at Yantai
Yuhuangding Hospital Affiliated to Qingdao University Medical
College (Yantai, China) for the present study. Among these, 57
patients had primary CML and had not received treatment. The
male/female ratio of these patients was 31/26 and their median age
was 56 years old. Based on the diagnostic results, the CML stages
were confirmed as follows: 41 cases of newly diagnosed chronic
phase CML (CML-CP), six cases of accelerated phase CML (CML-AP), 10
cases of blastic phase CML (CML-BP). Of the 79 patients, 22
patients were treated with imatinib, all of which had CML-CP. In
addition, the white blood cell (WBC) levels and the expression
levels of BCR/ABL in the patients with CML were determined. The
peripheral blood (PB) mononuclear cells of 30 healthy individuals
were also collected and used as controls. The Ethics Committee of
Qingdao University Medical College authorized the present study and
all patients provided signed informed consent.
Inclusion and exclusion criteria
Patients were enrolled if they met the following
criteria: i) confirmation by MICM; ii) no other types of cancer or
severe functional disease present; ii) no other blood disease
present; iv) newly diagnosed and untreated, with the exception of
those treated with imatinib; v) voluntarily participation and
ability to complete treatment. Patients were excluded if they were
<18 years of age or if their clinical information was
incomplete.
Imatinib treatment
To receive imatinib therapy, patients were required
to meet the following terms: i) Philadelphia chromosome-positive on
bone marrow chromosome cultivation; ii) BCR/ABL mutant positive;
iii) T315I mutant negative. The specification of imatinib in this
study was 100 mg per tablet. CML-CP and CML-AP patients orally
received 4 tablets of imatinib once a day (400 mg/day), and CML-BP
patients orally received 6 tablets of imatinib once a day (600
mg/day). During treatment, all patients were required to follow the
advice of their doctor and underwent hematological examination
every week, with genetic diagnosis and molecular diagnosis every 3
months. The observation period was adjusted until significant
blood, genetic or molecular activity was present, and the drug was
present from the initiation of treatment. Bone marrow samples were
collected from the patients at initial diagnosis and 3 months
following imatinib treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
To determine the mRNA levels of C/EBPα and SOX4,
lymphocyte separation medium (Tianjin Hao Yang Biological
Manufacture Co., Ltd., Tianjin, China) was used to separate
mononuclear cells from the bone marrow and PB samples. TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to extract RNA from the separated mononuclear cells and an
Easy Taq® PCR kit (Beijing Transgen Biotech Co., Ltd.,
Beijing, China) was used for cDNA production. Primers of SOX4,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), C/EBPα and
β-actin were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. (Table I), and the RT-PCR kit
was purchased from Applied Biosystems; Thermo Fisher Scientific,
Inc. The reactive sample for SOX4 was as follows: 10 µl 2X Mix, 0.5
µl each primer (10 µmol/l), 1.0 µl cDNA, and double distilled water
to 20 µl; and the reactive conditions were as follows: 95°C for 10
min; 39 cycles of 95°C for 31 sec, 60°C for 1 min; 65°C for 31 sec;
and 72°C for 10 min. The reactive sample for C/EBPα was as follows:
10.5 µl 2X Mix, 0.5 µl each primer (10 µmol/l), 1.0 µl cDNA and
double distilled water to 25 µl; and the reactive conditions were
as follows: 94°C for 5 min; 35 cycles of 94°C for 45 sec; 6°C for
45 sec; 72°C for 30 sec; and 72°C for 10 min. The reactive systems
of GAPDH and β-actin were as follows: 10 µl 2X Mix, 0.5 µl each
primer (10 µmol/l), 1.0 µl cDNA and double distilled water to 20
µl; and the reactive conditions were as follows: 94°C for 5 min; 35
cycles of 94°C, 30 sec; 55°C for 30 sec; 72°C for 1 min; and 72°C
for 10 min. Amplification was performed on a ABI 7500 PCR equipment
(Applied Biosystems, USA), and the recorded data were subjected to
statistical analysis to analyze the relative expression levels of
the genes using 2−ΔΔCq method (18). GAPDH was used as the internal
reference for SOX4 and β-actin was used as the internal reference
for C/EBPα.
 | Table I.Primers and forecasted product sizes
of genes detected using reverse transcription-quantitative
polymerase chain reaction analysis. |
Table I.
Primers and forecasted product sizes
of genes detected using reverse transcription-quantitative
polymerase chain reaction analysis.
| Gene | Primer | Product size
(bp) |
|---|
| SOX4
(NM_003107.2) | F:
5′-TTCAGCAACCAGCATTC-3′ | 104 |
|
| R:
5′-TCCCTCTCTCTCGCTCTCTC-3′ |
|
| GAPDH
(NM_001289746.1) | F:
5′-CCCACTCCTCCACCTTTGAC-3′ | 115 |
|
| R:
5′-ATGAGGTCCACCACCCTGTT-3′ |
|
| β-actin
(NM_001101.3) | F:
5′-GATCTGGCACCACACCTTCTAC-3′ | 182 |
|
| R:
5′-AGGCATACAGGGACAGCACA-3′ |
|
| C/EBPα
(NM_004364.4) | F:
5′-CACCGCTCCAATGCCTAC-3′ | 372 |
|
| R:
5′-CCCATCGCAGTGAGTTCCG-3′ |
|
Western blot analysis
In order to investigate the protein levels of C/EBPα
and SOX4, the separated mononuclear cells were dissociated in
protein lysis buffer to extract proteins, and the protein
concentrations were measured using the bicinchoninic acid method
(Thermo Fisher Scientific, Inc.). The electrophoresis of proteins
(20 ng) was performed on a 10% SDS-PAGE gel and then transferred
onto a nitrocellulose membrane. Following blocking with 5% skim
milk for 2 h, the nitrocellulose membrane was incubated with rabbit
anti-human SOX4 (cat. no. ab85204; 1:500; Abcam, Cambridge, MA,
USA) or rabbit anti-human GAPDH (cat. no. ab9485; 1:1,000; Abcam)
at 4°C overnight and then washed three times with
phosphate-buffered saline with Tween-20 (PBST). The nitrocellulose
membrane was then incubated with goat anti-rabbit IgG (H+L)-HRP
(cat. no. ab6721; 1:5,000; Abcam) at room temperature for 1 h and
washed with PBST three times. Finally, an electro-chemiluminescence
method was used to reveal the protein bands and Quantity One
software (version 4.6.3; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was utilized to analyze the gray values of protein bands.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM SPSS, Armonk, NY, USA). Comparison of
measurement data was performed using Student's t-test and the
results are expressed as the mean ± standard deviation. The
comparison of enumeration data was performed using a χ2
test, and the correlation between two samples in patients with
primary CML was determined using Spearman's method. P<0.05 was
considered to indicate a statistically significant difference.
Results
C/EBPα in patients with primary
CML
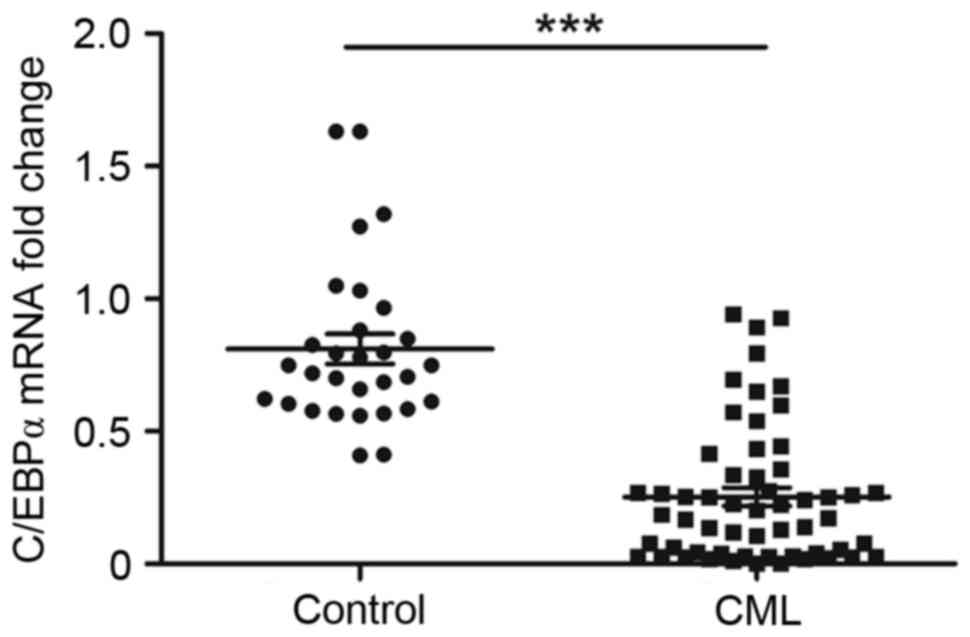
Based on the results of the RT-qPCR, the relative
mRNA level of C/EBPα was significantly lower in the patients with
primary CML, compared with that in the control group (0.253±0.034,
vs. 0.811±0.0563; P<0.01; Fig.
1).
Associations between the expression of
C/EBPα and gender, age, WBC and BCR/ABL in primary CML
To further elucidate the associations between the
expression of C/EBPα and known factors, including gender, age, WBC
levels and expression levels of BCR/ABL, the patients with primary
CML were divided into two groups according to
C/EBPα/β-actin<0.5, which was shown in all patients in the
control group, and Pearson's χ2 test was performed. The
results showed no significant association between the expression of
C/EBPα and gender (P>0.05). In addition, no significant
association was found between the expression of C/EBPα and age when
the patients were separated into two groups by their median age of
51.5 years (P>0.05). No significant association was identified
between the expression of C/EBPα and WBC levels when the patients
were divided into two groups by WBC>1×1010
(P>0.05). In addition, no significant association was found
between the expression of C/EBPα and BCR/ABL when the patients were
divided into two groups according to the expression of BCR/ABL
(P>0.05). The above results indicated that there were no
significant associations between the expression of C/EBPα and
gender, age, WBC levels or the expression of BCR/ABL in primary CML
(Table II).
 | Table II.Correlations between the expression
of C/EBPα and gender, age, WBCs and BCR/ABL. |
Table II.
Correlations between the expression
of C/EBPα and gender, age, WBCs and BCR/ABL.
| Index | Group | C/EBPα/β-actin
<0.5 | C/EBPα/β-actin
≥0.05 | χ2 | P-value |
|---|
| Gender | Male | 27 | 6 | 0.063 | 0.802 |
|
| Female | 19 | 5 |
|
|
| Age (years) | ≤51.5 | 22 | 7 | 1.774 | 0.183 |
|
| >51.5 | 25 | 3 |
|
|
| WBCs (n) |
≤1×1010 | 11 | 0 | 2.900 | 0.089 |
|
|
>1×1010 | 36 | 10 |
|
|
| BCR/ABL (%) | ≤100 | 25 | 4 | 0.574 | 0.449 |
|
| >100 | 22 | 6 |
|
|
Changes in the expression of C/EBPα in
primary CML following imatinib treatment
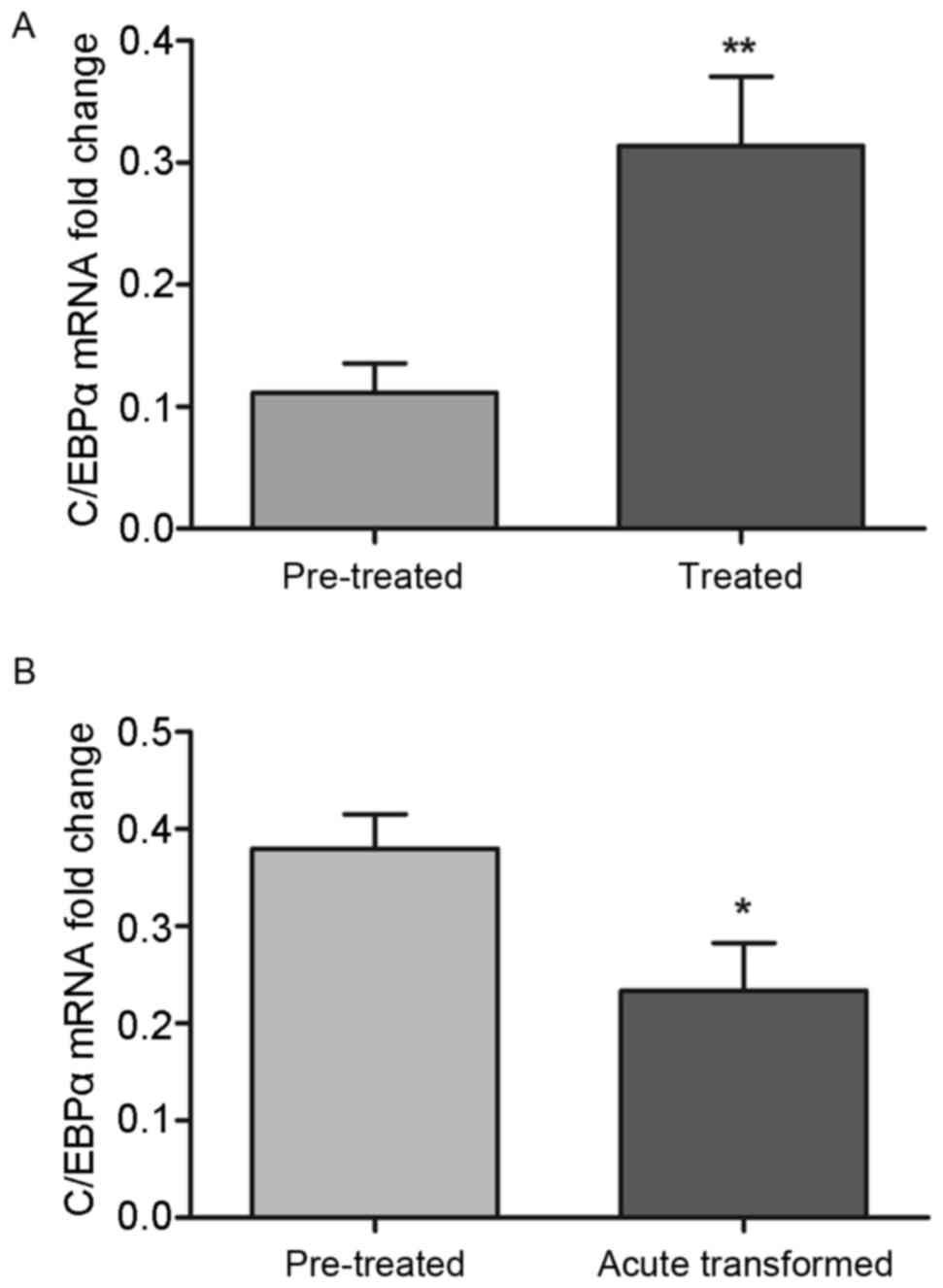
Among the enrolled patients, a total of 22 CML
patients accepted imatinib treatment. Following treatment, no
improvements in condition were observed in 13 patients with CML,
however, the expression of C/EBPα was significantly increased,
compared with that prior to treatment (0.314±0.0565, vs.
0.111±0.0242; P<0.01; Fig. 2A).
However, following treatment with imatinib, the expression of
C/EBPα remained lower than that in the control group (P<0.05).
In addition, nine cases of CML transformed into BP CML and the
expression of C/EBPα was significantly decreased following
treatment, compared with the control (0.234±0.0493, vs.
0.3798±0.0356; P<0.05; Fig.
2B).
SOX4 in patients with primary CML
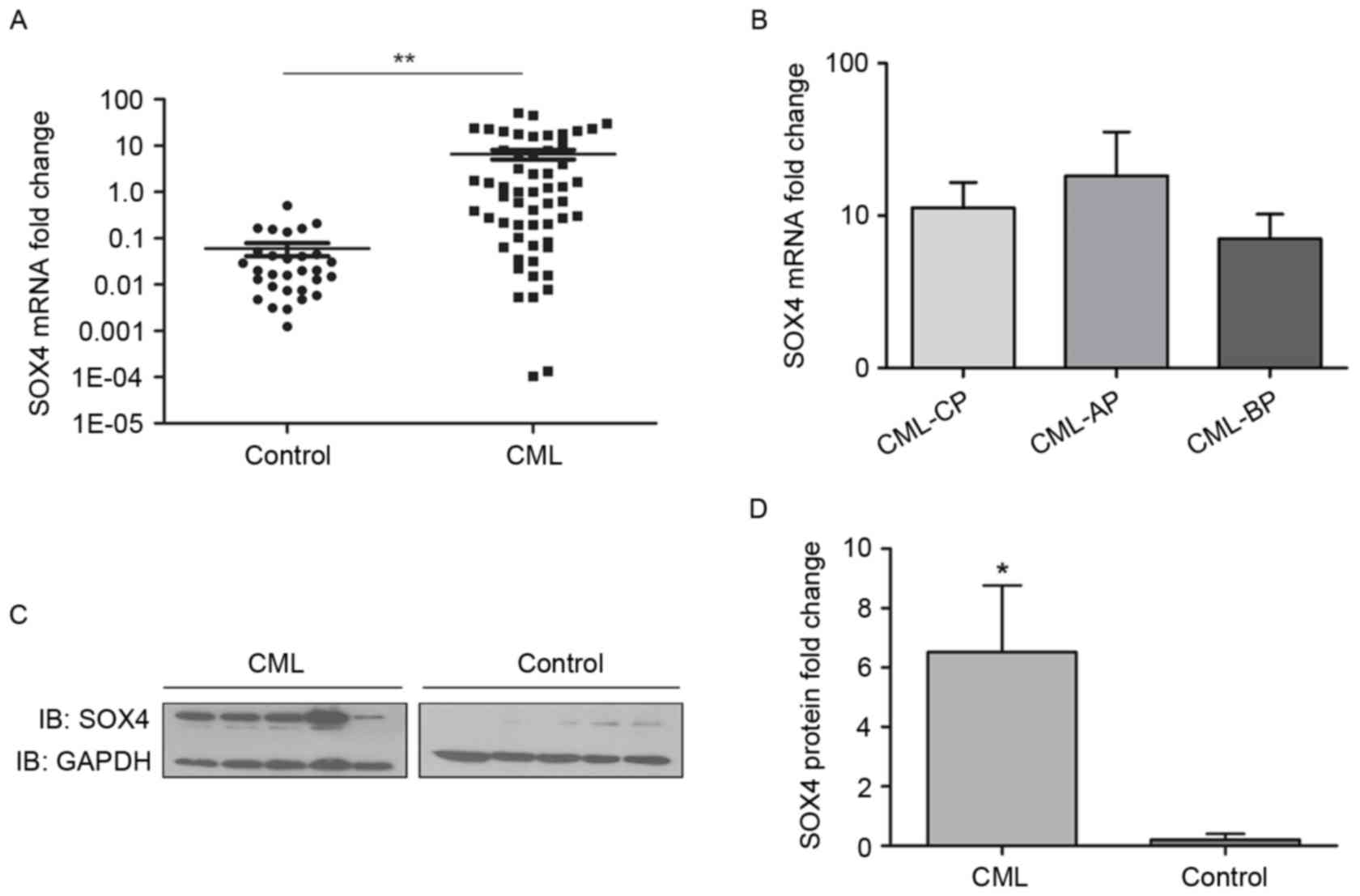
According to the results of the RT-qPCR analysis,
the mRNA level of SOX4 in the patients with primary CML was
significantly higher, compared with that in the control group
(6.546±1.495, vs. 0.0596±0.0187; P<0.01; Fig. 3A). Further subgroup investigation
found no significant differences in the mRNA levels of SOX among
the CML-CP, CML-AP and CML-BP patient groups (P>0.05; Fig. 3B). The protein level of SOX4 was
also determined using western blot analysis. Similar to the mRNA
levels, the protein expression of SOX4 in the primary CML group was
significantly higher, compared with that in the control group
(P<0.05; Fig. 3C and D).
Associations between the expression of
SOX4 and gender, age, WBC and BCR/ABL in primary CML
To investigate the associations between the
expression of SOX4 and known factors, including gender, age, WBC
levels and expression levels of BCR/ABL, the patients with primary
CML were divided into two groups according to SOX4/GAPDH <1,
which was shown in all patients in the control group. Pearson's
χ2 test was performed, and the results revealed there
was no significant association between the expression of SOX4 and
gender (P>0.05). In addition, no significant association was
found between the expression of SOX4 and age when the patients were
separated into two groups by the median age of 51.5 years
(P>0.05). In addition, no significant association was observed
between expression of SOX4 and WBC levels when the patients were
divided into two groups by WBC >1×1010 (P>0.05).
There was also no significant association between the expression of
SOX4 and BCR/ABL when the patients were divided into two groups
according to the expression of BCR/ABL (P>0.05). The above data
suggested that there were no significant associations between the
expression of SOX4 and gender, age, WBC levels or the expression
levels of BCLR/ABL in primary CML (Table III).
 | Table III.Correlations between the expression
of SOX4 and gender, age, WBCs and BCR/ABL. |
Table III.
Correlations between the expression
of SOX4 and gender, age, WBCs and BCR/ABL.
| Index | Group | SOX/GAPDH
<1 | SOX/GAPDH ≥1 | χ2 | P-value |
|---|
| Gender | Male | 15 | 16 | 0.211 | 0.646 |
|
| Female | 11 | 15 |
|
|
| Age (years) | ≤51.5 | 15 | 14 | 0.888 | 0.346 |
|
| >51.5 | 11 | 17 |
|
|
| WBCs (n) |
≤1×1010 | 3 | 9 | 2.604 | 0.107 |
|
|
>1×1010 | 23 | 22 |
|
|
| BCR/ABL (%) | ≤100 | 12 | 18 | 0.805 | 0.370 |
|
| >100 | 14 | 13 |
|
|
Changes in the expression of SOX4 in
primary CML following imatinib treatment
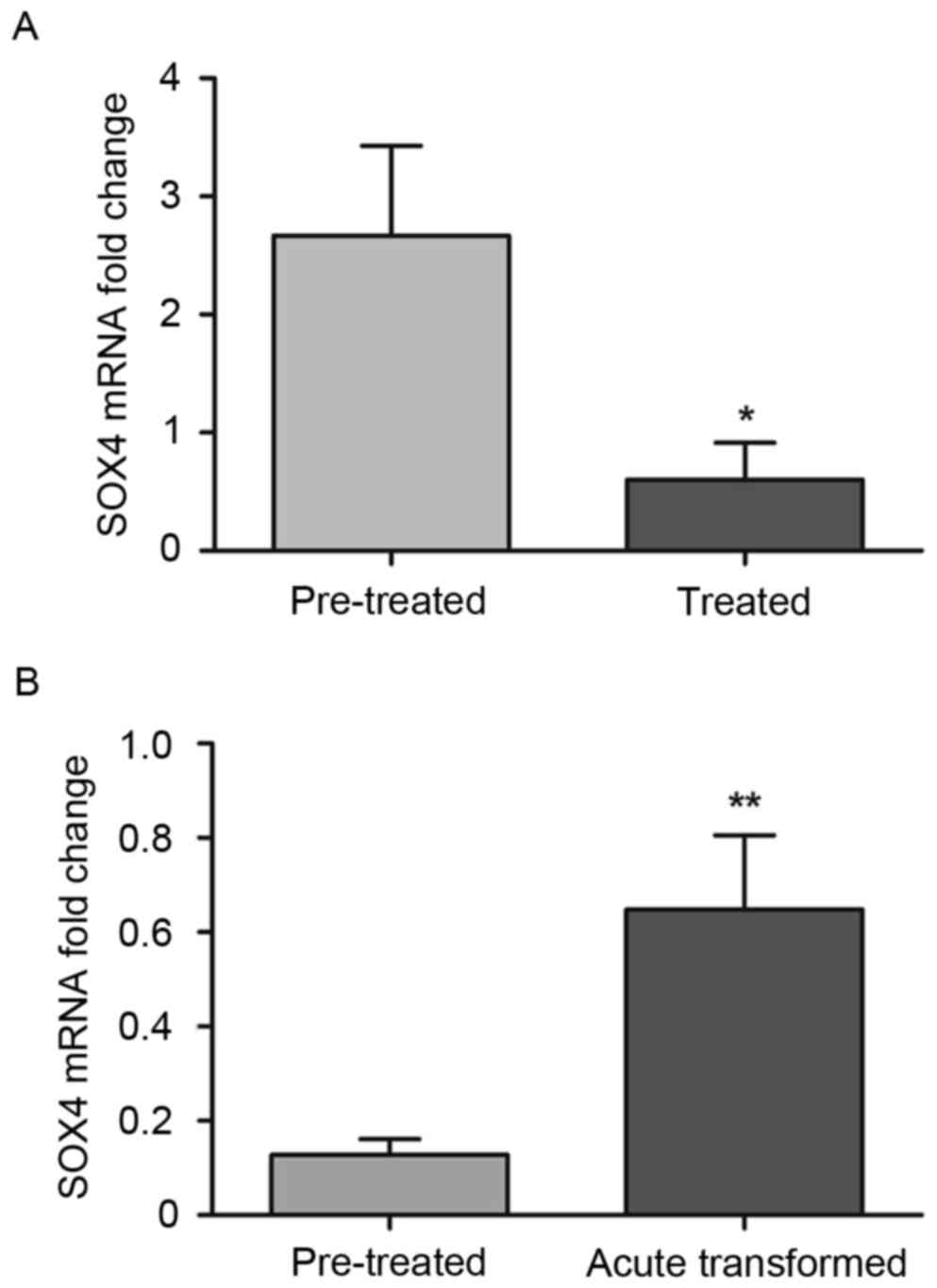
As with C/EBPα, the expression of SOX4 was also
detected following imatinib treatment. The expression of SOX4 was
significantly decreased following treatment, compared with
expression prior to treatment, in the 13 patients with CML who were
treated with imatinib (0.601±0.315, vs. 2.669±0.758; P<0.05;
Fig. 4A). However, following
treatment with imatinib, the expression of SOX4 was higher,
compared with that in the control group (P<0.05). The remaining
nine patients with CML developed BP CML following treatment, and
the expression of SOX4 was significantly increased compared with
the expression prior to treatment (0.648±0.157, vs. 0.128±0.0338;
P<0.01; Fig. 4B).
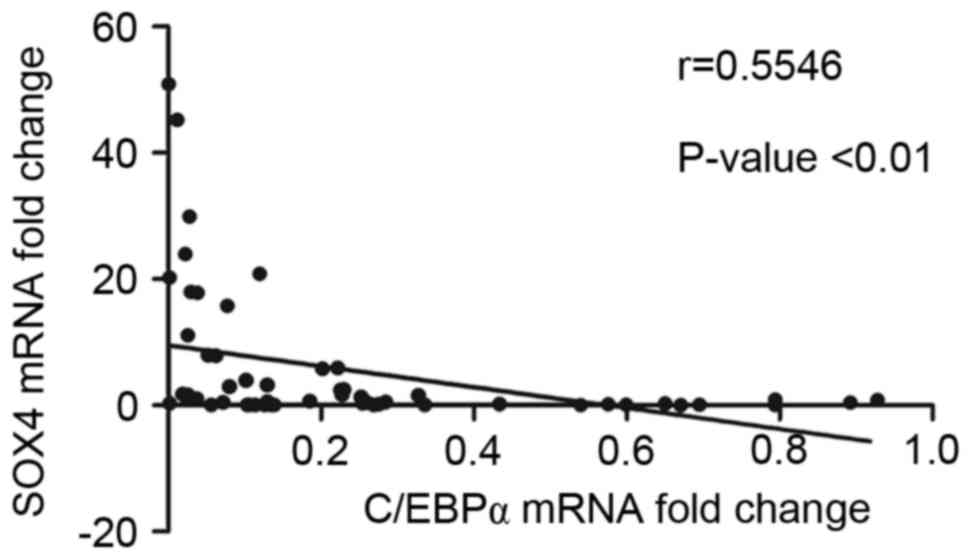
Correlation between the expression of
SOX4 and C/EBPα
To further determine the relevance of the expression
of SOX4 and C/EBPα, Spearman's correlation analysis was performed
in the 57 cases of primary CML. The analytical outcome revealed
that the expression of SOX4 was significantly negatively correlated
with the expression of C/EBPα in patients with CML (Fig. 5).
Discussion
CML is a myeloproliferative disorder originating
from hematopoietic stem cells with constitutive expression of the
BCR/ABL oncoprotein (19).
Imatinib was the first target drug approved by the US Food and Drug
administration for the treatment of CML, and remains a commonly
used drug in clinical therapy (20). In the present study, C/EBPα was
found to be markedly downregulated in patients with CML, compared
with healthy controls, whereas the opposite was found for SOX4. The
changes in the expression of C/EBPα and SOX4 were not associated
with the age, gender, WBC level or the expression of BCR/ABL.
Following treatment with imatinib, the expression of C/EBPα was
increased and the expression of SOX4 was decreased in 13/22
patients with CML, which were progression-free. The opposite was
observed in the expression levels of C/EBPα and SOX4 in the
remaining nine patients with CML, which developed into CML-BP. In
addition, the relative expression analysis showed that the
expression of C/EBPα was negatively correlated with that of SOX4 in
CML.
Myelopoiesis is the process by which myeloid
progenitor cells differentiate into myeloid cells, including
eosinophils, monocytes and granulocytes. C/EBPα is crucial in
addition to other myeloid transcription factors in this process
(21). A previous study reported
that mutation of the C/EBPα gene was present in ~5–14% of patients
with AML (10). The mutation or
deletion of C/EBPα may result in arrest of the transit of common
myeloid progenitors into granulocyte-monocyte progenitors, and lead
to a reduction in granulocytes and monocytes (22). In addition, deficiency of C/EBPα in
mice may induce disorder in myeloproliferation (19). In the present study, significantly
lower expression of C/EBPα was detected in patients with CML,
compared with that in normal controls. Epigenic modification is
recognized as an important mechanism in regulating the expression
of specific genes, which are associated with leukemogenesis
(23), and Chim et al
(24) reported that C/EBPα was
hypermethylated in its promoter in patients with AML. Therefore,
methylation may cause the downregulation of C/EBPα. This was
confirmed by Annamaneni et al (25), who reported that the aberrant
methylation of the C/EBPα promoter is a common event in CML. Theil
et al (26) demonstrated
that EZH2 acts as an oncogene to improve the methylation of C/EBPα,
and thus inhibit myeloid differentiation. Ubiquitination is another
essential mechanism for the downregulation of C/EBPα. Trib1 and 2
are two identified Tribbles family members, which can act as
adapters to recruit E3 ligases to mediate ubiquitin degradation and
inactivation (27). Therefore, the
downregulation of C/EBPα may be a result of methylation and
ubiquination, and this change may be crucial during the process of
CML.
SOX4 has been reported to be directly regulated by
C/EBPα, and to be important in the normal differentiation of
myeloid and lymphoid lineages (14). It has been shown that C/EBPα can
suppress the expression of SOX4 via directly binding to its
promoter, and the transition of leukemia caused by C/EBPα mutation
can be partially reversed by downregulating SOX4 (11,28).
The present study also revealed significantly upregulated mRNA and
protein expression levels of SOX in patients with CML, compared
with levels in normal controls, and SOX4 was negatively correlated
with C/EBPα. This suggested that the upregulated mRNA and protein
levels of SOX4 may have been caused by the downregulation of
C/EBPα. Aue et al (29)
demonstrated that the overexpression of SOX4 induces myeloid
leukemia via cooperating with the haplo-insufficiency of PU.1,
which is an important regulator of the proliferation and
differentiation of hematopoietic stem cells. The overexpression of
SOX4 can inhibit the differentiation of myeloid progenitor cells
(30). Ramezani-Rad et al
(17) revealed that SOX4 activates
the PI3K/AKT and MAPK signaling pathways to enhance survival
signaling, and these signaling pathways are required for the
survival, progression and proliferation of pre-B ALL. However,
whether this signaling pathway is associated with CML remains to be
elucidated and further investigations are required. Taken together,
these results indicate that aberrant hypermethylation of the C/EBPα
promoter may lead to the downregulation of C/EBPα, and this
downregulation may have a positive effect on the expression of
SOX4. The resulting excessive expression of SOX4 may lead to the
dys-proliferation of myeloid and lymphoid lineages, and result in
the occurrence and development of CML. In the present study, this
molecular pathway was referred to as the C/EBPα-SOX4 axis.
To further elucidate the correlation between the
C/EBPα-SOX4 axis and BCR/ABL, comparisons were made in 22 CML
patients who received imatinib treatment. The results showed that
the expression of SOX4 was decreased in the progression-free
patients, but increased in the acute transformed patients. By
contrast, the expression of C/EBPα was increased in the
progression-free patients, but decreased in the acute transformed
patients following treatment. Although the expression levels of
C/EBPα and SOX4 in 13 cases of CML improved, the stages of the
patients remained unchanged or worsened. It is known that imatinib
is a BCR/ABL-targeting drug, and the conditions of patients with
CML can be improved following therapy (31). However, the diagnostic results were
not in accordance with this. This indicates that the signaling
pathway of the C/EBPα-SOX4 axis involved in CML was not in
accordance with the signaling pathway of BCR/ABL. In addition, the
analyses of individual factors demonstrated that the expression
levels of C/EBPα and SOX4 were not correlated with the expression
of BCR/ABR. Therefore, the C/EBPα-SOX4 axis may be a novel
therapeutic target in CML, differing from the BCR/ABL target.
Another change in gene expression following imatinib
treatment requires mention. Following treatment for 3 months, the
expression of C/EBPα was significantly increased, compared with the
expression prior to treatment. However, the mean level remained
lower than that in the healthy controls. Although the expression of
SOX4 was decreased, the mean level remained higher than that in the
healthy controls. These results may indicate that SOX4 and C/EBPα
served critical roles in the treatment of CML with imatinib.
However, due to the short treatment duration, the overall condition
of the patients remained below that in the healthy controls.
Therefore, long-term evaluations of C/EBPα and SOX4 require
consideration in subsequent investigations.
In conclusion, the C/EBPα-SOX4 axis was found to be
important in the process of CML. Due to methylation, the expression
of C/EBPα was downregulated in CML and this downregulation induced
an upregulation in the expression of SOX4. However, these changes
were not correlated with the expression of BCR/ABL. Therefore, the
C/EBPα-SOX4 axis may be a novel therapeutic target for the
treatment of CML.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. ZR2015HM073).
The authors would like to thank the Biological Chip Laboratory of
Yantai Yuhuangding Hospital.
Glossary
Abbreviations
Abbreviations:
|
C/EBPα
|
CCAAT/enhancer binding protein α
|
|
CML
|
chronic myeloid leukemia
|
|
CP
|
chronic phase
|
|
AP
|
accelerated phase
|
|
BP
|
blastic phase
|
|
ALL
|
acute lymphoblastic leukemia
|
|
SOX4
|
SRY-related high mobility group box
containing transcription factor 4
|
|
CML-CP
|
chronic phase CML
|
|
CML-AP
|
accelerated phase CML
|
|
CML-BP
|
blastic phase CML
|
|
WBC
|
white blood cell
|
|
PB
|
peripheral blood
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S and Sawyers CL: Efficacy and safety of a specific
inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid
leukemia. N Engl J Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawyers CL: Chronic myeloid leukemia. N
Engl J Med. 340:1330–1340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pavlu J, Szydlo RM, Goldman JM and
Apperley JF: Three decades of transplantation for chronic myeloid
leukemia: What have we learned? Blood. 117:755–763. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howlader N, Noone AM, Krapcho M, Newman N,
Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2009 (Vintage 2009
Populations). National Cancer Institute; Bethesda, MD: Based on
November 2011 SEER data submission, posted to the SEER web site.
April. 2012, https://seer.cancer.gov/archive/csr/1975_2009_pops09/
|
|
5
|
Parker WT, Ho M, Scott HS, Hughes TP and
Branford S: Poor response to second-line kinase inhibitors in
chronic myeloid leukemia patients with multiple low-level
mutations, irrespective of their resistance profile. Blood.
119:2234–2238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apperley JF: Chronic myeloid leukaemia.
Lancet. 385:1447–1459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman AD: C/EBPα in normal and
malignant myelopoiesis. Int J Hematol. 101:330–341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeisig BB, Kulasekararaj AG, Mufti GJ and
So CW: SnapShot: Acute myeloid leukemia. Cancer Cell.
22:698.e12012. View Article : Google Scholar
|
|
9
|
Bhattaram P, Penzo-Méndez A, Sock E,
Colmenares C, Kaneko KJ, Vassilev A, Depamphilis ML, Wegner M and
Lefebvre V: Organogenesis relies on SoxC transcription factors for
the survival of neural and mesenchymal progenitors. Nat Commun.
1:92010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song G, Wang L, Bi K and Jiang G:
Regulation of the C/EBPα signaling pathway in acute myeloid
leukemia (Review). Oncol Rep. 33:2099–2106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Alberich-Jorda M, Amabile G, Yang
H, Staber PB, Di Ruscio A, Welner RS, Ebralidze A, Zhang J,
Levantini E, et al: Sox4 is a key oncogenic target in C/EBPα mutant
acute myeloid leukemia. Cancer Cell. 24:575–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuwahara M, Yamashita M, Shinoda K,
Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes
D, Iwamura C, et al: The transcription factor Sox4 is a downstream
target of signaling by the cytokine TGF-β and suppresses TH2
differentiation. Nat Immunol. 13:778–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aue G, Du Y, Cleveland SM, Smith SB, Davé
UP, Liu D, Weniger MA, Metais JY, Jenkins NA, Copeland NG and
Dunbar CE: Sox4 cooperates with PU.1 haploinsufficiency in murine
myeloid leukemia. Blood. 118:4674–4681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandoval S, Kraus C, Cho EC, Cho M, Bies
J, Manara E, Accordi B, Landaw EM, Wolff L, Pigazzi M and Sakamoto
KM: Sox4 cooperates with CREB in myeloid transformation. Blood.
120:155–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vervoort S, van Boxtel R and Coffer PJ:
The role of SRY-related HMG box transcription factor 4 (SOX4) in
tumorigenesis and metastasis: Friend or foe? Oncogene.
32:3397–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jafarnejad SM, Ardekani GS, Ghaffari M and
Li G: Pleiotropic function of SRY-related HMG box transcription
factor 4 in regulation of tumorigenesis. Cell Mol Life Sci.
70:2677–2696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramezani-Rad P, Geng H, Hurtz C, Chan LN,
Chen Z, Jumaa H, Melnick A, Paietta E, Carroll WL, Willman CL, et
al: SOX4 enables oncogenic survival signals in acute lymphoblastic
leukemia. Blood. 121:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suknuntha K, Ishii Y, Tao L, Hu K,
McIntosh BE, Yang D, Swanson S, Stewart R, Wang JY, Thomson J and
Slukvin I: Discovery of survival factor for primitive chronic
myeloid leukemia cells using induced pluripotent stem cells. Stem
Cell Res. 15:678–693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer S, Buchanan S and Ryan I: Tyrosine
kinase inhibitors for the treatment of chronic-phase chronic
myeloid leukemia: Long-term patient care and management. J Adv
Pract Oncol. 7:42–54. 2016.PubMed/NCBI
|
|
21
|
Porse BT, Bryder D, Theilgaard-Mönch K,
Hasemann MS, Anderson K, Damgaard I, Jacobsen SE and Nerlov C: Loss
of C/EBPα cell cycle control increases myeloid progenitor
proliferation and transforms the neutrophil granulocyte lineage. J
Exp Med. 202:85–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus
ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS,
Lekstrom-Himes JA, et al: Enhancement of hematopoietic stem cell
repopulating capacity and self-renewal in the absence of the
transcription factor C/EBP alpha. Immunity. 21:853–863. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baer C, Claus R, Frenzel LP, Zucknick M,
Park YJ, Gu L, Weichenhan D, Fischer M, Pallasch CP, Herpel E, et
al: Extensive promoter DNA hypermethylation and hypomethylation is
associated with aberrant microRNA expression in chronic lymphocytic
leukemia. Cancer Res. 72:3775–3785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chim C, Wong AS and Kwong YL: Infrequent
hypermethylation of CEBPA promotor in acute myeloid leukaemia. Br J
Haematol. 119:988–990. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Annamaneni S, Kagita S, Gorre M, Digumarti
RR, Satti V and Battini MR: Methylation status of CEBPA gene
promoter in chronic myeloid leukemia. Hematology. 19:42–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiel AT, Feng Z, Pant DK, Chodosh LA and
Hua X: The trithorax protein partner menin acts in tandem with EZH2
to suppress C/EBPα and differentiation in MLL-AF9 leukemia.
Haematologica. 98:918–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dedhia PH, Keeshan K, Uljon S, Xu L, Vega
ME, Shestova O, Zaks-Zilberman M, Romany C, Blacklow SC and Pear
WS: Differential ability of Tribbles family members to promote
degradation of C/EBPalpha and induce acute myelogenous leukemia.
Blood. 116:1321–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fung TK, Leung AY and So CW: Sox4you: A
new player in C/EBPα leukemia. Cancer cell. 24:557–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aue G, Du Y, Cleveland SM, Smith SB, Davé
UP, Liu D, Weniger MA, Metais JY, Jenkins NA, Copeland NG and
Dunbar CE: Sox4 cooperates with PU.1 haploinsufficiency in murine
myeloid leukemia. Blood. 118:4674–4681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du Y, Spence SE, Jenkins NA and Copeland
NG: Cooperating cancer-gene identification through
oncogenic-retrovirus-induced insertional mutagenesis. Blood.
106:2498–2505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanfstein B, Shlyaknto V, Lauseker M,
Hehlmann R, Saussele S, Dietz C, Erben P, Fabarius A, Proetel U,
Schnittger S, et al: Velocity of early BCR-ABL transcript
elimination as an optimized predictor of outcome in chronic myeloid
leukemia (CML) patients in chronic phase on treatment with
imatinib. Leukemia. 28:1988–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|