Introduction
Systemic lupus erythematosus (SLE) is a highly
complex autoimmune disease (1),
characterized by disorder of the generation of auto-antibodies to
the cell nucleus components (2).
Approximately 30–50% of SLE patients are diagnosed with lupus
nephritis (LN) (3), which is more
prevalent in Asians and Africans compared with other races, and the
5-year survival rate of patients with severe LN is <70-80%
(4,5). LN is determined by immune system
activation and the renal tissue response to inflammation with
substantial morbidity and mortality. Although many advances have
been made in its management, 10–30% of patients progress to
end-stage renal disease. Therefore, finding novel non-invasive
biomarkers at the early stages of SLE is of great interest.
MicroRNAs (miRNAs/miRs) are a group of highly
conserved small non-coding, single-stranded RNA molecules comprised
of ~18–25 bp in length (6). miRNAs
regulate gene expression at the post-transcriptional level by
binding to the 3′-untranslated region (UTR), which results in
degrading or blocking translation of messenger RNA (mRNA) (7,8).
miRNAs have been implicated to serve important roles in several
diseases due to their specificity, such as the pathogenesis of SLE
(9,10). Abnormal expression of miRNAs has
been demonstrated in LN patients, such as in the blood serum, urine
and renal tissue; however, these results have not been investigated
extensively. Therefore, the present study aimed to investigate the
expression and function of miR-198 in active LN, in order to
further understand the molecular mechanisms. These results bring
novel insight into understanding the mechanisms about the lupus
disease pathogenesis.
Materials and methods
Patients
Adult SLE patients (n=52) were enrolled between
January 2012 and December 2014 from the Department of Rheumatology
and Immunology, Nanfang Hospital. The present study was approved by
the Ethics Committee of Southern Medical University (Guangzhou,
China). Written informed consent was obtained from all patients.
Among them, 30 patients with consecutive SLE and active nephritis
underwent kidney biopsy, and 22 patients were diagnosed as inactive
SLE. Sections of the kidneys were surgically resected due to kidney
biopsy, and subsequently fixed in 4% formaldehyde at room
temperature overnight for in situ hybridization at 3–4 µm.
The diagnosis was according to the American College of Rheumatology
diagnostic criteria of SLE (11).
The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)
score was determined at the time of the blood draw; scores <4
were considered as inactive disease, and scores ≥4 was categorized
as active disease. Renal SLEDAI scores were determined as described
(12). The mesangial cell
proliferation was regarded as the main pathological change. A total
of 10 paired renal tissue samples served as the control.
Additionally, renal tissues were collected from 6 patients with
Behcet's disease to compare SLE with other autoimmune disease.
Patients with Behcet's disease were recruited from the Department
of Rheumatology and Immunology, Nanfang Hospital. Sections of the
kidneys were surgically resected due to pathological examination,
which were subsequently fixed in 4% formaldehyde at room
temperature overnight for in situ hybridization at 3–4
µm.
Cell culture and transfection
experiments
The MMC mouse mesangial cell line (cat. no.
CRL-1927) and human embryonic kidney 293 (HEK-293) cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA). The MMCs were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 (3:1; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.). HEK-293 cells were maintained in DMEM
supplemented with L-glutamine and 10% FBS (Thermo Fisher
Scientific, Inc.). Cells were transfected using the siPORTNeoFX
agent with 50 nM mirVana (Invitrogen; Thermo Fisher Scientific,
Inc.). hsa-miR-198 mimic, hsa-miR-198 inhibitor or their respective
negative controls [(NC); miR-NC, as-miR-NC] were used for
transfection (Ambion; Thermo Fisher Scientific, Inc.). Three
independent experiments were conducted. The transfected cells were
collected for total RNA or protein isolation at 48 h
post-transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse kidneys and
culture cell lysates using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The concentration and purity of the RNA was confirmed using an
Agilent 2100 system. RNA was reverse transcribed to cDNA using the
High Capacity RNA-to-cDNA kit or a PrimeScript 1st strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China). qPCR
was performed in 96-well plates on an ABI 7500 system using Taqman
Gene Expression assay according to the manufacturer's protocol
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The data were
normalized based on the expression of the endogenous control GAPDH
(mRNA), and U6 small nuclear RNA was used for miRNA normalization.
The primers used were as follows: GAPDH, forward
5′-GAGAAGTATGACAACAGCCTC-3′ and reverse 5′-ATGGACTGTGGTCATGAGTC-3′;
PTEN, forward 5′-AAGTCCAGAGCCATTTCCAT-3′ and reverse
5′-CAAGTCTAAGTCGAATCCATCCT-3′; miR-198, forward
5′-TCATTGGTCCAGAGGGGAGATAG-3′ and reverse 5′-GCAGGGTCCGAGGTATTC−3′;
U6, forward 5′-CTCGCTTCGGCAGCACA-3′, and reverse
5′-AACGCTTCACGAATTTGCGT-3′. The PCR amplification conditions were:
95°C for 10 min, followed by 35 cycles of 95°C for 10 sec, 60°C for
20 sec and 72°C for 30 sec, then 4°C for 5 min. Cq values were
normalized to the endogenous control and presented as the
fold-change relative to the control sample (2ΔΔCq)
(13).
Bioinformatics analysis
TargetScan 6.2 (http://www.targetscan.org) was used to screen the
potential targets of miR-198. PTEN was identified as one of the
miR-198 candidate targets.
Luciferase assay
The phosphatase and tensin homology deleted on
chromosome ten (PTEN) 3′-UTR luciferase reporter was cloned into
the pGL3 luciferase reporter vector between the SpeI and HindIII
sites. The mutant PTEN-3′UTR was generated as (TCTGGA to TCGGGA).
The sequence was confirmed by DNA sequencing. MMCs and HEK-293
cells were cultured in a 24-well plate the day before transfection.
Cells were co-transfected with the firefly luciferase-3-UTR
(pGL3-PTEN or pGL3-PTEN mutant; 500 ng) and the pRL-TK vector
(Promega Corporation, Madison, WI), along with the miR-198, miR-198
inhibitor or the control sequences. At 48 h after transfection,
cell lysates were prepared and luciferase assays were performed
using an HT microplate reader (BioTek China, Beijing, China).
Luciferase activities were normalized to Renilla luciferase
activity. All the experiments were repeated at least three
times.
Cell proliferation assays
Cell proliferation was examined using a Cell
Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen,
China) at 0, 24, 48, 72, 96 and 120 h after inoculation by means of
a cell proliferation assay according to the manufacturer's
protocol. The optical density was measured at a wavelength of 450
nm using a microplate reader.
Western blotting
Mouse kidneys or HEK-293T cells were lysed using
radioimmunoprecipitation assay buffer with complete protease
inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA).
Proteins (30 µg) were separated by 10% SDS-PAGE and then
transferred to a nitrocellulose membrane. After blocking with
non-fat milk for 30 min at room temperature, the membrane was
incubated overnight at 4°C with a PTEN primary antibody (MAB4037,
1:1,000 dilution, EMD Millipore, Billerica, MA, USA) or a mouse
monoclonal anti-β-actin primary antibody (MABT825, 1:1,000
dilution, EMD Millipore). Horseradish peroxidase (HRP)-conjugated
anti-rabbit (sc-2004, 1:3,000, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or anti-mouse IgG antibodies (sc-2005, 1:3,000,
Santa Cruz Biotechnology, Inc.) was used as the secondary antibody.
At last, the membranes were visualized with the ECL Western
Blotting Detection System (GE Healthcare Life Sciences).
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS Inc., Chicago, IL, USA). The results are
expressed as the mean ± standard deviation. Comparison of
parameters of the three groups (SLE, patients with Behcet's disease
and normal controls) was carried out using one way analysis of
variance/Kruskal-Wallis test. Multiple comparisons between the
groups were performed using Student-Newman-Keuls method. The
correlations between biomarker and the activity were calculated by
Spearman's rank correlation coefficient. P<0.05 was considered
to indicate a statistically significant difference.
Results
Higher expression of miR-198 is
observed in patients with SLE, and is correlated with disease
activity
RT-qPCR was used to examine miR-198 expression in
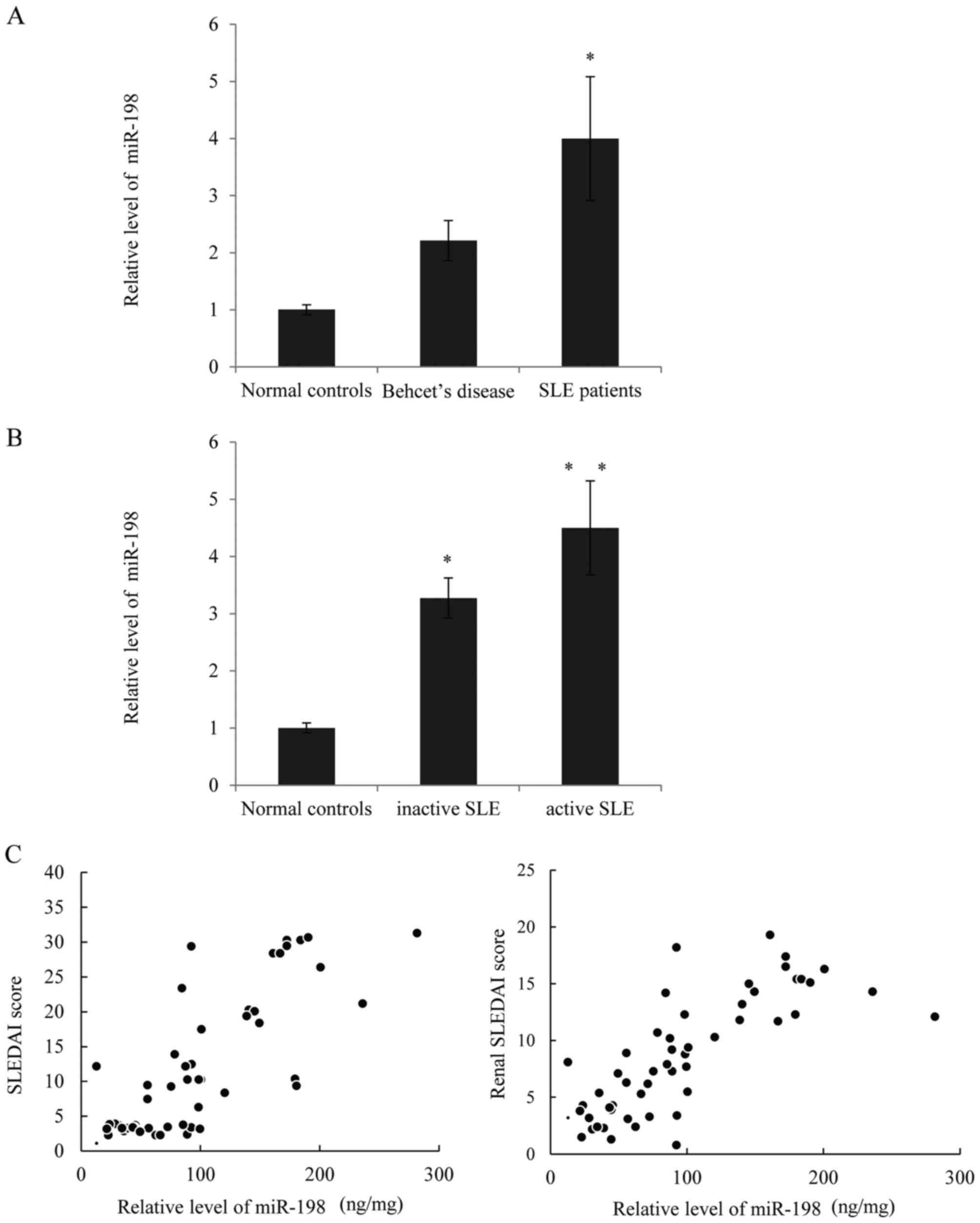
the SLE patients and those with active nephritis. As presented in
Fig. 1A, the expression of miR-198
was significantly higher in lupus patients compared with normal
controls, and although the miR-198 expression in patients with
Behcet's disease appeared higher than the controls, there was no
statistical differences. Next, correlational analysis was performed
to determine whether there was any correlation between the
expression of miR-198 and clinical features. As presented in
Fig. 1B, the expression of miR-198
was higher in patients with active SLE compared with those with
inactive SLE and the normal controls. Furthermore, a direct
positive correlation was observed between miR-198 levels and the
SLEDAI scores, as well as between the miR-198 levels and the renal
SLEDAI scores (Fig. 1C). Thus, it
was hypothesized that higher miR-198 expression positively
correlates with SLE disease activity.
miR-198 promotes glomeruli cell growth
and proliferation in LN
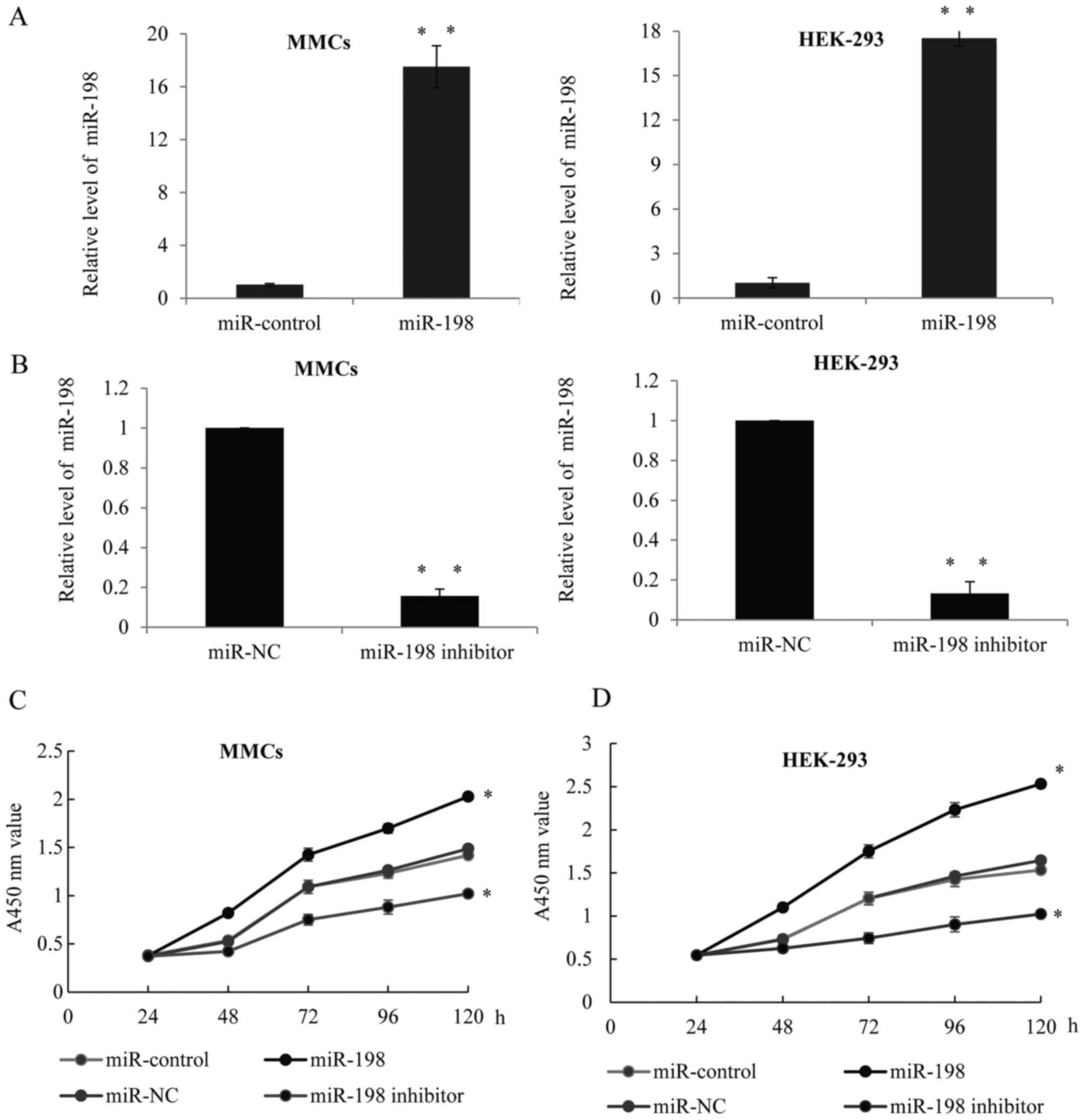
As high expression levels of miT-198 were observed
in the LN group, it was hypothesized that miR-198 may correlate
with glomeruli cell proliferation. To validate this hypothesis, a
hsa miR-198 mimic, or hsa-miR-198 inhibitor or their respective
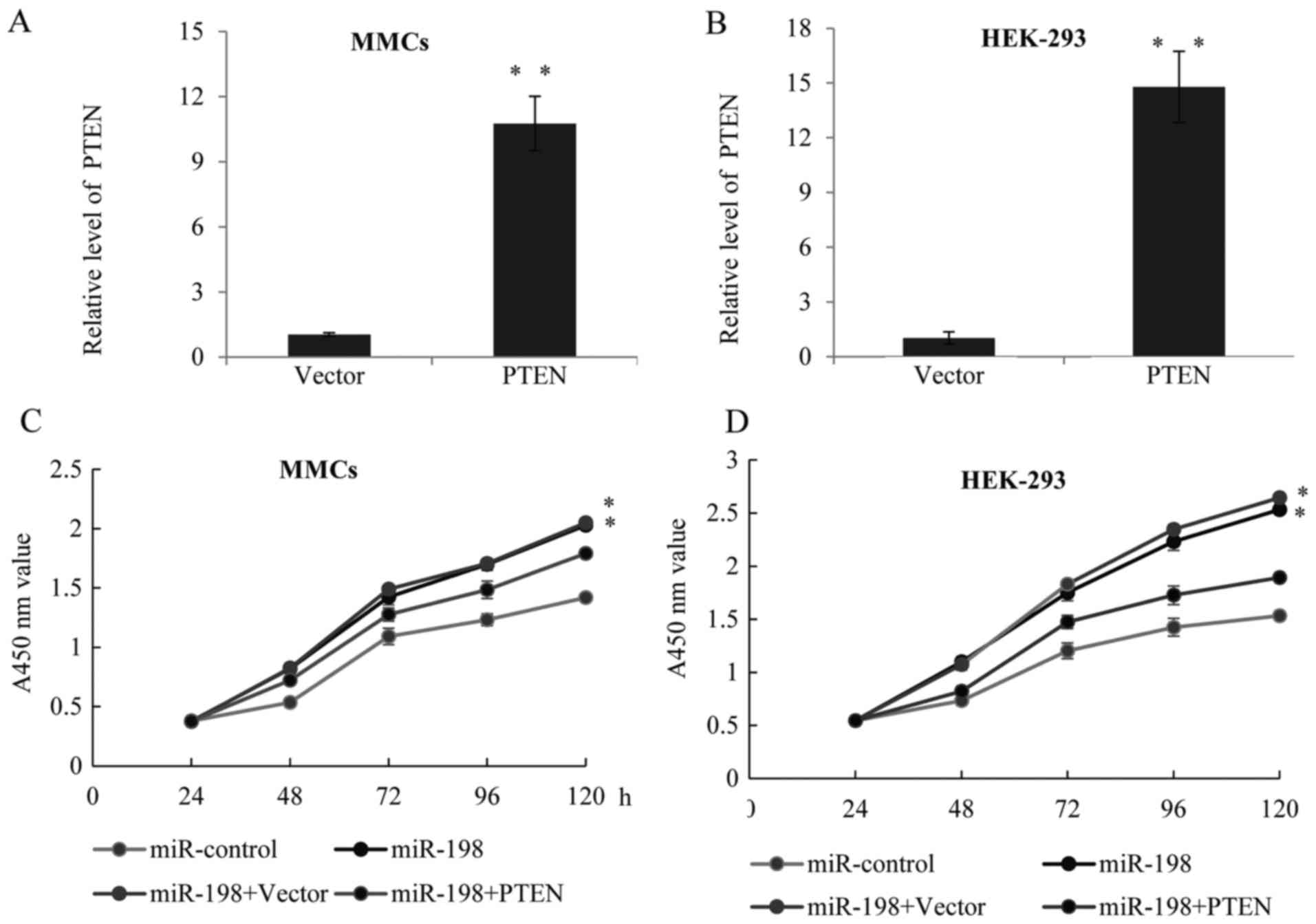
controls were transfected into MMCs or HEK-293 cells. Successful
overexpression (Fig. 2A) or
downregulation (Fig. 2B) of
miR-198 in the two cells was confirmed by RT-qPCR. Furthermore, a
CCK-8 assay was performed in the MMCs (Fig. 2C) or HEK-293 (Fig. 2D) cells transfected with
miR-control, miR-198, miR-NC or miR-198 inhibitor, and the growth
ratio was observed. Overexpression of miR-198 could significantly
increase the cell growth rates, while the knockdown of miR-198
remarkably inhibited the growth level.
Validation of PTEN as a direct target
of miR-198
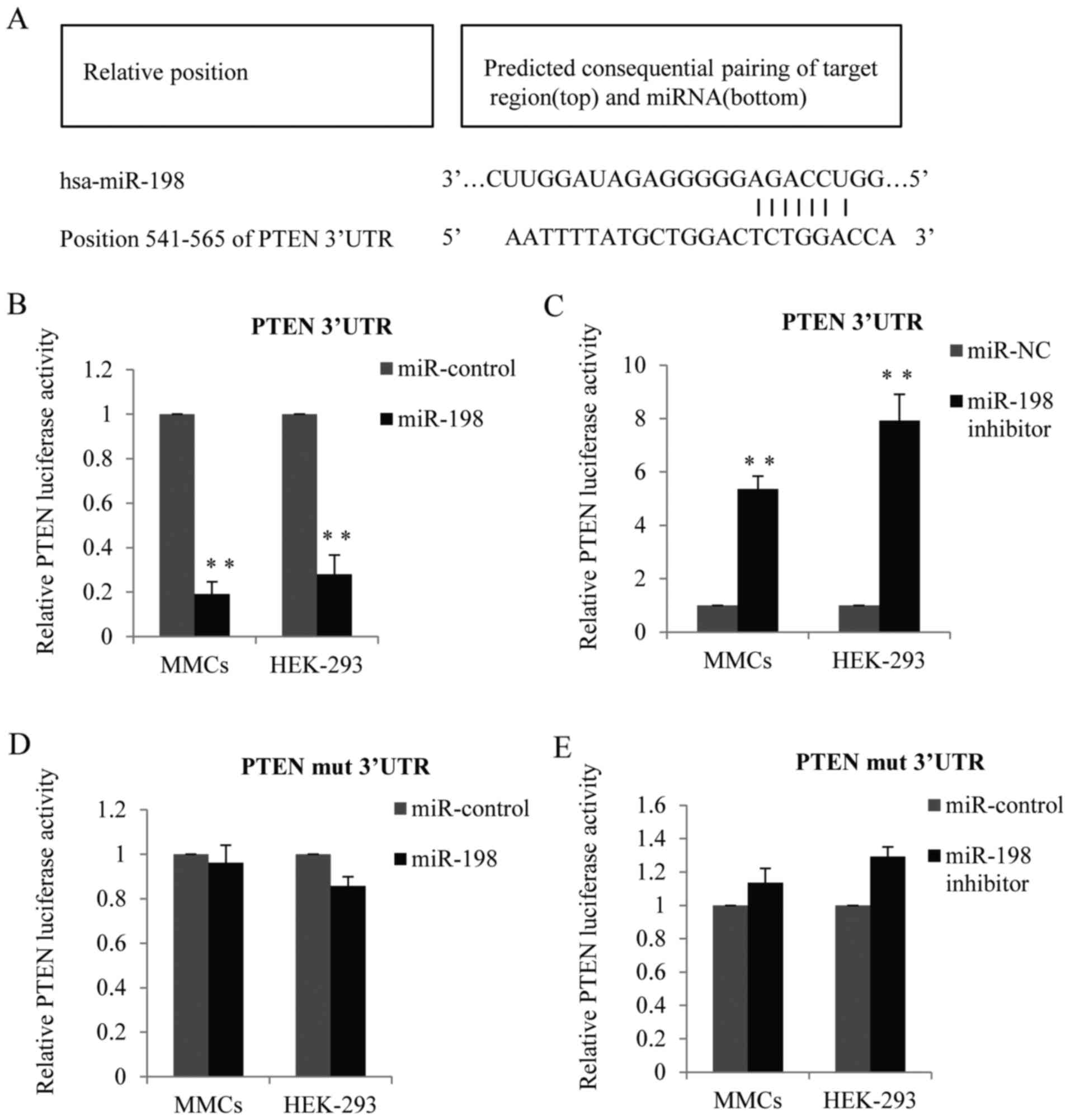
In order to elucidate the mechanism of miR-198 to
stimulate cell proliferation, bioinformatics analysis was used to
identify its target genes. TargetScan 6.2 (http://www.targetscan.org) was used to screen the
potential target and PTEN was identified as one of the miR-198
candidate targets (Fig. 3A). To
determine whether miR-198 can potentially bind to PTEN, a
luciferase reporter assay was performed in the MMCs and HEK-293
cell lines. As presented in Fig.
3B, in cells transfected with miR-198 mimic, the luciferase
activity of the PTEN-3′UTR was remarkably decreased compared with
the control. Conversely, when cells were transfected with miR-198
inhibitor, the PTEN-3′UTR luciferase activity was obviously
increased (Fig. 3C). However, when
cells were transfected with mutant PTEN-3′UTR (TCTGGA to TCGGGA),
the inhibition effect of miR-198 was almost abolished (Fig. 3D and E).
Forced expression of miR-198 inhibits
PTEN expression
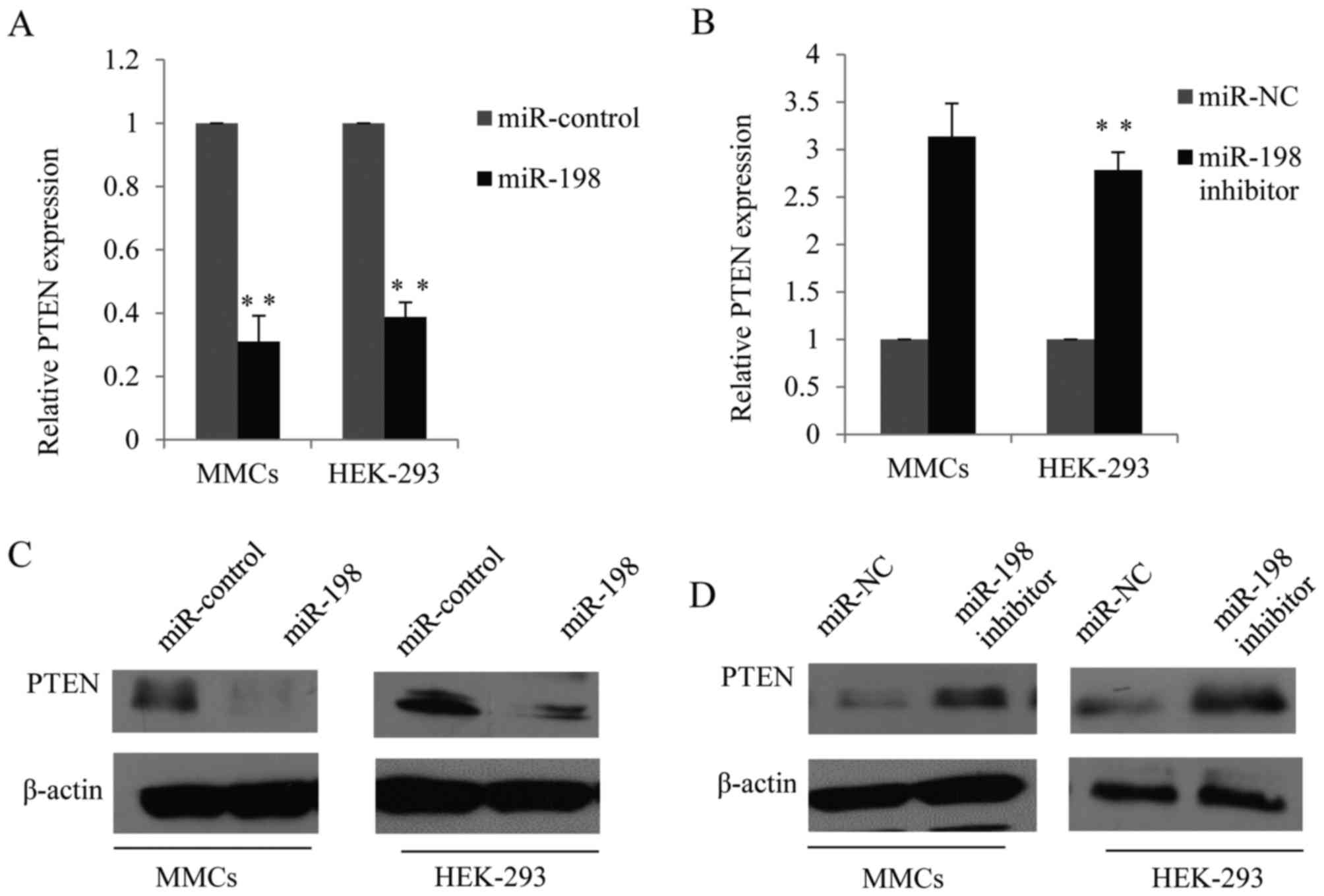
To investigate the possible mechanism underlying
miR-198 in cell proliferation, the present study detected the
function of miR-198 on the expression of PTEN using RT-qPCR.
miR-198 reduced the expression of PTEN compared with the control
cells (Fig. 4A) while the miR-198
inhibitor upregulated the expression of PTEN in MMCs and HEK-293
cells (Fig. 4B). Furthermore,
western blot analysis was performed to analyze the function of
miR-198 on PTEN protein expression. Forced expression of miR-198
mimics significantly reduced the expression of PTEN protein in MMCs
and HEK-293 cells (Fig. 4C),
whereas the expression of PTEN protein was upregulated after
miR-198 was inhibited in the two cell lines (Fig. 4D).
Suppression of PTEN is involved in the
miR-198-mediated MMC proliferation
In order to determine the importance of PTEN as a
functional target of miR-198, it was hypothesized that forced
expression of PTEN could circumvent the inhibitory effects of
miR-198 on cell proliferation. A lentiviral vector expressing PTEN
was constructed and transfected into the MMCs and HEK-293 cells,
and RT-qPCR was used to demonstrate that PTEN expression was
restored after PTEN lentiviral infection (Fig. 5A and B). Furthermore, CCK-8
analysis was performed in the above cell lines; compared with the
miR-198 groups, forced expression of PTEN rescued the function of
miR-198 on cell proliferation (Fig. 5C
and D).
Negative association between miR-198
and PTEN is observed in the patients with SLE
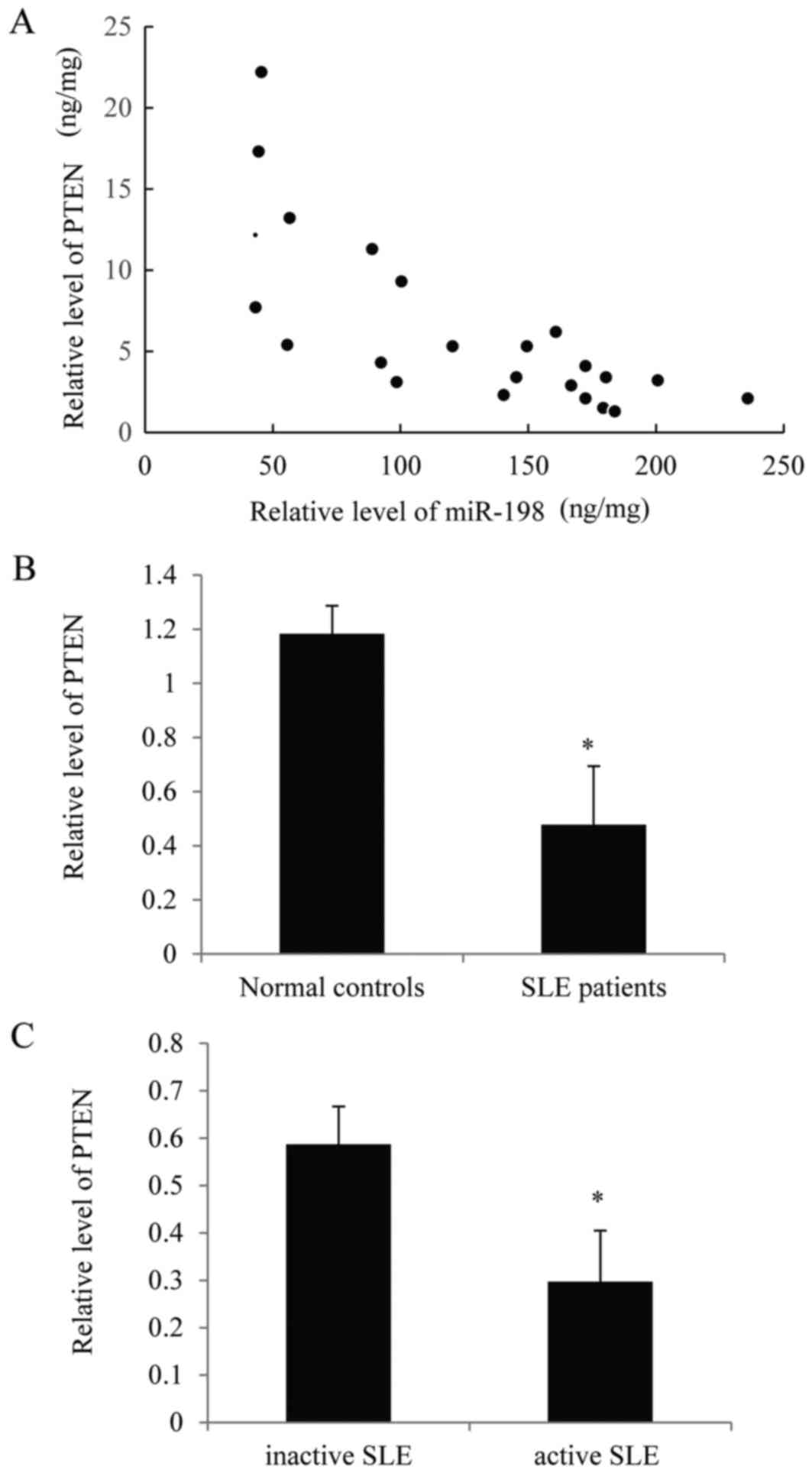
To better understand the functionality of miR-198 in
SLE progression, the present study assessed the correlation between
miR-198 and PTEN levels in the active SLE patients. As presented in
Fig. 6A, there was a negative
correlation between miR-198 and PTEN. Furthermore, the expression
of PTEN was significantly lower in SLE patients compared with
normal controls (Fig. 6B).
Additionally, the expression of PTEN was lower in patients with
active SLE compared with those with inactive SLE (Fig. 6C). These results further support
the hypothesis of the present study.
Discussion
Abnormal miRNA expression is correlated with
severity in clinical diseases such as SLE with active LN kidneys
(14,15). By interfering with target genes in
major intracellular pathways or necrosis and inflammation process,
miRNAs serve a critical role in the renal pathophysiology and work
as modulators of renal fibrosis (16).
The present study demonstrated an increase in
miR-198 expression in LN renal tissues and the MMCs cell line.
Furthermore, a novel regulatory mechanism by which PTEN expression
was regulated by miR-198 at the post-transcription level was
identified, as the mRNA expression level of PTEN remained unchanged
while there was a decrease in PTEN protein expression levels.
Therefore, miR-198 could accelerate MMC proliferation in
vitro while knockdown of miR-198 could suppress MMC
proliferation. This result is consistent with previous studies to
some extent. For example, miR-198 was identified to be upregulated
in the peripheral blood mononuclear cells derived from SLE patients
(17). Both glomerular and
tubulointerstitial expression of miR-198 is higher in LN patients
than controls (18), which is in
line with the present study (16).
PTEN is a well-known tumor suppressor gene located on chromosome 10
(19,20). The expression of PTEN is frequently
lost or mutated in a variety of human cancers, such as breast, lung
and brain cancer (21,22). The present study demonstrated that
low PTEN expression level was observed in the renal tissues during
LN, and the expression of PTEN was inhibited by miR-148a-3p.
In conclusion, the present study, to the best of our
knowledge, is the first to demonstrate that miR-198 is a negative
regulator of PTEN, as miR-148a was demonstrated to be previously
(23). miR-198 may decrease PTEN
expression by binding directly to the 3′UTR, and may serve as a
novel and critical factor in the pathogenesis of LN. However, there
is a major limitation in the present study in that miR-198
expression levels may change in response to immunosuppressive
therapy. Future more studies are required to measure the serial
change in the intra-renal expression of miRNAs. These results bring
novel insight into understanding the mechanisms about the lupus
disease pathogenesis.
References
|
1
|
Wahren-Herlenius M and Dörner T:
Immunopathogenic mechanisms of systemic autoimmune disease. Lancet.
382:819–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cook HT and Botto M: Mechanisms of
Disease: The complement system and the pathogenesis of systemic
lupus erythematosus. Nat Clin Pract Rheumatol. 2:330–337. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avihingsanon Y and Hirankarn N: Major
lupus organ involvement: Severe lupus nephritis. Lupus.
19:1391–1398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maroz N and Segal MS: Lupus nephritis and
end-stage kidney disease. Am J Med Sci. 346:319–323. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borchers AT, Leibushor N, Naguwa SM,
Cheema GS, Shoenfeld Y and Gershwin ME: Lupus nephritis: A critical
review. Autoimmun Rev. 12:174–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B and Farwell MA: microRNAs: A new
emerging class of players for disease diagnostics and gene therapy.
J Cell Mol Med. 12:3–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu LF and Liston A: MicroRNA in the immune
system, microRNA as an immune system. Immunology. 127:291–298.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rusek AM, Abba M, Eljaszewicz A, Moniuszko
M, Niklinski J and Allgayer H: MicroRNA modulators of epigenetic
regulation, the tumor microenvironment and the immune system in
lung cancer. Mol Cancer. 14:342015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Tam LS, Li EK, Kwan BC, Chow KM,
Luk CC, Li PK and Szeto C: Serum and urinary free microRNA level in
patients with systemic lupus erythematosus. Lupus. 20:493–500.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pitashny M, Schwartz N, Qing X, Hojaili B,
Aranow C, Mackay M and Putterman C: Urinary lipocalin-2 is
associated with renal disease activity in human lupus nephritis.
Arthritis Rheum. 56:1894–1903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorenzen JM, Haller H and Thum T:
MicroRNAs as mediators and therapeutic targets in chronic kidney
disease. Nat Rev Nephrol. 7:286–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai Y, Sui W, Lan H, Yan Q, Huang H and
Huang Y: Comprehensive analysis of microRNA expression patterns in
renal biopsies of lupus nephritis patients. Rheumatol Int.
29:749–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ichii O, Otsuka-Kanazawa S, Horino T,
Kimura J, Nakamura T, Matsumoto M, Toi M and Kon Y: Decreased
miR-26a expression correlates with the progression of podocyte
injury in autoimmune glomerulonephritis. PLoS One. 9:e1103832014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Te JL, Dozmorov IM, Guthridge JM, Nguyen
KL, Cavett JW, Kelly JA, Bruner GR, Harley JB and Ojwang JO:
Identification of unique microRNA signature associated with lupus
nephritis. PLoS One. 5:e103442010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Kwan BC, Lai FM, Tam LS, Li EK, Chow
KM, Wang G, Li PK and Szeto CC: Glomerular and tubulointerstitial
miR-638, miR-198 and miR-146a expression in lupus nephritis.
Nephrology (Carlton). 17:346–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Assis LV and Isoldi MC: The function,
mechanisms, and role of the genes PTEN and TP53 and the effects of
asbestos in the development of malignant mesothelioma: A review
focused on the genes' molecular mechanisms. Tumour Biol.
35:889–901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitagishi Y and Matsuda S: Redox
regulation of tumor suppressor PTEN in cancer and aging (Review).
Int J Mol Med. 31:511–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang LL, Mu GG, Ding QS, Li YX, Shi YB,
Dai JF and Yu HG: Phosphatase and Tensin Homolog (PTEN) represses
colon cancer progression through inhibiting paxillin transcription
via PI3K/AKT/NF-κB Pathway. J Biol Chem. 290:15018–15029. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qingjuan L, Xiaojuan F, Wei Z, Chao W,
Pengpeng K, Hongbo L, Sanbing Z, Jun H, Min Y and Shuxia L:
miR-148a-3p overexpression contributes to glomerular cell
proliferation by targeting PTEN in lupus nephritis. Am J Physiol
Cell Physiol. 310:C470–C478. 2016. View Article : Google Scholar : PubMed/NCBI
|