Introduction
Cardiopulmonary bypass (CPB) is considered
indispensable during heart operations, but the potential adverse
effects on sensitive organs, such as the brain or the kidneys,
cannot be ignored (1). In
particular, many of the patients who undergo CPB surgery suffer
from adverse cerebral outcomes, which may include stroke,
postoperative cognitive dysfunction and transient ischemic attacks
(2). The underlying molecular
mechanism of cerebral injuries induced by CPB is unknown; however,
the pathological changes may in part be due to microemboli and
impaired cerebral perfusion, as well as cerebral ischemia and
inflammatory damage (3,4).
It has been previously reported that CPB may
initiate systemic inflammatory reaction syndrome (SIRS) owing to
the blood comprehensive contact with non-biological materials
(5); CPB may also activate
cerebral inflammation in the presence of blood-brain barrier injury
or disruption (3,6). Therefore, inflammatory responses
serve important roles in the progression of cerebral injures
induced by CPB, and reducing inflammation would be of great benefit
for CPB surgery of (5,7). For example, ulinastatin treatment
exhibited neuroprotective effects on an animal model of CPB,
possibly through beneficial effects on anti-inflammatory systems
(8).
The cholinergic anti-inflammatory pathway (CAP) is
an endogenous neural feedback regulation mechanism and can regulate
peripheral inflammatory responses (9). Therefore, the physiological
regulation of CAP has been used to treat infectious or inflammatory
animal models (9–14). Stimulation of the efferent vagus
nerve releases the important neurotransmitter acetylcholine, which
acts through the α7 nicotinic acetylcholine receptor (α7nAchR)
expressed in the macrophages and the brain. Notably, it has been
revealed that activation of α7nAchR may effectively decrease the
expression of proinflammatory cytokines and inhibit the
inflammation process (10,15–18).
In addition, the α7nAchR agonist PHA568487 has been used to treat
neuroinflammation following tibia fracture and endotoxemia in mice
(15), as well as ischemic stroke
injury (16) and brain injury in a
subarachnoid hemorrhage model rats (19). Therefore, the α7nAchR agonist may
provide promising therapeutic effects for cerebral injuries.
However, it is still unclear whether activation of the α7nAchR
agonist is able to reduce cerebral injuries induced by CPB.
The present study evaluated the therapeutic effects
and the molecular mechanisms of the α7nAchR agonist on CPB-induced
brain injury in a rat model. The results indicated that the α7nAchR
agonist may effectively inhibit the inflammatory response and
reduce apoptosis by activating the Akt/GSK3β signaling pathway.
Materials and methods
Animals and ethical approval
A total of 96 adult male Sprague-Dawley rats (age,
8–9 weeks; weight, 350–450 g) were obtained from Shenyang Military
Region General Hospital Laboratory Animal Center [Shenyang, China;
license no. SCXK (Liao) 2012-00022012-0002]. Animals were housed at
a constant temperature (22±1°C), with 50% relative humidity and
a12-h light/dark cycle. The rats had access to food and autoclaved
water ad libitum. All animal procedures were approved by the
Animal Experiments Ethics Committee of the General Hospital of
Shenyang Military Region (Shenyang, China).
CPB animal model establishment
CPB surgery was performed as previously reported
(7), with minor modifications.
Briefly, rats received an intraperitoneal (i.p.) injection of 10%
chloral hydrate (300 mg/kg; Shanghai Ziyuan Pharmaceutical Co.,
Ltd., Shanghai, China) for anesthesia. Photopic oral intubation was
performed using a 16 G intravenous (i.v.) catheter, and animals
were mechanically ventilated with a small animal ventilator
(settings: Frequency, 60 beats/min; tidal volume, 3 ml/kg;
inspiratory to expiratory ratio, 1:1.5) connected to a monitor to
observe the heart rate, oxygen saturation and rectal temperature of
the rats. During surgery, anesthesia was maintained with i.v.
injection of pipecuronium bromide (0.1 mg/kg; Hangzhou Minsheng
Pharmaceutical Co., Ltd., Hangzhou, China).
The puncture site was sterilized with iodophor
(Shandong Lierkang Disinfection Technology Co., Ltd., Dezhou,
China), followed by exposure and puncture of the vein. Right
femoral vein catheterization (24G) was performed to open the fluid
path, which was transfused with 6% hydroxyethyl starch (Guangdong
Jiabao Pharmaceutical Co., Ltd., Qingyuan, China) and connected to
a microinfusion pump. The left femoral artery was catheterized
(22G) and used to monitor blood pressure. Coccygeal artery
catheterization (22G) and right internal jugular vein
catheterization (18G) were performed to drain blood for CPB. The
drainage tube, a homemade blood storage device, a constant
peristaltic pump (Baoding Longer Precision Pump Co., Ltd., Baoding,
China), silicone tubing (internal diameter, 4 mm) and a rat
membrane oxygenator (Guangdong Kewei Medical Instrument Co. Ltd.,
Dongguan, China) were installed between the two puncture sites to
establish the CPB circuit. Heparin sodium (300 IU/kg; Shenyang
Haitong Pharmaceutical Co., Ltd., Shenyang, China) was injected
into the left femoral vein once the activated clotting time reached
480 sec.
CPB was performed with the membrane oxygenator to
supply oxygen. The low-flow CPB velocity was 35 ml/kg/min, which
was later increased to 100–120 ml/kg/min at full-flow bypass. To
prevent air embolism, 1–2 ml of blood was retained in the blood
storage device. Mean arterial pressure was maintained at >60
mmHg, partial CO2 pressure at 35–45 mmHg, base excess at −3-3
mmol/l mmHg, pH at 7.35–7.45 and hematocrit at >0.25. Rats were
treated with 2–20 µg/100 g epinephrine hydrochloride (Wuhan Grand
Pharmaceutical Group Co., Ltd., Wuhan, China) and fluids during
surgery to maintain a stable circulation.
Groups and treatments
Rats were randomly divided into four groups
(n=24/group): i) The Sham group (S group), in which intubation and
mechanical ventilation were performed in the right femoral artery
only and the right internal jugular vein was catheterized without
bypass; ii) the CPB surgery group (C group), which received the CPB
surgery aforementioned; iii) the α7nAchR agonist group (P group),
which received an i.p. injection of the α7nAchR agonist PHA568487
(0.8 mg/kg; Tocris Bioscience; Bio-Techne, Minneapolis, MN, USA) 30
min prior to CPB establishment; and iv) the PHA568487 + α7nAchR
antagonist group (M group), which were also pretreated with
PHA568487 (0.8 mg/kg) for 30 min, followed by i.p. injection of the
α7nAchR antagonist methyllycaconitine (MLA; 6 mg/kg; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). CPB surgery was performed 60 min
after MLA injection.
Specimen collection and
processing
Arterial and venous blood samples were collected
prior to CPB (T0), upon completion of CPB surgery (T1), 2 h
post-CPB (T2) and 6 h post-CPB (T3); subsequently, rats were
sacrificed with 2% pentobarbital sodium (40 mg/kg by i.p.
injection; Merck Sharp & Dohme, Shanghai, China). The systemic
circulation system of the rats was infused with saline (250–400
ml), and the whole brain was collected on the ice and divided into
two halves along the median sagittal line. The hippocampus was
isolated from each of the two halves, the right half was fixed in
4% paraformaldehyde (PFA) at room temperature for 24 h, and the
left side was stored at −80°C for western blot analysis. Sera were
separated by centrifugation at 1,000 × g for 10 min at 4°C, and
stored at −80°C.
Histopathological assessment
Fixed hippocampal tissues were gradually dehydrated
with ethanol and embedded in paraffin. Paraffin blocks were
subsequently sectioned (5 µm) and stained with a Hematoxylin &
Eosin (H&E) staining kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Double-blind evaluation of hippocampal
injury was performed by two expert pathologists. Images of the
histopathological examination were captured by a light microscope
(Olympus Corporation, Tokyo, Japan) at ×400 magnification.
Tissue apoptosis assay
A terminal deoxynucleotidyl-transferase-mediated
dUTP nick-end labeling (TUNEL) Assay kit (Shanghai Fusheng
Industrial Co., Ltd., Shanghai, China) was used, according to the
manufacturer's protocol, to determine the effects of α7nAchR
agonist treatment on apoptosis in the fixed and mounted hippocampal
sections. DAPI was used as a nuclear stain, with sections stained
with 100 ng/ml DAPI for 5 min. Apoptotic rates were examined and
images captured using a light microscope (Olympus Corporation,
Japan) at a magnification of ×400, and the densitometric scanning
was finally analyzed by using the MetaMorph BX41 Image Analysis
System (Olympus Corporation, Japan). A total of 5 images were
captured randomly for each section at ×400 magnification and
integral optical density was calculated using Microscopic Image
Analyzer (MetaMorph BX41 Image Analysis System). Percentages of
TUNEL-positive cells above untreated controls were calculated as
follows: %apoptosis = (number of TUNEL-positive cells / number of
total cells) × 100.
Immunohistochemistry
To further determine the effects of α7nAchR on
apoptosis in the hippocampus, expression levels of the cellular
apoptosis maker Caspase 3 was examined by immunohistochemical
analysis. Briefly, dimethylbenzene was used to remove the paraffin
from the hippocampal sections, followed by immersion in distilled
water. Subsequently, antigen retrieval was conducted by placing the
slides in a microwave in 10 mmol/l citrate buffer, pH 6.0, for 15
min. The slides were washed with 0.01 mmol/l PBS (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) every 5 min for 3 times,
followed by incubating in TBS + 0.3% H2O2 +
0.1% saponin at room temperature for 15 min to block the endogenous
peroxidase. The slides were blocked with goat serum (Sigma-Aldrich;
Merck KGaA) in TBS + 0.1% saponin for 20 min at room temperature,
followed by incubating with polyclonal rabbit anti-Caspase 3
(1:300; ab13847; Abcam, Cambridge, UK) overnight at 4°C. The slides
were incubated with biotin-conjugated secondary antibody (1:2,000;
ab6720; Abcam) for 30 min, and 3,3′-diaminobenzidine stain (8 min
at room temperature) was used to visualize Caspase 3 expression in
the hippocampus. Images of Caspase 3 expression were captured with
a light microscope (Olympus, Japan) at a magnification of 400x. A
total of 5 images were captured randomly for each section at ×400
magnification and integral optical density was calculated using
Microscopic Image Analyzer (MetaMorph BX41 Image Analysis
System).
ELISA determination of S100β, tumor
necrosis factor (TNF)-α and interleukin (IL)-6 levels in rat
serum
Serum expression levels (in 100 µl) of S100β, TNF-α
and IL-6 were determined by ELISA kits (S100β, JM-E10007507; TNF-α,
JM-E10009363; IL-6, JM-E10004387; TSZ Biosciences, San Francisco,
CA, USA), according to the manufacturer's protocol. Optical density
was measured at 450 nm using a Spectra Max M5 Microplate Reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Western blot analysis
The frozen hippocampal tissues (100 mg) were ground
with a glass homogenizer and subsequently homogenized with
Radioimmunoprecipitation Assay Buffer (1 ml; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) using an IKA
T10 homogenizer (IKA-Werke GmbH & Co. KG, Staufen, Germany),
followed by centrifugation at 12,000 × g for 15 min at 4°C. The
supernatant was collected and protein quantification was performed
by bicinchoninic acid assay, and equal amounts of protein lysate
(40 µg) were separated by 12% SDS-PAGE. Proteins were transferred
to nitrocellulose membranes in transfer buffer [12 mM Tris base, 96
mM glycine (pH 8.3) and 15% methanol]. Membranes were blocked for 2
h in TBS + 0.5% Tween-20 (TBST) with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) at room temperature and subsequently
probed with polyclonal rabbit anti-Akt (1:500; ab8805; Abcam),
polyclonal rabbit anti-GSK3β (1:1,000; ab115774; Abcam), monoclonal
rabbit anti-p-Akt (1:500; 13038; Cell Signaling Technology, Inc.
Danvers, MA, USA), polyclonal rabbit anti-p-GSK3β ser9 (1:500;
ab131097; Abcam), polyclonal rabbit anti-Caspase 3 (1:300; ab13847;
Abcam) or monoclonal rabbit anti-β-actin antibody (1:100; 8457;
CST, USA) overnight at 4°C. Membranes were washed with TBST buffer
three times, followed by incubating with monoclonal goat
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibody (1:4,000; HS101; Beijing TransGen Biotech Co., Ltd.,
Beijing, China) for 1 h at room temperature. ECL chemiluminescence
was used to detect protein expression levels, which were visualized
by scanning densitometry (170–8070 Molecular Imager ChemiDoc XRS
System; Bio-Rad Laboratories, Inc. Hercules, CA, USA) using ImageJ
Software (version 1.37; National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Quantitative data were expressed as the mean ±
standard deviation. Statistical analyses were performed with
GraphPad Prism software, (version 6.00; GraphPad Software, Inc., La
Jolla, CA, USA). Multiple comparisons were analyzed with one-way
analysis of variance, followed by an appropriate multiple
comparison test (Tukey's procedure). P<0.05 was considered to
indicate a statistically significant difference.
Results
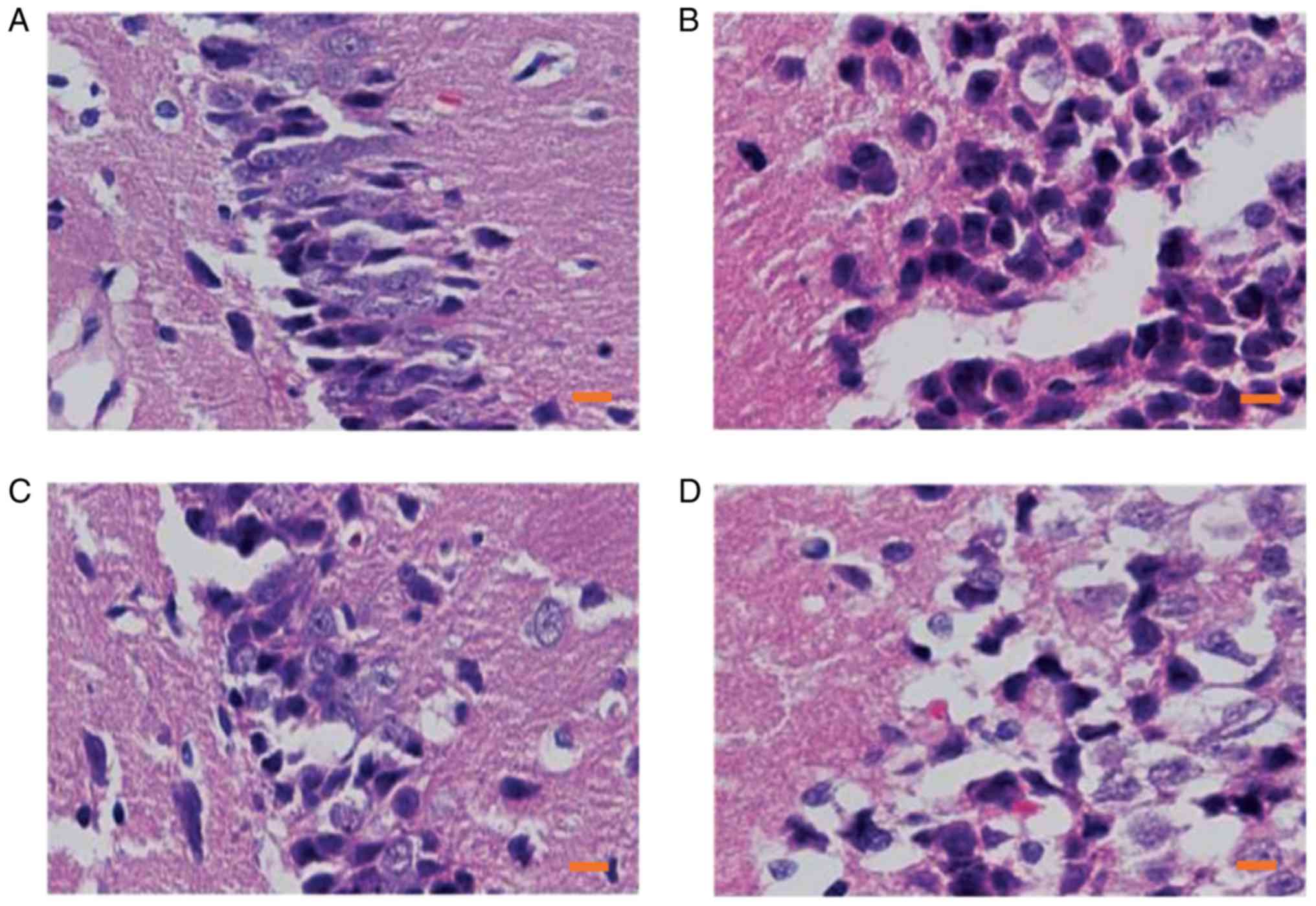
α7nAchR agonist alleviates
pathological injury caused by CPB
To determine the protective effects of α7nAchR
agonist on the morphological alterations of the hippocampus,
sections were evaluated at 6 h post-CPB, the T3 time point, by
H&E staining. There was no detectable morphological damage to
the hippocampal tissues in the S group (Fig. 1A), whereas clear cellular
degeneration and abnormal cell arrangements were observed in the
samples of CPB-injured rats (Fig.
1B), which indicated that the rat model of cerebral injury
caused by CPB was successfully established. Following pretreatment
with the α7nAchR agonist, only a slight morphological change was
observed in the P group as compared to those in the C group
(Fig. 1C), which suggested that
the α7nAchR agonist may have alleviated the pathological injury of
the CPB-injured rats; however, the typical vacuolated degenerations
in hippocampal neurons were observed in those co-treated with
α7nAchR antagonist (Fig. 1D),
indicating the protective effects of α7nAchR agonist may be
inhibited by MLA treatment. These results suggested that activation
of α7nAchR may alleviate CPB-induced pathological injury.
α7nAchR agonist inhibits CPB-induced
apoptosis of hippocampal neurons
To determine the effects of α7nAchR agonist
treatment on apoptosis in hippocampal neurons, the T3 sections were
also evaluated by TUNEL staining. Compared with the control neurons
in the S group, the neurons in the C group exhibited typical signs
of apoptosis (Fig. 2A); neuronal
apoptosis appeared to be lower in the P and M groups when compared
with the C group (Fig. 2A). To
further determine the effects of the α7nAchR agonist on hippocampal
neuron apoptosis, the integrated OD average of apoptosis positive
area was quantified in captured images from all experimental
groups. Compared with the S group, apoptosis was significantly
increased in CPB-injured rats in groups C, P and M, which suggested
that hippocampal cell apoptosis may be induced following CPB
surgery. Notably, a lower rate of neuronal apoptosis was observed
in rats pretreated with the α7nAchR agonist compared with the C
group (P<0.05; Fig. 2B);
however, apoptosis was significantly increased in rats co-treated
with the α7nAchR antagonist compared with the P group (Fig. 2B). These results indicated that
CPB-induced apoptosis of hippocampal neurons may be effectively
reduced by pretreatment with the α7nAchR agonist.
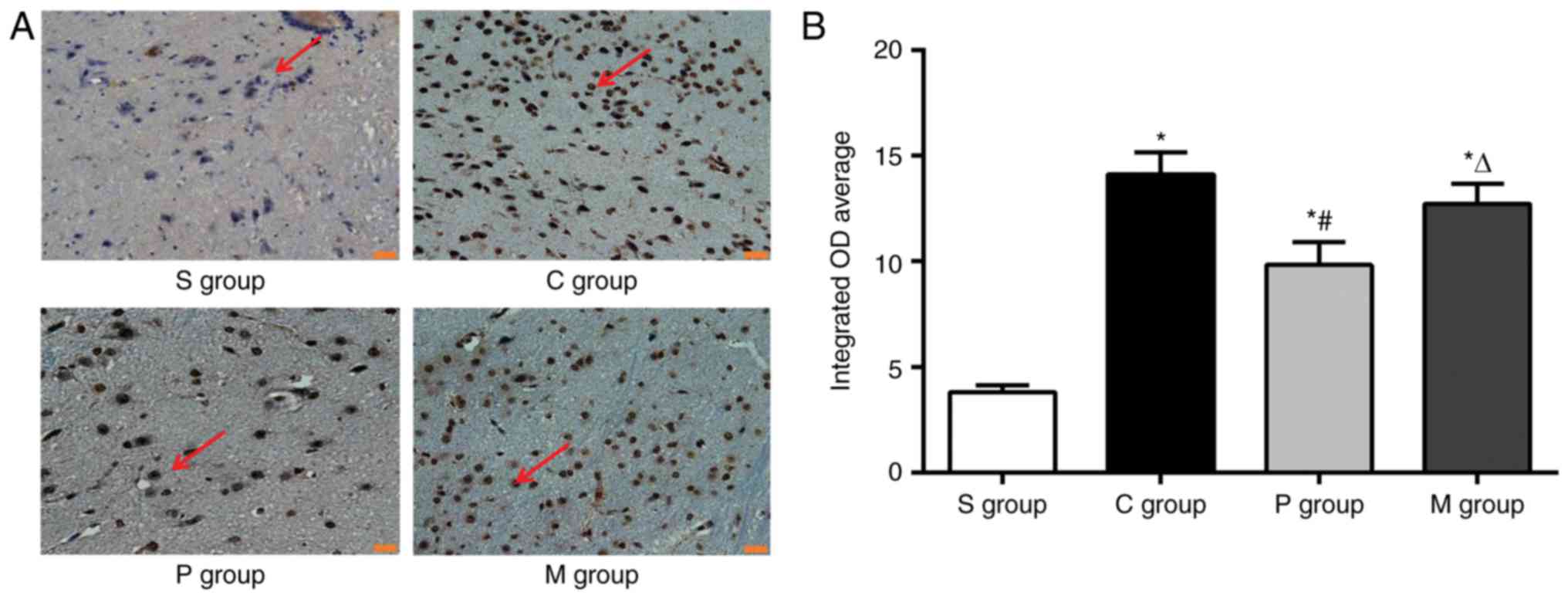 | Figure 2.α7nAchR agonist pretreatment inhibits
neuronal apoptosis in the hippocampus. Hippocampal tissues at T3
were examined by TUNEL assay to evaluate the effects of α7nAchR
agonist on apoptosis. (A) Hippocampal neurons exhibited typical
apoptosis, whereas a lower neuronal apoptosis can be observed after
pretreatment of α7nAchR agonist. Magnification, ×400; scale bar, 20
µm; red arrows indicated positive expressions. (B) Quantitative
results of TUNEL assay from part A. Data are presented as the mean
± standard deviation; n=24/group; *P<0.05 vs. S group;
#P<0.05 vs. C group; ∆P<0.05 vs. P
group. α7nAchR, α7 nicotinic acetylcholine receptor; C group, CPB
surgery only; CPB, cardiopulmonary bypass; M group, CBP + α7nAchR
agonist PHA568487 + α7nAchR antagonist methyllycaconitine; P group,
CBP + α7nAchR agonist PHA568487; S group, Sham operation; T3, 6 h
post-CPB. |
In the light of the inhibition of α7nAchR agonist on
apoptosis of hippocampal neurons, the protein expression levels of
Caspase 3, a key downstream inducer of apoptosis (20), was evaluated by western blot assay.
In the T0 and T1 tissue specimen, no significant differences were
detected in Caspase 3 expression between any of the groups, which
suggested that apoptosis was not induced at this period in time.
Conversely, tissues at T2 and T3 exhibited increased Caspase 3
expression in the CPB-injured rats in groups C, P and M compared
with expression in the S group rats (P<0.05; Fig. 3A and B), which implied that
apoptosis was activated 3–6 h post-CPB surgery. Caspase 3
expression was significantly decreased in P group rats following
pretreatment with α7nAchR agonist compared with the C group
(P<0.05), whereas this effect was reversed in M group rats
co-treated with the α7nAchR antagonist (P<0.05; Fig. 3A and B).
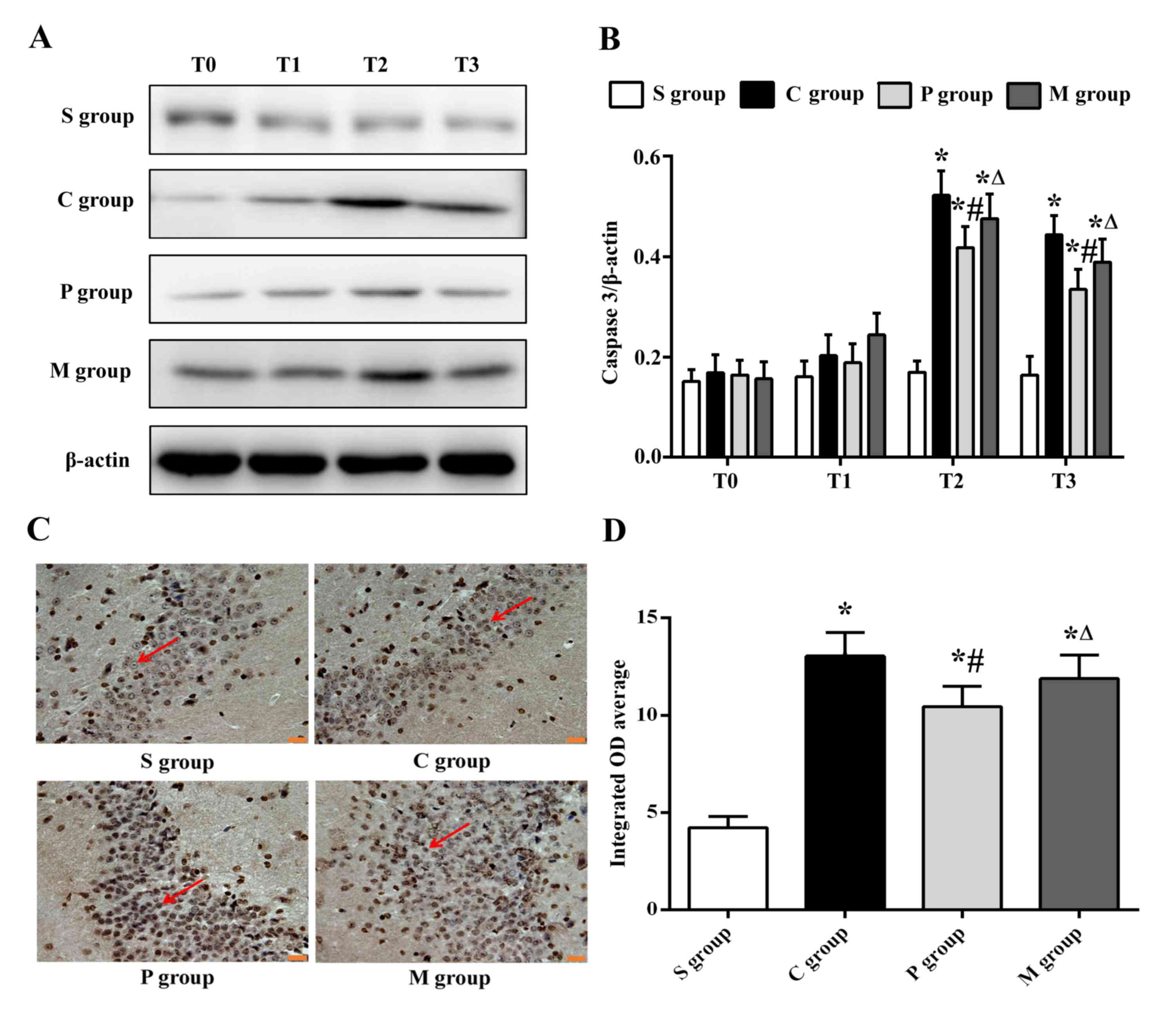 | Figure 3.α7nAchR agonist pretreatment inhibits
Caspase 3 protein expression in the hippocampus. (A) Western blot
analysis for Caspase 3 in hippocampus at different time points in
the different experiments groups. (B) Densitometric analysis
Caspase 3 expression presented in (A); decreased expressions of
Caspase 3 were observed in rats in the P group following
pretreatment of α7nAchR agonist compared with those in the C group
at T2 and T3. (C) Immunohistochemistry for Caspase 3 in hippocampus
at T2. Magnification, ×400; scale bar, 20 µm; red arrows indicate
positive expressions. (D) Integrated OD average analysis indicated
the decreased expression levels of Caspase 3 in P group rats
pretreated with the α7nAchR agonist compared with expression in the
C group model rats. Data are presented as the mean ± standard
deviation; n=24/group; *P<0.05 vs. S group;
#P<0.05 vs. C group; ∆P<0.05 vs. P
group. α7nAchR, α7 nicotinic acetylcholine receptor; C group, CPB
surgery only; CPB, cardiopulmonary bypass; M group, CBP + α7nAchR
agonist PHA568487 + α7nAchR antagonist methyllycaconitine; P group,
CBP + α7nAchR agonist PHA568487; S group, Sham operation; T0, prior
to CPB; T1, upon completion of CPB; T2, 3 h post-CPB; T3, 6 h
post-CPB. |
To confirm the location of Caspase 3 expression in
the hippocampus, immunohistochemical analysis was used to determine
the expression at T2, as the Caspase 3 expression reached a peak in
the CPB-injured rats at T2 according to the western blotting data
aforementioned. Caspase 3 expression was detected in the neurons of
hippocampus (Fig. 3C), and Caspase
3 expression was significantly inhibited in the P group compared
with the C group (P<0.05; Fig.
3D), which was consistent with western blotting results.
Therefore, these results indicated that the α7nAchR agonist may
effectively inhibit apoptosis in hippocampal neurons, which may
partly be accomplished by suppressing the expression of Caspase
3.
α7nAchR agonist pretreatment reduces
serum levels of S100β, TNF-α and IL-6 in CPB-injured rats
Serum expression levels of S100β, TNF-α and IL-6
were measured to evaluate the inflammatory response in rats with
CPB injury. Compared with the control S group, rats in the CPB
groups C, P and M exhibited significantly increased levels of
S100β, TNFα and IL6 at experimental time points T1-T3 (P<0.05;
Fig. 4A-C, respectively), which
was considered as an indicator of serious cerebral injury. The
levels of S100β, TNFα and IL6 were significantly decreased rats in
the P group following pretreatment with 7nAchR agonist compared
with the expression levels in CPB model rats in the C group at the
T1-T3 experimental time points (P<0.05); however, rats in the M
group exhibited an increase in serum expression levels compared
with the P group (P<0.05; Fig.
4A-C). The apparent improvement of inflammation suggested that
α7nAchR agonist pretreatment may have a beneficial effect on
anti-inflammatory systems of the rat model of CPB.
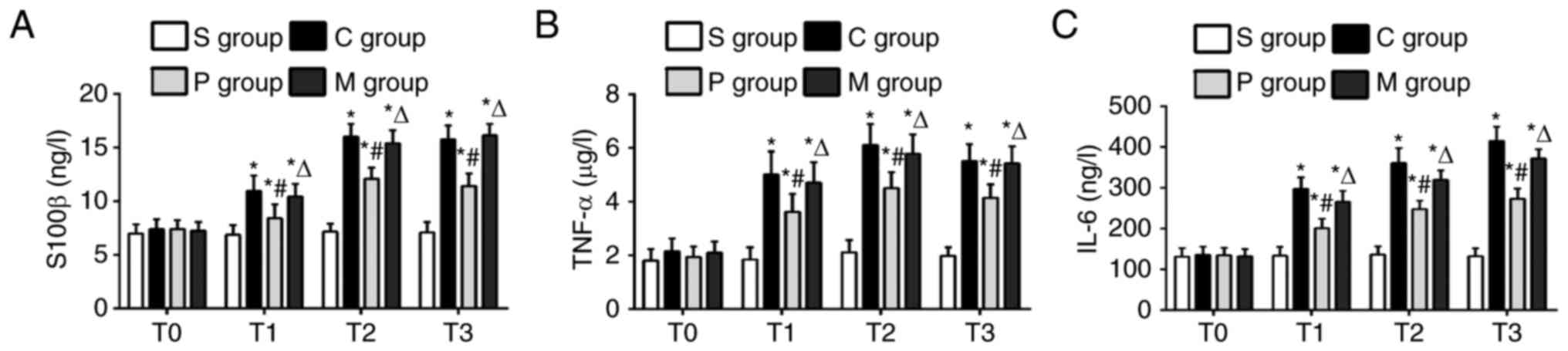 | Figure 4.α7nAchR agonist pretreatment reduces
the serum expression levels of S100β, TNF-α and IL-6 in CPB-injured
rats. The serum levels of (A) S100β, (B) TNF-α and (C) IL-6 were
measured to evaluate the anti-inflammation effects of α7nAchR
agonist on CPB-injured rats. Serum levels of S100β, TNFα and IL6
were significantly decreased in P group rats following pretreatment
with the α7nAchR agonist compared with levels in the C group model
rats. Data are presented as the mean ± standard deviation;
n=24/group; *P<0.05 vs. S group; #P<0.05 vs. C
group; ∆P<0.05 vs. P group. α7nAchR, α7 nicotinic
acetylcholine receptor; C group, CPB surgery only; CPB,
cardiopulmonary bypass; M group, CBP + α7nAchR agonist PHA568487 +
α7nAchR antagonist methyllycaconitine; IL, interleukin; P group,
CBP + α7nAchR agonist PHA568487; S group, Sham operation; T0, prior
to CPB; T1, upon completion of CPB; T2, 3 h post-CPB; T3, 6 h
post-CPB; TNF-α, tumor necrosis factor α. |
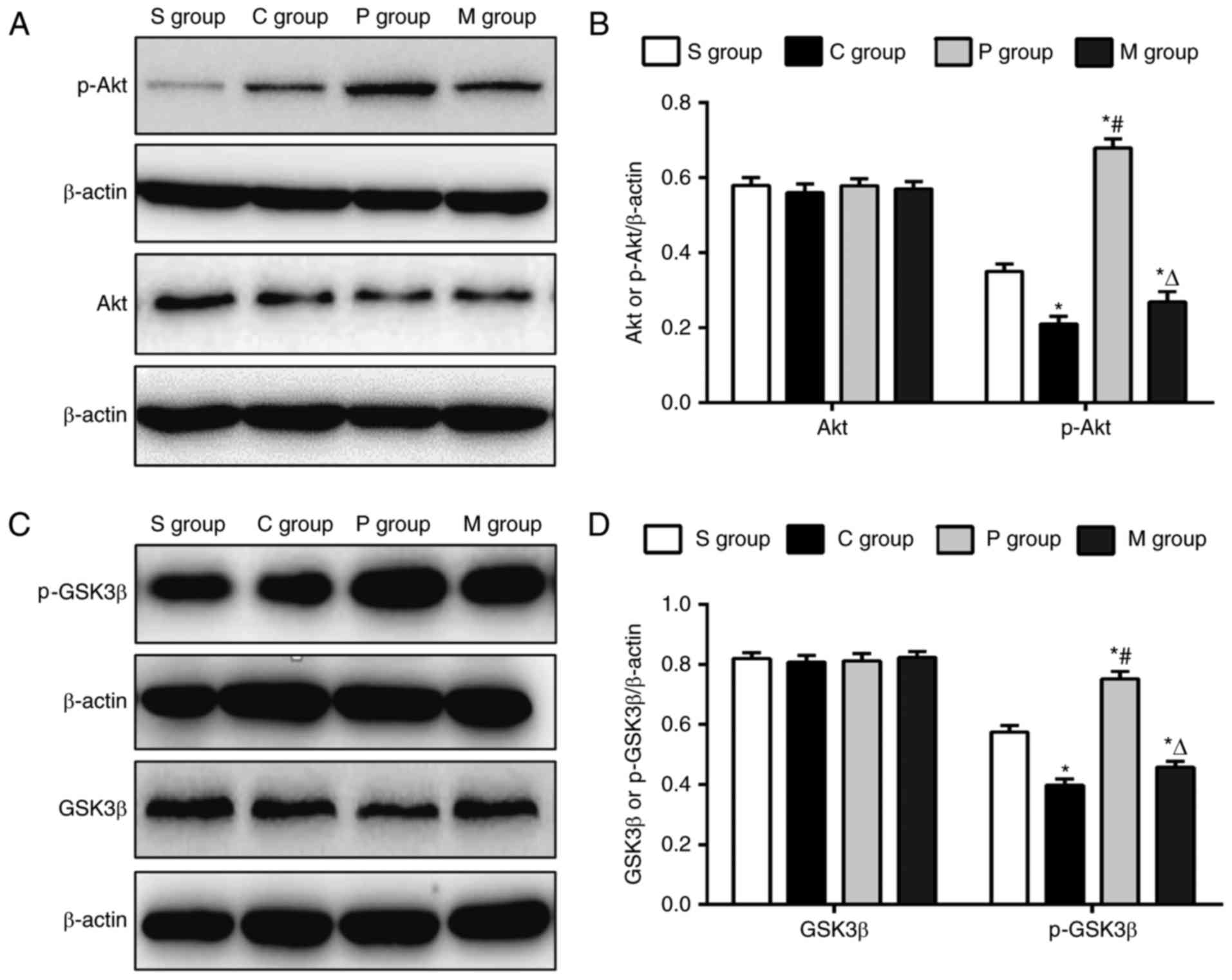
α7nAchR agonist pretreatment promotes
phosphorylation of Akt and GSK3β
To further explore the underlying mechanisms by
which α7nAchR agonist alleviated the cerebral injuries caused by
CPB, Akt/GSK3β pathway activation was examined to determine the
protective effects of α7nAchR agonist on the CPB-injured rats, as
the Akt/GSK3β pathway was previously identified as a significant
cell survival pathway (21). CPB
rats in the C group exhibited a significant increase in the
expression of p-Akt and p-GSK3β compared with expression levels in
the S group (P<0.05; Fig. 5);
whereas the expression levels of p-Akt and p-GSK3β were
significantly increased in the P group following pretreatment with
the α7nAchR agonist, compared with the S group and C group, which
suggested that the α7nAchR agonist may promote the phosphorylation
of Akt and GSK3β. Rats in the M group that were co-treated with the
α7nAchR antagonist exhibited a significant decrease in p-Akt and
p-GSK3β expression levels compared with the P group (Fig. 5). By contrast, no significant
differences in the expression levels of total Akt and total GSK3β
were identified between the groups (Fig. 5), which implied that the α7nAchR
agonist did not affect the expressions of Akt and GSK3β. Therefore,
these results indicated that the α7nAchR agonist may effectively
upregulate the activation of the Akt/GSK3β signaling pathway in the
CPB-injured rats.
Discussion
Cerebral injury is a serious complication following
the use of CPB in the cardiac surgery (2,22,23).
This pathological lesion may be due to several aspects, including
impaired cerebral perfusion and oxygenation, cerebral microemboli
and SIRS (3,4). Among these factors, SIRS is one of
great significance for CPB; therefore, minimizing SIRS is widely
considered as a prerequisite strategy for inhibiting the
inflammatory response (5). It is
generally accepted that proinflammatory cytokines such as TNF-α may
further increase the permeability of the blood-brain barrier and
subsequently promote the invasion of inflammatory cytokines and
immune cells (24). Results from
the present study demonstrated that expression levels of the
proinflammatory cytokines, including the TNF-α and IL-6, were
significantly increased in CPB-injured rats, which was consistent
with previous reports (7,25). Therefore, reducing proinflammatory
cytokines levels may alleviate neuronal injury and improve
functional recovery.
As a physiological regulation of the innate immune
system, CAP has been widely used to inhibit the expression of
proinflammatory cytokines for treating infectious and inflammatory
diseases (16). According to that
report, activation of the main regulatory target, α7nAchR, may aid
in the reduction of proinflammatory cytokines. Therefore, the
present study hypothesized that the α7nAchR agonist may effectively
inhibit the serum levels of TNF-α and IL-6 in the CPB-injured rats,
which suggested that the α7nAchR agonist may provide a promising
strategy for reducing SIRS post-CPB.
S100β is regarded as a reliable serum maker of
cerebral injury following the breakdown of the blood-brain barrier
(26–28). In the present study, an increased
serum level of S100β was observed in the CPB model rats compared
with normal rats, whereas the α7nAchR agonist was able to decrease
the serum level of S100β, which demonstrated that the CPB model was
successfully established and that the neuroprotective effects may
be achieved by pretreatment with the α7nAchR agonist.
Several previous reports suggested that the
hippocampus is sensitive to ischemia and reperfusion injury caused
by CPB (1,29). In the present study, clear
pathological damage and an increase in cell apoptosis and Caspase 3
expression levels in the hippocampus were observed in the
CPB-injured rats, which confirmed that pathological changes occur
in the hippocampus following CPB surgery. Notably, these
pathological injuries were effectively inhibited in rats pretreated
with the α7nAchR agonist, which demonstrated the protective effects
of the α7nAchR agonist on CPB rats.
Additional studies have demonstrated that the
Akt/GSK3β pathway serves a central role in cell survival in a
number of neurological diseases (30–32).
In particular, activation of the Akt/GSK3β pathway may attenuate
apoptosis, which is closely related to the regulation of Caspase 3
expression (33–35). Based on the present results that
demonstrated the inhibitory effects of the α7nAchR agonist on
apoptosis and Caspase 3 expression, activation of the Akt/GSK3β
pathway was further examined for the protective effects of α7nAchR
agonist on CPB. The results indicated that p-Akt and p-GSK3β
expressions were upregulated following α7nAchR agonist
pretreatment, which suggested that the α7nAchR agonist may be able
to inhibit hippocampal cell apoptosis by activating the Akt/GSK3β
pathway.
To further determine the protective effects of the
α7nAchR agonist on CPB, the α7nAchR antagonist was concurrently
administered in the present study. By contrast to pretreatment with
the α7nAchR agonist alone, co-treatment with the α7nAchR antagonist
resulted in significant increases in the serum levels of S100β,
TNF-α and IL-6, as well as the pathological damage, increased
apoptosis and increased Caspase 3 expression, and a significant
decrease in the expression levels of p-Akt and p-GSK3β. These
results further demonstrated the neuroprotective effects of α7nAchR
agonist on CPB-injured rats.
In conclusion, the present study demonstrated that
the α7nAchR agonist may reduce pathological damage and apoptosis in
the hippocampus by upregulating Akt/GSK3β signaling. The α7nAchR
agonist may provide a promising therapeutic approach for cerebral
injury caused by CPB.
Acknowledgements
This study was supported by The Natural Science
Foundation of China (grant nos. 81471121 and 3120175) and The
Teaching Project of China Medical University (grant no.
XZR20160036).
References
|
1
|
Salameh A and Dhein S: Strategies for
pharmacological organoprotection during extracorporeal circulation
targeting ischemia-reperfusion injury. Front Pharmacol. 6:2962015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roach GW, Kanchuger M, Mangano CM, Newman
M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH and
Ley C: Adverse cerebral outcomes after coronary bypass surgery.
Multicenter study of perioperative ischemia research group and the
ischemia research and education foundation investigators. N Engl J
Med. 335:1857–1863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Harten AE, Scheeren TW and Absalom AR:
A review of postoperative cognitive dysfunction and
neuroinflammation associated with cardiac surgery and anaesthesia.
Anaesthesia. 67:280–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao HJ, Sun YJ, Zhang TZ, Zhou J and Diao
YG: Penehyclidine hydrochloride attenuates the cerebral injury in a
rat model of cardiopulmonary bypass. Can J Physiol Pharmacol.
91:521–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evora PR, Bottura C, Arcêncio L,
Albuquerque AA, Evora PM and Rodrigues AJ: Key Points for curbing
cardiopulmonary bypass inflammation. Acta Cir Bras. 31 Suppl
1:S45–S52. 2016. View Article : Google Scholar
|
|
6
|
Ouk T, Amr G, Azzaoui R, Delassus L,
Fossaert E, Tailleux A, Bordet R and Modine T: Lipid-lowering drugs
prevent neurovascular and cognitive consequences of cardiopulmonary
bypass. Vascul Pharmacol. 80:59–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou J, Zhou N, Wu XN, Cao HJ, Sun YJ,
Zhang TZ, Chen KY and Yu DM: Role of the Toll-like receptor 3
signaling pathway in the neuroprotective effect of sevoflurane
pre-conditioning during cardiopulmonary bypass in rats. Mol Med
Rep. 12:7859–7868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Xue Q, Yan F, Li L, Liu J, Li S
and Hu S: Ulinastatin as a neuroprotective and anti-inflammatory
agent in infant piglets model undergoing surgery on hypothermic
low-flow cardiopulmonary bypass. Paediatr Anaesth. 23:209–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ulloa L: The vagus nerve and the nicotinic
anti-inflammatory pathway. Nat Rev Drug Discov. 4:673–684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu JS, Wei XD, Lu ZB, Xie P, Zhou HL,
Chen YY, Ma JM and Yu LZ: Liang-Ge-San, a classic traditional
Chinese medicine formula, protects against
lipopolysaccharide-induced inflammation through cholinergic
anti-inflammatory pathway. Oncotarget. 7:21222–21234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Z, Li-Sha G, Jing-Lin Z, Wen-Wu Z,
Xue-Si C, Xing-Xing C and Yue-Chun L: Protective role of the
cholinergic anti-inflammatory pathway in a mouse model of viral
myocarditis. PLoS One. 9:e1127192014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koopman FA, Vosters JL, Roescher N,
Broekstra N, Tak PP and Vervoordeldonk MJ: Cholinergic
anti-inflammatory pathway in the non-obese diabetic mouse model.
Oral Dis. 21:858–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Y, Li L, Liu B, Zhang Y, Chen Q and
Li C: Vagus nerve stimulation attenuates cerebral ischemia and
reperfusion injury via endogenous cholinergic pathway in rat. PLoS
One. 9:e1023422014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terrando N, Yang T, Ryu JK, Newton PT,
Monaco C, Feldmann M, Ma D, Akassoglou K and Maze M: Stimulation of
the α7 nicotinic acetylcholine receptor protects against
neuroinflammation after tibia fracture and endotoxemia in mice. Mol
Med. 20:667–675. 2015.PubMed/NCBI
|
|
16
|
Han Z, Shen F, He Y, Degos V, Camus M,
Maze M, Young WL and Su H: Activation of α-7 nicotinic
acetylcholine receptor reduces ischemic stroke injury through
reduction of pro-inflammatory macrophages and oxidative stress.
PLoS One. 9:e1057112014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su X, Matthay MA and Malik AB: Requisite
role of the cholinergic alpha7 nicotinic acetylcholine receptor
pathway in suppressing Gram-negative sepsis-induced acute lung
inflammatory injury. J Immunol. 184:401–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terrando N, Eriksson LI, Ryu JK, Yang T,
Monaco C, Feldmann M, Fagerlund M Jonsson, Charo IF, Akassoglou K
and Maze M: Resolving postoperative neuroinflammation and cognitive
decline. Ann Neurol. 70:986–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duris K, Manaenko A, Suzuki H, Rolland WB,
Krafft PR and Zhang JH: α7 nicotinic acetylcholine receptor agonist
PNU-282987 attenuates early brain injury in a perforation model of
subarachnoid hemorrhage in rats. Stroke. 42:3530–3536. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McComb S, Mulligan R and Sad S: Caspase-3
is transiently activated without cell death during early antigen
driven expansion of CD8(+) T cells in vivo. PLoS One. 5:e153282010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang G, Fang H, Zhen Y, Xu G, Tian J,
Zhang Y, Zhang D, Zhang G, Xu J, Zhang Z, et al: Sulforaphane
prevents neuronal apoptosis and memory impairment in diabetic rats.
Cell Physiol Bioche. 39:901–907. 2016. View Article : Google Scholar
|
|
22
|
Salazar JD, Wityk RJ, Grega MA, Borowicz
LM, Doty JR, Petrofski JA and Baumgartner WA: Stroke after cardiac
surgery: Short- and long-term outcomes. Ann Thorac Surg.
72:1195–1202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vedel AG, Holmgaard F, Rasmussen LS,
Paulson OB, Thomsen C, Danielsen ER, Langkilde A, Goetze JP, Lange
T, Ravn HB and Nilsson JC: Perfusion Pressure Cerebral Infarct
(PPCI) trial-the importance of mean arterial pressure during
cardiopulmonary bypass to prevent cerebral complications after
cardiac surgery: Study protocol for a randomised controlled trial.
Trials. 17:2472016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Li L, Wang L, Degos V, Maze M and
Su H: Alpha-7 nicotinic acetylcholine receptor agonist treatment
reduces neuroinflammation, oxidative stress, and brain injury in
mice with ischemic stroke and bone fracture. J Neurochem.
131:498–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YP, Huang J, Huang SG, Xu YG, Xu YY,
Liao JY, Feng X, Zhang XG, Wang JH and Wang J: The compromised
inflammatory response to bacterial components after pediatric
cardiac surgery is associated with cardiopulmonary
bypass-suppressed Toll-like receptor signal transduction pathways.
J Crit Care. 29:312.e7–e13. 2014. View Article : Google Scholar
|
|
26
|
Yuan SM: S100 and S100β: Biomarkers of
cerebral damage in cardiac surgery with or without the use of
cardiopulmonary bypass. Rev Bras Cir Cardiovasc. 29:630–641.
2014.PubMed/NCBI
|
|
27
|
Einav S, Shoshan Y, Ovadia H, Matot I,
Hersch M and Itshayek E: Early postoperative serum S100 beta levels
predict ongoing brain damage after meningioma surgery: A
prospective observational study. Crit Care. 10:R1412006. View Article : Google Scholar :
|
|
28
|
Zhang B, Yu JY, Liu LQ, Peng L, Chi F, Wu
CH, Jong A, Wang SF, Cao H and Huang SH: Alpha7 nicotinic
acetylcholine receptor is required for blood-brain barrier
injury-related CNS disorders caused by Cryptococcus neoformans and
HIV-1 associated comorbidity factors. Bmc Infect Dis. 15:3522015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chugani HT: Biological basis of emotions:
Brain systems and brain development. Pediatrics. 102(5 Suppl E):
S1225–S1229. 1998.
|
|
30
|
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL,
Yu J, Zhu MH, Wen CY, Yu LN and Yan M: Both PI3K/Akt and ERK1/2
pathways participate in the protection by dexmedetomidine against
transient focal cerebral ischemia/reperfusion injury in rats. Brain
Res. 1494:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ,
Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, et al: Exogenous basic
fibroblast growth factor inhibits ER stress-induced apoptosis and
improves recovery from spinal cord injury. CNS Neurosci Ther.
19:20–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krafft PR, Altay O, Rolland WB, Duris K,
Lekic T, Tang J and Zhang JH: α7 nicotinic acetylcholine receptor
agonism confers neuroprotection through GSK-3β inhibition in a
mouse model of intracerebral hemorrhage. Stroke. 43:844–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo
R, Jiang S, Nair S, Hu D and Ren J: Chronic Akt activation
attenuated lipopolysaccharide-induced cardiac dysfunction via
Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim
Biophys Acta. 1832:848–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan JJ, Chang QS, Wang X, Son YO, Liu J,
Zhang Z, Bi YY and Shi X: Activation of Akt/GSK3β and Akt/Bcl-2
signaling pathways in nickel-transformed BEAS-2B cells. Int J
Oncol. 39:1285–1294. 2011.PubMed/NCBI
|
|
35
|
Hong Y, Shao A, Wang J, Chen S, Wu H,
McBride DW, Wu Q, Sun X and Zhang J: Neuroprotective effect of
hydrogen-rich saline against neurologic damage and apoptosis in
early brain injury following subarachnoid hemorrhage: Possible role
of the Akt/GSK3β signaling pathway. PLoS One. 9:e962122014.
View Article : Google Scholar : PubMed/NCBI
|