Introduction
Chronic periodontitis is caused by a wide range of
microorganisms and involves an inflammatory process of bacterial
origin, which affects periodontal tissues and provokes the
destruction of supporting tissues of the teeth. The ultimate goal
of periodontal therapy is to regenerate structures diminished by
the disease (1); thus, development
of an antimicrobial agent and method to support regeneration of
periodontal supporting tissue to protect regenerated periodontal
tissue in infectious environments is required.
Defensins, a family of antimicrobial peptides, are
vital contributors to host immune responses due of their ability to
quickly and specifically recognize and neutralize invading
microorganisms. Among the known human β-defensins (HBDs), human
β-defensin-3 (HBD-3) is widely expressed in healthy individuals at
readily detectable levels, and exerts strong antibacterial and
immunomodulatory activities; whereas, its levels are markedly
reduced in the gingival crevicular fluid of individuals with
periodontitis (2). An in
vitro study conducted by Lee et al (3) demonstrated that the
periodontopathogens, P. intermedia and T. forsythia,
have distinct susceptibilities to HBD-3. Furthermore, we previously
reported that HBD-3 has important antibacterial activity and may
have a role in inducing fibroblast proliferation, promoting
periodontal regeneration (4).
Thus, it is reasonable to hypothesize that increasing HBD-3
expression in periodontal tissue may contribute to periodontal
regeneration.
Conventional treatments of periodontitis, including
proper oral hygiene and scaling/root planing, aim to prevent the
disease, and slow or stop its progress, however, they are usually
insufficient to promote the regeneration of damaged structures
(5). Cell sheet technology (CST),
a novel alternative to conventional cell delivery methods, is a
recently developed scaffold-free strategy that is also referred to
as ‘cell sheet engineering’ (6).
CST has been widely applied for tissue and organ reconstruction in
a diverse array of tissues, including the cornea (7), esophagus (8), trachea (9), liver (10) and heart (11). Cell-based therapies have also been
used to achieve periodontal regeneration, as periodontal ligament
cells (PDLCs) have the capacity to function as osteoblasts or
cementoblasts under regenerative conditions (12,13).
Antimicrobial gene therapy is useful for protecting
regenerated tissues/organs from infection, and reducing the need
for conventional antibiotics that induce drug resistance (14). HBD-3 gene transfection was
demonstrated to protect against bacterial invasion (15). Although the addition a drug is an
easy for clinical use, local gene therapy has the potential to
provide more sustained protein production, deliver protein in a
physiological manner and allow the development of biological
cellular delivery vehicles that can enhance bone repair (16). The combination of tissue
engineering and antimicrobial gene therapy could potentially work
hand-in-hand to make tissue repair more successful as infection is
always a major risk factor during tissue restoration (14).
The aim of the present study was to investigate
whether transfected PDLC sheets expressed HBD-3. Further,
anti-inflammatory activity of the transfected cell sheets were
evaluated in a beagle dog model of periodontitis to determine
whether they provide a suitable method of promoting periodontal
tissue healing and regeneration. Associated biomarkers were
evaluated via immunohistochemistry (IHC).
Materials and methods
Animals
Seven female beagle dogs (12 months of age) were
used in the current study. The study was approved by the Animal
Experiments Ethics Committee of Shanghai Jiao Tong University
School of Medicine (Shanghai, China). All of the experimental dogs
had optimal periodontal health prior to the study.
Cells isolation and culture
PDLCs were isolated from the anterior teeth of each
dog via enzyme digestion. The isolated PDLCs were cultured in
expansion medium composed of α-minimum essential medium (α-MEM)
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), ascorbic acid
phosphate ester and 1% antibiotic-antimycotic solution (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
of 95% air and 5% CO2.
Recombinant plasmid construction and
transfection
HBD-3 DNA was obtained from our previous study
(17). The HBD-3 gene and the
green fluorescent protein (eGFP) gene were cloned into the
pLV.Des3d. P/puro vector [all from Cyagen Biosciences (Guangzhou),
Inc., Guangzhou, China]. E. coli Stbl3 was used as the host.
For amplification, the primer sequences were as follows:
attB1-Kozak-Humacalx-F
5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGCCACCATGGGCTTCCAAAAGTTCTCCC-3′;
attB2-Humacalx-R,
5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTAGTTGGCATTCTGGGGCATG-3′;
attB1-Kozak-PTH-F,
5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGCCACCATGATACCTGCAAAAGACATGGC-3′;
attB2-PTH-R,
5′-GGGGACCACTTTGTACAAGAAGCTGGGTTCACTGGGATTTAGCTTTAGTTAATAC-3′
(Sangon Biotech Co., Ltd., Shanghai, China). The polymerase chain
reaction (PCR) amplification system included 5X primer STAR™ buffer
(Mg2+ Plus, 10 µl), dNTP mixture (4 µl) (both from
Takara Bio, Inc., Otsu, Japan), forward primer 1 µl, reverse primer
1 µl, DNA 1 µl, primer STAR™ HS DNA polymerase (0.5 µl; Takara Bio,
Inc.) and ddH2O. The PCR amplification conditions were
as follows: 98°C for 3 min, then 30 cycles of 98°C for 30 sec, 60°C
for 30 sec and 72°C for 1 min, and a final extension of 72°C for 5
min.
The recombinant plasmid pLV.EX3d.
P/puro-EF1A-Humacalx-IRES/eGFP was constructed using Gateway
Technology (Invitrogen; Thermo Fisher Scientific, Inc.). The
recombinant plasmid pLV.EX3d. P/puro-EF1A-PTH-IRES/eGFP was
constructed without HBD-3. The constructed expression plasmids were
amplified in the E. coli Stbl3 strain.
The 293T cells [American Type Culture Collection
(ATCC), Manassas, VA, USA] used in transfection experiments were
cultured and passaged in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% FBS (both from Gibco, Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C and 5% CO2. The lentiviral vector containing
HBD-3 was first transfected into 293T packaging cells. Following 8
h, the medium was replaced with fresh medium supplemented with 10%
FBS. The viral particles were collected 48 h following plasmid
transfection and were concentrated at 1,000 × g and 4°C for 15 min,
with filtration at 0.45 µm to remove cell debris in the culture
supernatant. The viral particles were then further concentrated by
centrifugation at 5,000 × g for 90 min at room temperature. The
obtained virus titer was approximately 6×109 TU/ml
(cells transfected with HBD-3 and GFP) and 2×109 TU/ml
(cells transfected with GFP only). The transfected PDLCs were
cultured in DMEM and incubated with cells in 5% CO2 at
37°C. The cells were then incubated for an additional 72 h. The
transfection efficiency was calculated using a fluorescence
microscope.
Western blot analysis
The GFP-HBD-3-PDLCs and GFP-PDLCs were cultured in
60 mm dishes until 80–90% confluency was reached. They were then
collected with radioimmunoprecipitation lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) and quantified using a
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). Total
proteins (20 µg/lane) were separated by 5–15% SDS-PAGE and
transferred to PVDF membranes. Membranes were blocked with 5%
nonfat milk for 1 h at room temperature and incubated overnight at
4°C with the corresponding primary antibodies: Anti-β-actin (cat
no. ab1801; 1:1,000) and anti-β-defensin-3 antibody (cat no.
ab19270; 1:1,000) (both from Abcam, Cambridge, UK). They were then
incubated with a goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (cat no. KC-RB-035;
1:5,000; Shanghai Kangchen Biotechnology Co., Ltd., Shanghai,
China) for 1 h at room temperature. After washing in PBST (PBS
containing 0.1% Tween-20), the membranes were developed using an
EZ-ECL detection kit following the manufacturer's instructions
(Biological Industries Israel Beit Haemek Ltd., Beit Haemek,
Israel) and were then imaged using a UVitec gel documentation
system (UVitec Limited, Cambridge, UK).
Generation of PDLC sheets
GFP-HBD-3-PDLCs and GFP-PDLCs were plated into
temperature-responsive culture dishes and cultured at 37°C in α-MEM
supplemented with 10% FBS, ascorbic acid phosphate ester and 1%
antibiotic-antimycotic solution. When the cells reached 100%
confluence, the dishes were transferred into an incubator set at
20°C to induce the formation of cell sheets. The resultant cell
sheets were harvested and fixed with 10% neutral formalin
solution.
ELISA
The culture supernatants were collected, and the
amount of secreted HBD-3 was detected using an HBD-3 ELISA kit (cat
no. JL19214; Shanghai Jiang Lai Biotechnology Co., Ltd., Shanghai,
China) according to the manufacturer's instructions. Absorbance at
490 nm was determined with a microplate reader (BioTek ELx800;
BioTek Instruments, Inc., Winooski, VT, USA). Each sample was
analyzed in triplicate.
Antimicrobial activity assay
Microbial strains and culturing
conditions
Actinomyces viscosus (ATCC 19246); Candida
albicans (ATCC 10231); Porphyromonas gingivalis (ATCC
33277) and Streptococcus mutans (UA 159) were used to test
the antimicrobial activity of the secreted HBD-3. All the strains
were gifts from the Shanghai Research Institute of Stomatology. The
strains were subcultured in brain heart infusion (BHI; BBL™
CHROMagar™; BD Biosciences, Franklin Lakes, NJ, USA) agar plates at
37°C in an aerobic (5% CO2 and 95% air) or anaerobic
(80% N2, 10% H2 and 10% CO2)
system for 24 h.
In vitro antimicrobial activity of
HBD-3 protein
Cell-free culture supernatants from the transfected
PDLC sheets were collected 48 h after transfection and were
serially diluted. Subsequently, 100 µl suspension of the four
microbial strains (106 CFU/ml) were inoculated in BHI
broth supplemented with 100 µl diluted supernatants at 37°C for 48
h. Microbial growth was assessed by measuring the optical density
(OD) at 590 nm with a microplate reader. All experiments were
performed in triplicate. Medium alone and untreated cells were used
in the control groups.
The four microbial strains in cell-free culture
supernatants from 35 mm dish were diluted 106, and 100
µl of the solution were spread on BHI agar plates at 37°C. The
number of colony-forming units (CFU) was counted after 48 h
incubation. All experiments were performed in triplicate. Medium
alone and untreated cells were used in the control groups.
Experimental periodontitis model
The experimental dogs were fed a high sugar, high
starch diet (daily diet of dog food mixed with 100 g sugar and 20 g
mashed potatoes). In addition, elastic ligatures were subgingivally
placed around the necks of all 3rd and 4th premolars (P3, P4) and
all 1st molars (M1) to induce experimental periodontitis. The
development and progression of periodontitis was monitored for 1
month.
Transplantation of PDLC sheets
Following intraperitoneal anesthesia and elevation
of the buccal mucoperiosteal flap, the alveolar bone of the dogs
was removed to create experimental periodontal bone defects. The
defect was 5 mm in width, 45 mm in length, and 3 mm in depth,
prepared from P3 to mesial root of M1.
The four sites of experimental periodontitis in each
dog were randomly assigned into the following three experimental
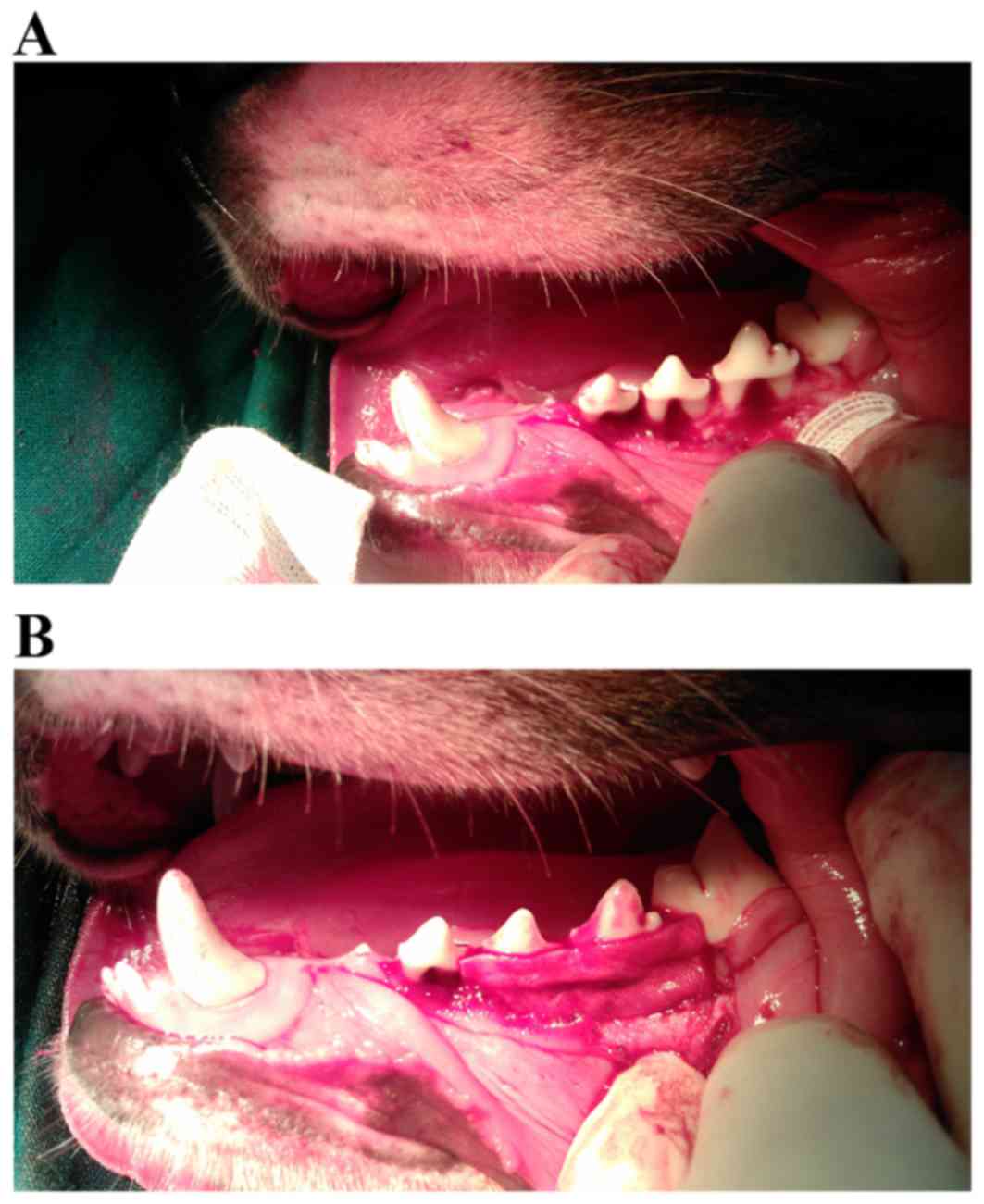
groups: Test group, transplanted with GFP-HBD-3-PDLC sheets
(Fig. 1); control group,
transplanted with GFP-PDLC sheets (Fig. 1); and untreated group, which
received no treatment. The grouping results were as follows: 28
sites were involved in the study and there were 9, 12 and 7 sites
assigned into the test group, the control group and the untreated
group, respectively. To ensure that a uniform thickness was
maintained between the different constructs, the same layers of
cell sheets were used, and all procedures were completed by the
same person. Following the surgery, all dogs used in this
experiment received 4×106 units amoxicillin
intramuscularly daily for 7 days.
Tissue preparation and IHC
Inflammation during the healing period was observed
2 weeks following transplantation in the diseased regions of the
untreated and control groups. Assessments were made by eye using
clinical observations including, gingival swelling, periodontal
overflow pus and loose teeth. At 8 weeks after the transplantation
with cell sheets, the periodontitis model dogs were sacrificed and
the alveolar bones of experimental sites were harvested. After
excision, fixation in 4% paraformaldehyde for 24 h at room
temperature and demineralization in 10% EDTA for 10 weeks at room
temperature, buccal-lingual histologic specimens from P3, P4 and
M1, each containing a tooth and its associated periodontium, were
embedded in paraffin wax. Serial tissue sections (3-µm-thick) from
the paraffin blocks were stained with hematoxylin and eosin for 5
min, respectively, at room temperature for cell visualization.
Following deparaffinization, antigen retrieval was
performed with 1 mM Tris-EDTA (pH 8.0) in a 100°C water bath for 20
min and endogenous peroxidase activity was blocked with 3% hydrogen
peroxide for 10 min at room temperature. All of the sections were
then incubated with primary antibodies against GFP (cat no.
ab183735; 1:200), tumor necrosis factor-α (TNF-α; cat no. ab199013;
1:200), interleukin-1β (IL-1β; cat no. ab34837; 1:200) and
osteocalcin (OCN; cat no. ab13418; 10 µg/ml) (all from Abcam) for 1
h at room temperature. An EnVision™ Detection Systems kit
(Peroxidase/DAB; cat no. K5007; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) was applied until the desired staining
intensity was achieved, which was observed under a light microscope
(Axio Scope A1; Carl Zeiss AG, Oberkochen, Germany). To ensure that
pathological diagnosis was standardized, the cellular localization
patterns, intensities, and numbers of cells with positive staining
were assessed by two experienced oral pathologists in a
double-blind manner. A consensus decision was reached in cases with
discrepancies. Staining intensity was defined as 0, 1, 2 and 3 for
no staining, light yellow staining, yellow-brown staining and brown
staining, respectively. Expression levels were semi-quantitatively
evaluated by multiplying the mean percentage of positive cells by
the intensity score. The mean percentage of positive cells was
determined in 4 random fields (magnification, ×400).
Statistical analysis
SPSS 19.0 software package (IBM Corp., Armonk, NY,
USA) was used for all statistical analyses. Significant differences
were calculated using one-way analysis of variance followed by the
least significant difference or Tamhane test. The ELISA and
antimicrobial activity assay data are expressed as the mean ±
standard error. The IHC are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Transfection efficiency
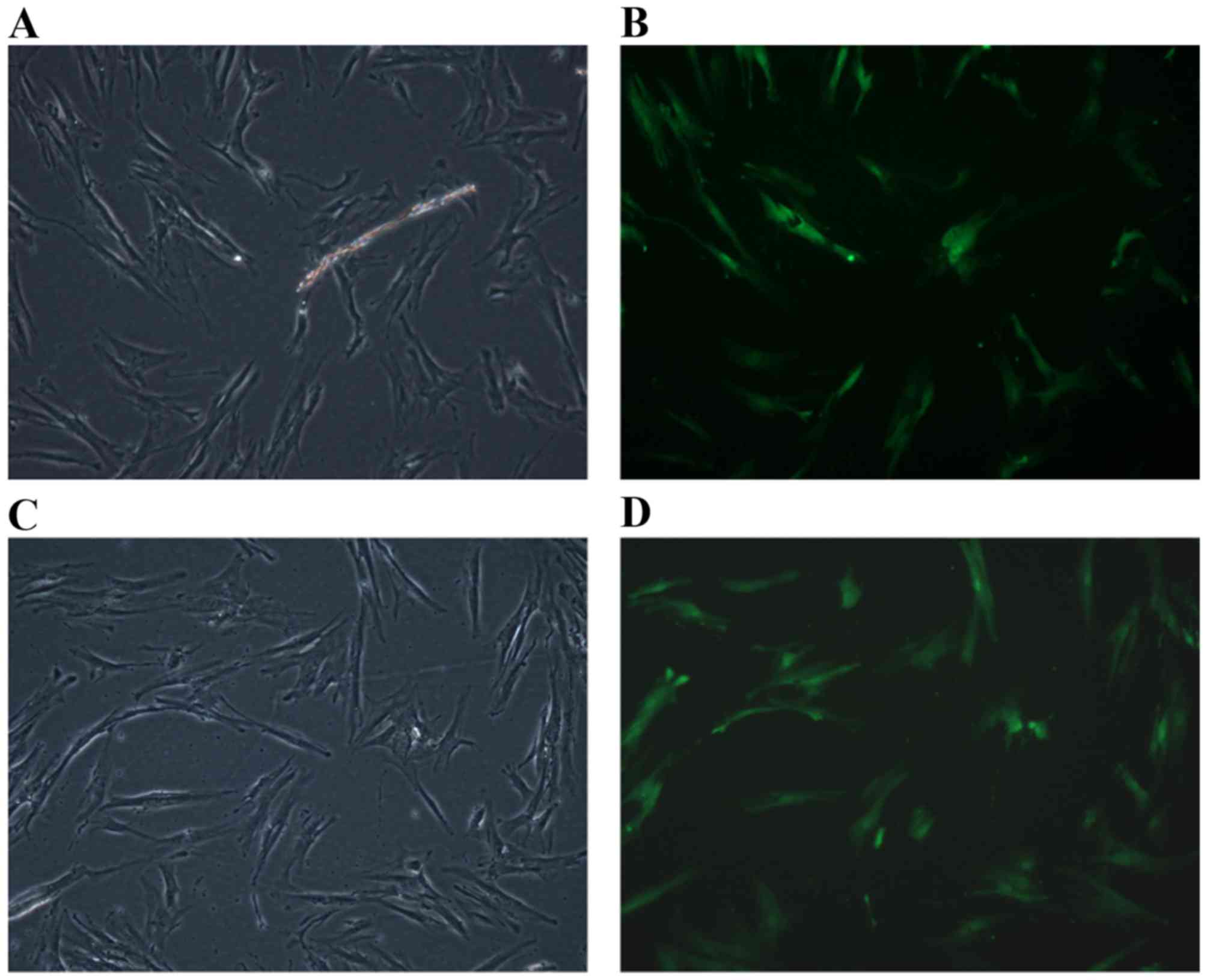
Transfection efficiency was calculated by counting
the cells that fluoresced green. The results demonstrated that the
rate of PDLC transfection with the GFP + HBD-3 vector was 78.98%
(Fig. 2A and B). The rate of PDLCs
transfection with the GFP only vector was 74.96% (Fig. 2C and D).
Western blot analysis
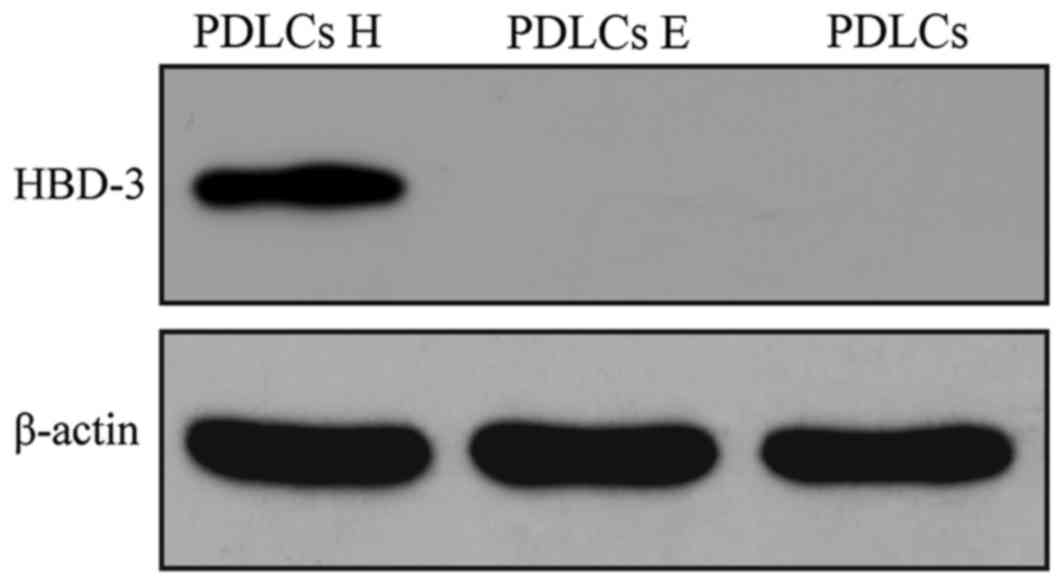
To examine the protein expression level of HBD-3 in
the transfected PDLCs, western blot analysis was performed.
Distinct positive bands were observed corresponding to the PDLCs
transfected with the HBD-3 + GFP vector. However, no positive bands
were observed in the untransfected groups and GFP only vector
(Fig. 3).
ELISA
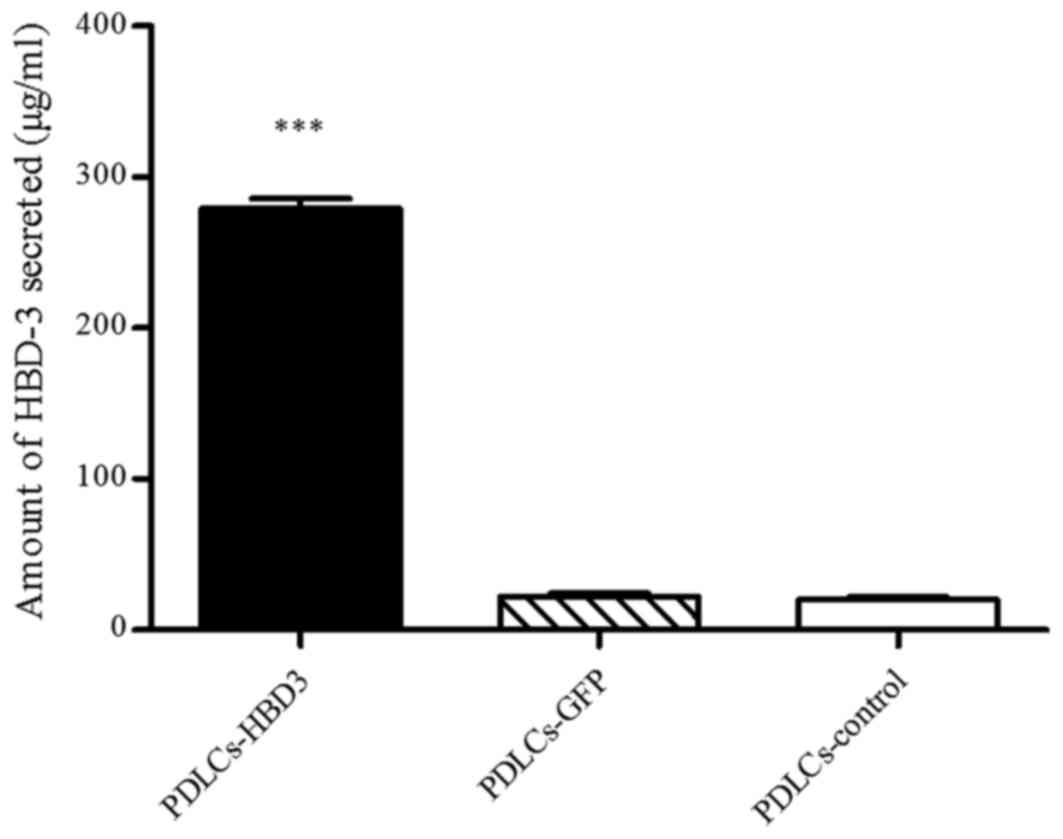
ELISA was performed to quantify the levels of HBD-3
in the PDLC sheets transfected with the HBD-3 and GFP genes. The
untreated cell sheets were used as a control. The results showed
that the concentrations of secreted HBD-3 were 279.22±2.17 µg/ml in
the supernatants of PDLC sheets transfected with HBD-3 (Fig. 4), which was significantly higher
than that of the corresponding cell sheets transfected with the
control vector and that of the untreated control group
(P<0.001).
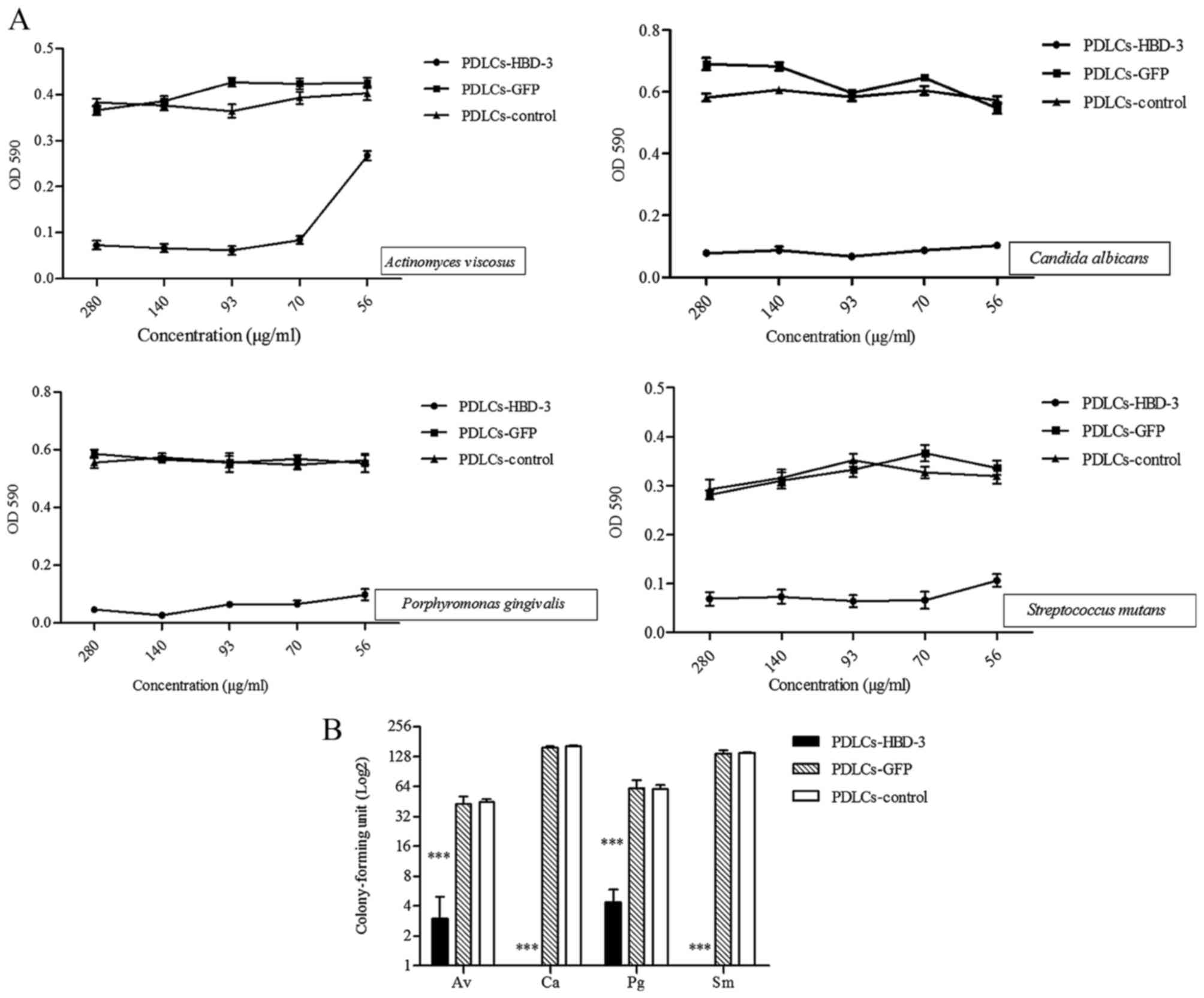
In vitro antimicrobial activity
As presented in Fig.
5A, Actinomyces viscosus, Candida albicans,
Porphyromonas gingivalis and Streptococcus mutans
were cultured with supernatants from PDLC sheets transfected with
the HBD-3 gene expression vector at various dilutions, and
exhibited lower OD values compared with those cultured with
supernatant from control vector and untransfected cells.
A CFU assay was also performed to evaluate the
effects of HBD-3 on the antimicrobial activity of the supernatant
of cell sheets against the microbial strains. Supernatant from the
PDLC sheets transfected with the HBD-3 vector demonstrated
significantly lower colony counts compared with those of the
control cell sheets (P<0.001; Fig.
5B).
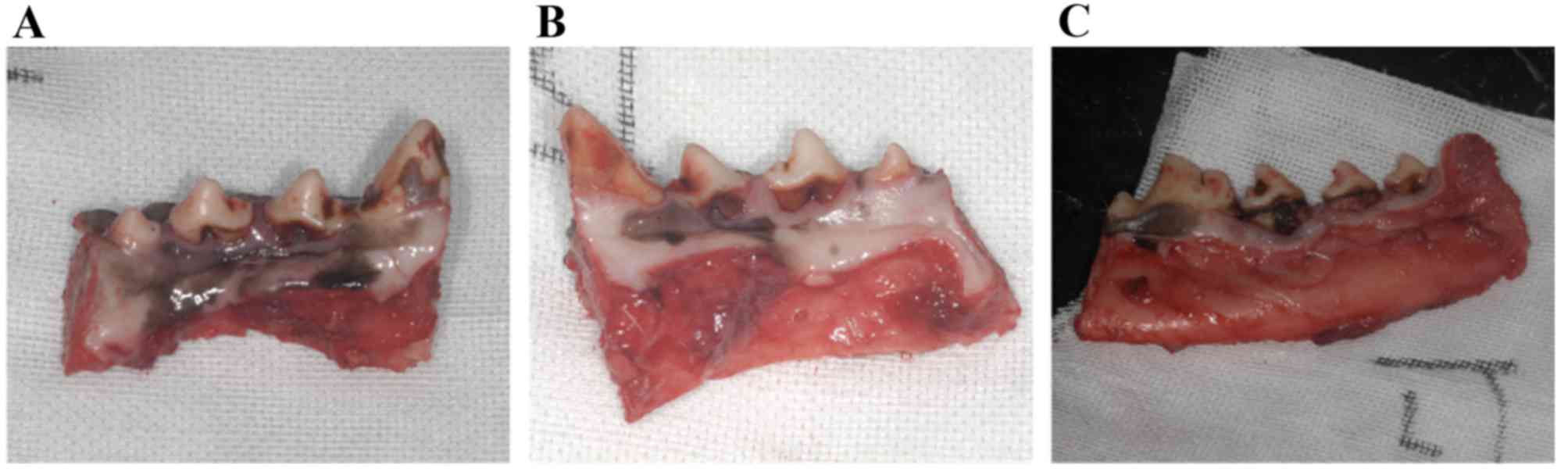
Clinical observations of animals
During the course of the experiment, no apparent
adverse reactions, such as severe infection and suppuration, were
detected around the periodontal tissue. Although root exposure was
observed in most dogs of all the groups, and gingival recession was
marginally more severe in the control group and the untreated group
than in the test group (Fig.
6).
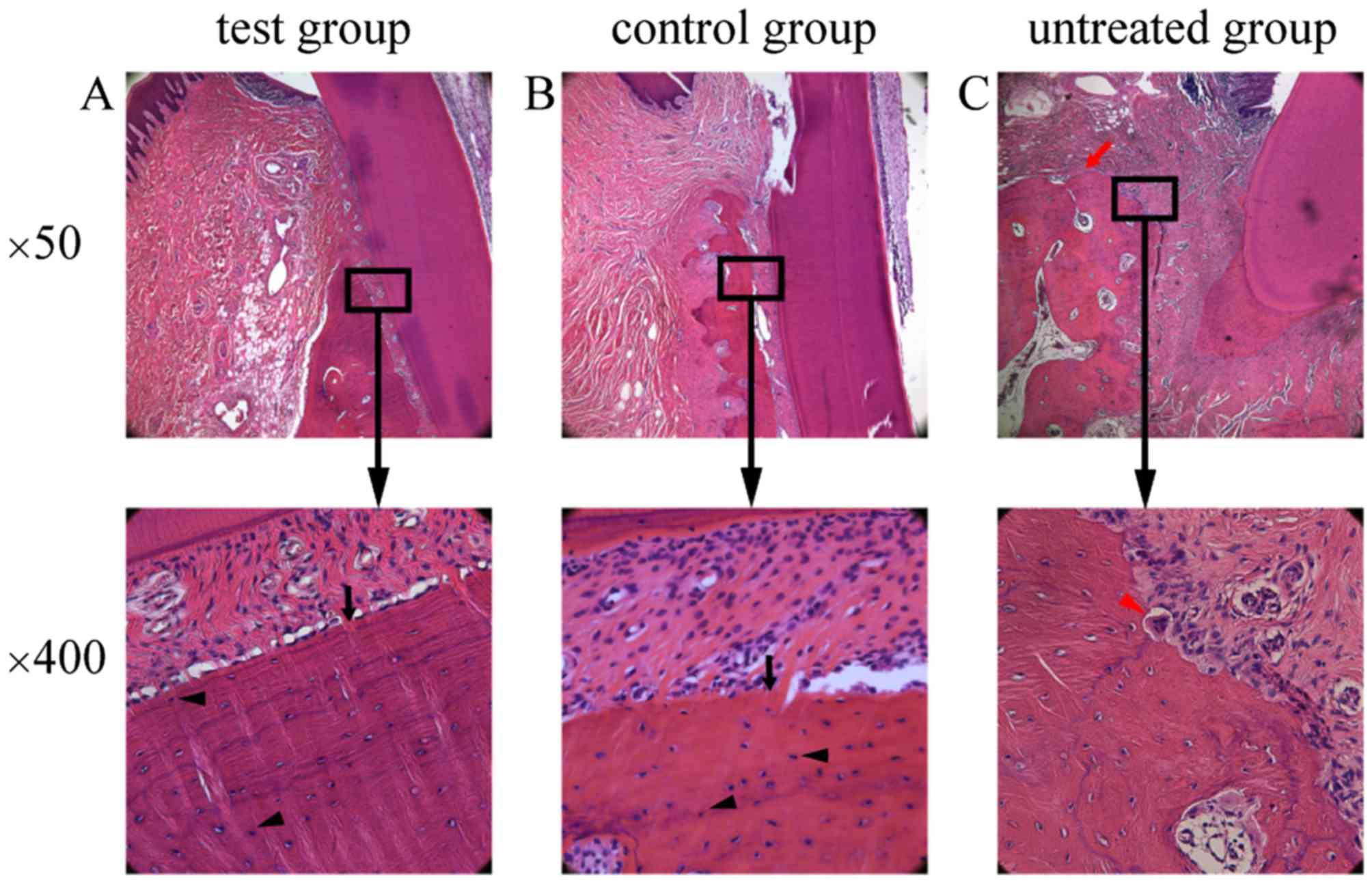
Histopathological analysis
Different degrees of epithelium attachment to the
cementum-enamel junction were observed among the three experimental
groups. Less inflammatory infiltration was observed in the
epithelium, and gingival and periodontal ligament fibers were
arranged in a more orderly and neat fashion in the test group
compared with those in the untreated group. In the test group
(Fig. 7A) and the control group
(Fig. 7B), a number of osteoblasts
and osteocytes, as well as newly formed alveolar bone with
trabeculae, were observed. By contrast, osteoclasts, visible
erosion of gingival epithelia and obvious absorption of the
alveolar crest were observed in the untreated group (Fig. 7C).
Immunohistochemical findings
In the test and the control group, a vast amount of
GFP-positive cells could be observed in the gingiva of the
experimental area (Fig. 8A) and
scattered small amounts in the alveolar bone (Fig. 8B).
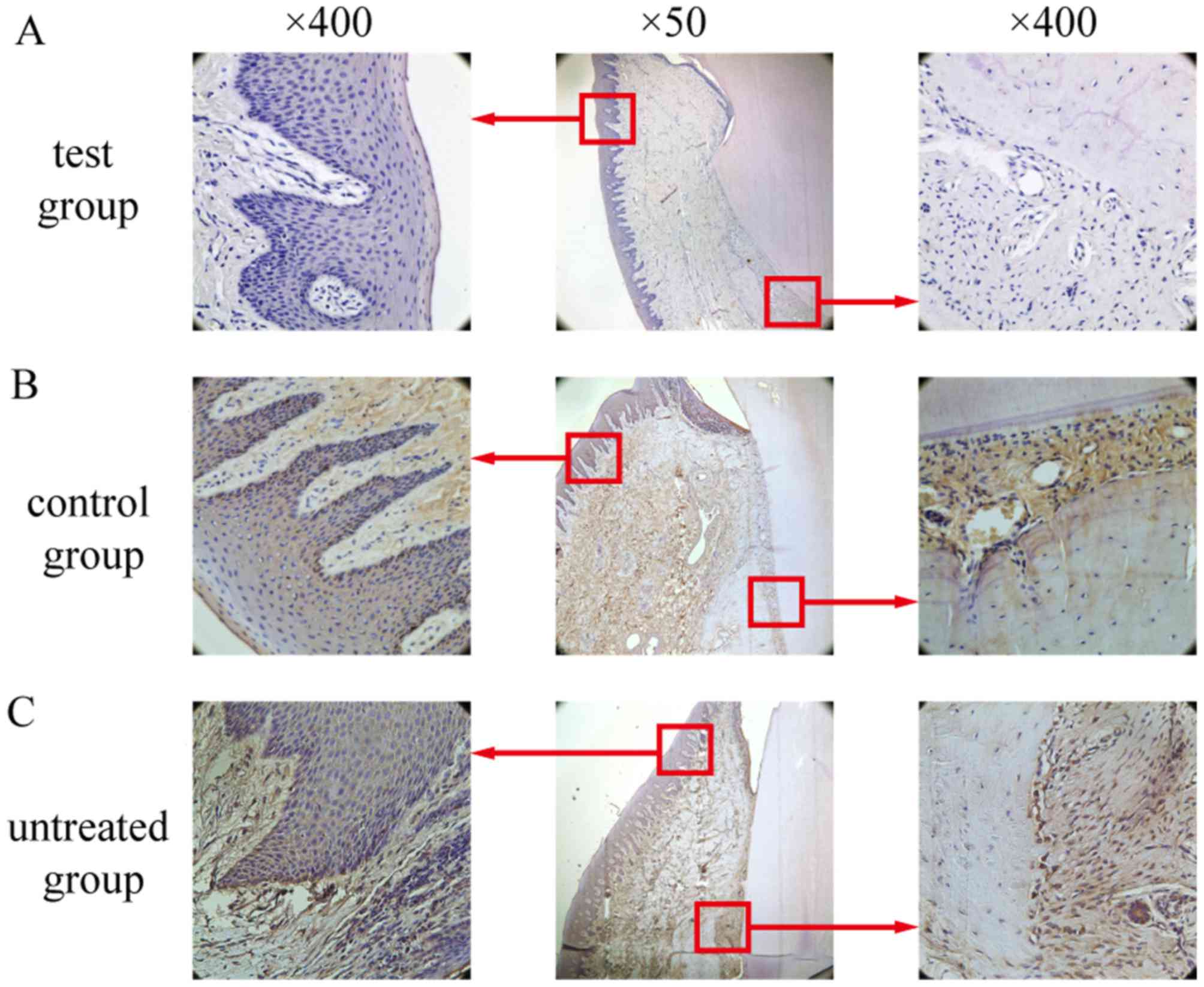
TNF-α-positive cells, predominantly macrophages,
were distributed throughout the gingival epithelium, periodontal
membrane matrix and osteoclasts. In the test group, only weak
positive staining was observed (Fig.
9A), whereas in the control group (Fig. 9B.) and the untreated group
(Fig. 9C.), TNF-α expression was
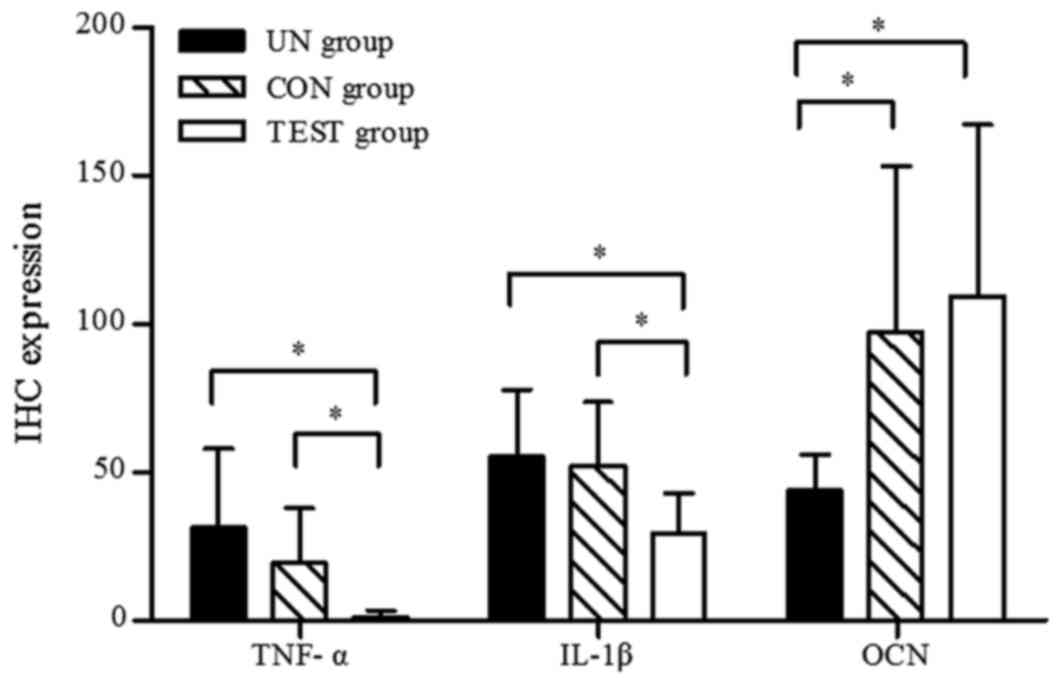
significantly elevated compared with the test group (P<0.05;
Fig. 10). There was no
significant difference between the untreated group and the control
group (P>0.05; Fig. 10).
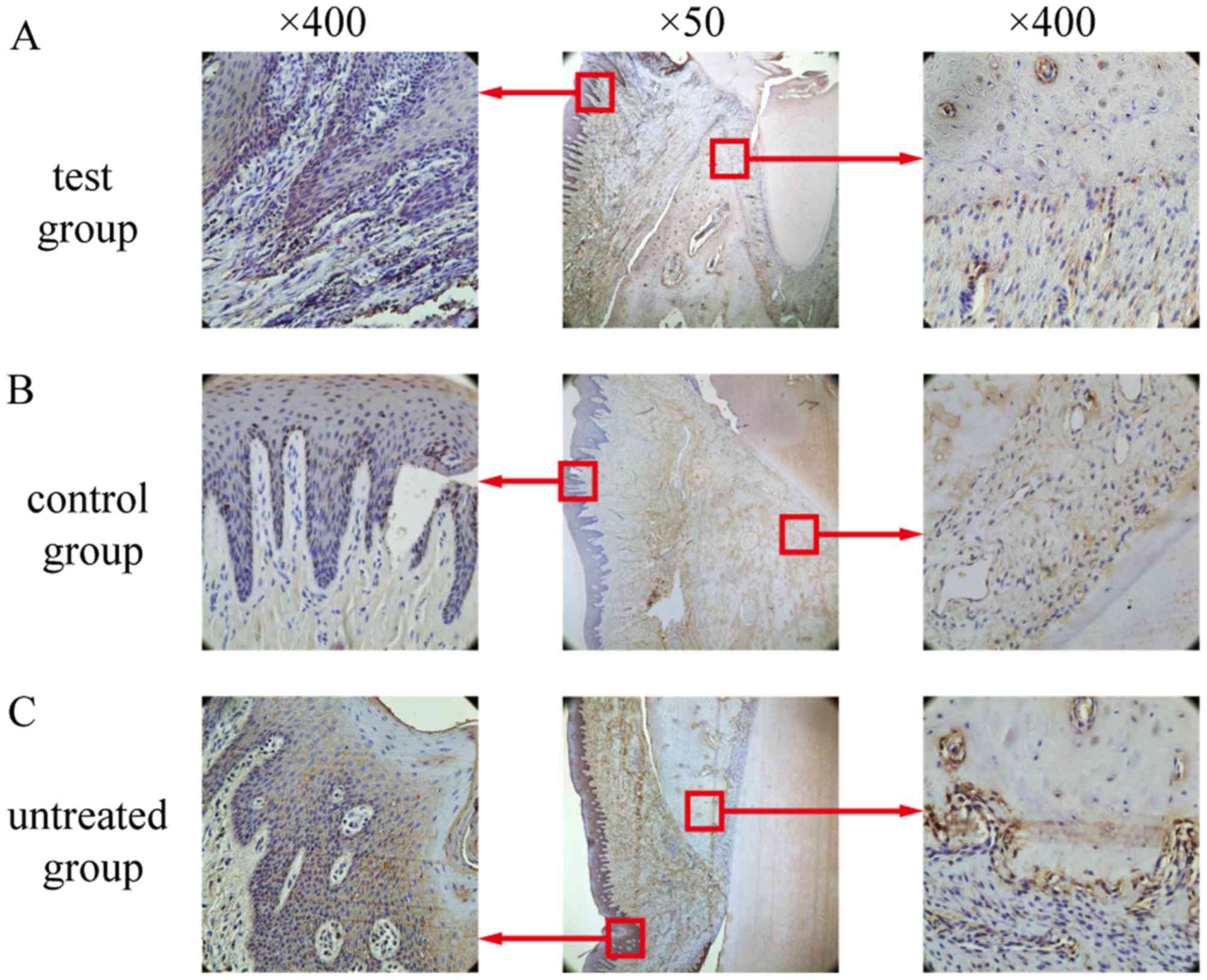
Similar to the TNF-α-positive staining patterns
described above, IL-1β-positive cells were primarily observed in
the gingival epithelium, periodontal membrane matrix, osteoblasts
and osteoclasts (Fig. 11). In the
test group, only weak IL-1β expression was observed, and this
expression was significantly lower than that in the control group
and the untreated group (Fig. 10;
P<0.05). No differences in IL-1β expression between the
untreated group and the control group were detected (P>0.05;
Fig. 10).
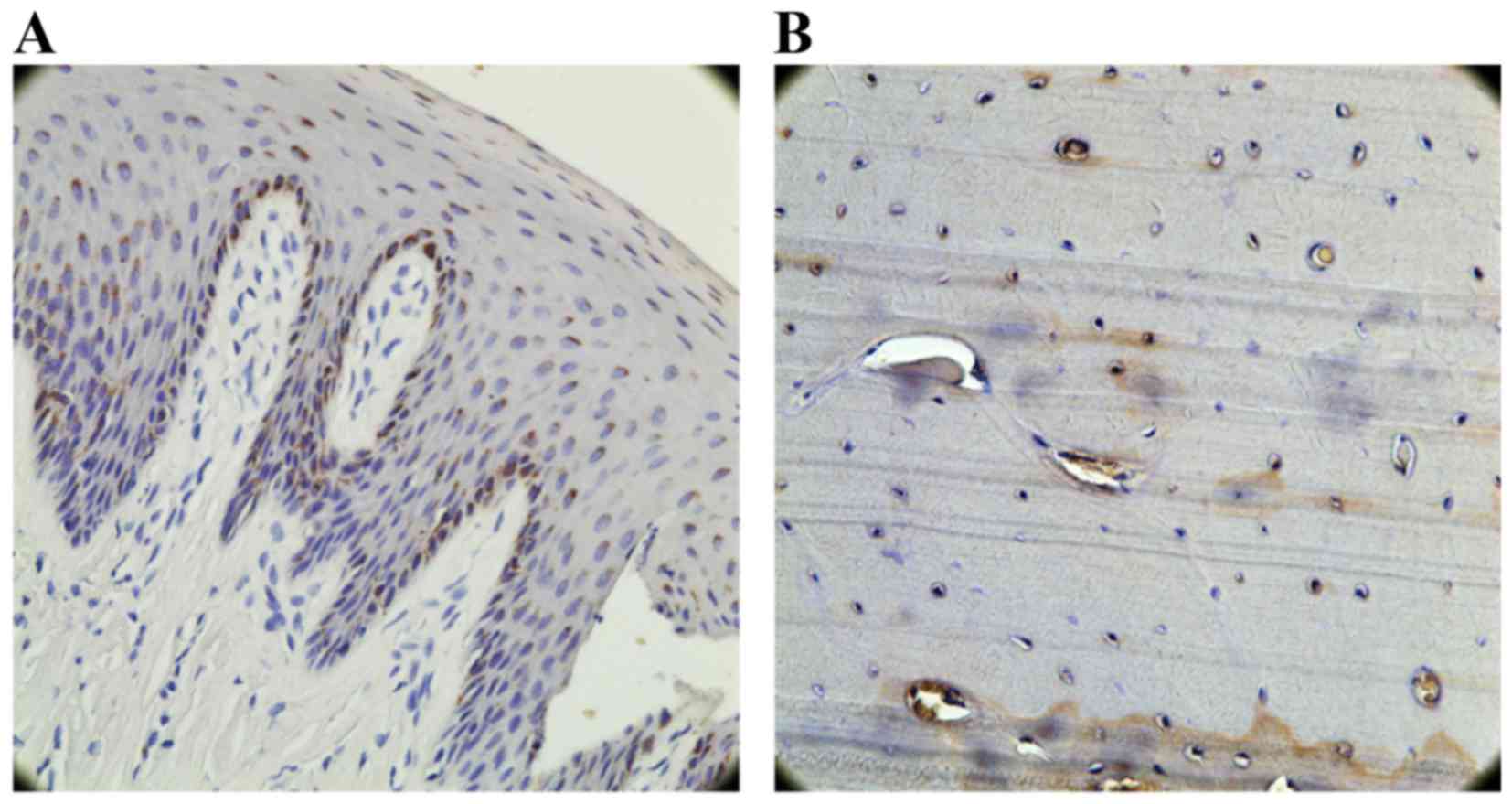
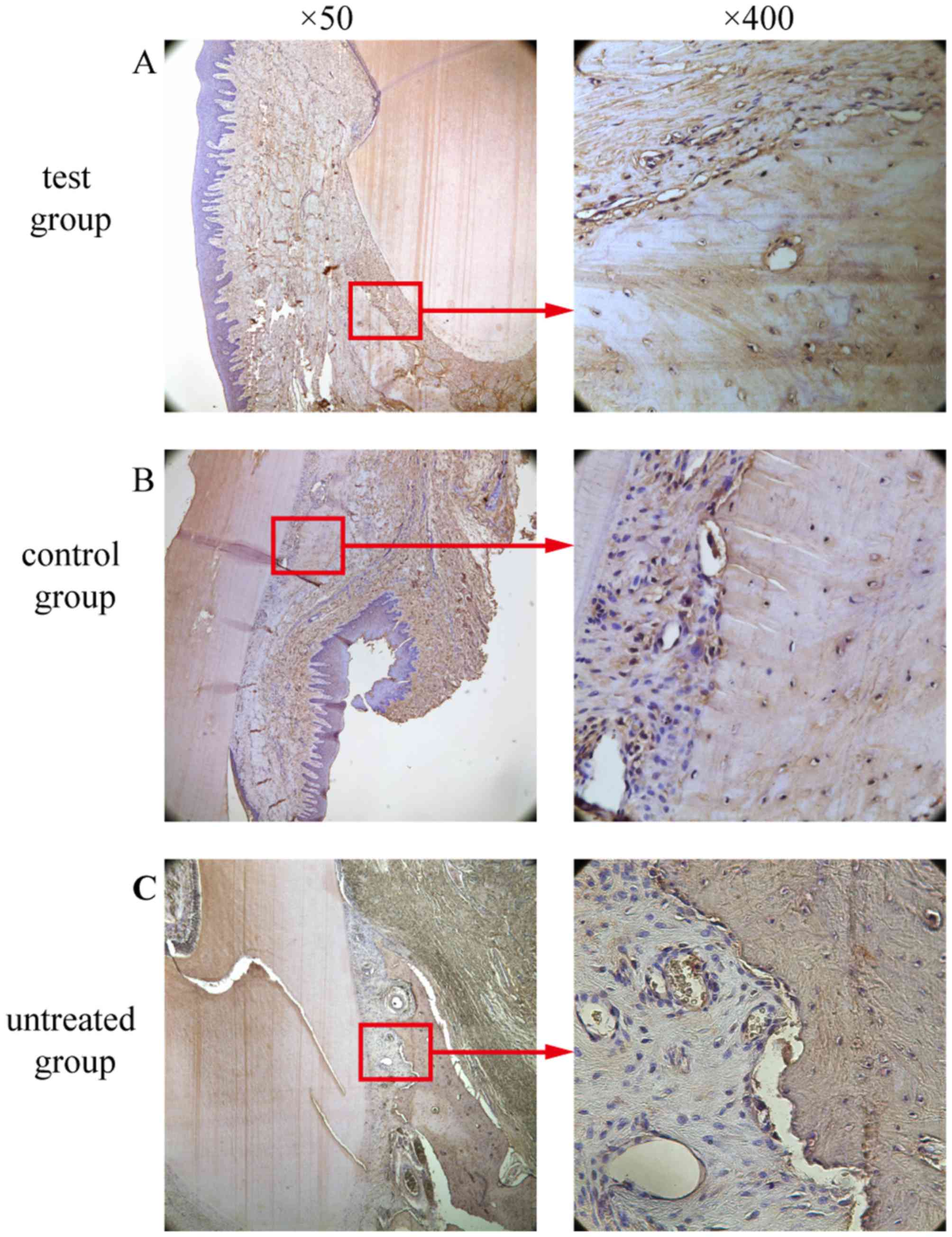
OCN-positive cell counting
A marker for bone tissue, OCN immunoreactivity was
observed in cells embedded in the alveolar bone and surrounding
tissues. In the test group (Fig.
12A) and the control group (Fig.
12B), the bone and the extracellular matrix of the periodontal
ligament showed moderate to strong OCN-positive staining. In the
untreated group, the extracellular matrix and some cells in the
alveolar bone exhibited weak staining for OCN (Fig. 12C). OCN staining was significantly
higher in the test group compared to the untreated group
(P<0.05; Fig. 10). There was
no significant difference in OCN staining between the test group
and the control group (P>0.05; Fig. 10).
Discussion
Periodontal diseases are highly prevalent and affect
up to 90% of the population worldwide (18). Pathogenic bacteria are widely
recognized to be a major cause of periodontal tissue destruction,
and the goals of periodontal treatments are to support good oral
hygiene and regenerate tissue integrity, which may have been
damaged by the inflammatory process (19).
Currently, periodontal regeneration is shifting
towards cell- and gene-based therapies (20). Certain studies have reported that
PDLCs have the capacity to function as osteoblasts or cementoblasts
under regenerative conditions, suggesting that they are candidates
for regeneration applications (12,21).
The crucial steps of gene therapy include the efficient transfer
and appropriate expression of the target gene. In the present
study, the efficiency of HBD-3 transfection into the PDLCs was
assessed. The HBD-3 protein levels in the transfected PDLCs were
sustained and were significantly higher than those of the control
group.
Furthermore, the antimicrobial activity of the PDLC
sheets transfected with a lentivirus containing the HBD-3 gene was
determined. In the present study, periodontal pathogens,
Actinomyces viscosus, Porphyromonas gingivalis, and
Candida albicans (22,23),
were demonstrated to be susceptible to the culture supernatant from
cells transfected with the HBD-3 vector compared with those in the
control vector group and the untreated group. The results indicated
that the transfected cell sheets expressed the HBD-3 and has the
capacity to inhibit broad-spectrum microbial activity in
vitro, which is consistent with the results of our previous
study (24) and with other reports
(25,26). Notably, the caries-causing bacteria
Streptococcus mutans (27)
was also susceptible to HBD-3 in the experimental group.
Previous studies have demonstrated that HBD is
susceptible to degradation and inactivation by both host and
bacterial proteases. It has also been reported that inflamed
gingival tissues expressed lower levels of HBD-3 mRNA than healthy
tissues (28,29). Brancatisano et al (2) detected HBD-3 using ELISA and observed
that the levels were inversely correlated with the severity of the
disease and with the degree of colonization by combinations of
bacterial species with elevated periodontopathogenic potential.
Based on these findings, it is reasonable to hypothesize that
aggressive inflammation and tissue destruction occur when the HBD-3
peptide cannot counteract the microbial activity. Thus, the
transfection of PDLC sheets with HBD-3 may have favorable effects
on antimicrobial activity by complementing the low levels of HBD-3
in aggressive periodontitis and other oral microbial diseases.
PDLC sheets have demonstrated osteogenic potential
in the reconstruction of periodontal tissues destroyed by chronic
inflammation (30,31). In the present study, multi-layer
PDLC sheets were produced using autologous cells collected from
beagle dogs. Following an 8-week experimental period, the alveolar
bone from the dogs in the test group and the control group was
examined. The bone exhibited newly formed tissue, and no teeth were
loosened or lost. By contrast, in the untreated group, root
exposure, gingival recession and tooth loss were observed.
Clinical transplantation is difficult to perform and
poses a risk of infection. It has been reported that an
inflammatory microenvironment reduces the osteogenic
differentiation potential of PDLCs (20,32).
In addition, inflammation of the periodontium leads to destruction
of the underlying ligament and alveolar bone and an imbalance in
bone remodeling that favors resorption. Few studies have examined
the role of HBD-3 in periodontal regeneration. In the present in
vivo study, PDLCs were transfected with HBD-3 and PDLC sheets
were created for use in an experimental periodontitis model. As
mediators of inflammatory responses, TNF-α and IL-lβ are two
pro-inflammatory cytokines involved in the process of bone
resorption under pathological conditions. The protein expression
levels of these two cytokines were detected in the current study
using IHC. The results demonstrated that TNF-α and IL-lβ expression
was significantly lower in the test group (PDLC sheets expressing
HBD-3) compared with the two control groups. These results
demonstrate the anti-inflammatory effects of HBD-3 in the healing
of periodontal disease. Following ~2 weeks after transplantation,
moderate inflammation was observed in diseased regions in the
untreated group and the control group, although intense
inflammation was not observed in any of the experimental groups by
the end of the study. The reason for this finding may be associated
with the function of HBD-3 in autologous periodontal tissues; based
on the above findings, HBD-3 has favorable anti-inflammatory
activity in the context of oral infectious disease, particularly
during the early stages of periodontitis.
Immunolocalization of OCN, an osteoblast-specific
marker, produced complementary results to the aforementioned
clinical observations and histopathological findings. There was
significantly greater positive OCN staining in the test group
compared with the untreated group. Stronger positive staining for
OCN was observed in the periodontal ligament and alveolar bone in
the test group than in the control group, although with no
significant differences between the groups. These data suggest that
HBD-3 can be used to neutralize toxins, and promote osteogenesis
via its anti-inflammatory effects within the inflammatory
microenvironment.
In conclusion, application of the lentiviral vector
containing HBD-3 has great potential as an efficient gene therapy
strategy to exert for anti-inflammatory activity in periodontitis.
Bone remodeling is a complex process that involves both bone
formation and bone resorption. Sheets of PDLC transfected with the
gene encoding HBD-3 may decelerate bone resorption caused by
inflammation, and this approach may offer a novel and safe method
for enhancing periodontal regeneration during the bone remodeling
process.
Acknowledgements
Dr Chunye Zhang and Dr Yuhua Hu (Department of Oral
Pathology, Shanghai Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine, Shanghai, China) are thanked for
their technical support. This study was supported by the National
Natural Science Foundation of China (grant no. 81271157).
References
|
1
|
Wang HL and Cooke J: Periodontal
regeneration techniques for treatment of periodontal diseases. Dent
Clin North Am. 49:637–659, vii. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brancatisano FL, Maisetta G, Barsotti F,
Esin S, Miceli M, Gabriele M, Giuca MR, Campa M and Batoni G:
Reduced human beta defensin 3 in individuals with periodontal
disease. J Dent Res. 90:241–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SH, Jun HK, Lee HR, Chung CP and Choi
BK: Antibacterial and lipopolysaccharide (LPS)-neutralising
activity of human cationic antimicrobial peptides against
periodontopathogens. Int J Antimicrob Agents. 35:138–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Watanabe H, Ogita M, Ichinose S
and Izumi Y: Effect of human beta-defensin-3 on the proliferation
of fibroblasts on periodontally involved root surfaces. Peptides.
32:888–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng X, Li M, Wang X, Wang Y and Ma D:
Both CD133+ and CD133− subpopulations of A549
and H446 cells contain cancer-initiating cells. Cancer Sci.
100:1040–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu WH, Qian NS, Li R and Dou KF:
Replacing Hoechst33342 with Rhodamine123 in isolation of cancer
stem-like cells from the MHCC97 cell line. Toxicol In Vitro.
24:538–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrand A, Sandrin MS, Shulkes A and
Baldwin GS: Expression of gastrin precursors by CD133-positive
colorectal cancer cells is crucial for tumour growth. Biochim
Biophys Acta. 1793:477–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bexell D, Gunnarsson S, Siesjö P, Bengzon
J and Darabi A: CD133+ and nestin+
tumor-initiating cells dominate in N29 and N32 experimental
gliomas. Int J Cancer. 125:15–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barcelos LS, Duplaa C, Kränkel N, Graiani
G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica
M, et al: Human CD133+ progenitor cells promote the
healing of diabetic ischemic ulcers by paracrine stimulation of
angiogenesis and activation of Wnt signaling. Circ Res.
104:1095–1102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boivin D, Labbé D, Fontaine N, Lamy S,
Beaulieu E, Gingras D and Béliveau R: The stem cell marker CD133
(prominin-1) is phosphorylated on cytoplasmic tyrosine-828 and
tyrosine-852 by Src and Fyn tyrosine kinases. Biochemistry.
48:3998–4007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Zhu B, DU HY, Sun TS, Zhang CH and
Yang LG: Detection and analysis of CD271, CD133 and CD34
expressions in bone marrow cells by flow cytometry with three color
fluorescence labelling. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
17:133–136. 2009.(In Chinese). PubMed/NCBI
|
|
12
|
Polimeni G, Xiropaidis AV and Wikesjö UM:
Biology and principles of periodontal wound healing/regeneration.
Periodontol 2000. 41:30–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi M, Ishikawa M, Kamei N, Nakasa T,
Adachi N, Deie M, Asahara T and Ochi M: Acceleration of skeletal
muscle regeneration in a rat skeletal muscle injury model by local
injection of human peripheral blood-derived CD133-positive cells.
Stem Cells. 27:949–960. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang GT, Zhang HB, Kim D, Liu L and Ganz
T: A model for antimicrobial gene therapy: Demonstration of human
beta-defensin 2 antimicrobial activities in vivo. Hum Gene Ther.
13:2017–2025. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawamura D, Goto M, Shibaki A, Akiyama M,
McMillan JR, Abiko Y and Shimizu H: Beta defensin-3 engineered
epidermis shows highly protective effect for bacterial infection.
Gene Ther. 12:857–861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu L, Chuanchang D, Wei L, Yilin C and
Jiasheng D: Enhanced healing of goat femur-defect using BMP7
gene-modified BMSCs and load-bearing tissue-engineered bone. J
Orthop Res. 28:412–418. 2010.PubMed/NCBI
|
|
17
|
Zhu M, Miao B, Zhu J, Wang H and Zhou Z:
Expression and antimicrobial character of cells transfected with
human β-defensin-3 against periodontitis-associated microbiota in
vitro. Mol Med Rep. 16:2455–2460. 2017.PubMed/NCBI
|
|
18
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HL, Greenwell H, Fiorellini J,
Giannobile W, Offenbacher S, Salkin L, Townsend C, Sheridan P and
Genco RJ: Research, Science and Therapy Committee: Periodontal
regeneration. J Periodontol. 76:1601–1622. 2005.PubMed/NCBI
|
|
20
|
Rios HF, Lin Z, Oh B, Park CH and
Giannobile WV: Cell- and gene-based therapeutic strategies for
periodontal regenerative medicine. J Periodontol. 82:1223–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JY, Jeon SH and Choung PH: Efficacy
of periodontal stem cell transplantation in the treatment of
advanced periodontitis. Cell Transplant. 20:271–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grant MM, Kolamunne RT, Lock FE, Matthews
JB, Chapple IL and Griffiths HR: Oxygen tension modulates the
cytokine response of oral epithelium to periodontal bacteria. J
Clin Periodontol. 37:1039–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Järvensivu A, Hietanen J, Rautemaa R,
Sorsa T and Richardson M: Candida yeasts in chronic periodontitis
tissues and subgingival microbial biofilms in vivo. Oral Dis.
10:106–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mimeault M, Johansson SL, Henichart JP,
Depreux P and Batra SK: Cytotoxic effects induced by docetaxel,
gefitinib, and cyclopamine on side population and nonside
population cell fractions from human invasive prostate cancer
cells. Mol Cancer Ther. 9:617–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ouhara K, Komatsuzawa H, Yamada S, Shiba
H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H and Sugai
M: Susceptibilities of periodontopathogenic and cariogenic bacteria
to antibacterial peptides, {beta}-defensins and LL37, produced by
human epithelial cells. J Antimicrob Chemother. 55:888–896. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia Z, Zhang C, Zeng Y, Wang T and Ai G:
Transplantation of BMSCs expressing hVEGF165 /hBD3 promotes wound
healing in rats with combined radiation-wound injury. Int Wound J.
11:293–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Costalonga M and Herzberg MC: The oral
microbiome and the immunobiology of periodontal disease and caries.
Immunol Lett. 162:22–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bissell J, Joly S, Johnson GK, Organ CC,
Dawson D, McCray PB Jr and Guthmiller JM: Expression of
beta-defensins in gingival health and in periodontal disease. J
Oral Pathol Med. 33:278–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hosokawa I, Hosokawa Y, Komatsuzawa H,
Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M,
Taubman MA and Kawai T: Innate immune peptide LL-37 displays
distinct expression pattern from beta-defensins in inflamed
gingival tissue. Clin Exp Immunol. 146:218–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shahrokhi S, Ebtekar M, Alimoghaddam K,
Pourfathollah AA, Kheirandish M, Ardjmand A, Shamshiri AR and
Ghavamzadeh A: Substance P and calcitonin gene-related
neuropeptides as novel growth factors for ex vivo expansion of cord
blood CD34(+) hematopoietic stem cells. Growth Factors. 28:66–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding G, Liu Y, Wang W, Wei F, Liu D, Fan
Z, An Y, Zhang C and Wang S: Allogeneic periodontal ligament stem
cell therapy for periodontitis in swine. Stem Cells. 28:1829–1838.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu
N, Ding B, Liu W, Liu Y, Shi H, et al: High levels of β-catenin
signaling reduce osteogenic differentiation of stem cells in
inflammatory microenvironments through inhibition of the
noncanonical Wnt pathway. J Bone Miner Res. 26:2082–2095. 2011.
View Article : Google Scholar : PubMed/NCBI
|