Introduction
Diabetes mellitus (DM) is characterized by chronic
high blood glucose, which causes injuries to vessels and lead to
the development of vascular complications in tissues, including the
heart, kidney and eye (1–4). The vascular complications of diabetes
have become a serious health concern in humans.
The damage of endothelial cells is observed in the
early stages of diabetic vascular complications (1,3–6). The
endothelial dysfunction, which is triggered by hyperglycemia,
includes increased endothelial oxidative stress, inflammation and
cell apoptosis, decreased nitric oxide (NO) bioavailability, and
high expression levels of hypoxia-inducible factor-1α (HIF-1α) and
vascular endothelial growth factor (VEGF) (1,5,6).
Increasing evidence indicates that endothelial dysfunction is
important in the pathogenesis of diabetic vascular complications
(2,3).
The levels of superoxide are elevated and the
activities of antioxidant defense substances are reduced in the
vessels of diabetic patients and rats, and in endothelial cells
exposed to high glucose (HG) (7–10).
Elevated oxidative stress may cause injuries in vascular
endothelial cells during the process of diabetic vascular
complications (11–13). Excessive reactive oxygen species
(ROS) and lipid peroxide initiate and promote endotheliocyte damage
(1,7). The antioxidant status of diabetic
patients is crucial in preventing oxidative stress and the process
of vascular complications. Antioxidative enzymes, including
superoxide dismutase (SOD), glutathione peroxidase (GPx) and
catalase (CAT), inhibit the generation of ROS (14–17),
which prevents against endothelial cell injury. Therefore, the
correlations among hyperglycemia, redox imbalance and oxidative
stress constitute the main pathological mechanism underlying
diabetic vascular complications (2).
There are several similarities between diabetic
vascular complications and chronic inflammatory diseases (7,10).
The inflammation triggered by hyperglycemia can cause damage to
endothelial cells, which then increases vascular permeability and
accelerates the release of proinflammatory mediators (18,19).
Nuclear factor-κB (NF-κB), activated by hyperglycemia, elevates the
levels of proinflammatory mediators, including intercellular
adhesion molecule-1 (ICAM-1), interleukins (ILs), VEGF and tumor
necrosis factor-α (TNF-α) (18–20).
Therefore, inflammation and diabetic vascular complications are
linked, and excessive inflammatory factors may predict the onset
and progression of diabetic vascular complications.
Danhong Huayu Koufuye (DHK) has long been used
clinically in China (21–24). It contains 29% Salvia
miltiorrhiza radix, 11.5% Angelicae sinensis radix, 15%
Chuanxiong rhizoma, 11.5% Persicae semen, 11.5%
Carthami flos, 11.5% Bupleuri radix and 10%
Aurantii fructus. DHK had the ability to promote blood
circulation to overcome blood stasis and, in Traditional Chinese
medicine, is believed to promote qi circulation and remove meridian
obstruction. Our previous studies showed that DHK prevented the
process of diabetic retinopathy in diabetic Sprague-Dawley (SD)
(22) and Zucker diabetic fatty
(ZDF) rats (23). It was also
found that DHK inhibited the formation of deep venous thrombosis
via anti-inflammatory activity in rats (24).
The present study investigated the protective effect
of DHK-medicated serum on HG-induced injury and apoptosis in EA.
hy926 cells, and examined whether the antioxidative and
anti-inflammatory activities of DHK were involved in the
mechanisms.
Materials and methods
Materials
DHK was provided by Hutchison Whampoa Guangzhou
Baiyunshan Chinese Medicine Co., Ltd. (Guangzhou, China).
Pentobarbital sodium salt and xylazine hydrochloride injection were
purchased from Merck Serono Co., Ltd. (Beijing, China) and Dunhua
Shengda Pharmaceutical Co., Ltd. (Dunhua, China), respectively.
Cell culture reagents, including Dulbecco's modified Eagle's medium
(DMEM), penicillin and streptomycin were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA), and fetal bovine
serum (FBS) was obtained from Biological Industries (Kibbutz Beit
Haemek, Israel). The Annexin V/Fluorescein Isothiocyanate Apoptosis
Detection kit was purchased from eBioscience, Inc. (San Diego, CA,
USA). 2′,7′-Dichlorodihydrofluorescein diacetate
(H2DCFDA) was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The SOD assay kit, thiobarbituric acid
reactive substance (TBARS) assay kit and GPx assay kit were
purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Rabbit
polyclonal antibodies against ICAM-1 (cat. no. ab7815), NF-κB (cat.
no. ab28835), HIF-1α (cat. no. ab82832), glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. ab37168), and goat anti-rabbit IgG
conjugated with horseradish peroxidase (cat. no. ab6721) were
purchased from Abcam (Cambridge, UK). The rabbit polyclonal
antibody against VEGF (cat. no. sc507) was purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Enhanced
chemiluminescence (ECL) reagent was purchased from Pierce; Thermo
Fisher Scientific, Inc.
Animals
Male SD rats (200–250 g; n=10) were obtained from
the Experimental Animal Center, Guangzhou University of Chinese
Medicine (Guangzhou, China). All rats (approval no. SCXK 2013–0020)
had free access to a standard diet and drinking water, and were
housed in a room at 24.0±0.5°C and with a 12:12 h light/dark
schedule. The experiments were performed in accordance with the
Animal Ethics Committee of Guangzhou University of Chinese
Medicine.
Cell culture
EA. hy926 cells, a hybrid human umbilical vein
endothelial cell line, were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in DMEM with 5 mM glucose, essential and
non-essential amino acids (8.9 mg/l L-alanine, 13.3 mg/l aspartic
acid, 15 mg/l L-asparagine, 14.7 mg/l monosodium glutamate and 11.5
mg/l proline), sodium selenite (0.02 mg/l), ascorbic acid (10
mg/l), 10% FBS (v/v) and antibiotics (100 Ul penicillin and 100
mg/l streptomycin) at 37°C in an atmosphere containing 5%
CO2 and 95% air. EA. hy926 cells at passages 3–5 were
used in all experiments.
Preparation of DHK-medicated
serum
The rats were randomly divided into two groups:
Vehicle group and DHK group, in which the rats were
intragastrically administered with distilled water or DHK (3.20
ml/kg), respectively, for 5 days consecutively (once per day).
Blood was collected from the carotid artery 1 h following the fifth
administration, and was centrifuged at 1,000 × g at room
temperature for 10 min to obtain serum. The serum was sterilized at
56°C for 30 min, and then mixed with complete medium to prepare the
DHK-medicated serum, according to the volume ratio (v/v) to give 1,
5, 10 and 20% solutions.
Cell viability assay
The viability of EA. hy926 cells treated with
various concentrations of glucose, DHK-medicated serum, or the two
in combination, was measured using a colorimetric MTT assay
(7,11). Briefly, the cells (3,000
cells/well) were seeded into 96-well plates and cultured in
complete DMEM. The cells were treated with glucose at
concentrations of 5, 25, 40 and 60 mM, isotonic mannitol at
concentrations of 35 and 55 mM, DHK-medicated serum (1, 2, 5, 10
and 20%) alone, and DHK-medicated serum (1, 5 and 10%) combined
with 40 mM glucose for 48, 72 and 96 h at 37°C in an atmosphere
containing 5% CO2 and 95% air. MTT solution (5 mg/ml; 10
µl) was added into each well containing the cells, and was
incubated at 37°C for 4 h in a humidified atmosphere containing 5%
CO2 and 95% air. Following removal of the medium,
formazan crystals were solubilized by adding 100 µl of
dimethylsulfoxide and oscillating for 10 min. The absorbance at 490
nm was measured using a microplate reader (Multiskan GO; Thermo
Fisher Scientific. Inc.).
Cell apoptosis analysis
The cells were divided into five experimental
groups: Normal group (5 mM glucose), model group (40 mM glucose),
and 1, 5 or 10% medicated serum + 40 mM glucose groups. Apoptosis
was evaluated with an Annexin V/fluorescein isothiocyanate
apoptosis detection kit using flow cytometry (25). The cells were detached and stained
according to the manufacturer's protocol and then measured using a
flow cytometer (FACSCanto™ II; BD Biosciences, Franklin Lakes, NJ,
USA).
Lipid peroxidation assay
Lipid peroxidation was assayed using a TBARS method
(7,18). The samples collected from the
culture medium were used to measure the malondialdehyde (MDA)
formed in a peroxidizing lipid system. The quantities of TBARS were
calculated according to a standard curve of 1,1,3,
3-tetramethoxypropane.
Measurement of the activities of GPx
and SOD
The activities of GPx and SOD in the culture medium
were measured using a spectrophotometric method according to the
manufacturer's protocol.
Measurement of intracellular ROS
Intracellular ROS levels were measured using the
oxidation-sensitive fluorescent probe dye H2DCFDA (Ex/Em
= 488 nm/525 nm). The cell suspension was inoculated into a 96-well
plate at a density of 1×104 cells/well. The cells were
rinsed with PBS and then incubated with 10 µM H2DCFDA at
37°C for 30 min according to the manufacturer's protocol. The
fluorescence intensities were detected using a multiscan spectrum
(2300–001M; PerkinElmer, Inc., Waltham, MA, USA) and images were
captured under an inverted light microscope (BDS 300; Optec
Instrument, Co., Ltd., Chongqing, China).
Western blot analysis
Western blot analysis was performed as previously
described (7,18,19,25).
Cells grown on 6-well plates were harvested using 200 µl ice-cold
Pierce Radioimmunoprecipitation Assay Buffer (Thermo Fisher
Scientific, Inc.) supplemented with 10 µl/ml protease inhibitor
cocktail (Sigma-Aldrich, Merck KGaA). Following centrifugation at
12,000 × g for 30 min at 4°C, the supernatants were collected and
the total protein content was quantified using a Bradford protein
assay. A total of 50 µg total protein extract was separated by
SDS-PAGE on a 10% gel and transferred onto a PVDF membrane using
Hoefer miniVE transfer system (170–4467; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with 5%
non-fat milk for 1 h at room temperature. The membrane was
incubated with ICAM-1 antibody (1:1,000), NF-κB p65 antibody
(1:1,000), VEGF antibody (1:1,000), HIF-1α antibody (1:1,000) or
GAPDH (1:1,000) antibody overnight at 4° following blocking. The
membrane was then incubated with secondary antibodies conjugated to
horseradish peroxidase at room temperature for 1 h. The bound
antibodies were detected using ECL reagent. The quantity of
immunoreactive protein was assessed using scanning densitometry
(1658001; Bio-Rad Laboratories, Inc). All blots were normalized
with an antibody against GAPDH and average band intensities
relative to total proteins were determined using Image J software
(version 1.43; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Each experiment was repeated at least three times.
All data are expressed as the mean ± standard error of the mean and
were analyzed using the Statistical Package for the Social Sciences
version 20.0 (IBM SPSS, Armonk, NY, USA). One-way analysis of
variance was performed and an LSD post hoc test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of DHK-medicated serum on the
viability of HG-treated EA. hy926 cells
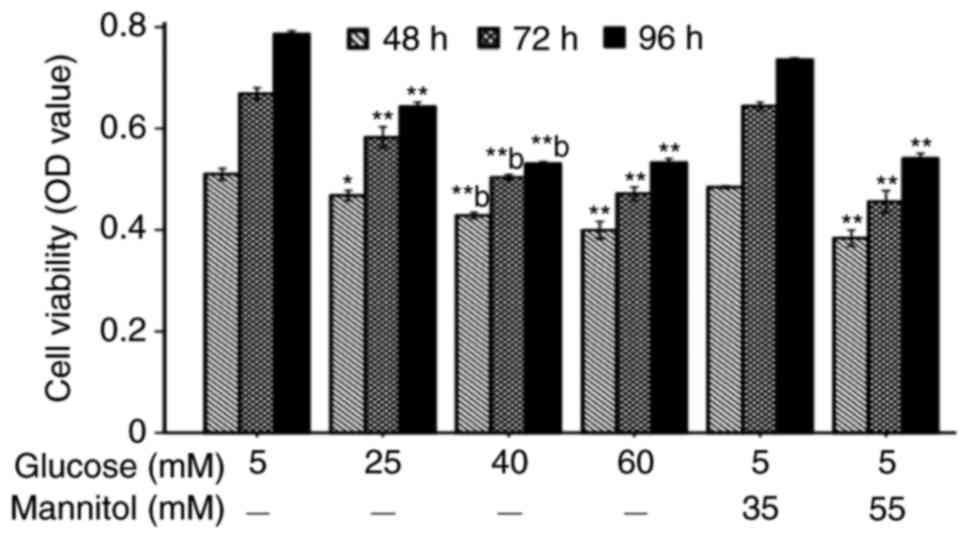
Compared with the normal cells, the inhibitory rates
of cell viability were 17.1, 24.9 and 37.7% when the cells were
incubated with 40 mM glucose for 48, 72 and 96 h, respectively
(Fig. 1). Compared with the
corresponding isotonic group, cell viability in the 40 mM glucose
group was significantly decreased (P<0.01). Therefore, the EA.
hy926 cells were cultured with 40 mM glucose for 96 h in the
following experiments.
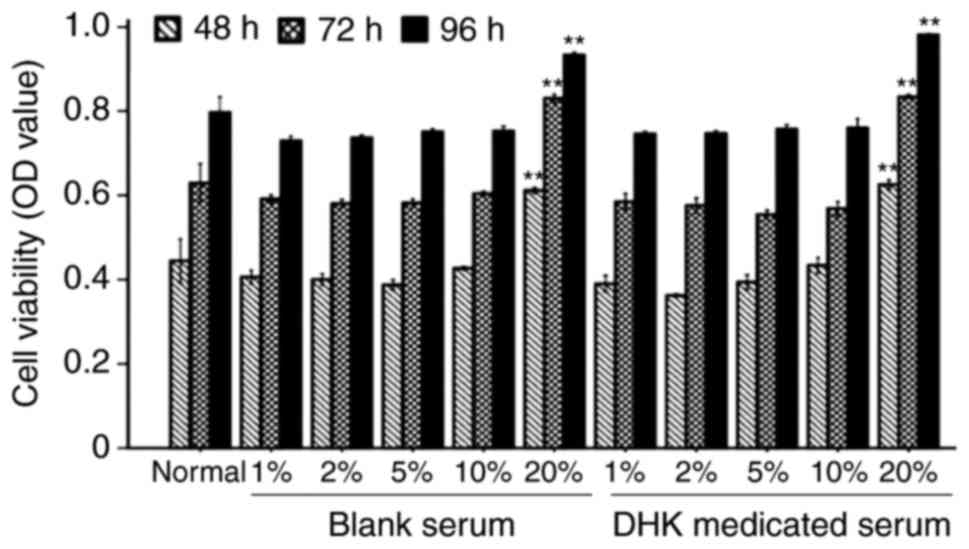
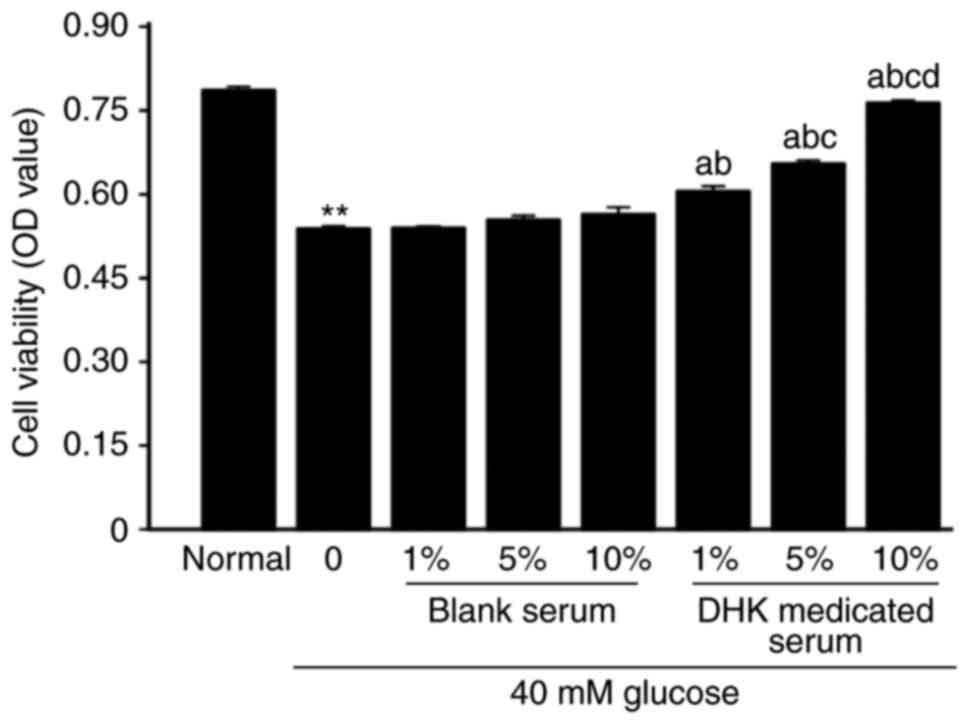
The viability of the normal cells was not altered by
DHK-medicated serum at concentrations of 1–10% for 48, 72 and 96 h
(Fig. 2). However, the viability
of cells incubated with 40 mM glucose for 96 h was increased by
DHK-medicated serum in a concentration-dependent manner (P<0.01,
vs. model group; Fig. 3).
Effect of DHK-medicated serum on
HG-induced apoptosis of EA. hy926 cells
The incubation of EA. hy926 cells with 40 mM glucose
for 96 h significantly increased apoptosis (P<0.01), compared
with that in the normal group, whereas DHK-medicated serum
significantly decreased the apoptotic rate in a
concentration-dependent manner. A 1% concentration of DHK-medicated
serum reduced the apoptotic rate by 42.8% (Fig. 4A-E).
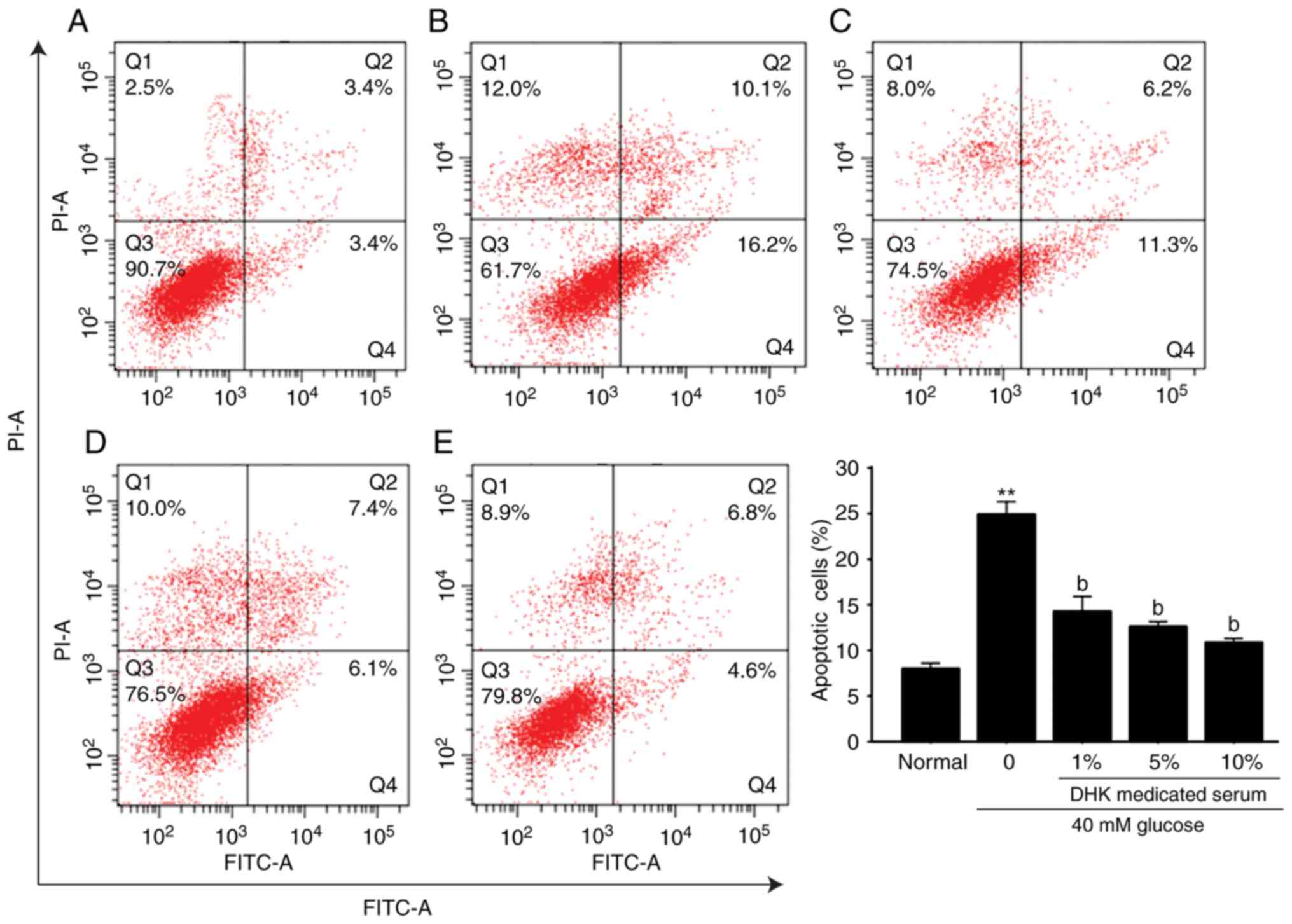 | Figure 4.Effect of DHK-medicated serum on the
high glucose-induced apoptosis of EA. hy926 cells. Q1, necrotic and
dead cells; Q2, late apoptotic cells; Q3, living cells; Q4, early
apoptotic cells. (A) normal group; (B) model group; (C) 1%
DHK-medicated serum group; (D) 5% DHK-medicated serum group; (E)
10% DHK-medicated serum group. Data are expressed as the mean ±
standard error of the mean (n=6). **P<0.01, vs. normal group;
bP<0.01, vs. model group DHK, Danhong Huayu Koufuye;
PI, propidium iodide; FITC, fluorescein isothiocyanate; Q,
quadrant. |
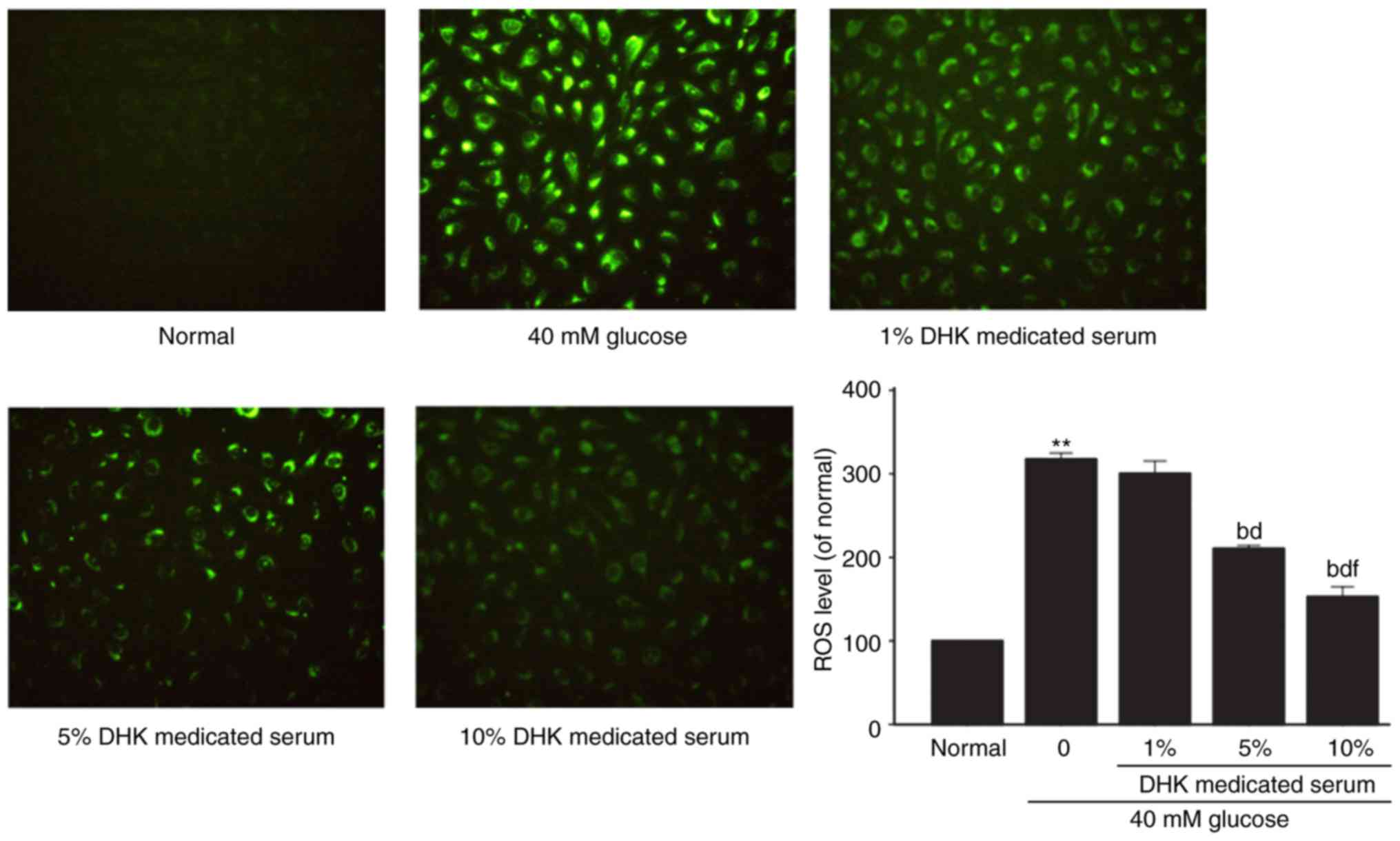
Effects of DHK-medicated serum on
levels of ROS and MDA in HG-treated EA. hy926 cells
The intracellular ROS level in the HG-treated cells
was significantly elevated by ~3-fold (P<0.01), compared with
that in the normal group. DHK-medicated serum
concentration-dependently reduced the ROS levels. DHK-medicated
serum at concentrations of 5 and 10% decreased the ROS levels by
33.7 and 51.9%, respectively (P<0.01, vs. model group; Fig. 5).
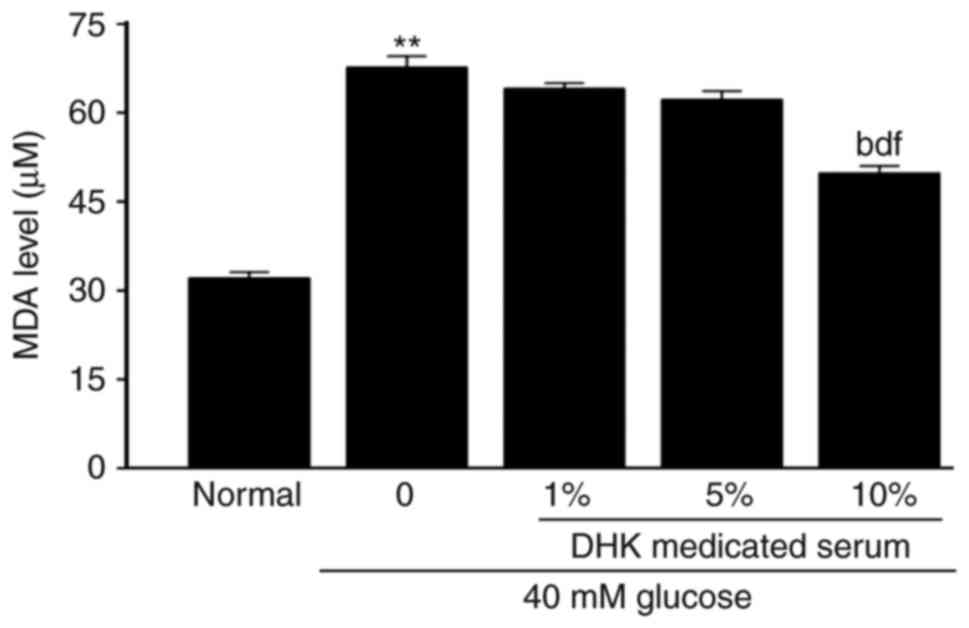
Compared with the normal cells, the MDA level in the
model group was significantly increased by ~2-fold (P<0.01).
DHK-medicated serum significantly decreased the level of MDA
(P<0.01, vs. model group; Fig.
6).
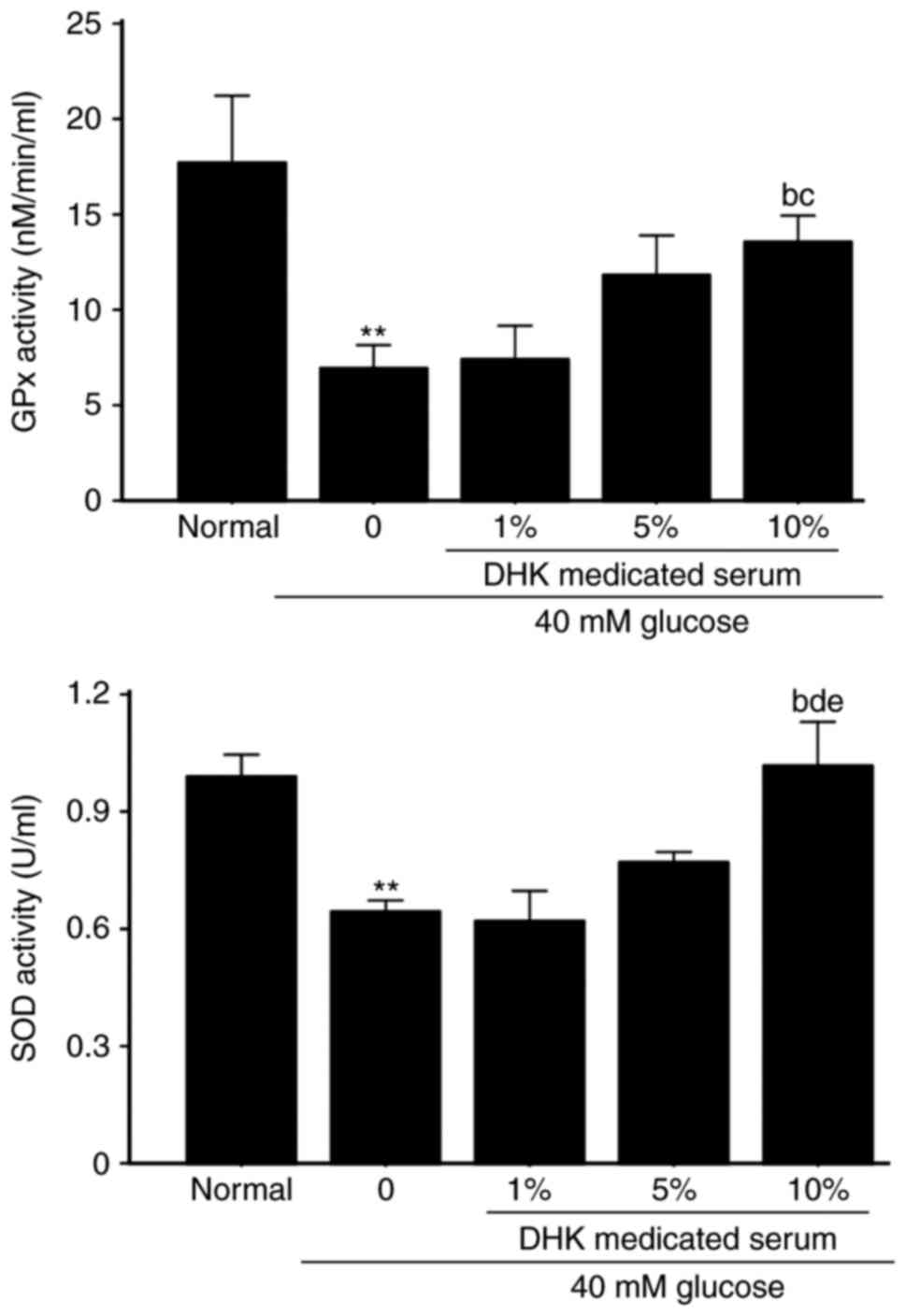
Effects of DHK-medicated serum on the
activities of GPx and SOD in HG-treated cells
As shown in Fig. 7,
the activities of GPx and SOD in the HG-treated EA. hy926 cells
were significantly reduced by 60.8 and 35.4%, respectively
(P<0.01, vs. normal group). A 10% concentration of DHK-medicated
serum significantly increased the activities of GPx and SOD by 95.5
and 59.4%, respectively (P<0.01, vs. model group).
Effects of DHK-medicated serum on
protein expression levels of ICAM-1, NF-κB, HIF-1α and VEGF in
HG-treated EA. hy926 cells
Compared with normal cells, the protein expression
levels of ICAM-1, NF-κB, HIF-1α and VEGF in the model group were
significantly increased by ~3-, 1.5-, 2.2- and 1.5-fold,
respectively (P<0.01). DHK-medicated serum
concentration-dependently decreased the protein expression levels
of ICAM-1, NF-κB, HIF-1α and VEGF (P<0.05 or P<0.01, vs.
model group; Fig. 8).
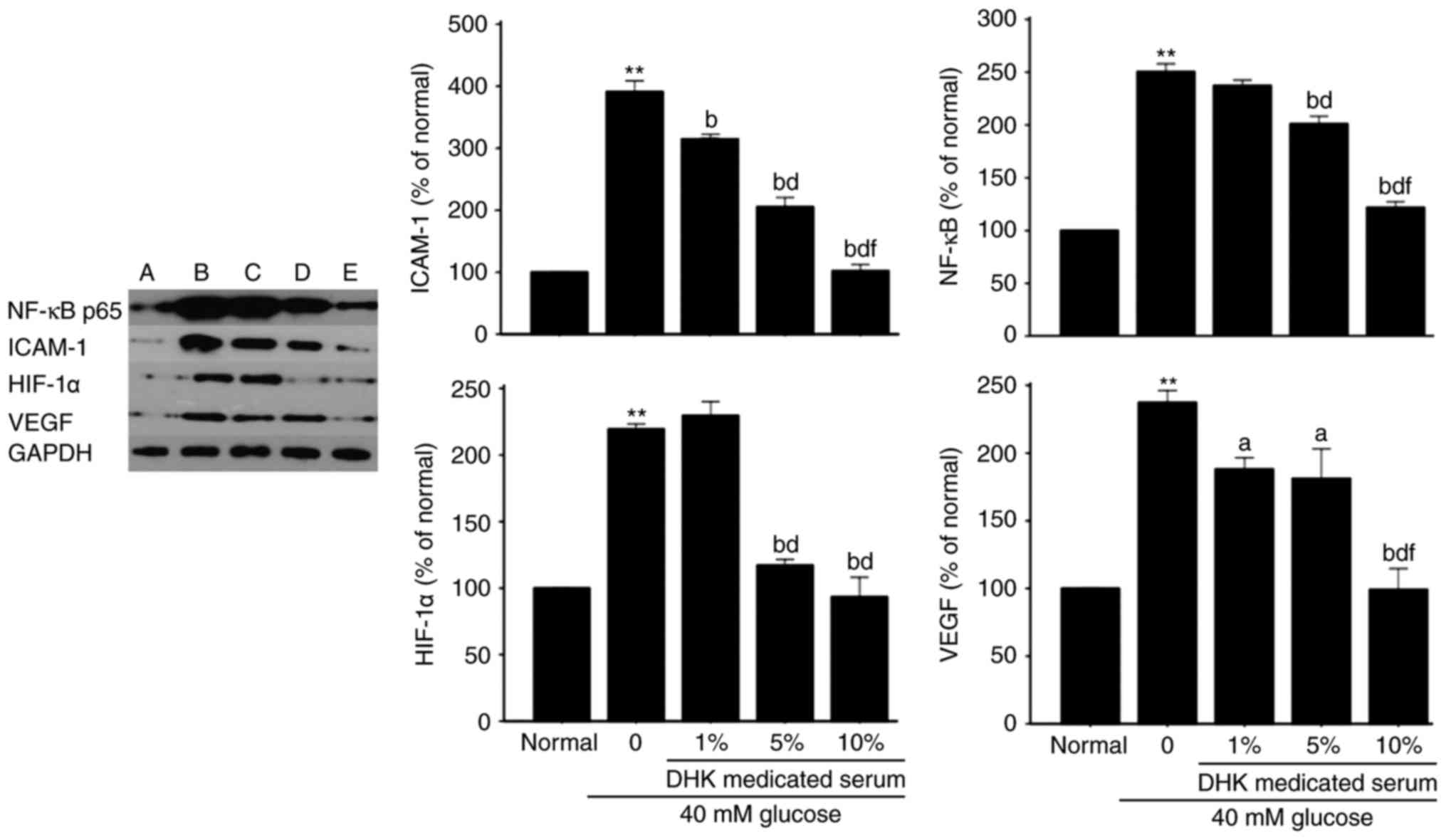 | Figure 8.Effects of DHK-medicated serum on
protein expression levels of ICAM-1, NF-κB, HIF-1α and VEGF in high
glucose-treated EA. hy926 cells. Data are expressed as the mean ±
standard error of the mean (n=6). (A) Normal group; (B) model
group; (C) 1% DHK-medicated serum group; (D) 5% DHK-medicated serum
group; (E) 10% DHK-medicated serum group. **P<0.01, vs. normal
group; aP<0.05, vs. model group;
bP<0.01, vs. model group; dP<0.01, vs.
1% DHK-medicated serum group; fP<0.01, vs. 5%
DHK-medicated serum group. DHK, Danhong Huayu Koufuye; ICAM-1,
NF-κB, nuclear factor-κB; HIF-1α, hypoxia-inducible factor-1α;
VEGF, vascular endothelial growth factor; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Discussion
Endothelial cell proliferation is inhibited and
apoptosis is induced when the cells are exposed to a HG environment
(7,18,26).
The results of the present study showed that cell viability was
significantly decreased (Fig. 3)
and apoptosis was increased (Fig.
4) when EA. hy926 cells were incubated with 40 mM glucose for
96 h, which suggested that the hyperglycemic cell model had been
successfully established.
Elevated oxidative stress can cause injury to
vascular endothelial cells during the process of diabetic vascular
complications (11–13). The imbalance between the increased
production of oxidants and decreased activity of antioxidants has
been a focus of attention in understanding the pathological
mechanisms of diabetic vascular complications (7,10,18,27).
The excessive production of ROS is one of the most
common contributors to endothelial damage. The
hyperglycemia-induced generation of ROS is considered to be causal
link between elevated glucose and the pathways of development of
diabetic vascular complications (27–30).
In the present study, DHK-medicated serum significantly decreased
the HG-induced generation of ROS (Fig.
5). This result suggested that DHK-medicated serum reduced cell
oxidative stress via inhibiting the generation of ROS and then
facilitating the inhibition of endothelial cell apoptosis.
Cellular lipid peroxidation, an activity induced by
oxidative stress, is known to be important in the complications of
DM. Lipid peroxidation is initiated when free radicals attack
membrane lipids. This attack generates increased quantities of
reactive products, which have been implicated in endotheliocyte
damage of tissues, including the heart, kidney and eye. MDA, a
secondary product of lipid peroxidation, is a common index used to
evaluate excess oxidative stress (7,8,25).
Excessive quantities of MDA in serum and tissues lead to the
development of diabetic vascular complications (14,15,31).
In the present study, DHK-medicated serum led to a reduction in the
level of MDA (Fig. 6), which
suggested that certain antioxidant components in DHK-medicated
serum contribute to the inhibition of lipid peroxide
production.
The free radicals created from the metabolic system
cause injury to cell membranes and ageing of the body, and induce
vascular diseases. The antioxidant status in diabetic patients is
crucial in preventing oxidative stress and vascular complications.
Antioxidative enzymes, including SOD, GPx and CAT, inhibit ROS
generation. SOD is a major antioxidant enzyme, which protects
endothelial cells from damage (32–35).
GPx reduces the numbers of free radicals, and SOD works in
conjunction with CAT and GPx to reduce ROS levels. The results of
the present study revealed that the activities of SOD and GPx were
significantly decreased in the HG-treated EA. hy926 cells, which
was consistent with previous reports (7,32).
The DHK-medicated serum increased the activities of these enzymes
(Fig. 7), which indicated that
DHK-medicated serum attenuated HG-induced oxidative stress to
maintain the stability of vascular endotheliocytes.
Endothelial function can be impaired by ROS via
numerous mechanisms, including lipid peroxidation, activation of
NF-κB, and inactivation of NO (7,8). In
addition to biomarkers of oxidative stress, inflammatory substances
are responsible for the development of vascular complications in
diabetic patients (10,18,20).
The NF-κB transcription factor has been recognized as an important
controller of the inflammatory process (33–36).
In the present study, the protein expression of NF-κB was high in
HG-treated cells and was inhibited by treatment with DHK-medicated
serum (Fig. 8). This indicated
that DHK suppressed the expression of NF-κB p65 in the diabetic
endothelial inflammation process.
The levels of ICAM-1 are increased through the
activation of NF-κB, which is a crucial mechanism of cell injury
when exposed to a HG environment, and an important event during the
inflammatory process of diabetic vascular complications (34,35,37).
In line with previous reports (35,37),
the present study found that the protein expression of ICAM-1 was
high in the HG-treated cells. DHK-medicated serum notably
downregulated the protein expression of ICAM-1 (Fig. 8), which suggested that
DHK-medicated serum protected vascular endothelial cells from
injury via its anti-inflammatory effects.
Diabetic factors result in the production of HIF-1α
and angiogenesis (38). The high
expression of VEGF, induced by hyperglycemia, is one of the most
important pathophysiological stimuli in diabetic vascular
complications (39,40). The expression of VEGF appears to be
regulated through two interdependent pathways: Directly via HIF-1α
and indirectly via the activation of NF-κB. In the present study,
the protein expression levels of VEGF and HIF-1α were upregulated
by HG in the cells, and this was reversed by DHK-medicated serum
(Fig. 8). These results suggested
that DHK protected the endothelial cells from damage via inhibiting
the activation of HIF-1α and VEGF.
In conclusion, the present study showed that
DHK-medicated serum had a marked effect on inhibiting HG-induced
oxidative stress and inflammation in EA. hy926 cells, which may be
important mechanisms underlying the effect of DHK in preventing
diabetic vascular complications in STZ-induced diabetic rats and
ZDF rats.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81303282) and the
Department of Education of Guangdong Province (grant no.
Yq2013044).
References
|
1
|
Ziberna L, Martelanc M, Franko M and
Passamonti S: Bilirubin is an endogenous antioxidant in human
vascular endothelial cells. Sci Rep. 6:292402016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi ES, Lee YJ, Seo CS, Yoon JJ, Han BH,
Park MC, Kang DG and Lee HS: Vascular protective role of Samul-Tang
in HUVECs: Involvement of Nrf2/HO-1 and NO. Evid Based Complement
Alternat Med. 2016:95802342016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daiber A, Steven S, Weber A, Shuvaev VV,
Muzykantov VR, Laher I, Li H, Lamas S and Münzel T: Targeting
vascular (endothelial) dysfunction. Br J Pharmacol. 174:1591–1619.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rask-Madsen C and King GL: Vascular
complications of diabetes: Mechanisms of injury and protective
factors. Cell Metab. 17:20–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sotníková R, Nedelčevová J, Navarová J,
Nosáĺová V, Drábiková K, Szöíková R, Nedelčevová J, Navarová J,
Nosáĺová V, Drábiková K, Szö;cs K, Křenek P, Kyseĺová Z, Bezek S,
Knezl V, et al: Protection of the vascular endothelium in
experimental situations. Interdiscip Toxicol. 4:20–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auger C, Said A, Nguyen PN, Chabert P,
Idris-Khodja N and Schini-Kerth VB: Potential of food and natural
products to promote endothelial and vascular health. J Cardiovasc
Pharmacol. 68:11–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jayakumar T, Chang CC, Lin SL, Huang YK,
Hu CM, Elizebeth AR, Lin SC and Choy CS: Brazilin ameliorates high
glucose-induced vascular inflammation via inhibiting ROS and CAMs
production in human umbilical vein endothelial cells. Biomed Res
Int. 2014:4037032014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain SK: Superoxide dismutase
overexpression and cellular oxidative damage in diabetes. A
commentary on ‘Overexpression of mitochondrial superoxide dismutase
in mice protects the retina from diabetes-induced oxidative
stress’. Free Radic Biol Med. 41:1187–1190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muscoli C, Mollace V, Wheatley J, Masini
E, Ndengele M, Wang ZQ and Salvemini D: Superoxide-mediated
nitration of spinal manganese superoxide dismutase: A novel pathway
in N-methyl-Daspartate-mediated hyperalgesia. Pain. 111:96–103.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pitocco D, Tesauro M, Alessandro R,
Ghirlanda G and Cardillo C: Oxidative stress in diabetes:
Implications for vascular and other complications. Int J Mol Sci.
14:21525–21550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanikarla-Marie P and Jain SK:
Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and
elevates oxidative stress, ICAM-1, and monocyte adhesivity in
endothelial cells. Cell Physiol Biochem. 35:364–373. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW,
Lin-Shiau SY and Liu SH: High glucose induces human endothelial
cell apoptosis through a phosphoinositide 3-kinase-regulated
cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol.
25:539–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ihnat MA, Thorpe JE, Kamat CD, Szabó C,
Green DE, Warnke LA, Lacza Z, Cselenyák A, Ross K, Shakir S, et al:
Reactive oxygen species mediate a cellular ‘memory’ of high glucose
stress signalling. Diabetologia. 50:1523–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palem SP and Abraham P: A Study on the
level of oxidative stress and inflammatory markers in type 2
diabetes mellitus patients with different treatment modalities. J
Clin Diagn Res. 9:BC04–BC07. 2015.PubMed/NCBI
|
|
15
|
Pereira EC, Ferderbar S, Bertolami MC,
Faludi AA, Monte O, Xavier HT, Pereira TV and Abdalla DS:
Biomarkers of oxidative stress and endothelial dysfunction in
glucose intolerance and diabetes mellitus. Clin Biochem.
41:1454–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fratantonio D, Speciale A, Canali R,
Natarelli L, Ferrari D, Saija A, Virgili F and Cimino F: Low
nanomolar caffeic acid attenuates high glucose-induced endothelial
dysfunction in primary human umbilical-vein endothelial cells by
affecting NF-κB and Nrf2 pathways. Biofactors. 43:54–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min SW and Han JS: Effect of Polyopes
lancifolia extract on oxidative stress in human umbilical vein
endothelial cells induced by high glucose. Prev Nutr Food Sci.
18:38–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y and Li Y, Ding Y, Dai X, Ma X, Bao
L, Zhang Z and Li Y: Grape seed proanthocyanidin extracts prevent
high glucose-induced endothelia dysfunction via PKC and NF-κB
inhibition. Biosci Biotechnol Biochem. 79:1493–1503. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mudaliar H, Pollock C, Ma J, Wu H, Chadban
S and Panchapakesan U: The role of TLR2 and 4-mediated inflammatory
pathways in endothelial cells exposed to high glucose. PLoS One.
9:e1088442014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li
J, Long R and Zhou Y: Protective effects of chronic resveratrol
treatment on vascular inflammatory injury in streptozotocin-induced
type 2 diabetic rats: Role of NF-kappa B signaling. Eur J
Pharmacol. 720:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin BQ, Zhou JY, Ma Y, Deng YJ, Zheng CJ
and Lin JL: Preventive effect of danhong huayu koufuye on diabetic
retinopathy in rats. Int J Ophthalmol. 4:599–604. 2011.PubMed/NCBI
|
|
22
|
Lin BQ, Zhong CM, Lin JL, Hu LY, Chen WP
and Zhang ZY: Effect of danhong huayu oral liquid combined with
insulin on prevention and progression of early diabetic
cardiomyopathy in rats. Zhong Yao Cai. 37:1218–1221. 2014.(In
Chinese). PubMed/NCBI
|
|
23
|
Chen WP, Wang YD, Ma Y, Zhang ZY, Hu LY,
Lin JL and Lin BQ: Danhong Huayu Koufuye combined with metformin
attenuated diabetic retinopathy in Zucker diabetic fatty rats. Int
J Ophthalmol. 8:1094–1100. 2015.PubMed/NCBI
|
|
24
|
Zhang Z, Hu L, Chen W, Zhou C, Gui G and
Lin B: Danhong huayu koufuye prevents deep vein thrombosis through
anti-inflammation in rats. J Surg Res. 201:340–347. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hao XL, Kang Y, Li JK, Li QS, Liu EL and
Liu XX: Protective effects of hyperoside against H2O2-induced
apoptosis in human umbilical vein endothelial cells. Mol Med Rep.
14:399–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YJ, Kang DG, Kim JS and Lee HS:
Buddleja officinalis inhibits high glucose induced matrix
metalloproteinase activity in human umbilical vein endothelial
cells. Phytother Res. 22:1655–1659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas SR, Witting PK and Drummond GR:
Redox control of endothelial function and dysfunction: Molecular
mechanisms and therapeutic opportunities. Antioxid Redox Signal.
10:1713–1765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Q, Zhang B, Li XM and Gao X:
Traditional Chinese medicine formula Qing Huo Yi Hao as superoxide
anion scavenger in high glucose-treated endothelial cells. Acta
Pharmacol Sin. 33:496–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng XL, Qu W, Wang LZ, Huang BQ, Ying CJ,
Sun XF and Hao LP: Resveratrol ameliorates high glucose and
high-fat/sucrose diet-induced vascular hyperpermeability involving
Cav-1/eNOS regulation. PLoS One. 9:e1137162014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Q, Xia P, Li X, Wang W, Liu Z and Gao
X: Tetramethylpyrazine ameliorates high glucose-Induced endothelial
dysfunction by increasing mitochondrial biogenesis. PLoS One.
9:e882432014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maithili Karpaga Selvi N, Sridhar MG,
Swaminathan RP and Sripradha R: Curcumin attenuates oxidative
stress and activation of redox-sensitive kinases in high fructose-
and high-fat-fed male Wistar rats. Sci Pharm. 83:159–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dogan A, Celik I and Kaya MS: Antidiabetic
properties of lyophilized extract of acorn (Quercus brantii Lindl.)
on experimentally STZ-induced diabetic rats. J Ethnopharmacol.
176:243–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koziel A, Sobieraj I and Jarmuszkiewicz W:
Increased activity of mitochondrial uncoupling protein 2 improves
stress resistance in cultured endothelial cells exposed in vitro to
high glucose levels. Am J Physiol Heart Circ Physiol.
309:H147–H156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Chen S, Cai X, Wang H, Wang X and
Wang W: TLR2 expression doesn't change in ox-LDL mediated
inflammation in Human umbilical vein endothelial cells under high
glucose culture. Int J Clin Exp Med. 8:22004–22010. 2015.PubMed/NCBI
|
|
35
|
Kim MH, Kang HM, Kim CE, Han S and Kim SW:
Ramipril inhibits high glucose stimulated up-regulation of adhesion
molecules via the ERK1/2 MAPK signaling pathway in human umbilical
vein endothelial cells. Cell Mol Biol Lett. 20:937–947. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu YS, Xu DL, Huang ZW, Hao L, Wang X and
Lu QH: Atorvastatin counteracts high glucose-induced Krüppel-like
factor 2 suppression in human umbilical vein endothelial cells.
Postgrad Med. 127:446–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haubner F, Lehle K, Münzel D, Schmid C,
Birnbaum DE and Preuner JG: Hyperglycemia increases the levels of
vascular cellular adhesion molecule-1 and
monocyte-chemoattractant-protein-1 in the diabetic endothelial
cell. Biochem Biophys Res Commun. 360:560–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu X, Chen P, Zheng Q, Wang Y and Chen W:
Effect of pioglitazone on diabetic nephropathy and expression of
HIF-1α and VEGF in the renal tissues of type 2 diabetic rats.
Diabetes Res Clin Pract. 93:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Zhao SZ, Wang PP, Yu SP, Zheng Z and
Xu X: Calcium mediates high glucose-induced HIF-1α and VEGF
expression in cultured rat retinal Müller cells through CaMKII-CREB
pathway. Acta Pharmacol Sin. 33:1030–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Zhang ZK and Liang S:
Epigallocatechin-3-gallate protects retinal vascular endothelial
cells from high glucose stress in vitro via the MAPK/ERK-VEGF
pathway. Genet Mol Res. 15:2016.
|