Introduction
Cardiac fibrosis can be caused by a variety of
cardiovascular disorders, such as hypertension, ischemic injury,
valvular heart disease (1–3). Cardiac fibrosis, which is
characterized by enhanced cardiac fibroblast (CF) proliferation and
excess production of extracellular matrix (ECM) such as collagen,
plays a pivotal role in pathological cardiac remodelling and is an
important determinant of many fatal cardiovascular events, such as
heart failure, severe arrhythmias and sudden cardiac death
(4–6). Pathologically, cardiac fibrosis is
characterized by excessive collagen accumulation and fibroblast
deposition in the heart, thereby leading to reduction of cardiac
muscle compliance, filling impairment, and ultimately congestive
heart failure (4,7). Therefore, preventing or slowing the
progression of cardiac fibrosis is beneficial for the prognosis of
cardiovascular disorders.
Cardiotrophin-1 (CT-1) is a member of the
interleukin 6 superfamily. Although initially, CT-1 was regarded as
an adaptive response factor mediating cell surviving diverse
adverse stimiuli, accumulating in vitro evidences suggest
that CT-1 also acts as a profibrotic cytokine in cardiac
fibroblasts (8,9). Meanwhile, existing in vivo
experimental and clinical data also show that CT-1 is an important
pro-fibrotic molecule in the heart (10). Regarding the molecular regulation
of CT-1 expression, a previous study has shown that reactive oxygen
species (ROS) acts as a pivotal mediator for the upregulation of
CT-1 (11).
As an important ‘Qi-invigorating’ medical herb,
A. membranaceus is widely used in traditional Chinese
medicine for the treatment of cardiovascular diseases, hepatitis,
kidney disease, and skin diseases (12–14).
Astragaloside IV (AsIV), a cycloartane triterpene saponin, is one
of the major active ingredients of this plant Astragalus
membranaceus (Huang Qi in Chinese). Several lines of evidence
suggested that AsIV has remarkable cardioprotective effects via its
anti-oxidative and anti-inflammatory activities (15,16).
AsIV also improved cardiac function through inhibiting
cardiomyocyte hypertrophy and apoptosis (13,15).
In addition, the anti-fibrotic effect of AsIV was also seen in
coxsackie virus-induced cardiomyopathy and in vitro cultured
fibroblasts (17,18). Since antioxidation represents as an
important mechanism for the anti-proliferative effect of AsIV on
cardiac fibroblasts (18), it is
thus reckoned that CT-1 inhibition may also be involved in this
pocess. Therefore, the aim of this study was to examine this
possibility.
Materials and methods
Animal experiment
A total of 30 male heathy Sprague-Dawley rats,
weighing 180–200 g were provided by the Animal Center, Jinzhou
Medical University (Jinzhou, China). All rats were maintained in a
temperature-controlled room (25±0.2°C) with a 12/12 h light/dark
cycle. These rats were fed with standard laboratory food and water.
These rats were randomly divided into 3 groups (n=10): i) Control
group, rats received the same volume of vehicle; ii) isoprenaline
(ISO) group, rats received ISO injection (10 mg/kg day−1
i.p.) for 4 weeks; and iii) ISO + AsIV group, rats received AsIV
treatment (80 mg/kg day−1 i.g.) 2 weeks before 4-week
ISO injection (10 mg/kg day−1 i.p.). ISO was dissolved
in normal saline and AsIV in 1% sodium carboxymethyl cellulose
solution. At the end of the treatment, left ventricular sections
were prepared and stained with Sirius Red according to the
instructions of the commercial kit (Beijing Leagene Biotech Co.,
Ltd., Beijing, China). All experimental procedures involving
animals were conducted in accordance with the animal care
guidelines of the National Institutes of Health (NIH) and Jinzhou
Medical University. All efforts were made to minimize animal
suffering and reduce the number of animals used.
CF culture
Primary cardiac fibroblasts (CFs) cultures were
prepared from 1- to 3-day-old SD rats as our previously described
method for culture of cardiomyocytes (12), except that pre-attached CFs but not
cardiac myocytes were used for experiment. The cells were grown and
maintained in Dulbecco's modified Eagle's medium (DMEM) (Corning
Inc., Corning, NY, USA) containing 10% fetal bovine serum (FBS;
Hyclone; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in 5%
CO2/95% air at 37°C.
CF proliferation assay
CFs were planted and cultured in 96-well plates and
challenged by ISO (Sigma-Aldrich; Merck KGaA (Darmstadt, Germany)
following pretreatment with AsIV (>98% purity; Nanjing Jingzhu
Bio-technology Co., Ltd., Nanjing, China), N-acetylcysteine
(NAC; 10 mM) and CT-1 siRNA transfection. DMEM (100 µl) with 10 µl
CCK-8 (Dojindo Laboratories, Kumamoto, Japan) was included in each
well for additional 4 h at 37°C. The optical density was measured
with an automated ELISA plate reader (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 450 nm. Based on a previous study
(4), a 24 h-stimulation by ISO
induced a significant elevation of CF proliferation.
Measurement of intracellular ROS
Intracellular ROS levels were detected by
dihydroethidium (DHE; Molecular Probes, Eugene, OR, USA). CFs were
first incubated in the dark with 10 µM DHE for 1 h at 37°C in an
incubator and then washed 3 times with phosphate-buffered saline
(PBS). ROS level of the DHE loaded CFs was examined by an inverted
fluorescence microscope (Leica, Wetzlar, Germany).
Knockdown of CT-1 by specific
siRNA
Transfection was performed as previously reported
(19), using siRNA sense sequence
for CT-1, 5′-CCAAUUGCUGGAGGAAUAUtt-3′, synthesized by GenePharma
(Suzhou, China). To allow incorporation of CT-1 siRNA into CFs, CFs
were incubated in serum-free DMEM for 24 h on the day before
transfection. Transfection solution A containing 2.5 µl (~660 ng)
siRNA and 40 µl Opti-MEMI, and solution B containing 2 µl
Oliogofectamine and 5.5 µl Opti-MEMI was firstly prepared and then
mixed to form transfection solution C for use. The culturing medium
was exchanged with 200 µl DMEM without serum, with transfection
solution C subsequently added and cultured at 37°C in a humidified
atmosphere of CO2/air (5:95%) for 8 h. Thereafter, 125
µl DMEM supplemented with 30% serum was added to the cultures.
Western blot analysis was performed to confirm the knockdown effect
three days after transfection.
Quantitative real-time PCR
Total mRNA was extracted by Trizol agent
(Invitrogen, Carlsbad, CA, USA). Reverse transcription was
performed using a PrimeScript RT reagent kit (Takara Bio, Inc.,
Dalian, China) according to the manufacturer's instructions. RNA
was then analyzed by Real-Time PCR using SYBR Premix Ex
Taq™ (Takara Bio, Inc). The sequences of the primers
used were as follows: CT-1 forward, 5′-GGAAGTCTGGAAGACCACCA-3′ and
reverse, 5′-TGCTGCACATATTCCTCCAG-3′; collagen I forward,
5′-TTCACCTACAGCACGCTTGT-3′ and reverse, 5′-TTGGGATGGAGGGAGTTTAC-3′;
and GAPDH forward, 5′-TGGCCTCCAAGGAGTAAGAAAC-3′ and reverse,
5′-GGCCTCTCTCTTGCTCTCAGTATC-3′. GAPDH gene was used as an internal
control. PCR parameters were as follows: 95°C for 10 sec; 35 cycles
of 95°C for 5 sec and 60°C for 20 sec. PCR amplification was
performed using the Mx3000P qPCR SYSTEM (Stratagene, La Jolla, CA,
USA) and comparative Ct (ΔΔCT) method was used to determine the
fold change in expression.
Western blotting
Total protein content was determined and equal
amounts of protein were subjected to sodium dodecyl
sulfate-polysacrylamide gel electrophoresis and blotted onto a
polyvinylidene fluoride membrane. The membrane was blocked with 1%
BSA for 1 h at room temperature and then incubated overnight at 4°C
with the primary rabbit anti-rat polyclonal antibodies against
collagen I (Col-I, 1:1,000, cat. no. ab34710; Abcam, Cambridge, MA,
USA), β-actin (1:5,000, cat. no. 40552; Signalway Antibody, College
Park, MD, USA) and mouse anti-rat monoclonal antibodies against
CT-1 (1:1,000, cat. no. ab13975; Abcam). Following incubation with
the polyclonal horseradish peroxidase-conjugated secondary goat
anti-rabbit antibodies (1:2,500, cat. no. sc-2004) or goat
anti-mouse antibodies (1:3,500, cat. no. sc-2005) (both from Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), the bands were detected
by an enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc.). Quantityone software (version 4.6.9; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to assess the
visualized optical density for each band.
Statistical analysis
All data are shown as mean ± standard error of mean
(SEM) from at least three independent experiments. The data were
subjected to one-way analysis of variance followed by Turkey's
post hoc comparisons. The level of significance was set at
P<0.05.
Results
ISO induced CF proliferation was
attenuated by increasing doses of AsIV
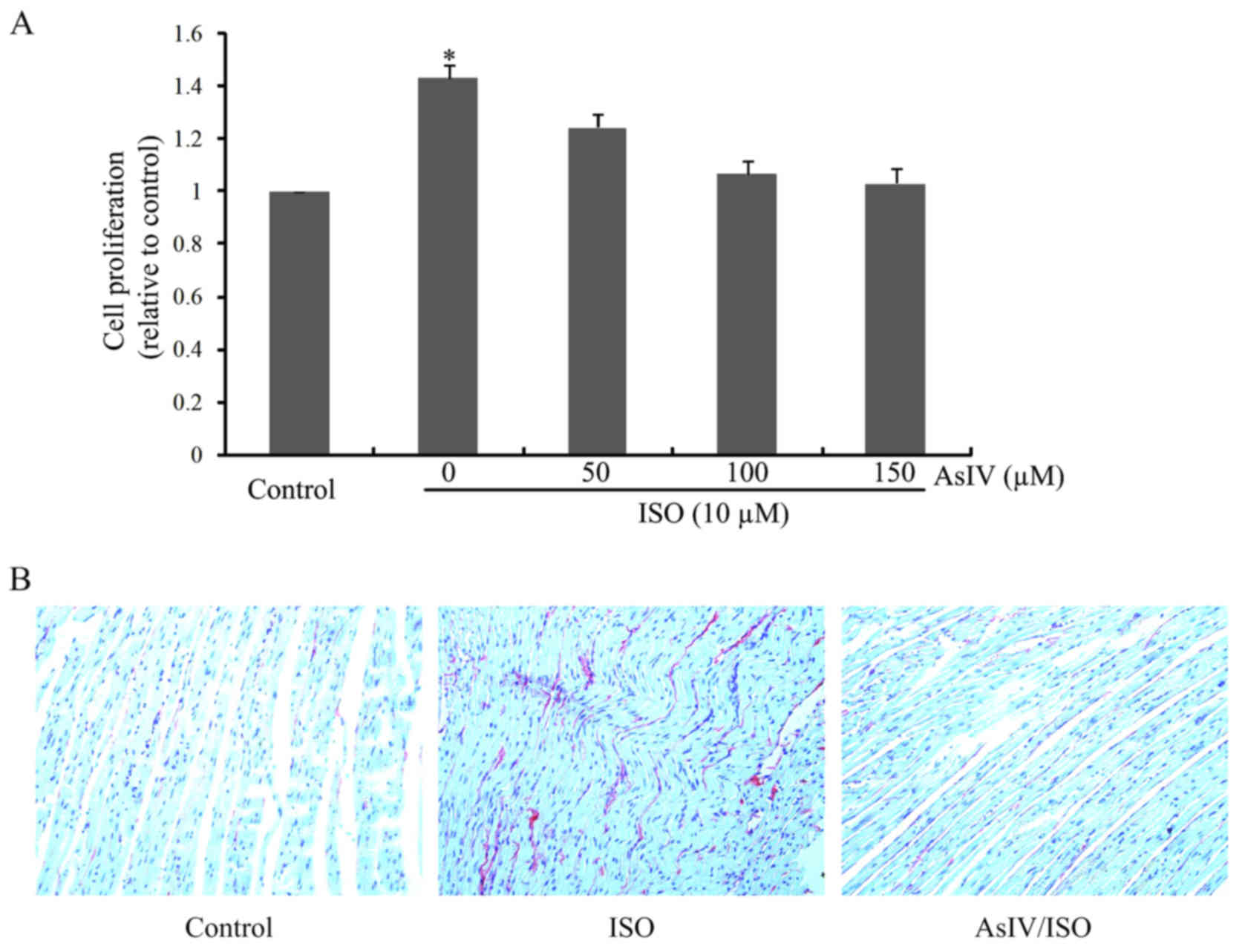
As shown in Fig.
1A, CF proliferation was significantly increased upon ISO
stimulation for 24 h. AsIV pretreatment remarkably inhibited ISO
triggered CF proliferation in a dose dependent manner. The
anti-fibrotic effect of AsIV was further confirmed by Sirius Red
stanning in vivo (Fig.
1B).
ISO induced ROS generation in CFs was
decreased by AsIV, but not by CT-1 siRNA
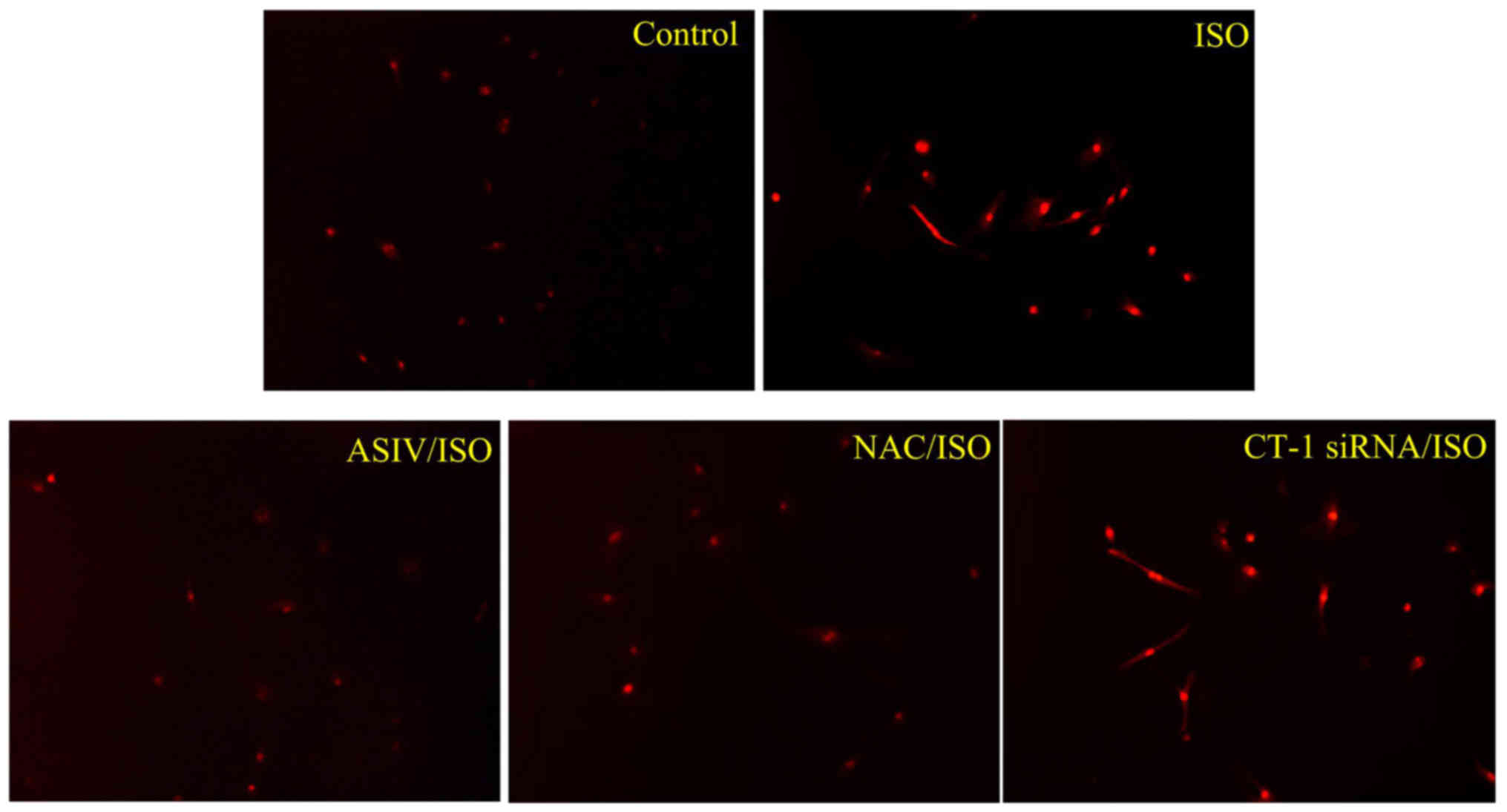
As shown in Fig. 2,
an elevated ROS production upon 30 min stimulation by ISO, which
was effectively blunted by pretreatment with AsIV (100 µM) or NAC
(10 mM) (20), the typical ROS
scavenger. Great reduced expression of protein of CT-1 was
confirmed by western blot analysis three days after transfection
(data not shown). This transfection, however, failed to produce any
effect on ISO-initiated ROS generation.
AsIV and NAC attenuated ISO induced
overexpression of CT-1
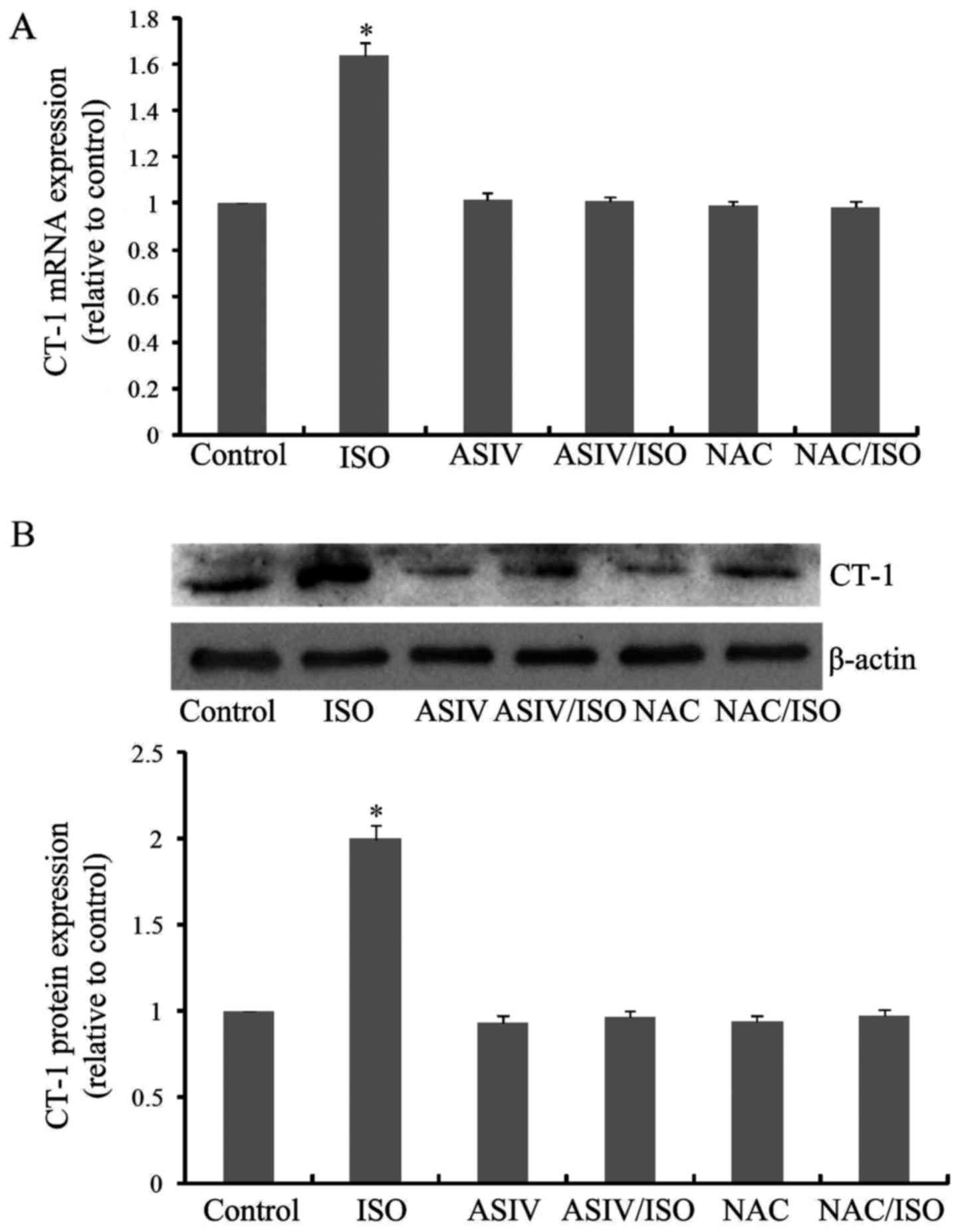
As shown in Fig. 3A and
B, ISO treatment for 24 h caused a significant elevation of
CT-1 expression, both at mRNA and protein levels. These
overexpressions of CT-1, however, were singnificantly inhibited by
AsIV pretreatment. In addition, NAC produced similar inhibitory
effects as AsIV on CT-1 overexpression.
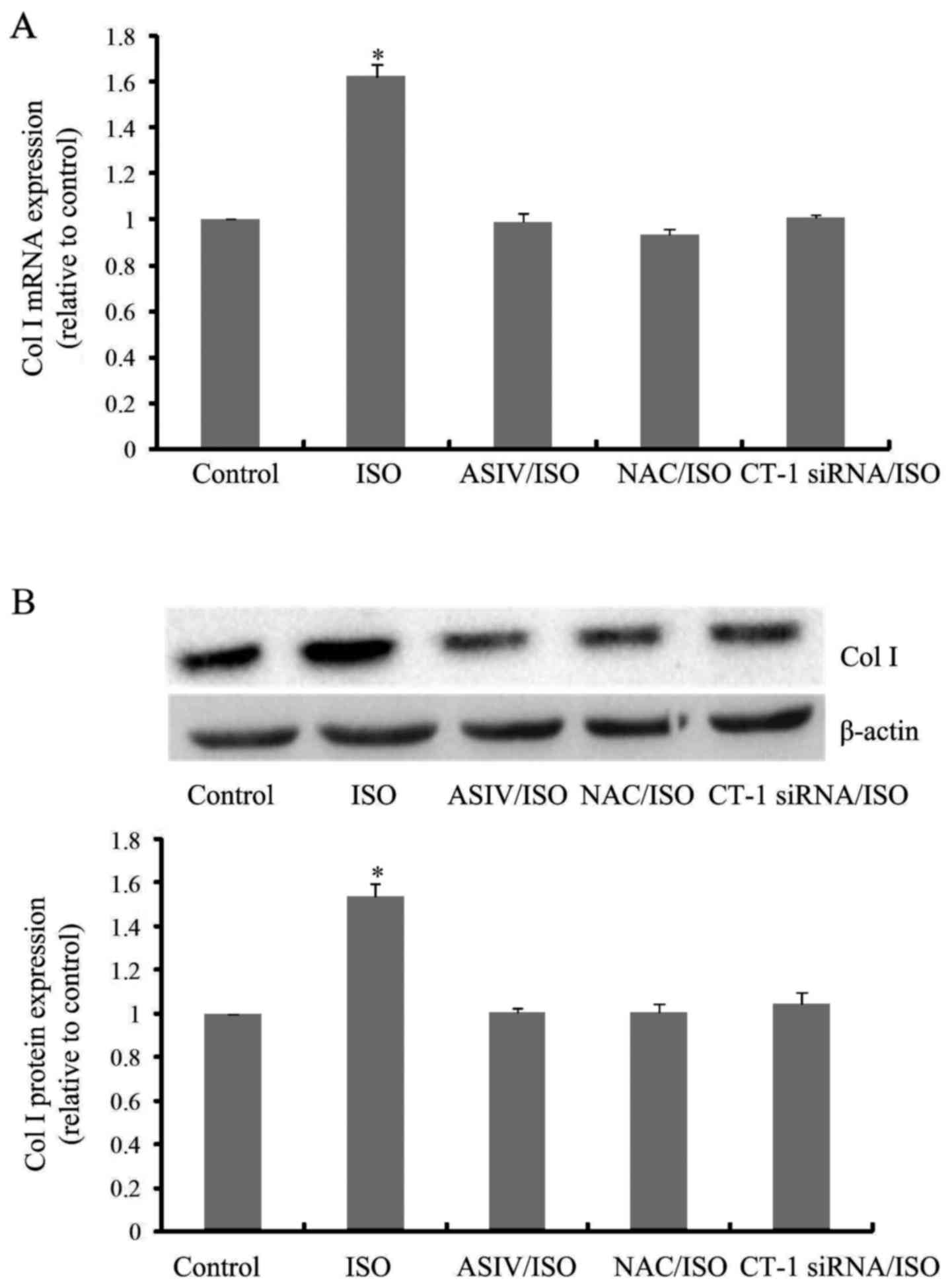
CF proliferation and type I collagen
synthesis induced by ISO was attenuated by AsIV, NAC and CT-1 siRNA
pretreatment
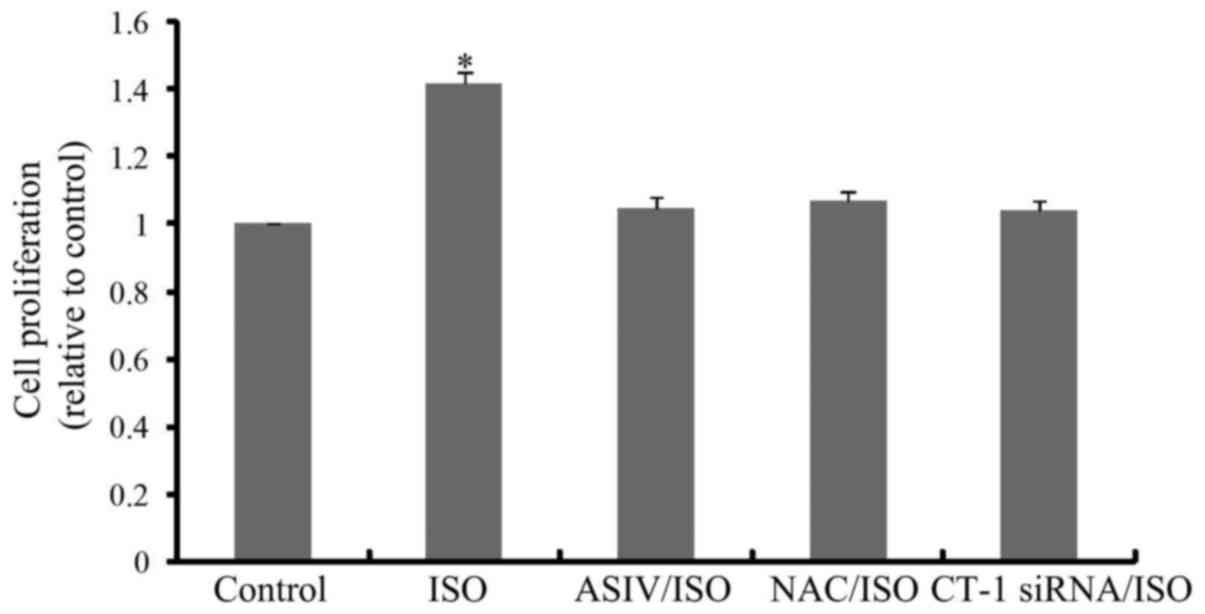
As shown in Fig. 4,
CF proliferation was significantly increased upon ISO stimulation
for 24 h. AsIV, NAC or CT-1 siRNA pretreatment remarkably inhibited
ISO triggered CF proliferation. Likewise, ISO-increased mRNA
(Fig. 5A) and protein (Fig. 5B) expressions of collagen I, the
most dominant component of extracellular matrix (21,22),
in cultured CFs were also blunted by application of AsIV, NAC or
CT-1 siRNA.
Discussion
Cardiac fibrosis is a pivotal phenomenon and a
hallmark in a variety of cardiovascular diseases (23). Enhanced CF proliferation and excess
production and deposition of ECM represent the key characteristics
of cardiac fibrosis. These pathological changes would eventually
lead to myocardial stiffness, impaired diastolic function, severe
arrhythmias, cardiac failure and even sudden cardiac death
(24,25). Therefore, early intervention in the
fibrosis process would effectively slow down or prevent the
procession of a variety of cardiovascular diseases.
Oxidative stress is a major pathogenesis mechanism
for diverse cardiac disorders, including cardiac fibrosis,
hypertrophy, apoptosis, inflammation, and resultant heart failure
(26–28). It is evidenced that ROS could
insult mitochondrial function and cause gene expression alteration
both in cardiomyocytes and cardiofibroblasts (29,30).
Anti-oxidation can prevent the procession of cardiovascular events
(15,31,32).
At present, the antioxidant property of AsIV has been demonstrated
by many previous reports (33,34).
Intriguingly, we herein showed besides by NAC, ISO-invoked ROS
overproduction was also blunted by application of AsIV, thereby
suggestive of a possible protection effect of AsIV on ISO-induced
damage of CFs via its anti-oxidation effect. Indeed, AsIV and NAC
pretreatment also alleviated ISO-induce CF proliferation and
collagen I synthesis. These data demonstrated an anti-oxidantion
activity dependent anti-fibrotic effect of ASIV. It is unclear
regarding how AsIV caused decreased production of ROS. In spite of
that, our recent study showed that AsIV effectively suppressed the
expression of mitochondrial NADPH oxidase 4 (mito-NOX4), and
enhanced mitochondrial superoxide dismutase (mito-SOD) and
mitochondrial catalase (mito-CAT) activity under ISO stimulation,
both in intact heart tissue and in vitro cultured H9C2 cells
(15). As such, it is reckoned
that AsIV's antioxidant activity as shown here is probably related
to its regulatory effect on mito-NOX4, mito-SOD, and mito-CAT,
which needs further investigation.
CT-1 is a member belonging to IL-6 superfamily
(35). By interacting with the
heterodimer composed of glycoprotein 130 and leukemia inhibitory
factor receptor-β, this cytokine exerts a series of cellular
effects (36). Accumulating
reports have delineated a key role for CT-1 in cardiomyocyte
survival and hypertrophy, vascular smooth muscle cell (VSMC)
proliferation, hypertrophy and extracellular matrix production. In
addition, CT-1 is also involved in the pathogenesis of cardiac
remodelling (37). Meanwhile, a
direct stimulation of CT-1 on CF proliferation and collagen type I
was also confirmed (9,38). In the present study, a significant
increase of CT-1 expression was seen in CFs upon ISO stimulation,
which is in compatible with the statement that CFs are the
predominant source of IL-6 in response to β-adrenergic receptor
stimulation (39). CT-1 knockdown
significantly reduced CF proliferation and collagen I production,
suggesting an indispensible role of CT-1 in ISO-triggered cardiac
fibrosis. As for the relationship between ROS and CT-1 pathways,
our present study indicates that ISO-induced ROS production is
needed for CT-1 overexpression, but not vice versa. A similar
sequence as in the present study has been observed for effect of
proxidant- and CoCl2-mediated upregulation of CT-1 in
mouse embryonic stem cells (11).
Both ROS production and CT-1 overexpression triggered by ISO were
remarkably suppressed by AsIV, thereby revealing an
ROS-CT-1-targeted anti-fibrotic effect of this natural occurring
substance. Since ROS operated upstream of CT-1 overexpression upon
ISO stimulation, and AsIV would effectively blunted ROS production,
it is argued that AsIV reduce CT-1 overexpression via, at least in
part, its antioxidant activity. But we are still unable to exclude
the possibility that AsIV may also have direct inhibitory effect on
CT-1 overexpression induced by ISO.
A. membranaceus has been widely used in
traditional Chinese medicine for the treatment of cardiovascular
diseases and AsIV represents one of the major active ingredients
thereof. Previous researches mainly paid more attention to the
cardiomyocytes, such as their hypertrophy or apoptosis, than
cardiofibroblasts (CFs) (15,40).
In contrast, our previous study has confirmed the beneficial effect
of AsIV on in vitro cultured CFs, including that of
anti-oxidation and anti-fibrosis (18). Our present study herein further
revealed that the anti-fibrotic effect of AsIV may be related to
ROS-mediated CT-1 overexpression. Although it is unclear regarding
how AsIV exerted its anti-fibrotic effect, β-receptor inhibition
seems not involved in this process, as AsIV is also able to prevent
cardiac fibrosis induced by stimuli irrelevant to β-receptor
activation, such as coxsackievirus (17). This finding, together with other
findings, would provide us comprehensive understanding of the
cardioprotective effect of this natural occurring substance. And
also, our present finding suggests that AsIV may serve as a useful
therapeutic treatment in patients with fibrosis- and
remodelling-related cardiovascular disorders, such as chronic heart
failure, diabetic cardiomyopathy, and iron overload cardiomyopathy
(41–43).
Acknowledgements
This study was carried out with the support of
National Natural Science Foundation of China (grant no.
81673632).
References
|
1
|
Yao Y, Shen D, Chen R, Ying C, Wang C, Guo
J and Zhang G: Galectin-3 predicts left ventricular remodeling of
hypertension. J Clin Hypertens (Greenwich). 18:506–511. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Y, Fang H, Lin S, Shen S, Tao L, Xiao
J and Li X: Qiliqiangxin protects against cardiac
ischemia-reperfusion injury via activation of the mTOR pathway.
Cell Physiol Biochem. 37:454–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decker JA: Arrhythmias in paediatric
valvar disease. Cardiol Young. 24:1064–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu H, Tian A, Wu J, Yang C, Xing R, Jia P,
Yang L, Zhang Y, Zheng X and Li Z: Danshensu inhibits β-adrenergic
receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol
Pharm Bull. 37:961–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Löfsjögård J, Persson H, Díez J, López B,
González A, Edner M, Mejhert M and Kahan T: Atrial fibrillation and
biomarkers of myocardial fibrosis in heart failure. Scand
Cardiovasc J. 48:299–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sirish P, Li N, Liu JY, Lee KS, Hwang SH,
Qiu H, Zhao C, Ma SM, López JE, Hammock BD and Chiamvimonvat N:
Unique mechanistic insights into the beneficial effects of soluble
epoxide hydrolase inhibitors in the prevention of cardiac fibrosis.
Proc Natl Acad Sci USA. 110:pp. 5618–5623. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Zou XJ, Gao X, Chen H, Luo JL,
Wang ZH, Liang QS and Yang GT: Sodium tanshinone IIA sulfonate
attenuates angiotensin II-induced collagen type I expression in
cardiac fibroblasts in vitro. Exp Mol Med. 41:508–516. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freed DH, Chilton L, Li Y, Dangerfield AL,
Raizman JE, Rattan SG, Visen N, Hryshko LV and Dixon IM: Role of
myosin light chain kinase in cardiotrophin-1-induced cardiac
myofibroblast cell migration. Am J Physiol Heart Circ Physiol.
301:H514–H522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freed DH, Borowiec AM, Angelovska T and
Dixon IM: Induction of protein synthesis in cardiac fibroblasts by
cardiotrophin-1: Integration of multiple signaling pathways.
Cardiovasc Res. 60:365–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López B, González A, Querejeta R, Larman
M, Rábago G and Díez J: Association of cardiotrophin-1 with
myocardial fibrosis in hypertensive patients with heart failure.
Hypertension. 63:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ateghang B, Wartenberg M, Gassmann M and
Sauer H: Regulation of cardiotrophin-1 expression in mouse
embryonic stem cells by HIF-1alpha and intracellular reactive
oxygen species. J Cell Sci. 119:1043–1052. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai H, Jia G, Liu X, Liu Z and Wang H:
Astragalus polysaccharide inhibits isoprenaline-induced cardiac
hypertrophy via suppressing Ca2+-mediated
calcineurin/NFATc3 and CaMKII signaling cascades. Environ Toxicol
Pharmacol. 38:263–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Wang HX, Zhang YJ, Yang YH, Lu ML,
Zhang J, Li ST, Zhang SP and Li G: Astragaloside IV attenuates
inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway
in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol.
Oct 25–2013.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Jiang X, Cao X, Huang Y, Chen J, Yao X,
Zhao M, Liu Y, Meng J, Li P, Li Z, et al: Effects of treatment with
Astragalus membranaceus on function of rat leydig cells. BMC
Complement Altern Med. 15:2612015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mei M, Tang F, Lu M, He X, Wang H, Hou X,
Hu J, Xu C and Han R: Astragaloside IV attenuates apoptosis of
hypertrophic cardiomyocyte through inhibiting oxidative stress and
calpain-1 activation. Environ Toxicol Pharmacol. 40:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao P, Wang Y, Zeng S, Lu J, Jiang TM and
Li YM: Protective effect of astragaloside IV on
lipopolysaccharide-induced cardiac dysfunction via downregulation
of inflammatory signaling in mice. Immunopharmacol Immunotoxicol.
37:428–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang
CB, Yang Y, Zou Y, Ge J, Chen R and Chen H: Astragaloside IV
attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in
coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol.
658:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai H, Jia G, Lu M, Liang C, Wang Y and
Wang H: Astragaloside IV inhibits isoprenaline-induced cardiac
fibrosis by targeting the reactive oxygen species/mitogen-activated
protein kinase signaling axis. Mol Med Rep. 15:1765–1770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai H, Song D, Xu J, Li B, Hertz L and
Peng L: Ammonia-induced Na,K-ATPase/ouabain-mediated EGF receptor
transactivation, MAPK/ERK and PI3K/AKT signaling and ROS formation
cause astrocyte swelling. Neurochem Int. 63:610–625. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu M, Zheng Y, Sun HX and Yu DJ:
Inhibitory effects of enalaprilat on rat cardiac fibroblast
proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules.
17:2738–2751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye Y, Lv X, Wang MH, Zhu J, Chen SQ, Jiang
CY and Fu GS: Alendronate prevents angiotensin II-induced collagen
I production through geranylgeranylation-dependent RhoA/Rho kinase
activation in cardiac fibroblasts. J Pharmacol Sci. 129:205–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu LM and Xu Y: Epigenetic regulation in
cardiac fibrosis. World J Cardiol. 7:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Putten S, Shafieyan Y and Hinz B:
Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol.
93:133–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chimura M, Kiuchi K, Okajima K, Shimane A,
Sawada T, Onishi T, Yamada S, Taniguchi Y, Yasaka Y and Kawai H:
Distribution of ventricular fibrosis associated with life
threatening ventricular tachyarrhythmias in patients with
nonishcemic dilated cardiomyopathy. J Cardiovasc Electrophysiol.
Jul 29–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu H, Li GN, Xie J, Li R, Chen QH, Chen
JZ, Wei ZH, Kang LN and Xu B: Resveratrol ameliorates myocardial
fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in
STZ-induced diabetic mice. BMC Cardiovasc Disord. 16:52016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghule AE, Kandhare AD, Jadhav SS, Zanwar
AA and Bodhankar SL: Omega-3-fatty acid adds to the protective
effect of flax lignan concentrate in pressure overload-induced
myocardial hypertrophy in rats via modulation of oxidative stress
and apoptosis. Int Immunopharmacol. 28:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An LP, An SK, Wei XH, Fu SY and Wu HA:
Atorvastatin improves cardiac function of rats with chronic cardiac
failure via inhibiting
Rac1/P47phox/P67phox-mediated ROS release.
Eur Rev Med Pharmacol Sci. 19:3940–3946. 2015.PubMed/NCBI
|
|
29
|
Bartz RR, Suliman HB and Piantadosi CA:
Redox mechanisms of cardiomyocyte mitochondrial protection. Front
Physiol. 6:2912015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schunke KJ, Coyle L, Merrill GF and
Denhardt DT: Acetaminophen attenuates doxorubicin-induced cardiac
fibrosis via osteopontin and GATA4 regulation: Reduction of oxidant
levels. J Cell Physiol. 228:2006–2014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen O, Ye Z, Cao Z, Manaenko A, Ning K,
Zhai X, Zhang R, Zhang T, Chen X, Liu W and Sun X: Methane
attenuates myocardial ischemia injury in rats through
anti-oxidative, anti-apoptotic and anti-inflammatory actions. Free
Radic Biol Med. 90:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mathur P, Ding Z, Saldeen T and Mehta JL:
Tocopherols in the prevention and treatment of atherosclerosis and
related cardiovascular disease. Clin Cardiol. 38:570–576. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu DM, Lu PH, Zhang K, Wang X, Sun M, Chen
GQ and Wang Q: EGFR mediates astragaloside IV-induced Nrf2
activation to protect cortical neurons against in vitro
ischemia/reperfusion damages. Biochem Biophys Res Commun.
457:391–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu JY, Han J, Chu ZG, Song HP, Zhang DX,
Zhang Q and Huang YS: Astragaloside IV attenuates hypoxia-induced
cardiomyocyte damage in rats by upregulating superoxide dismutase-1
levels. Clin Exp Pharmacol Physiol. 36:351–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng ZZ, Fu X Tian, Liang J and Guo Z
Bing: CT-1 induces angiogenesis by regulating the ADMA/DDAH
pathway. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
159:540–546. 2015.PubMed/NCBI
|
|
36
|
Pennica D, King KL, Shaw KJ, Luis E,
Rullamas J, Luoh SM, Darbonne WC, Knutzon DS, Yen R, Chien KR, et
al: Expression cloning of cardiotrophin 1, a cytokine that induces
cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 92:pp.
1142–1146. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
López-Andrés N, Martin-Fernandez B,
Rossignol P, Zannad F, Lahera V, Fortuno MA, Cachofeiro V and Díez
J: A role for cardiotrophin-1 in myocardial remodeling induced by
aldosterone. Am J Physiol Heart Circ Physiol. 301:H2372–H2382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsuruda T, Jougasaki M, Boerrigter G,
Huntley BK, Chen HH, D'Assoro AB, Lee SC, Larsen AM, Cataliotti A
and Burnett JC Jr: Cardiotrophin-1 stimulation of cardiac
fibroblast growth: Roles for glycoprotein 130/leukemia inhibitory
factor receptor and the endothelin type A receptor. Circ Res.
90:128–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin F, Li P, Zheng M, Chen L, Xu Q, Chen
K, Wang YY, Zhang YY and Han C: Interleukin-6 family of cytokines
mediates isoproterenol-induced delayed STAT3 activation in mouse
heart. J Biol Chem. 278:21070–21075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Tang F, Yang Y, Lu M, Luan A,
Zhang J, Yang J and Wang H: Astragaloside IV protects against
isoproterenol-induced cardiac hypertrophy by regulating
NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One.
10:e01187592015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giam B, Chu PY, Kuruppu S, Smith AI,
Horlock D, Kiriazis H, Du XJ, Kaye DM and Rajapakse NW:
N-acetylcysteine attenuates the development of cardiac fibrosis and
remodeling in a mouse model of heart failure. Physiol Rep.
4:e127572016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Faramoushi M, Sasan R Amir, Sarraf V Sari
and Karimi P: Cardiac fibrosis and down regulation of GLUT4 in
experimental diabetic cardiomyopathy are ameliorated by chronic
exposures to intermittent altitude. J Cardiovasc Thorac Res.
8:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Wang H, Cui L, Zhang Y, Liu Y,
Chu X, Liu Z, Zhang J and Chu L: Continuing treatment with Salvia
miltiorrhiza injection attenuates myocardial fibrosis in chronic
iron-overloaded mice. PLoS One. 10:e01240612015. View Article : Google Scholar : PubMed/NCBI
|