Introduction
In recent years, there has been a marked increase in
the number of joint replacement surgeries. According to Food and
Drug Administration records, ~400,000 hip arthroplasty procedures
are performed every year in the US alone (1). As metals and metal prosthesis exhibit
remarkable stability and abrasion resistance, they have been widely
used in surgeries involving joint replacement and bone damage
repair (2). However, abrasion and
invasion of the prosthesis have been demonstrated to release large
amounts of metal particles and ions, with the major components
being cobalt (Co) and chromium (Cr) particles (3). Several recent studies have discussed
the toxic effects of increased whole blood levels of Co and Cr
following metal-on-metal hip arthroplasties and other orthopedic
surgical procedures that involve prosthetic implants (4–7).
However, the potential risks of increased Co-Cr levels and their
safety levels remain to be established (8).
It is understood that no biomaterials are completely
inert, and an interaction between the material and human body often
elicits an immune response. Metallic implants have been reported to
break down or corrode over time, leading to release of soluble ions
that may have both local and systemic effects (9). It is therefore important to identify
the safe levels of these byproducts, and the risks associated with
their elevated levels.
Several studies have monitored Co ion concentrations
in patients following hip arthroplasties (4,5).
Certain patients were suspected to exhibit symptoms of cobalt
toxicity (mostly neurological dysfunction), as identified by
previous cases of inhalation and ingestion of excess cobalt
(8). However, the mechanism of
cobalt toxicity remains to be identified, and there is no
comparative data on symptomatic and asymptomatic patients with
cobalt-containing metal alloy prosthetics.
In vitro studies have demonstrated that at
concentrations higher than 100 µ/l, Co nanoparticles (CoNPs) and Co
ions reduce the viability of MG-63 cells, inhibit cell growth and
induce apoptosis (10–14). Both Co(2+) and Cr(6+) cause a
reduction in numbers of and resorption in mature osteoclasts
(15,16). A study on SaOS-2 osteoblast-like
cells has demonstrated that exposure to Co and Cr ions and
nanoparticles affect osteoblast function and mineralization of the
prosthesis surface (17). CoNPs
and ions have also exhibited biological toxicity against monocytes
(18), and affect osteogenic
differentiation of mesenchymal stem cells (19). Intraperitoneal administration of
cobalt has been revealed to alter serum parameters associated with
bone metabolism, and may potentially induce bone resorption in
adult rats (20). Mice osteoblasts
treated with Co for 48 h in vitro exhibited changes in
morphology and an increased secretion of the cytokine receptor
activator of nuclear factor κ-B ligand (RANKL), which may activate
osteolysis (21).
Several key transcription factors regulating
different stages of skeletogenesis, and their modes of action and
regulation by other factors have been studied in the past (22). It may be worthwhile to examine the
effects of CoNPs on some of these key transcription factors.
To the best of our knowledge, there are no previous
studies investigating whether low doses of CoNPs affect growth and
differentiation of human osteoblasts. In the present study, MG-63
cells were treated with CoNPs and cell growth, survival and death
were examined. Additionally, the expression levels of genes
responsible for osteogenic differentiation, alkaline phosphatase
(ALP), osteocalcin (BGLAP), collagen I (COL I) and osteoprotegerin
(OPG) were detected.
Materials and methods
Cell line and reagents
The MG-63 human osteosarcoma cell line was purchased
from the Shanghai Institute for Life Science, Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 100 µg/ml
streptomycin sulfate (Sigma-Aldrich; Merck KGaA). The culture
medium was changed every alternate day. Sterilized CoNPs (Shanghai
ChaoWei Nanotechnology Co., Ltd, Shanghai, China) were prepared in
PBS to obtain a concentration of 5 mM and stored at room
temperature. The solution was further diluted with PBS after
ultrasonic oscillation to obtain different concentrations required
for the experiment. Ultra-pure water was used to prepare 5 mM
CoCl2 solution, which was sterilized by filtration, and
stored at 4°C until use.
CoNP treatment
To assess alterations in cell morphology, MG-63
cells were seeded into 24-well culture plates at a density of
4×105 cells/well, and treated with 0.01, 0.1, 0.3 or 0.5
mg/ml of CoNPs for 12 or 24 h. The control cells were treated with
PBS. Cells were analyzed under an inverted microscope (Olympus
Corporation, Tokyo, Japan).
MTT assay
MG-63 cells were seeded into 96-well culture plates
at a density of 1×105 cells/well, and treated 0.01, 0.1,
1, 10 or 100 mg/ml CoNPs or Co2+ for 6, 12, 24 and 48 h
in triplicate. Control cells were treated with PBS. A total of 20
µl 5 mg/ml 3-(4,5)-dimethylthiahiazo
(−z-y1)-3,5-di-phenytetrazoliumromide (Sigma-Aldrich; Merck KGaA)
was added into each well after the treatment and cultured for 2 h.
The cells were then centrifuged at 94 × g for 20 min at room
temperature, the supernatant was discarded and 50 µl dimethyl
sulfoxide was added into each well. The cells were oscillated at
low frequency for 10 min until the crystals dissolved completely.
The optical density at a wavelength of 570 nm was read by a
microplate reader (calibrated at 630 nm).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cellular RNA was extracted using RISO RNA isolation
reagent (Biomics USA, Co., Ltd., San Francisco, CA, USA) according
to the manufacturer's protocol. cDNA preparation was carried out by
reverse transcription as follows: 2 µg total RNA, 1 µl oligo (dT)
18 primer, 4 µl 5X Reaction Buffer, 1 µl Ribolock™ RNase inhibitor
(Fermentas; Thermo Fisher Scientific, Inc.), 2 µl 10 mM dNTP Mix, 1
µl RevertAid™ M-MLV Reverse Transcriptase (Takara Biotechnology Co.
Ltd., Dalian, China), and 9 µl diethyl pyrocarbonate. The mixture
was incubated at 42°C for 60 min, followed by incubation at 70°C
for 5 min to end the reaction. The products were stored at −20°C
until use.
For qPCR, a SYBR Green kit (Roche Diagnostics GmbH,
Mannheim, Germany) was used with an Applied Biosystems 7500 Real
Time PCR instrument. The 20 µl reaction mix was prepared as
follows: 10 µl 2X Master Mix buffer, 1.2 µl forward and reverse
primers each (10 mM), 1 µl cDNA and 6.6 µl double distilled water.
The primer sequences for ALP, BGLAP, COL I, OPG, RANKL and GAPDH
are listed in Table I. The PCR
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by denaturation at 95°C for 10 sec, and annealing and
extension at 60°C for 30 sec for 40 cycles. The RNA levels were
calculated by the 2−ΔΔCq method using β-actin as an
internal reference (23).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence |
|---|
| ALP | F:
5′-GCCCTAAGGTCCATTCCA-3′ |
|
| R:
5′-CTTTGTGTTTCCCAGAAGAATG-3′ |
| BGLAP | F:
5′-CAACCCCAGTTCTGCTCCT-3′ |
|
| R:
5′-CCTCTTCTGGAGTTTATTTGGG-3′ |
| COL I | F:
5′-TCAATCCCTTGTGCCGC-3′ |
|
| R:
5′-CACATCAAGACAAGAACGAGGTA-3′ |
| OPG | F:
5′-GCAGCGGCACATTGGAC-3′ |
|
| R:
5′-CCCGGTAAGCTTTCCATCAA-3′ |
| RANKL | F:
5′-AGAGCGCAGATGGATCCTAA-3′ |
|
| R:
5′-TTCCTTTTGCACAGCTCCTT-3′ |
| β-actin | F:
5′-ATGACTTAGTTGCGTTACACCCTT-3′ |
|
| R:
5′-GCTGTCACCTTCACCGTTCC-3′ |
Western blotting
The cells were lysed on ice with 1X
radioimmnoprecipitation assay buffer [20 mM Tris-HCl (pH 7.5); 150
mM NaCl; 1 mM Na2EDTA; 1 mM EGTA; 1% NP-40, 1% sodium
deoxycholate; 2.5 mM sodium pyrophosphate; 1 mM β-glycerophosphate;
1 mM Na3VO4; 1 µg/ml leupeptin] for 30 min,
and then centrifuged at 15,871 × g for 30 min at room temperature.
The supernatant was collected and stored at −80°C until use. The
protein concentration was determined by the Pierce BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). Total protein (30 µg)
was separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. Following blocking in PBS + 5% non-fat milk
for 1 h at room temperature, the membranes were incubated with ALP,
COL I, BGLAP and β-actin primary antibodies at 4°C overnight, as
follows: ALP, 1:1,000, cat. no. SAB2500128; COL I, 1:1,000, cat.
no. SAB4200678; BGLAP, 1:1,000, cat. no. WH0000632M1; β-actin,
1:5,000, cat. no. A1978; all purchased from Sigma-Aldrich; Merck
KGaA. The membranes were washed with TBS + 0.1% Tween-20 and
incubated with the following peroxidase-conjugated secondary
antibodies for 2 h at room temperature: Goat anti-mouse
immunoglobulin (Ig)G (1:5,000; cat. no. A3682) and rabbit anti-goat
IgG (1:5,000; cat. no. A8919) (both from Sigma-Aldrich; Merck
KGaA). An Enhanced Chemiluminescence assay (EMD Millipore,
Billerica, MA, USA) was used to detect proteins, and images were
captured using a gel imaging system (VersaDoc Imaging System,
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the densitometry
was quantified using ImageJ 1.41 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
The above experiments were all repeated in
triplicate. SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used for the statistical analysis. All the data are presented as
the mean ± standard deviation. For comparison of more than two
groups, one-way analysis of variance followed by a Bonferroni-Dunn
post hoc test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of CoNPs
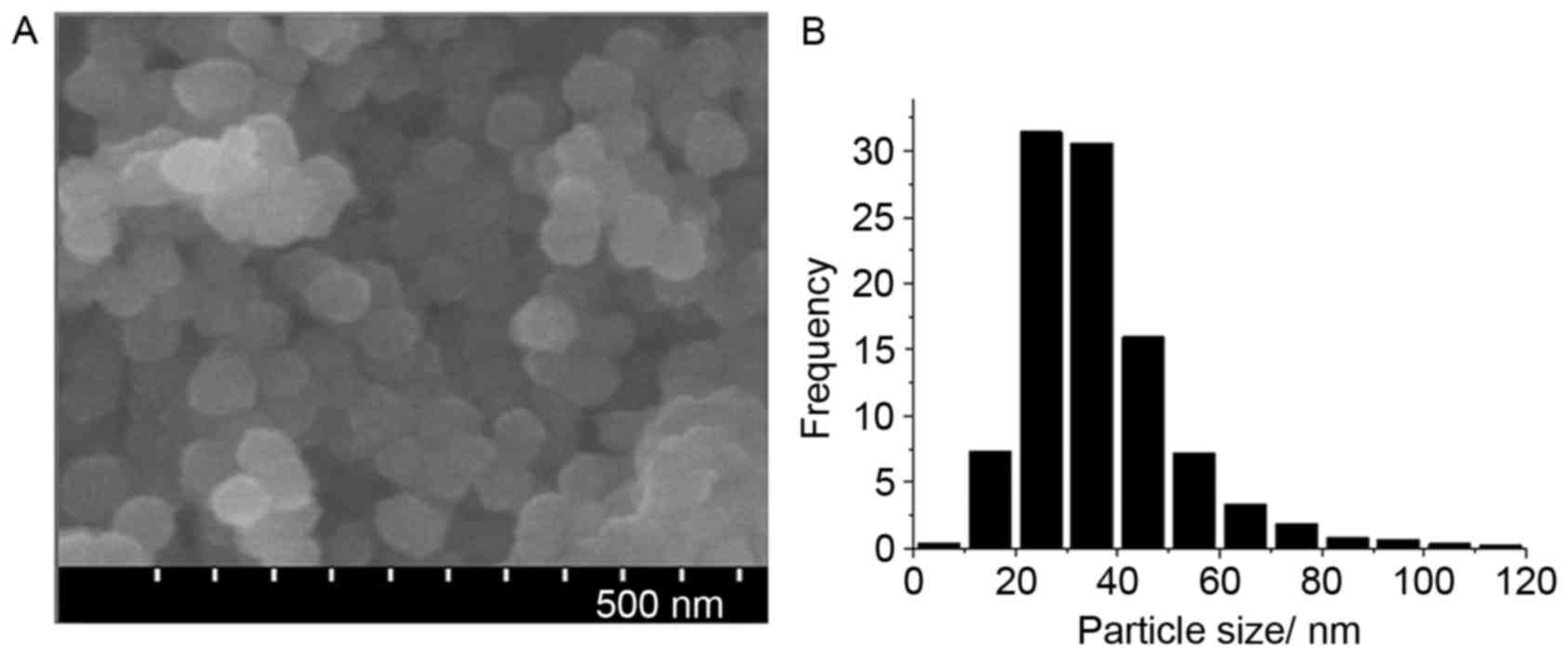
CoNPs were observed via electron microscopy. The
CoNPs were of homogenous size with the mean diameter being 50 nm.
X-ray diffraction demonstrated that the sizes of the particles were
mainly between 20 and 40 nm, and the percentage of Co was
99.9±0.001%, while the other components including iron, silver,
copper, tungsten and nickel were all <0.01% (Table II; Fig. 1).
 | Table II.X Ray fluorescence analysis of cobalt
nanoparticles. |
Table II.
X Ray fluorescence analysis of cobalt
nanoparticles.
| Element | Content/wt.% | Standard
deviation |
|---|
| Co | 99.9 | 0.001 |
| Fe |
0.002 | 0.003 |
| Ag |
0.001 | 0.001 |
| Cu |
0.002 | 0.003 |
| W |
0.004 | 0.006 |
| Ni |
0.009 | 0.002 |
| N | 0.01 | 0.007 |
| O | <0.07 |
|
Effects of CoNPs on the morphology and
viability of MG-63 cells
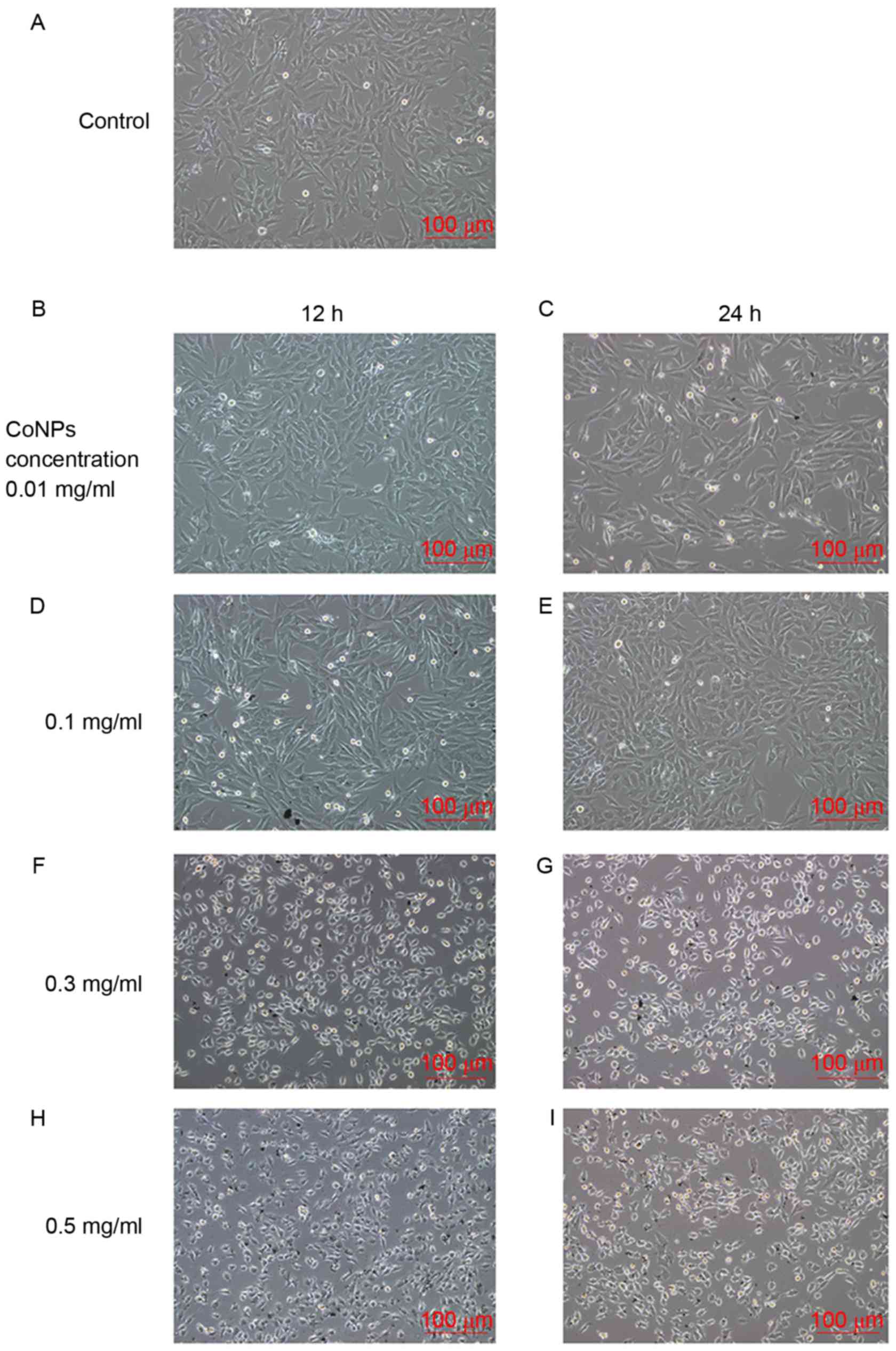
The MG-63 cells were treated with 0.01, 0.1, 0.3 or
0.5 mg/ml of CoNPs for 12 or 24 h and then analyzed
microscopically. The results indicated that the cell morphologies
changed slightly after treatment with 0.01 and 0.1 mg/ml CoNPs.
When the cells were treated with CoNPs at concentrations of 0.3
mg/ml or higher, retraction of cellular pseudopods, pyknosis of the
cytoplasm and cell death was observed. Cell death was observed when
incubated with CoNPs for 12 h, and it increased further after 24 h
and was accompanied by an increase in CoNP concentration. The
highest cell death was observed in cells treated with 0.5 mg/ml of
CoNPs for 24 h (Fig. 2).
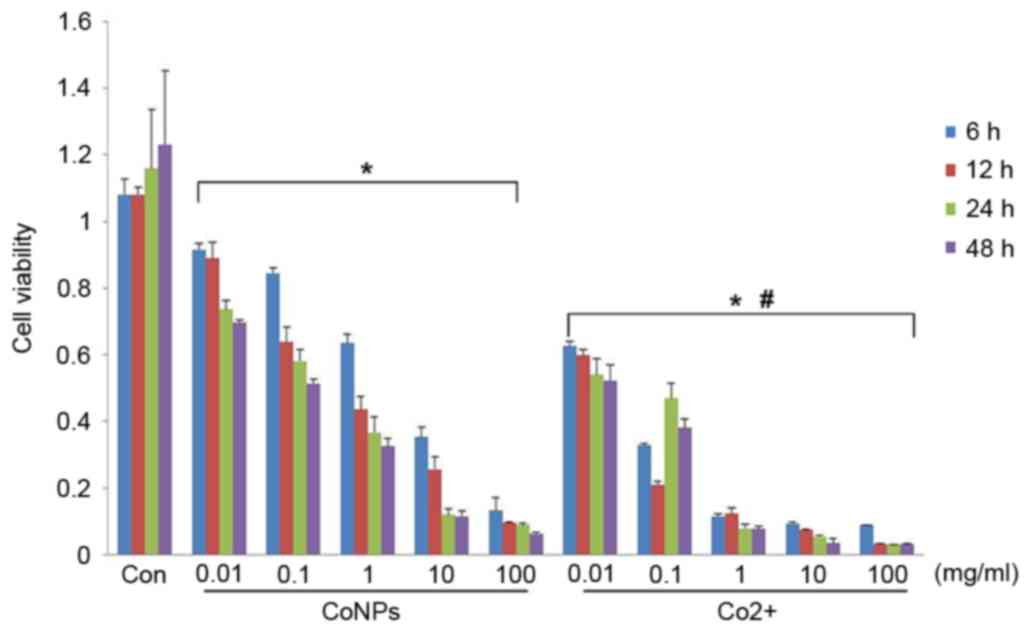
MTT assay was used to evaluate cell viability after
treatment with different concentrations of CoNPs or Co2+
for 6, 12, 24 or 48 h. After treatment with different
concentrations of CoNPs or Co2+ for 6 h, the viability
of the cells was significantly reduced compared with the control
group (P<0.05). Cell viability decreased in a dose-dependent
manner. In comparison to CoNPs, a reduction in cell viability was
more pronounced at the same concentration of Co2+
(P<0.05). The reduction in cell viability increased with an
increase in the duration of treatment, and peaked at 48 h (Fig. 3).
Effects of CoNPs on mRNA and protein
expression levels of ALP, BGLAP, Col I, OGP and RANKL
RT-qPCR was performed to evaluate the mRNA
expression of ALP, BGLAP, Col I, OGP and RANKL in MG-63 cells
treated with different concentrations of CoNPs. After the cells
were treated with 0.01, 0.1 and 0.3 mg/ml CoNPs for 12 or 24 h, the
mRNA expression levels of ALP, BGLAP, COL I and OPG decreased
significantly (P<0.05) in a concentration-dependent manner. An
upward trend in RANKL mRNA levels were observed at a CoNP
concentration of 0.3 mg/ml; however, this increase was not
statistically significant (Fig.
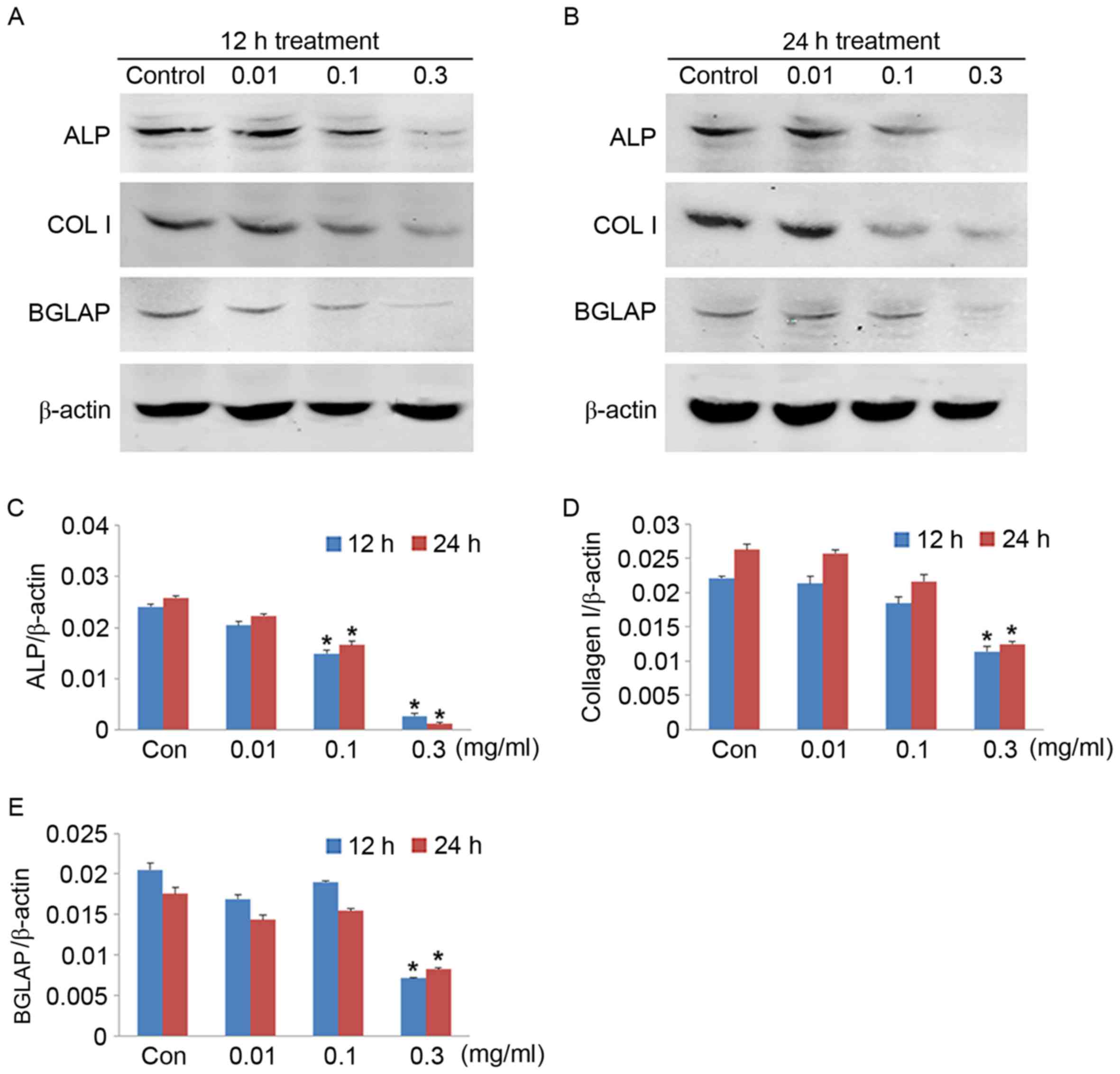
4). The reduction in mRNA expression levels was also confirmed
by measuring the protein expression levels by western blotting
(Fig. 5). The ALP, BGLAP and COL I
protein expression levels decreased significantly when the cells
were treated with 0.3 mg/ml CoNPs for 12 h. Thus, CoNPs affected
the mRNA and protein expression levels of ALP, BGLAP and COL I in
MG-63 cells in a concentration-dependent manner, and may affect
osteogenesis.
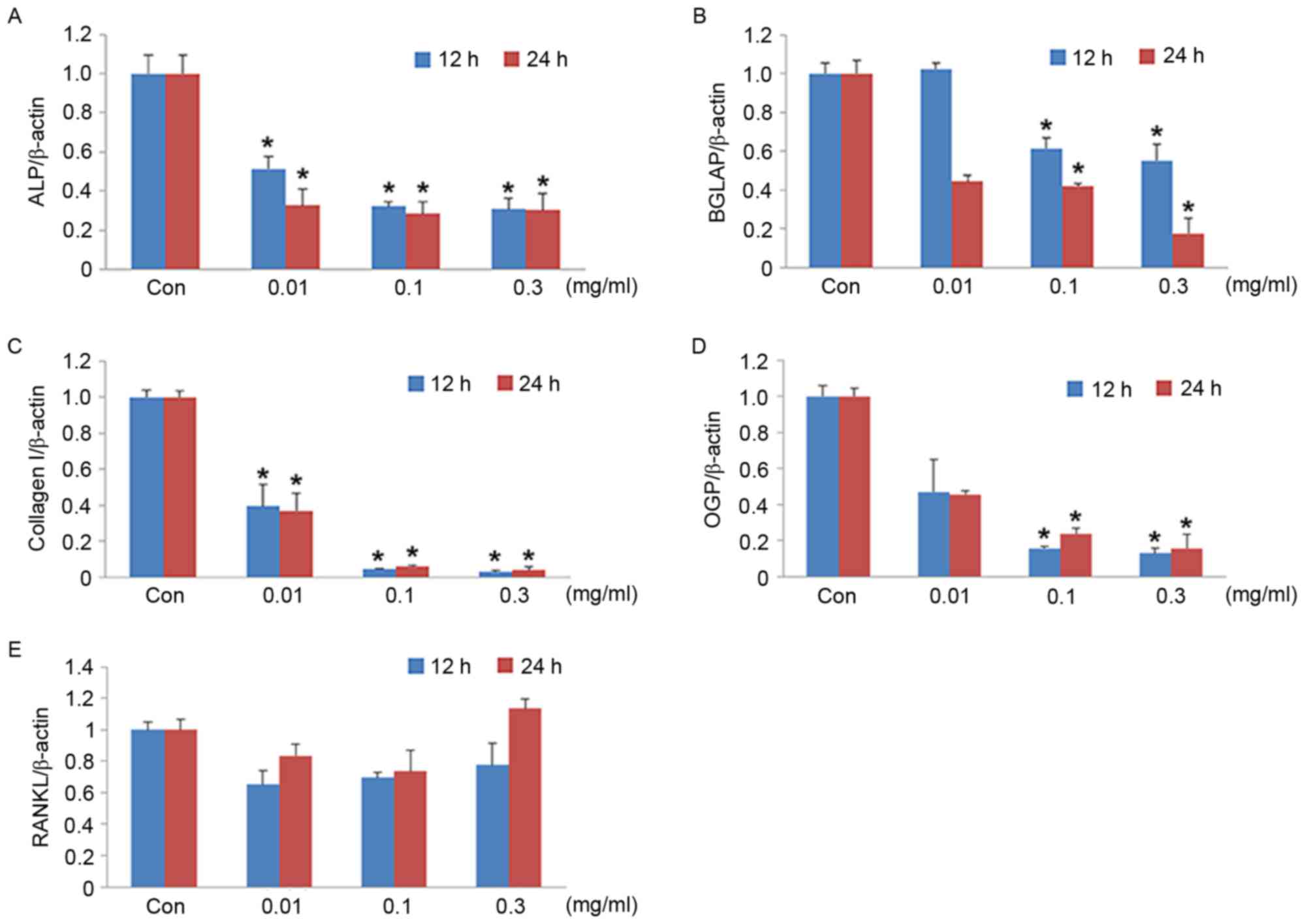 | Figure 4.mRNA expression levels in MG-63 cells.
Quantification of (A) ALP, (B) BGLAP, (C) COL I, (D) OPG AND (E)
RANKL mRNA expression levels following treatment with 0.01, 0.1 and
0.3 mg/ml of cobalt nanoparticles for 12 and 24 h. β-actin served
as an internal control. Data are expressed as the mean ± standard
deviation. *P<0.05 vs. control group at the same time point.
ALP, alkaline phosphatase; BGLAP, osteocalcin; COL I, collagen I;
OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor
κ-B ligand; Con, control. |
Discussion
The findings of the present study show demonstrated
increased cell death and decreased survival in CoNP-treated MG-63
cells. In addition, CoNP treatment affected the mRNA and expression
levels of ALP, BGLAP, COL I and OPG, genes responsible for
osteogenic differentiation, and the protein expression levels of
ALP, COL I and BGLAP.
Previous studies on mice osteoblasts and animal
models have revealed that Co2+ and Cr3+ may
inhibit the proliferation of mice osteoblasts, and induce cytotoxic
effects on cells. In human osteoblasts, CoNPs and Co2+
may also inhibit the proliferation of osteoblasts, cause cell
apoptosis and induce the release of inflammatory cytokines such as
tumor necrosis factor-α and interleukin-6 (13,24,25).
The present study also confirmed that CoNPs at concentrations
between 0.01 and 0.5 mg/ml may inhibit the growth of
osteoblasts.
High concentrations of CoNPs have been demonstrated
to induce cell apoptosis (16).
Further investigation on the functions of osteoblasts and
expression of relevant genes revealed that CoNPs could inhibit the
functions of osteoblasts, reduce the expression levels of ALP and
COL I, genes that are required for bone matrix maturation, and
decrease the expression of the BGLAP gene that is required for
mineralization (26). The process
of osteolysis has also been identified to be regulated by CoNPs,
which may downregulate the inhibitory RANKL ligand OPG to increase
bone absorption of osteoclasts (27). The results of the present study
also indicated that in MG-63 cells, both mRNA and protein
expression levels of genes responsible for osteogenic
differentiation, ALP, BGLAP, COL I and OGP, are affected by low
levels of CoNP treatment. Therefore, CoNPs may not only affect the
growth and differentiation of osteoblasts, but also indirectly
upregulate the lysis and absorption of the bone by osteoclasts.
Metal prostheses can release large amounts of
Co2+ and CoNPs due to biological and chemical corrosion,
mechanical abrasion and oxidation of the metals (16). Andrews et al (16) demonstrated that Co2+
potentially had a greater inhibitory effect on the growth of MG-63
cells compared with CoNPs, and the IC50 for inhibiting the growth
of MG-63 cells was lower than CoNPs. Co2+ can diffuse in
solutions more easily compared to CoNPs, which may be why there are
more pronounced effects post-treatment with Co2+.
In conclusion, the present study demonstrated that
CoNPs induce cytotoxic effects on MG-63 cells by markedly reducing
cell viability and inducing cell death at high concentrations. In
addition, CoNPs may inhibit the function and differentiation of
osteoblasts by affecting the mRNA and protein expression levels of
associated genes. These results revealed the potential negative
effect of CoNPs on prosthesis aseptic loosening following total
joint replacement surgery. Inhibition of this effect should be
further investigated.
References
|
1
|
Administration TUSFaD: Information for all
health care professionals who provide treatment to patients with a
metal-on-metal hip implant. 2015 https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241744.htmAccessed.
January 9–2015.
|
|
2
|
Fathi M, Ahmadian M and Bahrami M: Novel
bioactive Co-based alloy/FA nanocomposite for dental applications.
Dent Res J (Isfahan). 9:173–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacQuarrie RA, Chen Y Fang, Coles C and
Anderson GI: Wear-particle-induced osteoclast osteolysis: The role
of particulates and mechanical strain. J Biomed Mater Res B Appl
Biomater. 69:104–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devlin JJ, Pomerleau AC, Brent J, Morgan
BW, Deitchman S and Schwartz M: Clinical features, testing, and
management of patients with suspected prosthetic hip-associated
cobalt toxicity: A systematic review of cases. J Med Toxicol.
9:405–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jantzen C, Jorgensen HL, Duus BR, Sporring
SL and Lauritzen JB: Chromium and cobalt ion concentrations in
blood and serum following various types of metal-on-metal hip
arthroplasties: A literature overview. Acta Orthop. 84:229–236.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pizon AF, Abesamis M, King AM and Menke N:
Prosthetic hip-associated cobalt toxicity. J Med Toxicol.
9:416–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradberry SM, Wilkinson JM and Ferner RE:
Systemic toxicity related to metal hip prostheses. Clin Toxicol
(Phila). 52:837–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald SJ: Can a safe level for metal
ions in patients with metal-on-metal total hip arthroplasties be
determined? J Arthroplasty. 19 8 Suppl 3:S71–S77. 2004. View Article : Google Scholar
|
|
9
|
Caicedo MS, Pennekamp PH, McAllister K,
Jacobs JJ and Hallab NJ: Soluble ions more than particulate
cobalt-alloy implant debris induce monocyte costimulatory molecule
expression and release of proinflammatory cytokines critical to
metal-induced lymphocyte reactivity. J Biomed Mater Res A.
93:1312–1321. 2010.PubMed/NCBI
|
|
10
|
Allen MJ, Myer BJ, Millett PJ and Rushton
N: The effects of particulate cobalt, chromium and cobalt-chromium
alloy on human osteoblast-like cells in vitro. J Bone Joint Surg
Br. 79:475–482. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anissian L, Stark A, Dahlstrand H,
Granberg B, Good V and Bucht E: Cobalt ions influence proliferation
and function of human osteoblast-like cells. Acta Orthop Scand.
73:369–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hallab NJ, Vermes C, Messina C, Roebuck
KA, Glant TT and Jacobs JJ: Concentration- and
composition-dependent effects of metal ions on human MG-63
osteoblasts. J Biomed Mater Res. 60:420–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown C, Lacharme-Lora L, Mukonoweshuro B,
Sood A, Newson RB, Fisher J, Case CP and Ingham E: Consequences of
exposure to peri-articular injections of micro- and
nano-particulate cobalt-chromium alloy. Biomaterials. 34:8564–8580.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sansone V, Pagani D and Melato M: The
effects on bone cells of metal ions released from orthopaedic
implants. A review. Clin Cases Miner Bone Metab. 10:34–40.
2013.PubMed/NCBI
|
|
15
|
Patntirapong S, Habibovic P and Hauschka
PV: Effects of soluble cobalt and cobalt incorporated into calcium
phosphate layers on osteoclast differentiation and activation.
Biomaterials. 30:548–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andrews RE, Shah KM, Wilkinson JM and
Gartland A: Effects of cobalt and chromium ions at clinically
equivalent concentrations after metal-on-metal hip replacement on
human osteoblasts and osteoclasts: Implications for skeletal
health. Bone. 49:717–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah KM, Wilkinson JM and Gartland A:
Cobalt and chromium exposure affects osteoblast function and
impairs the mineralization of prosthesis surfaces in vitro. J
Orthop Res. 33:1663–1670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YK, Ye J, Han QL, Tao R, Liu F and
Wang W: Toxicity and bioactivity of cobalt nanoparticles on the
monocytes. Orthop Surg. 7:168–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schrock K, Lutz J, Mändl S, Hacker MC,
Kamprad M and Schulz-Siegmund M: Co (II)-mediated effects of plain
and plasma immersion ion implanted cobalt-chromium alloys on the
osteogenic differentiation of human mesenchymal stem cells. J
Orthop Res. 33:325–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moshtaghie AA, Malekpouri P, Moshtaghie E
and Rahnamaie F: Cobalt induces alterations in serum parameters
associated with bone metabolism in male adult rats. Turk J Biol.
38:1–567. 2014. View Article : Google Scholar
|
|
21
|
Dai M, Yuan X, Fan H, Cheng M and Ai J:
Expression of receptor activator of nuclear factor kappaB ligand
and osteoprotegerin of mice osteoblast induced by metal ions.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 24:292–295. 2010.(In
Chinese). PubMed/NCBI
|
|
22
|
Karsenty G: Transcriptional control of
skeletogenesis. Annu Rev Genomics Hum Genet. 9:183–196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt C, Steinbach G, Decking R, Claes
LE and Ignatius AA: IL-6 and PGE2 release by human osteoblasts on
implant materials. Biomaterials. 24:4191–4196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanaji A, Caicedo MS, Virdi AS, Sumner DR,
Hallab NJ and Sena K: Co-Cr-Mo alloy particles induce tumor
necrosis factor alpha production in MLO-Y4 osteocytes: A role for
osteocytes in particle-induced inflammation. Bone. 45:528–533.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stein GS and Lian JB: Cellular and
Molecular Biology Of Bone. 1st. Academic Press; Tokyo: 1993,
View Article : Google Scholar
|
|
27
|
Zijlstra WP, Bulstra SK, van Raay JJ, van
Leeuwen BM and Kuijer R: Cobalt and chromium ions reduce human
osteoblast-like cell activity in vitro, reduce the OPG to RANKL
ratio, and induce oxidative stress. J Orthop Res. 30:740–747. 2012.
View Article : Google Scholar : PubMed/NCBI
|