Introduction
Chromosome diseases are usually caused by a change
in chromosome number and structural abnormalities. Chromosome
number abnormalities include autosomal and sex chromosome
abnormalities (1). Autosomal
abnormalities include aneuploidy abnormalities, such as trisomy 21,
trisomy 18 and trisomy 13, chimera, such as 46,XX/47,XX and +21,
and polyploid abnormalities, such as single triploid and tetraploid
chimera. Sex chromosome abnormalities include Turner's syndrome and
sex chromosome triploid abnormalities. Chromosome structure
abnormalities include chromosome deletion, translocation,
inversion, circular chromosome and isochromosome (2). Chromosome aberration can occur during
the process of germ cell meiosis and the development of the
fertilized egg cells, or in human cells. Chromosome abnormalities
account for 50–60% of spontaneous miscarriages, and occur in
0.5–0.73% of newborns (3,4). Chromosome abnormalities usually
result in developmental arrest and spontaneous miscarriage. The
first confirmed human chromosome number abnormality, trisomy 21,
also known as Down's syndrome (5,6), it
is one of the most common chromosomal abnormalities (occurs in
~1.5% of newborns). Its clinical features include a characteristic
face, mental retardation, multiple malformations and derma to glyph
changes (5).
Due to of environmental pollution and hereditary
factors, the rates of birth defects including neural tube defects,
congenital heart disease and Down's syndrome are high (7), Down's syndrome has been reported to
occur at a rate of approximately 1.28 in every 1,000 live births in
China (8), The etiology of birth
defects is complex, including chromosome abnormalities, single
genetic defects, polygenic inheritance and teratogens, but
chromosome abnormalities account for ~6% of birth defects (9). Currently, there is no effective
method for preventing and treating chromosome diseases.
Prenatal examinations are performed to screen for
and diagnose chromosomal abnormalities. Screening involves
serological and ultrasound screening (10). Chorionic villus biopsy (11), amniocentesis, fetal umbilical cord
blood puncture and biopsy, and fetal endoscopy are used for the
diagnosis of chromosomal abnormalities (11–14).
Fetal karyotype analysis is the gold standard for prenatal
diagnosis. In developed countries, antenatal examination is common
even in the first trimester (15),
but in developing country, the majority of pregnant women register
for obstetric examination in the second trimester. Additionally,
invasive prenatal diagnosis can cause miscarriage, and the rate can
reach 1% (7). Currently, prenatal
diagnosis methods are commonly performed for pregnant women
identified as high-risk in the prenatal screening (16). Therefore, developing safe,
effective and economical prenatal screening methods is of important
clinical significance.
Second trimester maternal serologic trigeminy
screening of α-fetoprotein (AFP), free β-human chorionic
gonadotropin (HCG) and unconjugated-estriol (uE3) at 15–20 weeks is
a popular method (17). Pregnant
women with a risk rate of Down's syndrome <1/270 and trisomy 18
risk rate <1/350, but with abnormal serum markers [AFP Multiples
of the Median (MoM) ≥2.5; AFP MoM <0.7; HCG MoM >2.5; HCG MoM
<0.25; or uE3 MoM <0.7) were involved in the present study.
The aim of the current study was to screen out the chromosome
abnormalities of these pregnant women. Systematic fetal sonography
was performed at the Third Xiangya Hospital of Central South
University (Changsha, China) and Xiangtan Center Hospital
(Xiangtan, China). If ultrasound abnormalities were observed,
amniotic cavity puncture or umbilical cord blood tube centesis were
performed, combined with chromosome karyotype analysis. In this
process ultrasound screening was useful for effectively screening
for chromosome abnormalities. Furthermore, preliminary analysis was
performed on the association between different pregnancy histories
and the risk of chromosomal abnormalities, and the association
between the detection of abnormal features on the fetal ultrasound
and the detection of chromosomal abnormalities, which may provide a
more effective method to screen for fetal chromosomal diseases.
Materials and methods
Subjects
Between June, 2013 and April, 2015, 8,469 pregnant
women were recruited to this study (3,456 from Xiangtan Center
Hospital and 5,014 from The Third Xiangya Hospital of Central South
University, Hunan, China), aged 20–35. At 16–28 weeks' gestation,
second trimester serologic trigeminy screening was performed; 1,217
cases with a Down's syndrome risk rate <1/270 and 18-trisomy
risk rate <1/350, exhibited single abnormal serum markers (AFP
MOM ≥2.5, AFP MOM <0.7, HCG MOM >2.5, HCG MOM <0.25 or uE3
MOM <0.7). Of the patients with a single abnormal serum marker,
1,012 were primipara, and 205 were multipara. Systematic fetal
ultrasound was performed at 18–32 weeks' gestation. In the group
with abnormal markers, fetal ultrasound abnormalities were observed
in 99 women, therefore, prenatal diagnosis was performed using
amniotic fluid or umbilical cord blood. Abnormalities were
confirmed by pathological examination following birth or induced
labor. Pregnant women with abnormal markers but without fetal
ultrasound abnormalities (1,118 in total) were used as the control,
95 underwent prenatal diagnosis. All study methods were approved by
the Ethics Committee of the Third Xiangya Hospital of Central South
University and Xiangtan Center hospital. All subjects provided
written formal consent.
Ultrasonography
At 18–32 weeks, pregnant women were examined by
systematic ultrasound examination (abdominal probe 3.5–6 MHz) with
attention paid to the fetal head, face, spine, internal organs,
limbs, placenta, amniotic fluid, umbilical cord, the measurement of
biparietal diameter, head circumference, cerebellar diameter,
vertical pool depth, abdominal perimeter, femur and humerus. If an
abnormality was identified, detailed inspection was performed on
other areas or organs. If a surface deformity, such as facial or
foot deformity was identified, three-dimensional ultrasound imaging
was performed to provide more intuitive, visual and accurate
information.
Ultrasound examination assessment
standard
Prenatal ultrasound screening for fetal chromosomal
disease aimed to screen for two types of abnormality: i) Anatomical
malformations of the fetus, such as cleft lip palate and cardiac
abnormalities; and ii) Markers of chromosomal abnormalities, such
as neck soft tissue layer thickening and lateral ventricle mild
expansion. These indicators are also termed soft marker ultrasound
anomalies.
Amniotic fluid cells chromosome
analysis
Amniotic fluid (20 ml) was women at 18–24 weeks by
amniotic cavity puncture under ultrasound guidance, then was
transferred into two 15 ml sterile centrifuge tubes. Centrifugation
was performed at 800 × g and 25°C for 10 min, the supernatant was
discarded and the remainder (0.5–1 ml) was used to prepare a
single-cell suspension. The cells were transferred to a culture
bottle with 3 ml F10 media (pH 6.8) and 1 ml fetal bovine serum
(25%) (both Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) added.
Cells were maintained at 37°C in a 5% CO2 incubator.
Fiber or epithelioid cell growth was observed within 5–6 days in
culture, and cells entered a vigorous growth period in 8–14 days.
When 4–6 colonies formed in each bottle, the culture medium was
refreshed, and culture was continued for 2 days. Under a light
microscope (magnification, ×30-40), 3–5 round cells were observed
in each view, then thymidine (0.3 mg/ml) was added. Following
treatment for 14–18 h, cells were washed twice with F10 medium, and
then 2.5 ml F10 and 1.5 ml calf serum (25%) (Sigma-Aldrich; Merck
KGaA), and cultured at 37°C for ~10 h. When numerous round large
cells were observed, actinomycin D (2 µg/ml) or ethidium bromide
(10 µg/ml) were added and cells were incubated with colchicine
(0.2–0.3 µg/ml) for 1.5 h before the cells were harvested. Adherent
cells were removed with a bamboo peeling tool and the cell
suspension was transferred to a 10 ml centrifuge tube and
centrifuged at 2,000 × g for 10 min and at 25°C. The supernatant
was discarded and cells were resuspended in a 3 ml mixture of 0.4%
potassium chloride and 0.4% sodium citrate (1:1) and incubated in a
water bath for 4–6 min at 37°C. Cells were fixed in a mixture of
methanol and glacial acetic acid (3:1; 0.5 ml) and centrifuged for
8 min at 2,500 × g and 25°C. The supernatant was discarded and
fixed with the new fixative for >30 min in two changes. After
using ice to cool slides, small droplets of the cell suspension
were placed onto slides and passed through a flame two to three
times, then heated to 75°C for 2–3 h and naturally cooled to
~37°C.
Umbilical cord blood cell chromosome
analysis
Cord blood centesis was performed at 24–32 weeks
gestation, except onesubject whose fetal amniotic fluid chromosome
analysis result (detected by both amniotic fluid and cord blood)
was trisomy 21 syndrome; her cord blood was retained for further
consultation before induced labor. Following the addition of
heparin (0.5 U/ml) anticoagulant, cord blood was cultured for 72 h
in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) containing 25% calf
serum (Sigma-Aldrich; Merck KGaA). Thymine nucleoside (0.3 mg/ml)
was added and incubated at 37°C for 17 h then washed with RPMI-1640
twice to remove excess thymine. RPMI-1640 medium (5 ml) and
bromodeoxyuridine (12 µg/ml) was added and incubated at 37°C for 5
h. Actinomycin D (6 mg/ml) was added and cultured for 60 min.
Finally, colchicine (0.4 µg/ml) was added and after 10 min the
cells were transferred into a 10 ml centrifuge tube and centrifuged
at 1,600–2,000 × g for 10 min at room temperature and the
supernatant was removed. Preheated (37°C) 0.4% potassium chloride
and 0.4% sodium citrate (1:1; 8 ml) was added and incubated at 37°C
for 15 min. The fixative of methanol and glacial acetic acid (3:1;
1 ml) was added, gently mixed, and centrifuged at 2,400 × g for 10
min at room temperature. The supernatant was removed, and 8 ml of
the fixative was added; fixative was changed every 30 min at least
twice, with 2,400 × g centrifugation for 10 min performed between
fixations. The upper layer of fixative was removed and 8–10 drops
of new fixative was added. The cell aggregates were combined into a
single cell suspension. Slides were precooled and 1–2 drops of the
cell suspension were added to slides, which were then immediately
passed through a flame, then heated to 75°C for 2–3 h and allowed
to cool to 37°C.
G banding
The sample slides were incubated for 2–3 h at 37°C
then 0.025% preheated trypsin was added for 30–75 sec. Slides were
rinsed in phosphate buffer solution (pH 6.8) for ~15 sec. Slides
were dyed with Giemsa dye solution (1:10) for 30–40 min, washed for
a few seconds with tap water and air-dried.
Microscopy
Middle division phase cells were observed using a
light microscope and a certain number cells (~50 cells) with a
distinct chromosome spread were chosen, and the chromosome number
was calculated to determine the diploid chromosome number. Three
mitosis phases were observed under an oil immersion lens to
determine whether the chromosome zone was normal.
Statistical analysis
SPSS statistics software version 12.0 (SPSS, Inc.,
Chicago, IL, USA) is used for statistical analysis; χ2
analysis was used to comparatively analyze the rate of fetal
chromosomal abnormalities between the observed group and the
control group. Comparisons among groups were performed by
χ2 and t test. Data are expressed as the mean ± standard
error. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ultrasound screening results
Ultrasounds revealed that 99 of 1,217 pregnant women
with single abnormal serum markers had prenatal abnormalities, all
of which were confirmed by pathological examination of the fetus.
Only certain minor deformities were missed by ultrasound diagnosis,
such as malformation of the urinary or reproductive tract, and
finger/toe malformation.
Cytogenetic results from umbilical
cord blood and amniotic fluid specimens
Amniotic fluid cell culture is the safer method of
prenatal diagnosis at 18–24 weeks compared with umbilical cord
blood analysis. The amniotic fluid is >200 ml and easy to
replenish, and the cells are abundant and easy to culture. However,
in long term culture, the amniotic fluid cells are vulnerable to
contamination, therefore a strictly sterile culture must be
observed.
Of the 99 pregnant women with abnormalities revealed
by ultrasound, the culture of two amniotic fluid specimens failed,
and 97 cases underwent chromosome karyotype analysis. One of the 97
women had a history of two problematic pregnancies, 37 cases had
had one problematic pregnancy; none had a history of trisomy 21. Of
the 38 cases with abnormal gestation and parturition history, seven
fetal chromosomal abnormalities were identified in this study: Two
trisomy 18, one trisomy 21, three trisomy 13 and one 45,X (Turner
syndrome). Two fetal chromosomal abnormalities were identified in
the 59 women without abnormal gestation and parturition history:
One trisomy 18 and one trisomy 21.
Samples were taken from 95 pregnant women with
abnormal serum markers but no fetal ultrasound abnormalities as the
control group. One umbilical cord blood specimen became
contaminated and the culture of two amniotic fluid specimens
failed. Complete karyotype analysis was performed on 92 controls
(96.8%; 88 cases using amniotic fluid cell, 7 cases using cord
blood cell) with no chromosomal abnormalities identified.
In patients with abnormal serum markers and
ultrasound abnormalities, chromosome analysis was performed
successfully for 97 cases (Table
I); 57 had single abnormalities on the ultrasound and 40 had
multiple abnormalities. Of those with a single abnormality, 2 cases
of chromosomal abnormalities were identified, and of those with
multiple abnormalities, 9 chromosome abnormalities were identified
(Table II). In the group with
abnormalities observed on the ultrasound, 88 cases had a normal
chromosome karyotype (90.7%) and nine cases had abnormal karyotype
(9.28%); two cases with trisomy 21 (2.06%), three cases with
trisomy 18 (3.09%), three cases with trisomy 13 (3.09%) and one
case with 45,X (Turner syndrome; 1.03%). Ultrasound images of 5
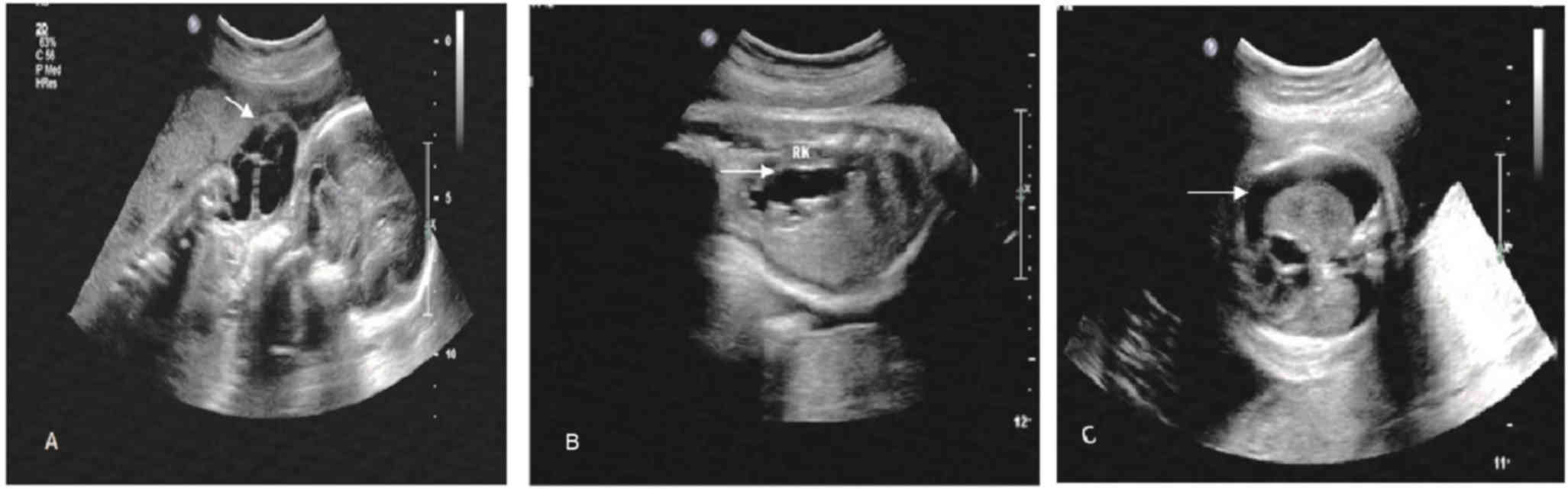
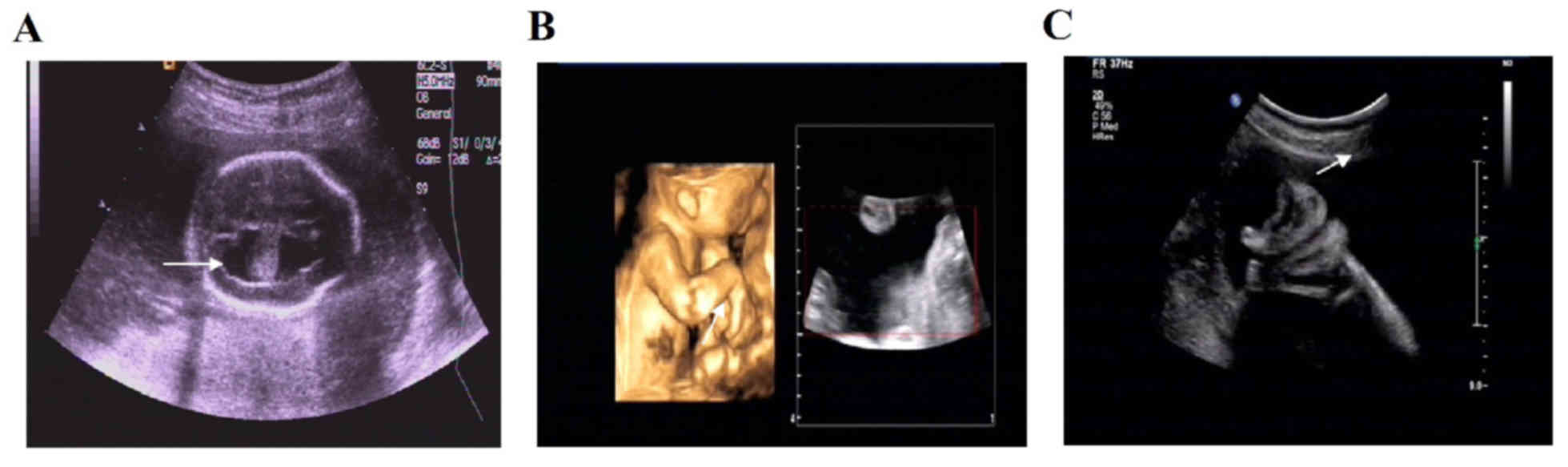
cases with ultrasound and chromosomal abnormalities (Figs. 1–5). Of the 9 cases with chromosomal
abnormalities, 5 of the pregnant womenwereaged >30 years old
(55.6%). Representative normal and abnormal chromosome karyotype
maps are presented in Fig. 6.
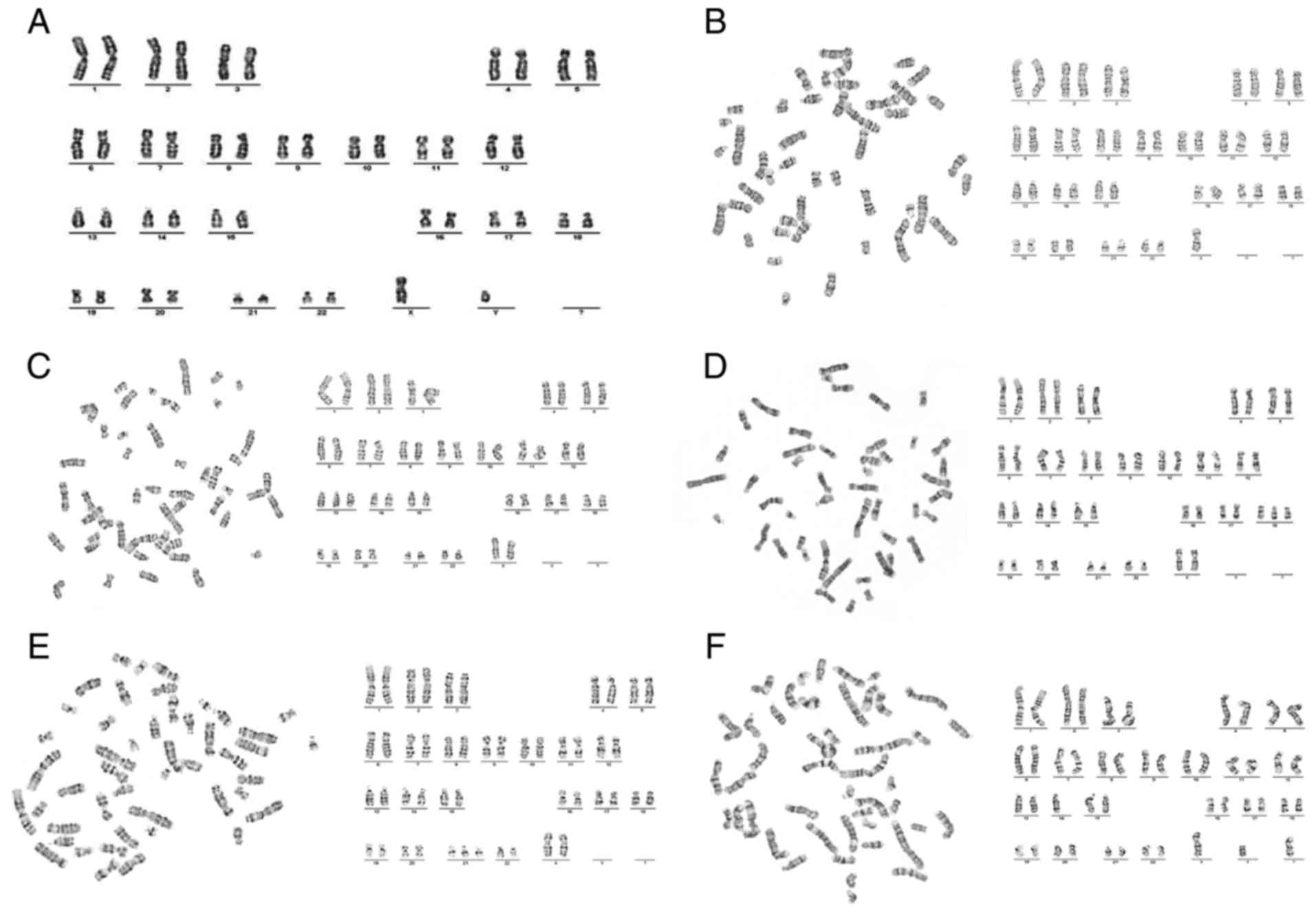 | Figure 6.Karyotype analysis. (A) Karyotype
analysis of normal karyotype: 46,XY. (B) Karyotype analysis of
Turner syndrome: 45,X (C) Karyotype analysis of trisomy 13: 47,XX,
+13. (D) Karyotype analysis of trisomy 18: 47,XX,+18. (E) Karyotype
analysis of trisomy 21: 47,XX,+21. (F) Karyotype analysis of
trisomy 21: 46,XX, der (14,21),
+21 (×400 magnification). |
 | Table I.Chromosomal abnormalities of 97 cases
with abnormalities identified by ultrasound. |
Table I.
Chromosomal abnormalities of 97 cases
with abnormalities identified by ultrasound.
| Group | Number of cases | Number of chromosomal
abnormalities | Rate of chromosomal
abnormalities (%) |
|---|
| Single ultrasound
abnormalities (Each abnormality occurs once: a, b, c, d) | 57 | 2 | 3.51 |
| Multiple ultrasound
abnormalities (Each abnormality occurs twice or more: a, b, c,
d) | 40 | 7 | 17.5 |
| Cardiac
structural abnormalities + other abnormalities | 25 | 4 | 16% |
| Cleft lip
and palate + other abnormalities | 3 | 1 | 33% |
| Neck
lymph cyst + hydramniosa (a) + other abnormalities | 2 | 1 | 50% |
| Lateral
ventricle broad (b) or posterior fossa pool broad | 10 | 1 | 20% |
| (c) or
separation of the renal pelvis (d) + other anomalies |
| Total | 97 | 9 | 9.28 |
 | Table II.Results of prenatal serum screening,
chromosome examination and sonographic findings of the nine cases
with fetal chromosomal abnormalities. |
Table II.
Results of prenatal serum screening,
chromosome examination and sonographic findings of the nine cases
with fetal chromosomal abnormalities.
| Patient number | Age of mother | Gestational
weeks | Single abnormal serum
marker | Ultrasound
abnormalities | Chromosome
examination | Pathological
examination |
|---|
| 1a | 35 | 22 | AFP MoM <0.7 | Neck lymph cyst,
pleural effusion in great quantities, right kidney seeper | 45,X | As prenatal
ultrasound diagnosis |
| 2a | 34 | 20 | HCG MoM <0.25 | Cleft of lip and
palate, cardiac structural abnormalities, single umbilical artery,
polyhydramnios, intrauterine growth restriction | 47,XX,+13 | As prenatal
ultrasound diagnosis and hypospadia |
| 3 | 32 | 22 | HCG MoM <0.25 | Hydrocephalus,
cardiac structural abnormalities, posterior fossa pool broadening,
little magenblase, double kidney seeper | 47,XX,+18 | As prenatal
ultrasound diagnosis |
| 4a | 26 | 23 | HCGMoM <0.25 | Fetus diaphragmatic
hernia, cardiac structural abnormalities, choroid plexus cyst,
polyhydramnios | 47,XX,+18 | As prenatal
ultrasound diagnosis |
| 5a | 29 | 24 | AFP MoM <0.7 | Fetal edema, pleural
effusion, hydramnios, double renal pelvis separation | 47,XX,+21 | As prenatal
ultrasound diagnosis |
| 6 | 32 | 26 | HCG MoM <0.25 | Cardiac structural
abnormalities | 47,XY,+18 | As prenatal
ultrasound diagnosis |
| 7a | 33 | 25 | AFP MoM <0.7 | Cerebellum lateral
ventricle broadening, cardiac structural abnormalities, limbs
deformities, polyhydramnios | 46,XY,der
(14;21),+21 | As prenatal
ultrasound diagnosis, eyes wide, tongue slightly larger to the
lips, through the palm (left), genital malformations |
| 8 | 28 | 18 | HCG MoM
<0.25 | Fetal umbilical
bulging | 47,XY,+13 | As prenatal
ultrasound diagnosis |
| 9 | 29 | 24 | uE3 MoM
<0.7 | Cerebellar lateral
ventricle broadening, local intestinal canalstrong echo, single
umbilical artery, oligohydramnios,intrauterine growth
restriction | 47,XY,+13 | As prenatal
ultrasonic diagnosis |
Comparison of chromosome abnormality
rate in different groups
Comparison of fetal chromosomal abnormality rate
between the control group and those with abnormalities identified
by ultrasound is presented in Table
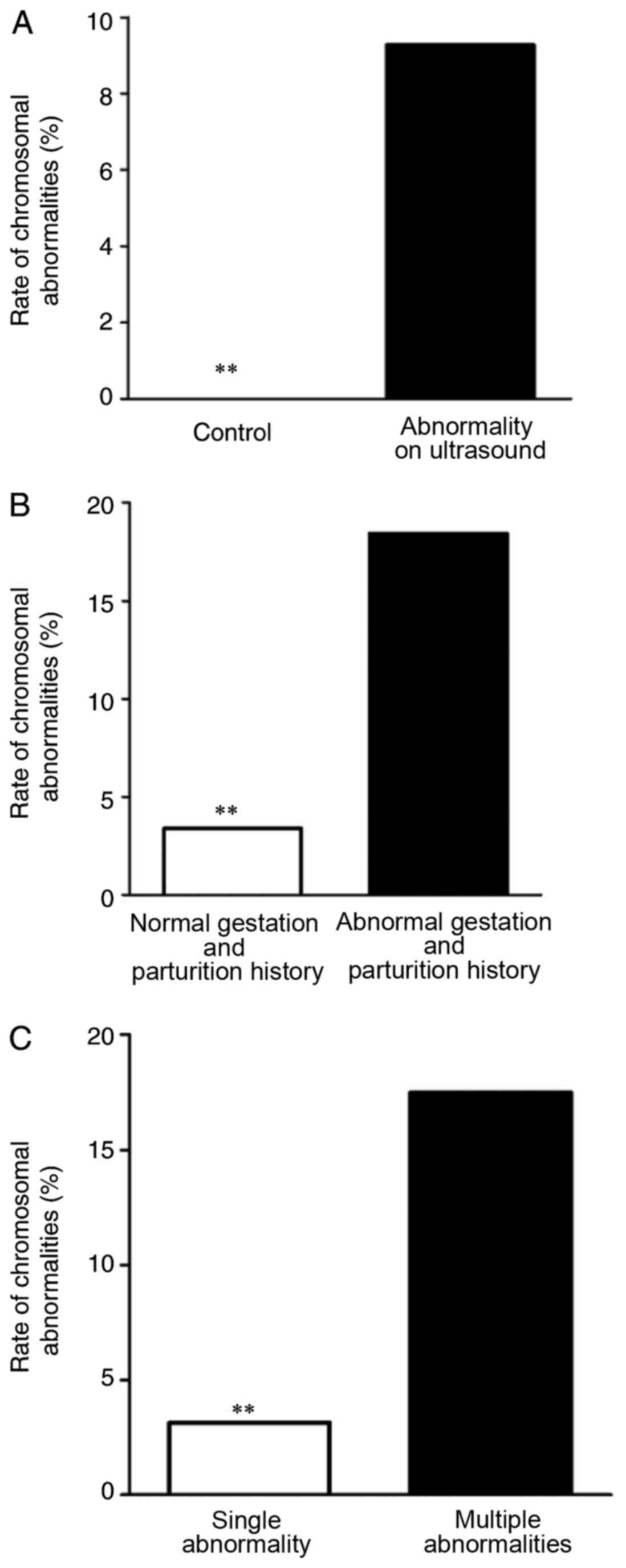
III and Fig. 7A. The rate of
fetal chromosomal abnormalities of in cases with abnormalities
identified by ultrasound is significantly higher than that of the
control group (P<0.01). Comparison of the fetal chromosomal
abnormality rate of the 97 cases with abnormalities identified by
ultrasound with different gestation and birth histories is
presented in Table IV and
Fig. 7B. In cases with
abnormalities identified by ultrasound, there were 38 pregnant
women with abnormal gestation and birth history, including previous
fetal abnormalities, stillbirth and habitual miscarriage, [seven
chromosomal abnormalities (18.4%), they were two trisomy 18, one
trisomy 21, three trisomy 13 and one 45,X (Turner syndrome)]. Among
the 59 cases with normal gestation and birth, there were two
chromosomal abnormalities (3.39%); one trisomy 18 and one trisomy
21. The rate of fetal chromosomal abnormalities was higher in women
with a history of abnormal gestation and parturition than those
with a normal history (P<0.01). The fetal chromosomal
abnormality rates between pregnant women with a single abnormal
indicator on the ultrasound and those with multiple indicators are
compared in Table V and Fig. 7C. The rate of fetal chromosomal
abnormalities was higher in cases where multiple abnormalities were
observed on the ultrasound compared with cases where a single
abnormality was observed on the ultrasound (P<0.05).
 | Table III.Comparison of fetal chromosomal
abnormalities rate between those with abnormal ultrasound
indicators and the control group. |
Table III.
Comparison of fetal chromosomal
abnormalities rate between those with abnormal ultrasound
indicators and the control group.
| Group | Abnormal
karyotype | Normal
karyotype | Total | Abnormality rate
(%) |
|---|
| Control | 0 | 92 | 92 | 0 |
| Observed | 9 | 88 | 97 | 9.28a |
| Total | 9 | 180 | 189 | 4.76 |
 | Table IV.Comparison of the fetal chromosomal
abnormalities rate of the 97 pregnant women (those with
abnormalities on ultrasound) with different gestation and
parturition history. |
Table IV.
Comparison of the fetal chromosomal
abnormalities rate of the 97 pregnant women (those with
abnormalities on ultrasound) with different gestation and
parturition history.
| Group | Abnormal
karyotype | Normal
karyotype | Total | Abnormality rate
(%) |
|---|
| No AGPH | 2 | 57 | 59 |
3.39 |
| AGPH | 7 | 31 | 38 | 18.42a |
| Total | 9 | 88 | 97 |
9.28 |
 | Table V.Comparison of fetal chromosomal
abnormalities rate between single and multiple abnormal ultrasound
indicators (AUI). |
Table V.
Comparison of fetal chromosomal
abnormalities rate between single and multiple abnormal ultrasound
indicators (AUI).
| AUI | Abnormal
karyotype | Normal
karyotype | Total | Abnormality rate
(%) |
|---|
| Single
abnormality | 2 | 55 | 57 |
3.51 |
| Multiple
abnormalities | 7 | 33 | 40 | 17.5a |
| Total | 9 | 85 | 97 |
9.28 |
Discussion
Second trimester antenatal examination is a popular
method to screen for chromosomal diseases. The examination is
generally divided into two forms: Invasive prenatal examination,
including amniotic cavity puncture and umbilical cord blood tube
centesis, with confirmation of by chromosomal disease by chromosome
karyotype analysis; and non-invasive examination, including
peripheral blood fetal cell enrichment, serologic screening and
ultrasound screening (6,10,18).
Because the invasive prenatal examination method can increase the
high risk of miscarriage, intrauterine infection, fetal
malformation and fetal intrauterine death, while enrichment of
peripheral blood fetal cells is complex and expensive. Therefore,
these methods are not suitable for screening of large populations.
As ultrasound examination combined with serologic screening is
simple, non-invasive, economical and reproducible, it is becoming
more and more appealing option (19,20).
Ultrasound screening for pregnant women with a
single abnormal serum marker makes up for the limitations of the
serological screening. The current study confirmed there is a risk
of fetal chromosomal abnormalities among pregnant women with a
single abnormal serum marker, and that ultrasound is useful for
detecting indicators of chromosomal abnormalities.
The present study demonstrated that trisomy 18,
trisomy 13, trisomy 21 and Turner syndrome non-integral chromosomal
abnormalities was commonly, which is similar to a previous report
(21). In the current study, among
7 cases with cardiac structural abnormalities accompanying other
abnormalities observed on the ultrasound, 4 (57%) had chromosomal
abnormalities, which was also in accordance with the previous
report (22). Cleft lip and palate
combined with other deformities are strong indicators of
chromosomal abnormalities. In the present study, 1 out of 2 cases
of cleft lip with other malformations was confirmed as trisomy 13
syndrome. A lymphatic hygroma on the neck is a typical indicator of
the deadly Turner syndrome, which is a common sex chromosome
abnormality that occurs in 0.27% of female newborns (23); cystic lymphangioma and edema in the
extremities are the main indictors of Turner syndrome in prenatal
ultrasound screening. In the current study, the 2 cases with fetal
neck lymphatic hygroma and the case with Turner syndrome chose to
terminate the pregnancy. The optimal time to undergo prenatal
ultrasound screening is at 18–24 weeks. The present study confirmed
that other fetal chromosomal abnormalities, in addition to trisomy
21, also existed in those with a single abnormal serum marker,
which is in accordance with a previous report (24). However, whatever screening method
is used only assesses the risk of fetal chromosome abnormalities,
which is a disadvantage of ultrasound screening (19), thus, for diagnosis, karyotype
analysis is indispensable.
Additionally, age is also a risk factor for
pregnancy with chromosome abnormalities (Table II), as has been reported
previously (25,26). Pregnant women older than 35 years
carry a high risk of Down's syndrome. In this study, of the 9
abnormal fetuses, all chose termination of the pregnancy. This
study also confirmed that pregnant women with abnormal gestation
and birth history had a high risk of fetal chromosome aberration
(Table IV), which may be closely
associated with any chromosome abnormality of the couple (27). Therefore, chromosome analysis of
the parents may be important for those with abnormal gestation and
birth histories. Additionally, the chromosome abnormality rate of
with multiple abnormal features observed on the ultrasound as
higher than when a single abnormal feature was observed on the
ultrasound, which is consistent with the report by Nicolaides
(28). The results of the present
study indicate that ultrasound screening combined with serological
screening is an effective method for identifying a risk of fetal
chromosomal abnormalities.
A deficiency of the current study is that not 100%
of chromosome abnormalities produce features that can be screened
for using ultrasound. In the control group, with an abnormal serum
marker and no abnormalities observed on the ultrasound, no
chromosomal abnormalities were identified by karyotype analysis;
but considering the sample size is small, the relationship cannot
be confirmed confidently, thus the further studies sound include a
greater number of patients.
In conclusion, ultrasound examination can be
effectively used to assist in screening for fetal chromosome
abnormalities in pregnant women that have a risk of Down's syndrome
risk rate >1/270 and trisomy 18 risk rate >1/350 (serologic
trigeminy screening) and a single abnormal serum marker. These
pregnant women have a high risk of fetal chromosomal
abnormalities.
References
|
1
|
Benn P: Expanding non-invasive prenatal
testing beyond chromosomes 21, 18, 13, X and Y. Clin Genet.
90:477–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gardner RJM and Sutherland GR: Chromosome
abnormalities and genetic counseling. 3rd. Oxford University Press;
Oxford: 2004
|
|
3
|
Nielsen J and Wohlert M: Chromosome
abnormalities found among 34,910 newborn children: Results from a
13-year incidence study in Arhus, Denmark. Hum Genet. 87:81–83.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abramsky L, Hall S, Levitan J and Marteau
TM: What parents are told after prenatal diagnosis of a sex
chromosome abnormality: Interview and questionnaire study. BMJ.
322:463–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roizen NJ and Patterson D: Down's
syndrome. Lancet. 361:1281–1289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alfirevic Z and Neilson JP: Antenatal
screening for Down's syndrome. BMJ. 329:811–812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botto LD, May K, Fernhoff PM, Correa A,
Coleman K, Rasmussen SA, Merritt RK, O'Leary LA, Wong LY, Elixson
EM, et al: A population-based study of the 22q11.2 deletion:
Phenotype, incidence, and contribution to major birth defects in
the population. Pediatrics. 112:101–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao ZY, Liu X, Shi TK, Xu Y, Song QH and
Tang SH: First trimester, second trimester, and integrated
screening for Down's syndrome in China. J Med Screen. 19:68–71.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hansen M, Kurinczuk JJ, Bower C and Webb
S: The risk of major birth defects after intracytoplasmic sperm
injection and in vitro fertilization. N Engl J Med. 346:725–730.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mademont-Soler I, Morales C, Soler A,
Martínez-Crespo JM, Shen Y, Margarit E, Clusellas N, Obón M, Wu BL
and Sánchez A: Prenatal diagnosis of chromosomal abnormalities in
fetuses with abnormal cardiac ultrasound findings: Evaluation of
chromosomal microarray-based analysis. Ultrasound Obstet Gynecol.
41:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfirevic Z, Sundberg K and Brigham S:
Amniocentesis and chorionic villus sampling for prenatal diagnosis.
Cochrane Database Syst Rev: CD00325. 2003. View Article : Google Scholar
|
|
12
|
Pallister PD, Pallister AB, South S,
Toydemir R, Johnson JP, Beischel L and Opitz JM: A deletion
13q34/duplication 14q32.2–14q32.33 syndrome diagnosed 50 years
after neonatal presentation as infantile hypercalcemia. Am J Med
Genet A. 155A:1–839. 2011.PubMed/NCBI
|
|
13
|
Harrison MR, Keller RL, Hawgood SB,
Kitterman JA, Sandberg PL, Farmer DL, Lee H, Filly RA, Farrell JA
and Albanese CT: A randomized trial of fetal endoscopic tracheal
occlusion for severe fetal congenital diaphragmatic hernia. N Engl
J Med. 349:1916–1924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Socié G, Gluckman E, Carosella E, Brossard
Y, Lafon C and Brison O: Search for maternal cells in human
umbilical cord blood by polymerase chain reaction amplification of
two minisatellite sequences. Blood. 83:340–344. 1994.PubMed/NCBI
|
|
15
|
Hromadníková I, Sedlácková L, Mrstinová M,
Stechová K, Karamanov S, Kofer J and Macek M: Levels of peripheral
circulating nucleated erythrocytes in pregnant women for
noninvasive prenatal diagnosis. Ceska Gynekol. 65:33–37. 2000.(In
Czech). PubMed/NCBI
|
|
16
|
Oneda B, Steindl K, Masood R, Reshetnikova
I, Krejci P, Baldinger R, Reissmann R, Taralczak M, Guetg A, Wisser
J, et al: Noninvasive prenatal testing: More caution in counseling
is needed in high risk pregnancies with ultrasound abnormalities.
Eur J Obstet Gynecol Reprod Biol. 200:72–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frank M, Maymon R, Wiener Y, Neeman O,
Kurzweil Y and Bar J: The effect of hereditary versus acquired
thrombophilia on triple test Down's syndrome screening. Prenat
Diagn. 33:191–195. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hui WW and Chiu RW: Noninvasive prenatal
testing beyond genomic analysis: What the future holds. Curr Opin
Obstet Gynecol. 28:105–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baillie C, Smith J, Hewison J and Mason G:
Ultrasound screening for chromosomal abnormality: Women's reactions
to false positive results. Br J Health Psychol. 5:377–394. 2000.
View Article : Google Scholar
|
|
20
|
Shipp TD and Benacerraf BR: Second
trimester ultrasound screening for chromosomal abnormalities.
Prenat Diagn. 22:296–307. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nyberg DA and Souter VL: Sonographic
markers of fetal trisomies: Second trimester. J Ultrasound Med.
20:655–674. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moran CJ, Tay JB and Morrison JJ:
Ultrasound detection and perinatal outcome of fetal trisomies 21,
18 and 13 in the absence of a routine fetal anomaly scan or
biochemical screening. Ultrasound Obstet Gynecol 2. 20:482–485.
2002. View Article : Google Scholar
|
|
23
|
Bondy CA: Turner Syndrome Study Group:
Care of girls and women with Turner syndrome: A guideline of the
Turner syndrome study group. J Clin Endocrinol Metab. 92:10–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massé J, Summers A, Cherian G and Forest
J: Transportation of maternal serum specimens for screening for
chromosomal aneuploidies: Effect of seasonal variations, distance,
and freezing on the stability of the biological markers. Clin
Biochem. 33:273–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sancken U and Bahner D: Comparison of
triple-risk assessment of fetal trisomy 21 including total human
choriogonadotropin (hCG) or its free β-subunit (free βhCG). Fetal
Diag Ther. 18:122–127. 2003. View Article : Google Scholar
|
|
26
|
Goetzl L, Krantz D, Simpson JL, Silver RK,
Zachary M, Pergament E, Platt LD, Mahoney MJ and Wapner RJ:
Pregnancy-associated plasma protein A, free beta-hCG, nuchal
translucency, and risk of pregnancy loss. Obstet Gynecol.
104:30–36. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung MC, Goldberg JD and Kan YW:
Prenatal diagnosis of sickle cell anaemia and thalassaemia by
analysis of fetal cells in maternal blood. Nat Genet. 14:264–268.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicolaides K: Screening for chromosomal
defects. Ultrasound Obstet Gynecol. 21:313–321. 2003. View Article : Google Scholar : PubMed/NCBI
|