Introduction
Hepatitis B is the most common serious liver
infection worldwide. Chronic hepatitis B (CHB) is a major public
health concern, affecting ~240,000,000 individuals with chronically
infection, and leading to ~650,000 individuals each year succumbing
to CHB-associated mortality worldwide (1). The major complications of CHB are
cirrhosis and hepatocellular carcinoma (HCC) (2,3).
Antiviral agents with activity against hepatitis B virus (HBV) are
available. CHB can be treated with drugs, including interferons,
and antiviral agents, including nucleos(t)ide analogue (NA), which
have been shown to suppress HBV replication, prevent its
progression to cirrhosis, and reduce the risk of HCC (4). However, current available treatment
methods fail to eradicate the virus in the majority of those
treated. In addition, long-term NA therapy does not guarantee
protection against the development of HCC or CBH-associated
mortality in patients with cirrhosis (5).
Traditional Chinese medicine (TCM) is widely used in
China and is becoming increasingly prevalent in Europe and America.
Since ~1950, the scientific evaluation of herbs used for the
treatment of viral hepatitis has been undertaken in China,
including clinical investigations of complex formulas and isolated
components, isolation of active constituents, and assessment of
crude herb extracts and purified components in laboratory animals.
Several complex formulas and crude materials used in Chinese
medicines have been shown to have measurable activity against viral
hepatitis, including Xiaochaihu decoction (XCHD) (6–8).
XCHD, a TCM clinical prescription, has advanced the
treatment of hepatobiliary diseases in China (9,10).
Modified XCHD (mXCHD) has been used to treat CHB and has shown
clinical efficacy (11,12). XCHD is supplemented with
Phyllanthus urinaria (YeXiaZhu in Chinese), Largehead
atractylodes rhizome (BaiZhu in Chinese), Poria cocos
(FuLing in Chinese), white peony root (BaiShao in Chinese) and
Fructus Schisandrae (WuWeiZi in Chinese) to produce
mXCHD.
In clinical trials performed in China during the
last 20 years, the standards of effectiveness have usually
comprised a reduction in the serum levels of alanine transaminase
(ALT) and aspartate transaminase (AST) liver enzymes and HBV DNA,
and seroconversion from hepatitis B surface antigen
(HBsAg)+ or hepatitis B e antigen (HBeAg+ to
HBsAg− and HBeAg−, in addition to various
measures of symptom reduction. The actions of XCHD may be
subdivided into two categories: Hepatoprotective and antiviral
(13–18).
To further elucidate the possible molecular
mechanism underlying the effect of mXCHD in the treatment of CHB, a
serum pharmacological method was used in the present study to
investigate the effects of mXCHD on the proliferation of HepG2.2.15
cells, and on factors associated with the Janus kinase
(JAK)2/signal transducer and activator of transcription (STAT)3
signaling pathway in vitro.
Materials and methods
Materials and reagents
Minimum essential medium (MEM), fetal bovine serum
(FBS), sodium pyruvate, penicillin-streptomycin, sodium bicarbonate
solution and trypsin-EDTA were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). RNA Isolater Total RNA
Extraction Reagent, HiScript® II Q RT Supermix for qPCR
(+gDNA wiper), AceQ® qPCR SYBR®−Green Master
Mix, RIPA lysis buffer and a BCA protein quantification kit were
obtained from Vazyme Biotech Co., Ltd. (Nanjing, China). The
following antibodies were purchased from ProteinTech Group, Inc.
(Chicago, IL, USA): JAK2 polyclonal antibody, STAT3 monoclonal
antibody, and β-actin monoclonal antibody. Secondary antibodies,
including horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG (H+L) and HRP-conjugated goat anti-mouse IgG (H+L), were
obtained from Vazyme Biotech Co., Ltd.
Participants
The participants involved in the present study were
recruited among volunteers receiving a physical check-up,
outpatients and inpatients between January 2015 and May 2015 at the
Second Affiliated Hospital of the Fujian University of Traditional
Chinese Medicine (Fuzhou, China; 30 cases and 30 healthy
volunteers) and Xiamen Hospital of Traditional Chinese Medicine
(Xiamen, China; 30 cases).
Diagnosis, inclusion and exclusion
criteria for CHB and TCM differentiation
CHB was diagnosed according to the Guideline of
Prevention and Treatment for Chronic Hepatitis B (19) established by the Chinese Society of
Hepatology and Chinese Society of Infectious Diseases of the
Chinese Medical Association. The inclusion criteria for CHB were as
follows: Meets the diagnostic criteria of CHB; provision of a
signed informed consent form; age range between 18 and 60 years
old. The exclusion criteria were as follows: Cases of CHB combined
with another hepatitis virus; cases of chronic severe hepatitis and
cirrhosis; pregnant or lactating women; and individuals unable to
express their feelings clearly.
The standards of the liver stagnation and spleen
deficiency syndrome (LSSDS) for the use of TCMs in CHB were as
defined in the TCM syndrome differentiation standards of viral
hepatitis (Trial) (20) issued by
the Internal Medicine Department Committee of Liver Disease of the
Traditional Chinese Medicine Association. The major features
include distending pain of the lateral thorax, and abdominal
distension and loose stools. The minor features include chest
distress and depression, lassitude and fatigue, and a pink and
tooth-marked tongue. The inclusion criteria for LSSDS were as
follows: Cases with all major features; cases with the major
feature of chest distress and depression, and the minor features of
lassitude and fatigue, and a pink and tooth-marked tongue; and
cases with the major feature of abdominal distension and loose
stools, and the minor feature of chest distress and depression.
Ethics and consent
The study protocol conformed to the Helsinki
Declaration (21) and the research
regulations for Chinese clinical trials. The Ethics Committee of
Fujian University of Traditional Chinese Medicine reviewed and
approved the study protocol, and all participants signed informed
consent prior to participation in the study. Patients with CHB, who
met the LSSDS diagnostic criteria and were enrolled. All patients
were randomly divided into the mXCHD group and the western medicine
group, with 30 patients in each group.
Preparation of drugs
The components of XCHD and mXCHD are listed in
Table I. The Chinese herbs were
processed into formula granules at Beijing Tcmages Pharmaceutical
Co., Ltd. (Beijing, China). The production procedure was as
follows: Selection of the genuine regional drug; implementation of
modern pharmaceutical technology; use of Chinese herbal fragments
as raw materials according to traditional processing methods;
preparation of the granular formulation following single-herb
extraction; concentration and drying with the decoction as a
standard. As presented in Table
II, the quality of the granules met the Codex standard for
enterprise internal control standards (22).
 | Table I.Components of XCHD and mXCHD. |
Table I.
Components of XCHD and mXCHD.
| Chinese name | English name | Scientific name | mXCHD (g) | XCHD (g) |
|---|
| Chai Hu | Chinese thorowax
root | Bupleurum
chinense DC | 15 | 15 |
| Huang Qin | Scutellaria
baicalensis | Scutellaria
baicalensis Georgi. | 10 | 10 |
| Ban Xia | Pinellia
ternate | Pinellia
ternate (Thunb.) Breit. | 10 | 10 |
| Sheng Jiang | Ginger | Zingiber
officinale Rosc. | 10 | 10 |
| Ren Shen | Ginseng | Panax ginseng
C. A. Mey. | 10 | 10 |
| Da Zao | Ziziphi
Jujubae | Ziziphus
Jujubae Mill. var. inermis (Bge.) Rehd. | 10 | 10 |
| Gan Cao | Liquorice root | Radix
Glycyrrhizae | 10 | 10 |
| Ye Xia Zhu | Phyllanthus
urinaria | Phyllanthus
urinaria L. | 15 | – |
| Bai Zhu | Largehead
atractylodes rhizomze | Atractylodes
macrocephala Koidz. | 15 | – |
| Fu Ling | Poria
cocos | Poria cocos
(Schw.) Wolf. | 15 | – |
| Bai Shao | White peony
root | Paeonia
lactiflora Pall. | 10 | – |
| Wu Wei Zi | Fructus
schisandrae | Schisandra
chinensis (Turcz.) Baill. | 10 | – |
 | Table II.Quality standards of the
granules. |
Table II.
Quality standards of the
granules.
| Granule | Active
ingredients | Control
standard |
|---|
| Chinese thorowax
root | 95% ethanol
extract | 15% minimum |
| Pinellia
ternate | 95% ethanol
extract | 1%
minimum |
| Ginger | 95% ethanol
extract | 3%
minimum |
| Ziziphi
jujubae | 95% ethanol
extract | 20% minimum |
| Largehead
atractylodes rhizome | 95% ethanol
extract | 7%
minimum |
| Poria
cocos | 95% ethanol
extract | 1.5%
minimum |
| Scutellaria
baicalensis | Baicalin | 10% minimum |
| Ginseng | Total ginsenoside
Rb1 | 0.18%
minimum |
| Liquorice root | Ammonium
glycyrrhizinate | 5%
minimum |
| White peony
root | Paeoniflorin | 3%
minimum |
| Fructus
schisandrae | Schisandrin | 0.03%
minimum |
| Phyllanthus
urinaria | Total phenols | 5%
minimum |
Preparation of mXCHD-containing
serum
The participants in the mXCHD group were treated
with mXCHD (Table I) twice a day,
using one bag (10 g) each time, 30 min following breakfast and
dinner. The patients in the western medicine group were treated
with 0.5 mg of entecavir orally as a dispersible tablet (Chiatai
Tianqing Pharmaceutical Group, Lianyungang, China; cat. no.
141226201) daily. After 7 days, venous blood was obtained from all
individuals. An additiona l30 healthy volunteers donated their
venous blood for the study as a control. The sera were separated by
centrifugation at 1,006 × g at room temperature for 5 min and
stored at −80°C.
Cell culture
The HepG2.2.15 human hepatoma cell line stably
transfected with the HBV genome was obtained from the China Center
for Type Culture Collection (Wuhan, China). The cells were
maintained in MEM supplemented with 10% (vol/vol) FBS (Thermo
Fisher Scientific, Inc.), 1 µmol/ml sodium pyruvate (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Thermo Fisher Scientific, Inc.), adjusted to pH 7–7.2 with 7.5%
sodium bicarbonate. A humidified incubator with 5% CO2
was used to maintain the cells at 37°C.
Cell treatment and grouping
The HepG2.2.15 cells were randomly divided into the
following four groups: Healthy group, entecavir group, 10% mXCHD
group and 20% mXCHD group. The cells of the first three groups were
maintained in 10% (vol/vol) serum, respectively. The cells of the
fourth group were maintained in 20% (vol/vol) mXCHD-containing
serum. Following treatment with the appropriate serum, cell
proliferation was detected using an MTT assay. The mRNA and protein
levels of JAK2 and STAT3 were measured using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses, respectively.
Determination of HepG2.2.15 cell
viability using an MTT assay
The HepG2.2.15 cell suspension was inoculated into a
96-well plate at a density of 4.0×104 cells/ml in 0.1 ml
of medium. The cells were divided into the four groups: Healthy
group; entecavir-treated group; 10% mXCHD-treated group; 20%
mXCHD-treatedgroup. As described above, each group was treated with
the indicated human serum. The cells in each group were treated for
48 and 144 h at 37°C. At the end of the treatment, 20 µl of 5 mg/ml
MTT was added to each well, and the samples were incubated for an
additional 4 h at 37°C. The purple/blue MTT formazan precipitate
was dissolved in 100 µl of dimethylsulfoxide (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) in each well, and the optical
density value of each well was measured at 490 nm wavelength using
an ELISA plate reader (EXL800; BioTek Instruments, Inc., Winooski,
VT, USA).
RT-qPCR analysis
The HepG2.2.15 cells were cultured in a 6-well
culture plate (Costar) at a density of 4.0×104 cells/ml
in 2 ml of medium. The grouping of cells was as previously
described (healthy, entecavir-treated, 10% mXCHD-treated, and 20%
mXCHD-treated). Each group was treated with human serum. Following
treatment with the appropriate serum for 48 h, the cells in each
group were washed with PBS, and the total RNA was isolated using
Total RNA Extraction reagent (Vazyme Biotech Co., Ltd.). cDNA was
synthesized from 1.5 µg of the total RNA using a cDNA synthesis kit
(Vazyme Biotech Co., Ltd.). RT-qPCR was performed using a 20-µl
reaction mixture, which contained 10 µl of SYBR-Green Master Mix
(Vazyme Biotech Co., Ltd.) and 2 µl of cDNA with 0.2 µM of each of
the forward and reverse primers. The qPCR conditions were as
follows: 95°C preheating for 5 min, followed by 40 cycles of 95°C
for 10 sec and 60°C for 35 sec. To confirm the amplification
specificity, each qPCR product was evaluated by melting curve
analysis. The reaction was performed using an Applied Biosystems
7500 FastReal-Time PCR system (Thermo Fisher Scientific, Inc.). The
relative expression of the mRNA was calculated using the
2−ΔΔCq method (23).
β-actin mRNA was used as an internal standard, and each result was
normalized to the level of β-actin. The sequences of the primers
used in the present study are presented in Table III.
 | Table III.Primer sequences. |
Table III.
Primer sequences.
| Gene | Primer sequence
(5′-3′) | Amplicon length
(bp) | Annealing
temperature (°C) |
|---|
| JAK2 | Forward:
GCCTTCTTTCAGAGCCATCAT | 143 | 55 |
|
| Reverse:
GTGTAGGATCCCGGTCTTCAA |
|
|
| STAT3 | Forward:
GGAGGAGGCATTCGGAAAGTA | 120 | 55 |
|
| Reverse:
CTGCAGGTCGTTGGTGTCA |
|
|
| β-actin | Forward:
CCTGGCACCCAGCACAAT | 156 | 55 |
|
| Reverse:
GGGCCGGACTCGTCATAC |
|
|
Western blot analysis
Following treatment with the medicated serum for 48
h, the HepG2.2.15 cells were lysed with RIPA lysis buffer
containing protease and phosphatase inhibitor cocktails (Vazyme
Biotech Co., Ltd.). Following centrifugation at 13,000 × g for 15
min at 4°C, the supernatants were collected and the total protein
content was quantified using a bicinchoninic acid protein assay.
Proteins (30 µg per well) were separated by 8% SDS-PAGE, under the
following conditions: 25 V for 10 min, 80 V for 30 min and 120 V
for 70 min. Following electrophoresis, the proteins were
transferred onto polyvinylidine fluoride membranes (EMD Millipore,
Billerica, MA, USA) in 1-Step™ transfer buffer (Thermo Fisher
Scientific Inc.) using a semidry blotting system. The membranes
were blocked for 2 h with western blot blocking buffer (Beyotime
Institute of Biotechnology, Shanghai, China). The membranes were
then exposed to the primary antibodies against JAK2 (1:2,000; cat.
no. 17670-1-AP; ProteinTech Group, Inc.) and STAT3 (1:1,000; cat.
no. 60199-1-lg; ProteinTech Group, Inc) overnight at 4°C. β-actin
(1:1,000; cat. no. 60008-1-lg; ProteinTech Group, Inc) was used as
an internal control for protein loading. The membranes were then
washed in TBST and incubated with secondary HRP-conjugated
antibodies (Vazyme Biotech Co. Ltd; cat. no. SA00001-2) at a
1:5,000 dilution for 1 h at room temperature, and the membranes
were washed again in TBST. Finally, the antibody-bound protein
bands were detected using enhanced chemiluminescence, and images
were captured using a ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The greyscale value ratio
of the target protein to the internal control was used to measure
the relative concentrations of JAK2 and STAT3.
Enzyme-linked immunosorbent assay
(ELISA)
The cell culture supernatants in the four groups
were collected immediately and at 48 h post-intervention, and were
separated by centrifugation at 1,006 × g at room temperature for 3
min. The expression of HBsAg in the supernatant was detected using
ELISA according to the manufacturer's protocol by determining the
absorbance rate at 450 nm.
Statistical analysis
The values are expressed as the mean ± standard
deviation. Statistical analyses of the data were performed using a
paired t-test and a one-way analysis of variance with SPSS, version
23.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of mXCHD serum on HepG2.2.15
cell viability
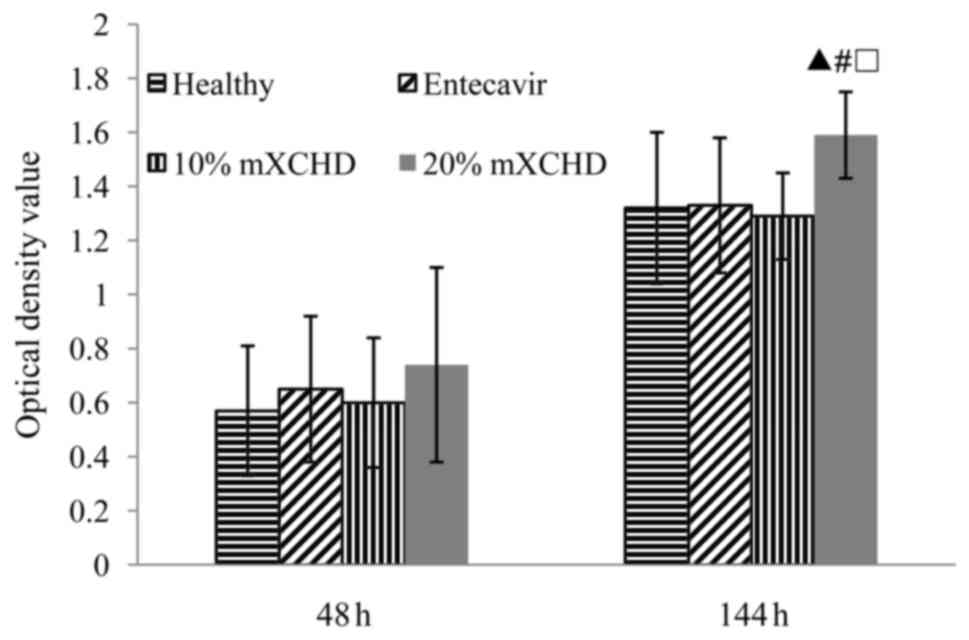
As shown in Fig. 1,
HepG2.2.15 cell proliferation was significantly higher in the 20%
mXCHD serum group in a time-dependent manner (P<0.01), compared
with the healthy group and 10% mXCHD group.
Effect of mXCHD serum on the
concentration of HBsAg in the cell supernatant
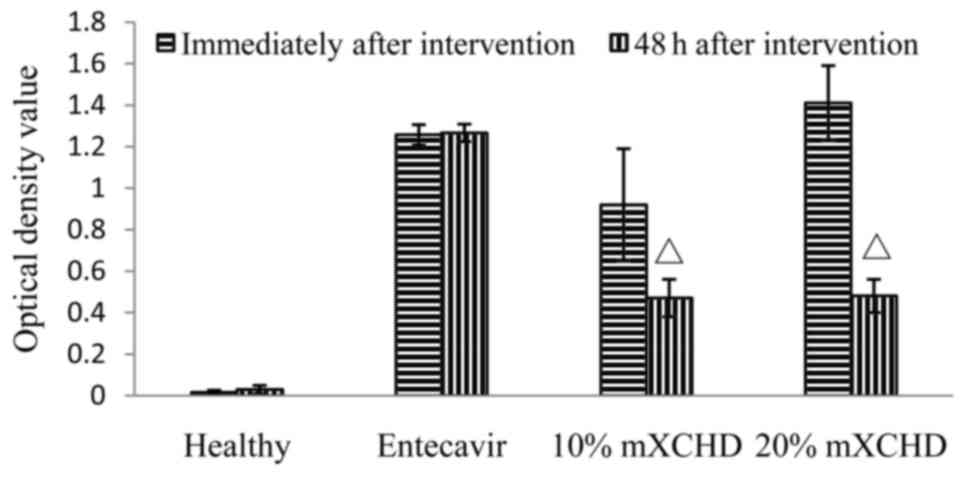
As shown in Fig. 2,
treatment with 10 and the 20% mXCHD serum led to decreases in the
concentration of HBsAg in the supernatants of the HepG2.2.15 cells.
The difference between the two time points, immediately following
and 48 h following intervention was significant in the 10 and 20%
mXCHD groups (P<0.01). These results suggested that mXCHD serum
inhibited the expression of HBV.
Effect of mXCHD serum on the mRNA
expression levels of JAK2 and STAT3 in HepG2.2.15 cells
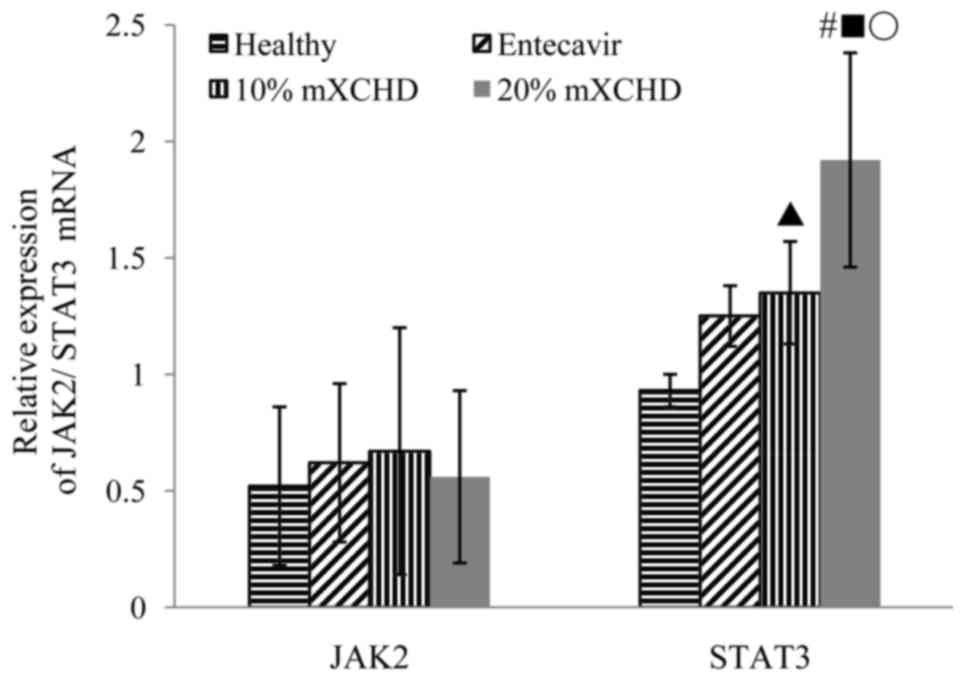
As shown in Fig. 3,
the mRNA expression levels of STAT3 were markedly higher in the 10
and 20% mXCHD groups, compared with those in the healthy group
(P<0.05 and P<0.01), whereas no significant differences in
JAK2 were observed in the four groups. In the 20% mXCHD group, the
mRNA expression level of STAT3 was higher, compared with that in
the entecavir group following treatment for 48 h (P<0.01). The
mRNA expression level of STAT3 in the 20% mXCHD group was highest.
These results suggested that mXCHD serum promoted the mRNA
expression of STAT3.
Effect of mXCHD serum on the protein
expression levels of JAK2 and STAT3 in HepG2.2.15 cells
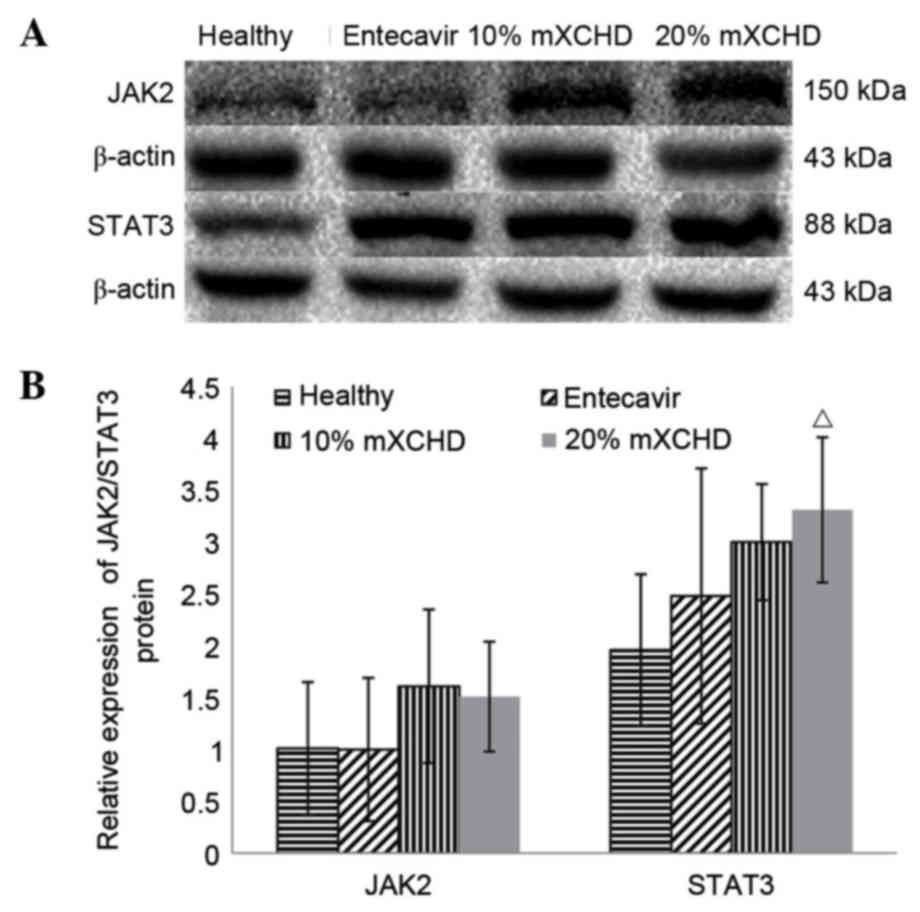
As shown in Fig. 4A and
B, the protein expression levels of JAK2 and STAT3 were similar
to their respective mRNA levels (Fig.
3) following treatment for 48 h. No significant differences
were observed in the protein expression levels of JAK2 among the
four groups. The protein expression level of STAT3 in the 20% mXCHD
group was significantly higher, compared with that in the healthy
group (P<0.05).
Discussion
CHB is a major disease, which affects millions of
individuals. The immune response induced by HBV is the primary
mechanism contributing to liver cell injury and inflammation. The
aims of current treatment for CHB are to achieve the sustained
suppression of HBV replication, remission of hepatic inflammation,
necrosis and fibrosis, and prevention of cirrhosis and HCC. A
clinical cure for CHB has always been the aim of clinicians,
involving a sustained virological response, the disappearance of
HBsAg, normalizing of ALT levels, and pathological improvements in
liver histology (24). Antiviral
treatment has progressed in previous years, however, its
application remains limited due to its high cost, prolonged
therapy, frequent relapse following therapy cessation, and various
side effects (25). As
investigations of traditional Chinese medicine have progressed, the
identification of drugs and therapies from TCMs with high
efficiency, low toxicity and anti-HBV effects has been a focus of
interest in medicine (26).
XCHD, derived from the classic TCM described in the
Treatise on Febrile Diseases (27)
has shown positive effects in the treatment of CHB in China, and it
is a classic treatment for promoting bile flow. Bupleurum
root and Scutellaria root are the core paired-component of
XCHD. These two herbs have notable effects against infection and
have antipyretic effects. There are other herbs in XCHD, including,
Pinellia ternate and ginger, which can regulate
gastrointestinal function. In addition, ginseng, Ziziphi
jujubae and liquorice root can improve immunity. Qin et
al (6) reported that,
according to the methods of a systematic review, XCHD appeared to
be effective at improving liver function and in the clearance of
serum HBV markers in patients with CHB. XCHD can regulate the
immune response, improve suppression and clearance of viruses, and
alleviate liver inflammation, and has an antifibrotic effects in
the liver.
mXCHD contains Chinese thorowax root, Scutellaria
baicalensis, Pinellia ternate, ginger, ginseng,
Ziziphi jujubae, liquorice root, Phyllanthus
urinaria, Largehead atractylodes rhizome, Poria cocos,
white peony root and Fructus schisandrae. Modern
pharmacology indicates that Phyllanthus urinaria possesses
significant anti-HBV and anticancer activities, in addition to
hepatoprotective effects. It is used primarily for the treatment of
HBV, and infection of the intestinal and urinary systems. Largehead
atractylodes rhizome, Poria cocos, white peony root and
Fructus schisandrae have hepatoprotective and antifibrotic
effects (28). In the present
study, HepG2.2.15 cells were cultured with the sera of patients
treated with mXCHD. Following treatment with the drug-containing
serum for 48 h, the cell viability, level of HBsAg, and expression
levels of JAK2 and STAT3 of the JAK/STAT signaling pathway were
detected.
Serum pharmacology is a novel method used to
investigate traditional Chinese herbs. This technique overcomes the
various disadvantages of applying drugs directly to cells.
Following the oral administration of traditional Chinese herbs,
various ingredients are absorbed into the blood through the
gastrointestinal tract and are transformed into bioactive
ingredients. Cells treated with serum containing TCMs in
vitro provide conditions similar to those of cells found in
vivo. Therefore, serum pharmacology experiments on Chinese
herbal medicines performed in vitro may produce results with
reliable consistency to corresponding experiments in vivo.
In the majority of associated investigations into serum
pharmacology, experimental animals, including rats and rabbits, are
administered with the drug through their feed, and animal serum
containing the drug is used to treat cells in culture in
vitro. In the present study, human serum containing the drug
was used under the preconditions of medical ethics approval and
provision of informed consent. Drugs may produce novel active
substances or stimulate the body to produce other active substances
by metabolism following absorption. Therefore, theresults of adding
the drug-containing serum in vitro are similar to those
observed in vivo, particularly for Chinese herbs. Following
oral absorption and metabolism of the anti-HBV TCM compound, the
human serum containing the drug was added to the HepG2.2.15 cell
culture, to more closely resemble the real status of drug
metabolism in vivo.
In the present study, the proliferation of
HepG2.2.15 cells was detected using an MTT assay following
treatment with a range of concentrations of mXCHD serum for 48 h.
The results demonstrated that serum containing mXCHD was not toxic
towards the cells, and it increased cell activity. Without drug
intervention, the level of HBV in the supernatant of cell cultures
increased with proliferation of the HepG2.2.15 cells. The
mXCHD-containing serum inhibited the expression of HBsAg at 10 and
20%. Following intervention with mXCHD, normal growth of the
HepG2.2.15 cells was maintained with no effect on cell
proliferation, and the HBsAg content of HBV in the supernatant was
also reduced. This indicated that HBV was effectively suppressed,
which is consistent with the results expected in the clinical
treatment of CHB. As drug-containing serum from HBV-infected
patients was used for cell culture in the present study, the HBsAg
concentration in the supernatant of the cell cultures in the mXCHD
group were not only from the HepG2.2.15 cells, but also the human
drug-containing serum of the patients with CHB. There was no HBV or
HBsAg in the sera of healthy volunteers, and their sera was used in
one of the cell groups as a healthy control group. The effect of
mXCHD on HBsAg was analyzed by comparing the changes in the level
of HBsAg prior to and following treatment with 10 and 20%
mXCHD-containing serum.
The JAK2/STAT3 signaling pathway transmits
information from extracellular chemical signals to the nucleus,
resulting in DNA transcription and the expression of genes involved
in immunity, proliferation, inflammation, apoptosis and
oncogenesis. The protein expression levels of several members of
this pathway can be observed in liver tissues. These proteins are
also involved in the wound-healing process in the liver. The liver
has a regenerative function, in which the proliferative and
regenerative function of hepatocytes promote repair of the injured
liver. It has been shown that STAT3 is closely associated with the
liver. With further investigations, the importance of STAT3 in the
protection of the liver is likely to be gradually elucidated. STAT3
has notable anti-apoptotic and mitogenic abilities, and it can
upregulate the expression of several genes associated with cell
survival and proliferation. When liver cells are damaged, the
expression of STAT3 increases following acute stress, the binding
force to DNA strengthens, and the expression of Cyclin D1 is
activated directly or indirectly. Cell mitosis then accelerates and
stimulates liver cell proliferation (29–36).
In the present study, the mRNA and protein
expression levels of STAT3 in cells were significantly upregulated
by culture in serum containing mXCHD, whereas those of JAK2 were
unaffected. It was hypothesized that mXCHD can promote the
upregulation of STAT3, and the latter is involved in the
wound-healing process in the liver. The promotion of the
proliferation and regeneration of hepatocytes through the
JAK2/STAT3 signaling pathway may be one of the important mechanisms
underlying the effect of mXCHD against CHB. The JAK2/STAT3
signaling pathway, particularly STAT3, has a regulatory role in
protection of the liver, which merits further investigation.
In conclusion, the present study demonstrated that
mXCHD not only inhibited HBV, but also stimulated hepatocyte
proliferation by upregulating the expression of STAT3. In the
present study, the two detection time points of 48 and 144 h
post-drug intervention were used. Due to limited funding, the
extent of the experiments was limited. Therefore, further
discussions and investigations are required, with the inclusion
ofadditional time points for detection. The data obtained improves
understanding of the effects and mechanisms of mXCHD in the
treatment of CHB. However, which of the components of this herbal
medicine contribute to these effects remains to be elucidated.
Therefore, further investigations of the individual components of
mXCHD are required in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81403331) and the
Natural Science Foundation of Fujian Province (grant no.
2014J01363).
References
|
1
|
World Health Organization: Guidelines for
the prevention, care and treatment of persons with chronic
hepatitis B infection. Geneva: WHO. 1–11. 2015.
|
|
2
|
Varbobitis I and Papatheodoridis GV: The
assessment of hepatocellular carcinoma risk in patients with
chronic hepatitis B under antiviral therapy. Clin Mol Hepatol.
22:319–326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Intaraprasong P, Siramolpiwat S and
Vilaichone RK: Advances in management of hepatocellular carcinoma.
Asian Pac J Cancer Prev. 17:3697–3703. 2016.PubMed/NCBI
|
|
4
|
Liaw YF, Kao JH, Piratvisuth T, Chan HL,
Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al:
Asian-Pacific consensus statement on the management of chronic
hepatitis B: A 2012 update. Hepatol Int. 6:531–561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai MC, Chen CH, Hu TH, Lu SN, Lee CM,
Wang JH and Hung CH: Long-term outcomes of hepatitis B
virus-related cirrhosis treated with nucleos (t)ide analogs. J
Formos Med Assoc. 115:883–889. 2016.PubMed/NCBI
|
|
6
|
Qin XK, Li P, Han M and Liu JP: Xiaochaihu
Tang for treatment of chronic hepatitis B: A systematic review of
randomized trials. Zhong Xi Yi Jie He Xue Bao. 8:312–320. 2010.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao F, An LF, Ma YQ and Yang KF: Efficacy
of singly Chinese herbal drugs for hepatitis B virus in vitro and
in vivo: A systematic review. Chinese Traditional Patent Medicine
(Chin). 37:2282–2285. 2015.(In Chinese).
|
|
8
|
Bai Y, Xia FN, Zhou SH, Yu KH and Liu JH:
Regulating effect of traditional Chinese medicines for Enh II and
core promoter of hepatitis B virus. Lishizhen Medicine and Materia
medica Research (Chin). 26:1534–1536. 2015.(In Chinese).
|
|
9
|
Wang Y: Effect of Xiaochaihu decoction
combined with western medicine on liver function and HBV DNA copies
in patient with chronic hepatitis B. Journal of New Chinese
Medicine (Chin). 48:74–76. 2016.(In Chinese).
|
|
10
|
Chen DD and Gong ZJ: The clinical study of
Xiaochaihu decoction in treatment of chronic hepatitis B patients
with liver fibrosis. Modern Journal of Integrated Traditional
Chinese and Western Medicine (Chin). 20:3928–3929. 2011.(In
Chinese).
|
|
11
|
Zhao CH: Clinical observation on 40 cases
of HBeAg-positive chronic hepatitis B treated with modified
Xiaochaihu decoction combined with adefovior. Henan Traditional
Chinese Medicine (Chin). 34:1924–1925. 2014.(In Chinese).
|
|
12
|
Wang MH, Zheng DR and Cheng Q: Clinical
Research of modified Xiaochaihu decoction combined with adefovior
in treatment of chronic hepatitis B patients. Journal of Community
Medicine (Chin). 12:30–31. 2014.(In Chinese).
|
|
13
|
Xiao-qiu Liu, Xiao-jian Hu, Hong-Xing Xu
and Xiao-Ying Zeng: Xiaochaihu decoction attenuates the vicious
circle between the oxidative stress and the ALP inactivation
through LPS-catecholamines interactions in gut, liver and brain
during CCI4+ethanol-induced mouse HCC. BMC Complement
Altern Med. 13:3752013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XB, Wang J, Zhang QQ, Liu T, Dang TM
and Cao YM: The protective effect of XD in ConA-induced liver
injury. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 26:1193–1194. 2010.(In
Chinese). PubMed/NCBI
|
|
15
|
Li J, Xie M and Gan Y: Effect of
Xiaochaihu decoction and different herbal formulation of component
on inhibiting H22 liver cancer in mice and enhancing immune
function. Zhongguo Zhong Yao Za Zhi. 33:1039–1044. 2008.(In
Chinese). PubMed/NCBI
|
|
16
|
Sun MY, Xie M, Zhang N, Yi L and Wang S:
Effect of xiaochaihu decoction and different herbal formulations of
its components on cytokines of carrageenan induced pleuritis in
rats. Zhongguo Zhong Xi Yi Jie He Za Zhi (Chin). 24:628–631.
2004.(In Chinese).
|
|
17
|
Liu Z, Xiong M and Zhang H: Experimental
study on inhibitory effect of xiaochaihu decoction on duck
hepatitis B virus. Zhongguo Zhong Xi Yi Jie He Za Zhi. 20:853–855.
2000.(In Chinese). PubMed/NCBI
|
|
18
|
Sun XH, Wang ZL and Liu ZJ: Effect of
modified Xiaochaihu decoction on HBV-DNA levels in sera and liver
tissue in transgenic mice. Chinese Journal of Integrated
Traditional and Western Medicine on Digestion (Chin). 19:91–93.
2011.(In Chinese).
|
|
19
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases, Chinese Medical Association: The
guideline of prevention and treatment for chronic hepatis B (2010
version). Zhonghua Gan Zang Bing Za Zhi. 19:13–24. 2011.(In
Chinese). PubMed/NCBI
|
|
20
|
The Internal Medicine Department Committee
of Liver Disease in Chinese Traditional Chinese Medicine
Association: TCM syndrome differentiation standards of Viral
hepatitis (Trial). Journal of Traditional Chinese Medicine (Chin).
5:39–40. 1992.(In Chinese).
|
|
21
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China (volume 4, 2015
version). Beijing: China Medical Science Press; pp. 7–151. 2015,
(In Chinese).
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boucle S, Bassit L, Ehteshami M and
Schinazi RF: Toward elimination of Hepatitis B virus using novel
drugs, approaches and combined modalities. Clin Liver Dis.
20:737–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeng WJ, Chen YC, Sheen IS, Lin CL, Hu TH,
Chien RN and Liaw YF: Clinical relapse after cessation of tenofovir
therapy in Hepatitis B e antigen-negative patients. Clin
Gastroenterol Hepatol. 14:1813–1820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia J, Inagaki Y, Song P, Sawakami T,
Kokudo N, Hasegawa K, Sakamoto Y and Tang W: Advance in studies on
traditional Chinese medicines to treat infection with the hepatitis
B virus and hepatitis C virus. Biosci Trends. 10:327–336. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong MQ, Wang QG, Guan QZ, et al: Science
of Exogenous Febrile Diseases. Beijing: China Press of Traditional
Chinese Medicine; pp. 263–272. 2003, (In Chinese).
|
|
28
|
Gu JP, Wang CL, Zhang FF, et al:
Pharmacology of Traditional Chinese Medicine. Shanghai: East China
University of Science and Technology Press; pp. 110–229. 2015, (In
Chinese).
|
|
29
|
Li L, Lei QS, Zhang SJ, Kong LN and Qin B:
Suppression of USP18 potentiates the anti-HBV activity of
interferon Alpha in HepG2.2.15 cells via JAK/STAT signaling. PLoS
One. 11:e01564962016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu X, Xie C, Li YM, Huang ZL, Zhao QY, Hu
ZX, Wang PP, Gu YR, Gao ZL and Peng L: TMEM2 inhibits hepatitis B
virus infection in HepG2 and HepG2.2.15 cells by activating the
JAK-STAT signaling pathway. Cell Death Dis. 7:e22392016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan SH, Yang K, Lu MJ, Lu YP and Yang DL:
The influence of HBV and its antigens on the expressions of
JAK-STAT signal transduction pathway molecules and antiviral
proteins of IFN alpha. Zhonghua Gan Zang Bing Za Zhi. 19:440–444.
2011.(In Chinese). PubMed/NCBI
|
|
32
|
Bock CT, Toan NL, Koeberlein B, Song le H,
Chin R, Zentgraf H, Kandolf R and Torresi J: Subcellular
mislocalization of mutant hepatitis B X proteins contributes to
modulation of STAT/SOCS signaling in hepatocellular carcinoma.
Intervirology. 51:432–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Churin Y, Roderfeld M, Stiefel J, Würger
T, Schröder D, Matono T, Mollenkopf HJ, Montalbano R, Pompaiah M,
Reifenberg K, et al: Correction: Pathological impact of hepatitis B
virus surface proteins on the liver is associated with the host
genetic background. PLoS One. 10:e01273752015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Liang X, Kellendonk C, Poli V and
Taub R: STAT3 contributes to the mitogenic response of hepatocytes
during liver regeneration. J Biol Chem. 277:28411–28417. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwabe RF, Bradham CA, Uehara T, Hatano
E, Bennett BL, Schoonhoven R and Brenner DA: c-Jun-N-terminal
kinase drives cyclin D1 expression and proliferation during liver
regeneration. Hepatology. 37:824–832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen XL, Meng Q and Liu KX: Role of STAT3
signaling pathway in repair of liver injury. World Chinese Journal
of Digestology (Chin). 22:1051–1057. 2014.(In Chinese). View Article : Google Scholar
|