Introduction
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a potential anticancer agent.
Numerous cancer cells have exhibited sensitivity to TRAIL-induced
death, which has not been observed in normal cells. TRAIL binds to
at least five receptors, including death receptors 4 and 5, which
are functional TRAIL receptors. However, decoy receptors 1 and 2,
and osteoprotegerin are decoy TRAIL receptors that inhibit the
death-inducing activity of TRAIL (1). The application of TRAIL in cancer
therapy is hindered due to TRAIL-associated resistance in cancer
cells. The mechanisms of resistance may be associated with the
dysfunction of TRAIL-induced signaling and/or high expression
levels of anti-apoptotic molecules. TRAIL activates several
cellular signaling pathways, including those involving
mitogen-activated protein kinases (MAPKs), which result in
apoptosis (2–4). Combining TRAIL with other agents to
modulate these molecules or pathways may help to overcome
TRAIL-associated resistance and therefore improve the therapeutic
applications of TRAIL (2,3).
Scutellaria baicalensis Georgi is a common
Chinese medicinal herb, which contains various flavonoids,
including baicalin, wogonin, wogonoside, oroxylin A and oroxylin
A-7 (5). These flavonoids have
various activities. Baicalin (5, 6-dihydroxy-7-o-glucuronide
flavone) has been reported to possess a diverse range of
pharmacological properties, including antioxidant,
anti-inflammatory, antiviral and anticancer activities (6). Human leukemia, myeloma, breast, lung,
bladder and lymphoma cancer cells were potently suppressed by this
flavone as reported in previous studies (7–9). The
molecular mechanisms underlying these effects are thought to
involve alterations in oxidation/reduction status, cell cycle
inhibition and the induction of apoptosis (6). However, the effect of baicalin on the
anticancer activity of TRAIL has yet to be explored.
In the present study, non-small cell lung cancer
cell lines A549 and H2009 were treated with the combination of
baicalin and TRAIL. Baicalin potently sensitized TRAIL-induced
apoptosis of cancer cells via p38 MAPK activation and induction of
intracellular reactive oxygen species (ROS) accumulation.
Materials and methods
Reagents
Glutathione S-transferase-TRAIL was purchased from
SinoBio Biotech Ltd., (Shanghai, China). Baicalin was purchased
from the National Institute of the Control Pharmaceutical and
Biological Products (Beijing, China). Z-VAD-FMK was obtained from
Calbiochem (EMD Millipore, Billerica, MA, USA). Butylated
hydroxyanisole (BHA) and N-acetyl-L-cysteine (NAC) were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
4-(4-fluo-rophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-imidazole
(SB203580) (cat. no. 152121-47-6) was obtained from Gene Operation
(Ann Arbor, MI, USA). The JNK inhibitor SP600125 (cat. no.
129-56-6) was from EMD Millipore. The ERK inhibitor U0126 (cat. no.
#9903) was from Cell Signaling Technology Inc., (Danvers, MA, USA).
CellROX Deep Red reagent was purchased from Thermo Fisher
Scientific, Inc., (Waltham, MA, USA). The antibody poly
(ADP-ribose) polymerase (PARP; cat. no. AP102) was obtained from
Beyotime Institute of Biotechnology, Haimen, China). Anti-p38 MAPK
(cat. no. #9212) and anti-phosphorylated-p38 MAPK (Thr180/Tyr182)
antibodies (cat. no. #9211) were purchased from Cell Signaling
Technology Inc., (Danvers, MA, USA). All of the above antibodies
were diluted 1:1,000 in 5% milk. Anti-GAPDH antibody (1:2,000; cat.
no. 10494-1-AP) was obtained from Proteintech Group, Inc. (Chicago,
IL, USA).
Cell culture
Two non-small cell lung cancer cell lines A549 and
H2009 were from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and cultured in RPMI 1640 medium (cat. no.
SH30809.01; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences), 1 mmol/l glutamate, 100 U/ml penicillin and 100
µg/ml streptomycin under standard incubator conditions at 37°C with
5% CO2.
Cell death assay
Cells were seeded in a 96-well plate 24 h prior to
treatment and were then treated with baicalin, TRAIL alone or in
combination for 72 h. Then culture medium from each well was
collected and transferred to 96-well flat-bottomed plates. Lactate
dehydrogenase (LDH) activity was determined by adding equal volumes
of the reaction mixture to each well and incubating for 30 min at
22°C. The absorbance of the samples was measured at 490 nm using a
plate reader (Tecan Infinite F200). Cell death was detected
quantitatively via lactate dehydrogenase (LDH) release using a
cytotoxicity detection kit (Promega Corporation, Madison, WI, USA)
as described previously (10). All
experiments were repeated three to five times; the average is
presented in each figure. Cell death was calculated using the
formula: Cytotoxicity (%) = (experimental value-spontaneous LDH
release) / (maximum LDH release - spontaneous LDH release) ×
100%.
Analysis of apoptosis by flow
cytometry
Apoptosis was detected by flow cytometry. A549 cells
were seeded into a 6-well plate 24 h prior to treatment and were
then treated with baicalin (75 µM), TRAIL (30 ng/ml) alone or in
combination for 48 h. Subsequently, the cells were double stained
with Annexin V-fluorescein isothiocyanate (V-FITC) and propidium
iodide (PI) using an Annexin V-FITC Apoptosis Detection kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to the
manufacturer's protocol. Early apoptosis is defined by Annexin
V+/PI− staining (Q4) and late apoptosis is
defined by Annexin V+/PI+ staining (Q2,) as
determined by fluorescence-activated cell sorting (Beckman Coulter,
Inc., Brea, CA, USA).
Western blot analysis
A549 cells were seeded in a 6-well plate 24 h prior
to treatment and were then treated with baicalin (75 µM), TRAIL (30
ng/ml) alone or in combination for 72 h. Cell extracts were then
prepared by lysing cells in M2 buffer [20 mmol/l Tris-HCL (pH 7.6),
0.5% NP-40, 250 mmol/l NaCl, 3 mmol/l EDTA, 3 mmol/l EGTA, 2 mmol/l
dithiothreitol, 0.5 mmol/l phenylmethylsulfonyl fluoride, 20 mmol/l
β-glycerophosphate, 1 mmol/l sodium vanadate and 1 µg/ml leupeptin]
and homogenizing them on ice. The extracts were subsequently
incubated on ice for 30 min. Protein concentration was determined
using a Bradford protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. Protein samples (50 µg) were separated by 10% SDS-PAGE.
The proteins were transferred to a nitrocellulose filter membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) following
separation. The membrane was blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) in 1X Tris-buffered saline and 0.05%
Tween-20 (TBST) for 1 h at room temperature with agitation. The
membrane was then incubated with primary antibodies (1:1,000) at
4°C overnight, after which the membrane was washed three or five
times with 1X TBST and was then incubated with the horse radish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no.
ZB 2301; 1:2,000; OriGene Technologies, Inc., Rockville, MD, USA)
for 1 h at room temperature with agitation. The proteins were
visualized by enhanced chemiluminescence (Merck KGaA) using Bio-Rad
Image Station (Bio-Rad Laboratories Inc.). Each experiment was
repeated at least three times and representative results are shown
in each figure.
Detection of ROS
Cells were cultured in 12-well plates overnight and
subsequently treated with baicalin (75 µM), TRAIL (30 ng/ml) alone
or in combination for 3 h. Cells were then stained for 30 min with
5 µM CellROX Deep Red reagent (Thermo Fisher Scientific, Inc.),
washed three times with precooled PBS, and analyzed using a
Multiscan Spectrum plate reader (Thermo Fisher Scientific, Inc.).
The wavelengths were set as follows: Excitation, 640 nm; emission,
665 nm. All the experiments were repeated at least three times, and
representative results are shown in each figure (11,12).
Statistical analysis
The presented data and results were confirmed in at
least three independent experiments and were expressed as the mean
± standard deviation. Student's t-test or one-way analysis of
variance and a Tukey's post hoc test were used for statistical
analyses. The calculations were performed with SPSS 19.0 statistics
software package (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Baicalin enhances TRAIL-induced cell
death in cancer cells
The present study investigated whether baicalin was
able to enhance the anticancer activity of TRAIL in A549 cells.
A549 cells were treated with 75 µM baicalin, 30 ng/ml TRAIL alone,
or a combination of both for 72 h. Following treatment, cell death
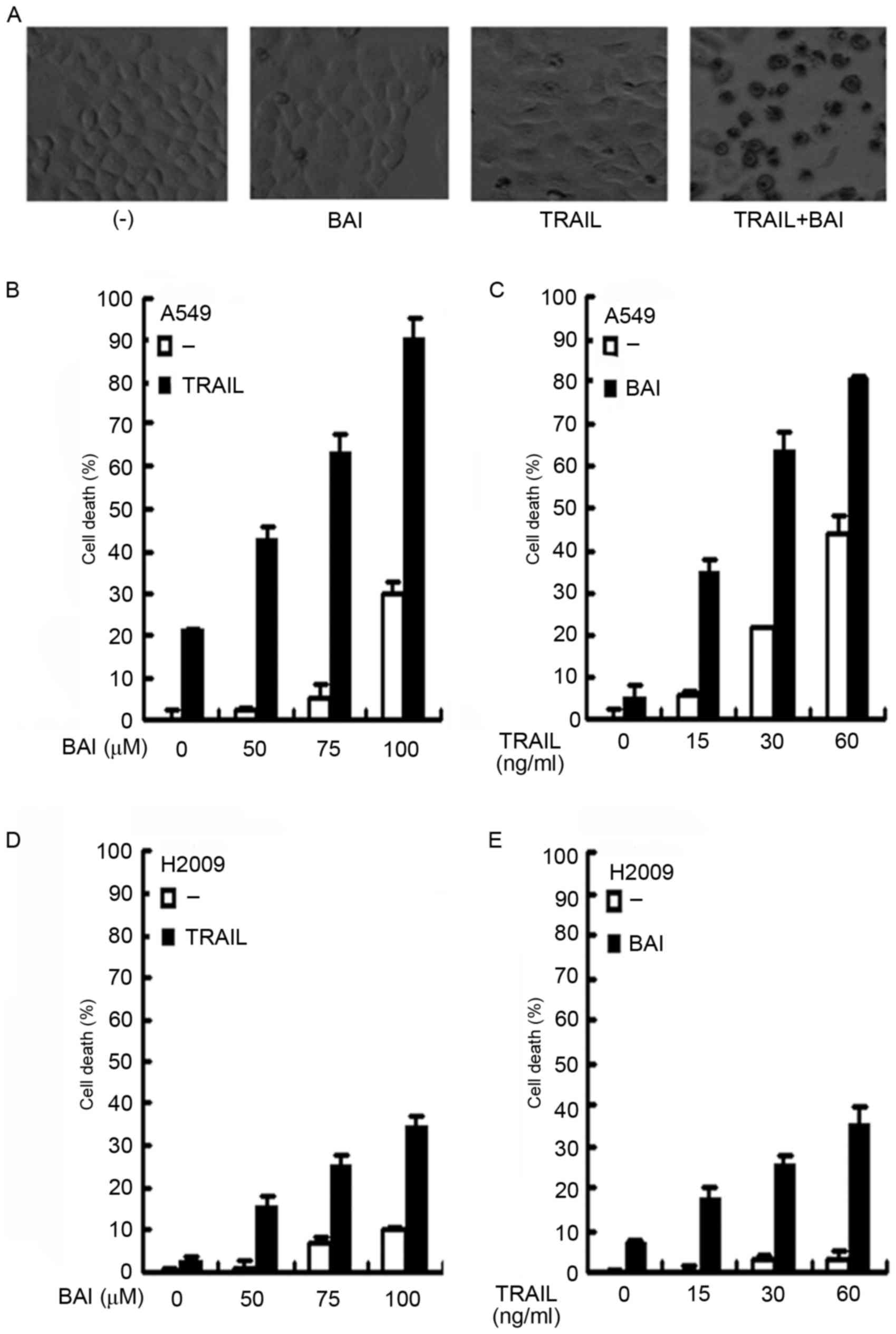
was observed microscopically. As demonstrated in the representative
images (Fig. 1A), TRAIL or
baicalin alone could induce only limited cell death, compared with
the observed increase in cytotoxicity following co-treatment with
baicalin and TRAIL. To quantitatively measure cell death, A549
cells were treated with increasing concentrations of baicalin
(50–100 µM) and a fixed concentration of TRAIL (30 ng/ml). Cell
death was detected using a LDH release assay. While TRAIL alone
caused ~20% cell death, baicalin synergistically sensitized
TRAIL-induced cell death in a dose-dependent manner (Fig. 1B). The synergism that killed ~90%
of cells was detected following treatment with TRAIL and the
highest dose of baicalin (100 µM), whereas this concentration of
baicalin alone, was responsible for ~30% of cell death. In
addition, a similar dose-dependent synergistic effect of baicalin
and TRAIL co-treatment was also observed with a fixed baicalin dose
(75 µM) and increasing concentrations of TRAIL (Fig. 1C). To exclude potential cell
line-specific biases, the anticancer activity-associated
sensitization of TRAIL was further validated in H2009 cells. As
expected, a similar dose-dependent synergism with either a fixed
concentration of TRAIL or baicalin was observed (Fig. 1D and E). These results suggested
that baicalin sensitized cancer cells to TRAIL-induced
cytotoxicity.
Baicalin enhances TRAIL-induced
apoptosis of cancer cells
Baicalin and TRAIL are able to induce apoptosis
(4,13,14).
The present study investigated whether the increased cell death
observed in lung cancer cells co-treated with baicalin and TRAIL
was achieved through the potentiation of apoptosis. A549 cells were
treated with baicalin, TRAIL alone or in combination. Subsequently,
the cells were stained with Annexin V-FITC and PI; apoptosis was
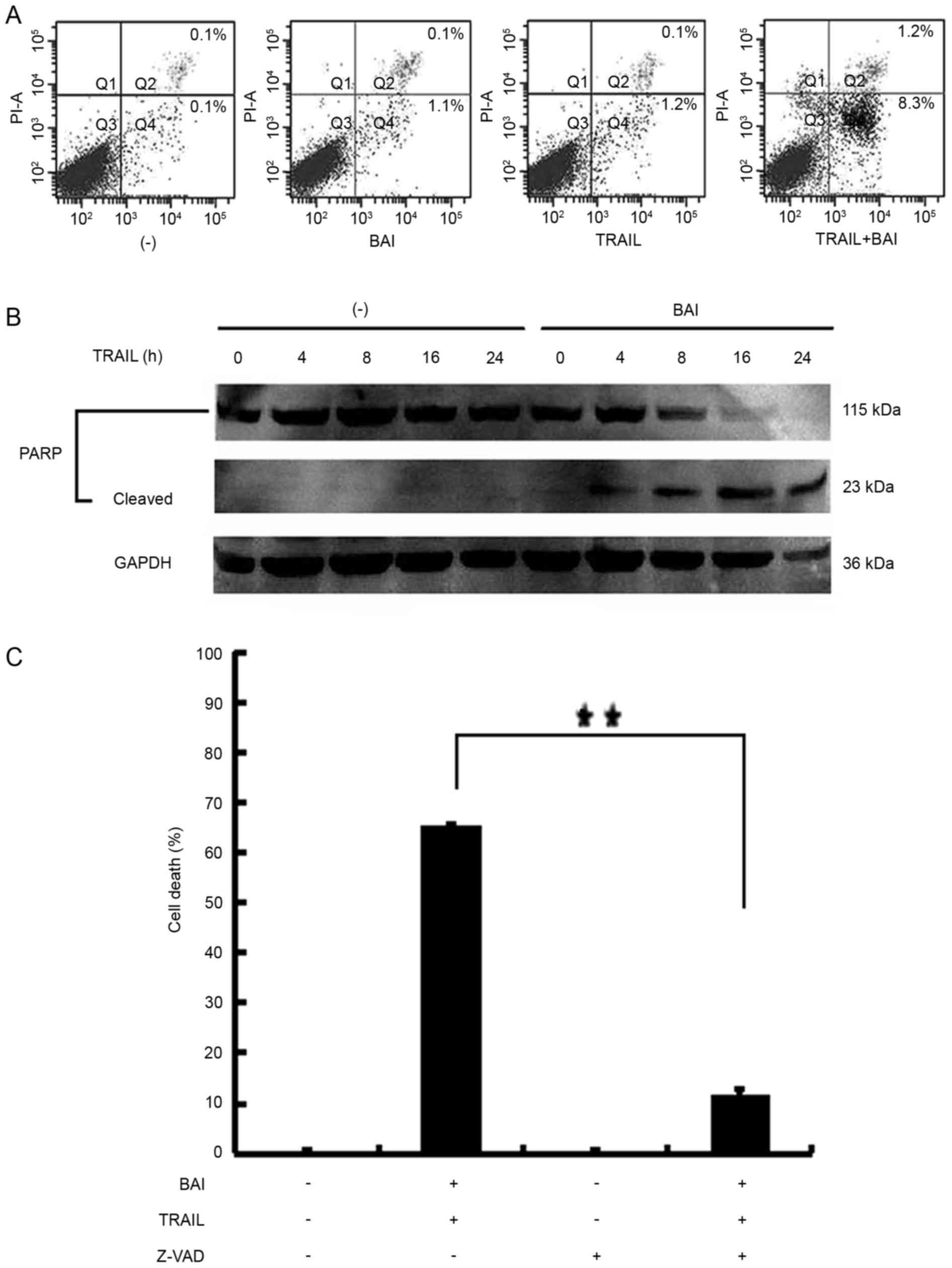
analyzed by flow cytometry. As shown in Fig. 2A, early apoptotic and late
apoptotic cell populations were markedly increased with baicalin
and TRAIL co-treatment. This finding indicated that the observed
increased cell death stems from enhancing apoptosis. Western blot
analysis also detected the activation of apoptosis. As depicted in
Fig. 2B, the cleavage of the
caspase substrate PARP was markedly increased in A549 cells treated
with TRAIL and baicalin. As exhibited in Fig. 2C, A549 cells were pretreated with
or without Z-VAD-FMK (20 µM) for 1 h, followed by baicalin (75 µM)
and TRAIL (30 ng/ml) for 72 h. The enhancement of TRAIL-induced
apoptosis by baicalin was significantly suppressed the synergistic
cytotoxicity induced by co-treatment with TRAIL and baicalin
(P<0.01).
p38 MAPK activation contributes to
increased cytotoxicity induced by TRAIL and baicalin
co-treatment
The apoptotic pathway is tightly regulated within
the cell through multistep regulatory mechanisms. To investigate
the mechanisms underlying increased cytotoxicity induced by
baicalin and TRAIL co-treatment, several inhibitors were employed:
a JNK inhibitor, SP600125; an ERK inhibitor, U0126; and a p38
inhibitor, SB203580. These inhibitors were used to block
corresponding pathways in A549 cells treated with baicalin and
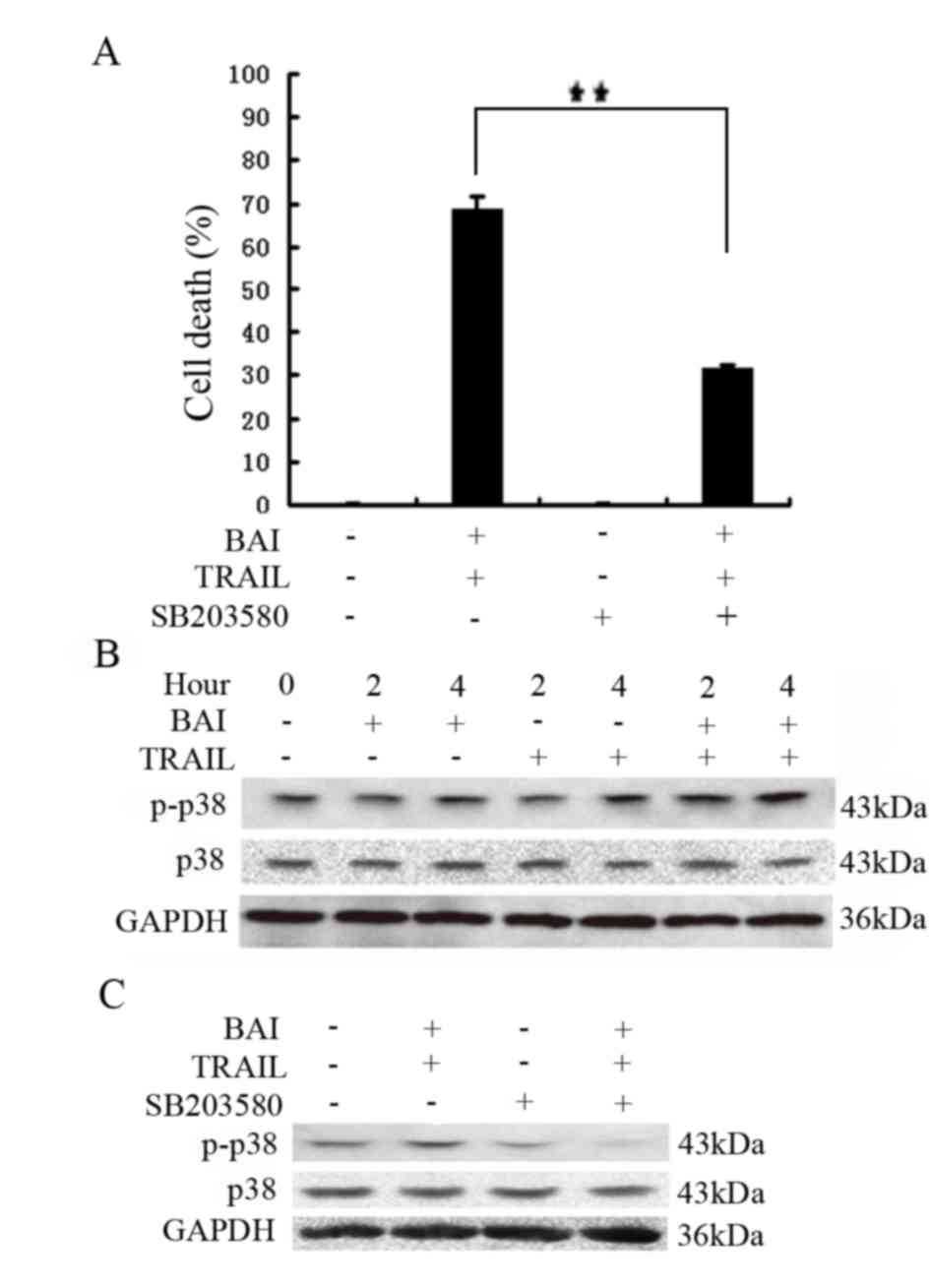
TRAIL. The results of the present study revealed that only the p38
inhibitor SB203580 significantly inhibited the increased
cytotoxicity induced by baicalin and TRAIL co-treatment; therefore,
activation of the p38 pathway may be involved (Fig. 3A and data not shown). Consistently,
whereas baicalin or TRAIL alone weakly activated p38, baicalin and
TRAIL co-treatment markedly activated p38 (Fig. 3B), which could be suppressed by
SB203580 (Fig. 3C). Altogether,
these results suggested that the cytotoxic synergy of baicalin and
TRAIL combination may be mediated by p38.
ROS accumulation contributes to the
synergistic cytotoxicity induced by baicalin and TRAIL
co-treatment
Since flavonoids affect cellular ROS status and
excessive ROS are cytotoxic to cells, the role of ROS in baicalin
and TRAIL-induced synergistic cytotoxicity was examined. ROS
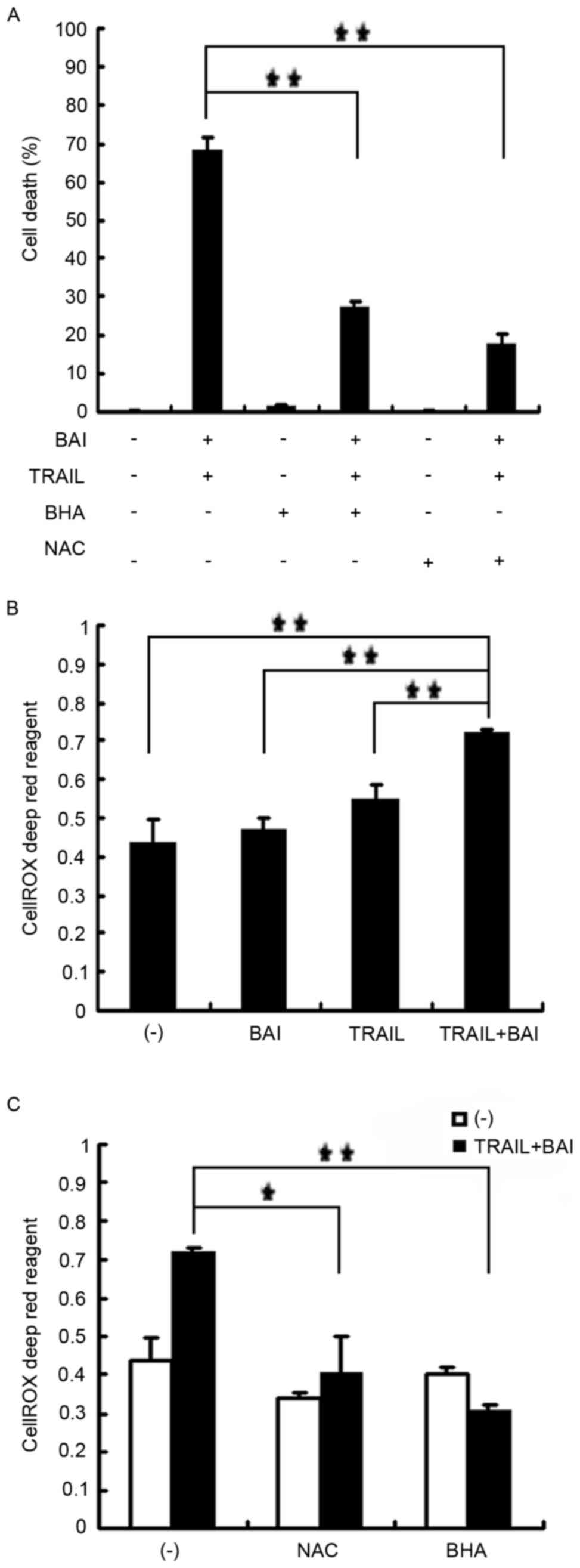
scavengers BHA and NAC were applied to A549 cells as a pretreatment
prior to co-treatment with baicalin and TRAIL. The result
demonstrated that BHA and NAC effectively suppressed the
synergistic cytotoxicity in A549 cells co-treated with baicalin and
TRAIL (Fig. 4A). The combination
of baicalin and TRAIL induced significant ROS accumulation within
the A549 cells, as detected by the CellROX Deep Red reagent
(Fig. 4B); however, BHA and NAC
effectively suppressed ROS accumulation (Fig. 4C). However, the ROS scavengers had
little effect on p38 activation (data not shown), suggesting that
p38 and ROS may be independently involved in cell death caused by
baicalin and TRAIL co-treatment.
Discussion
TRAIL is considered the most promising therapy
within the TNF family for anticancer treatment, as it selectively
induces death within tumor cells but not in normal cells (15). The combination of TRAIL with
conventional chemotherapy drugs or radiotherapy can enhance
cytotoxicity toward cancer cells. Previous studies have
demonstrated that alone baicalin has numerous anticancer activities
(7–9).
In the present study, whether baicalin could
effectively potentiate TRAIL-induced cytotoxicity in cancer cells
was examined. Treating cancer cell lines with baicalin and TRAIL
resulted in synergistic cytotoxicity in a dose-dependent manner.
Furthermore, the synergistic cytotoxicity induced by baicalin and
TRAIL was revealed to be due to increased apoptosis, as determined
by Annexin V staining, detection of PARP cleavage and cell death
inhibition by the pan-caspase inhibitor Z-VAD-FMK.
The involvement of p38 and ROS in baicalin and
TRAIL-induced cell death was subsequently investigated. It is known
that MAPKs, including p38, serve an important role in drug response
during cancer chemotherapy (16–18).
It was demonstrated in the present study that p38 MAPK was
significantly activated in TRAIL and baicalin co-treated cancer
cells; however, the p38 inhibitor SB203580 was able to suppress
increased cell death. ROS are important modulators of cellular
signaling for apoptosis. Baicalin and TRAIL co-treatment
significantly induced ROS accumulation, whereas ROS scavengers BHA
and NAC markedly suppressed the potentiated cytotoxicity caused by
baicalin and TRAIL co-treatment. Consistently, our previous study
demonstrated that wogonin, another important component of S.
baicalensis Georgi, activates ROS to promote TRAIL-induced
apoptosis (13). It is also known
that the activation of p38 is often mediated by ROS (19–22).
However, the results of the present study revealed that baicalin
and TRAIL-induced activation of p38 is independent of ROS.
Therefore, the present study demonstrated that
baicalin could be used as a potential TRAIL sensitizer for cancer
therapy by regulating ROS degradation and the p38 MAPK signaling
pathway. Further studies are required to elucidate the mechanisms
of the ROS and p38 MAPK pathway interaction in baicalin and
TRAIL-induced cell death. The results of the present study add to
the knowledge of the anticancer value of these naturally occurring
compounds and indicate novel therapeutic targets for lung
cancers.
Acknowledgements
The present study was supported by grants from the
Science & Technology Department of Sichuan Province (grant no.
2014JY0035) and the Chengdu University of TCM (grant nos.
ZRYY201209 and ZRYY201310).
References
|
1
|
Emery JG, McDonnell P, Burke MB, Deen KC,
Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, et
al: Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J
Biol Chem. 273:14363–14367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grotzer MA, Eggert A, Zuzak TJ, Janss AJ,
Marwaha S, Wiewrodt BR, Ikegaki N, Brodeur GM and Phillips PC:
Resistance to TRAIL-induced apoptosis in primitive neuroectodermal
brain tumor cells correlates with a loss of capase-8 expression.
Oncogene. 19:4604–4610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Chen W, Zeng W, Bai L, Tesfaigzi
Y, Belinsky SA and Lin Y: Akt-mediated eminent expression of c-FLIP
and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity
to lung cancer cells. Mol Cancer Ther. 7:1156–1163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Li G and Zhang K: Pro-survival
effects by NF-κB, Akt and ERK(1/2) and anti-apoptosis actions by
Six1 disrupt apoptotic functions of TRAIL-Dr4/5 pathway in ovarian
cancer. Biomed Pharmacother. 84:1078–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li K and Sheu S: Determination of
flavonoids and alkaloids in the scute-coptis herb couple by
capillary electrophoresis. Anal Chim Acta. 313:113–120. 1995.
View Article : Google Scholar
|
|
6
|
Srinivas NR: Baicalin, an emerging
multi-therapeutic agent: Pharmacodynamics, pharmacokinetics, and
considerations from drug development perspectives. Xenobiotica.
40:357–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee D, Ko WK, Hwang DS, Heo DN, Lee SJ,
Heo M, Lee KS, Ahn JY, Jo J and Kwon IK: Use of baicalin-conjugated
gold nanoparticles for apoptosis induction of breast cancer cells.
Nanoscale Res Lett. 11:3812016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orzechowska B, Chaber R, Wiśniewska A,
Pajtasz-Piasecka E, Jatczak B, Siemieniec I, Gulanowski B, Chybicka
A and Błach-Olszewska Z: Baicalin from the extract of Scutellaria
baicalensis affects the innate immunity and apoptosis in leukocytes
of children with acute lymphocytic leukemia. Int Immunopharmacol.
23:558–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng
Z and Chen Y: Down-regulation of the PI3K/Akt signaling pathway and
induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin.
J Exp Clin Cancer Res. 31:482012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Ju W, Renouard J, Aden J, Belinsky
SA and Lin Y: 17-allylamino-17-demethoxygeldanamycin
synergistically protentiates tumor necrosis factor-induced lung
cancer cell death by blocking the nuclear factor-kappaB pathway.
Cancer Res. 66:1089–1095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ju W, Wang X, Shi H, Chen W, Belinsky SA
and Lin Y: A critical role of luteolin-induced reactive oxygen
species in blockage of tumor necrosis factor-activated nuclear
factor-kappaB pathway and sensitization of apoptosis in lung cancer
cells. Mol Pharmacol. 71:1381–1388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saidani C, Hammoudi-Triki D,
Laraba-Djebari F and Taub M: In vitro studies with renal proximal
tubule cells show direct cytotoxicity of Androctonus australis
hector scorpion venom triggered by oxidative stress, caspase
activation and apoptosis. Toxicon. 120:29–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Wang Q, Li D, Zhou Y, Zheng X, Yan
J, Zhang L, Lin Y and Wang X: Wogonin enhances antitumor activity
of tumor necrosis factor-related apoptosis-inducing ligand in vivo
through ROS-mediated downregulation of cFLIPL and IAP proteins.
Apoptosis. 18:618–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Refaat A, Abdelhamed S, Saiki I and
Sakurai H: Inhibition of p38 mitogen-activated protein kinase
potentiated the apoptotic effect of berberine/tumor necrosis
factor-related apoptosis-inducing ligand combination therapy. Oncol
Lett. 10:1907–1911. 2015.PubMed/NCBI
|
|
15
|
Walczak H, Miller RE, Arial K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galan-Moya EM, de la Cruz-Morcillo Ma,
Valero M Llanos, Callejas-Valera JL, Melgar-Rojas P, Losa J
Hernadez, Salcedo M, Fernández-Aramburo A, y Cajal S Ramon and
Sánchez-Prieto R: Balance between MKK6 and MKK3 mediates p38 MAPK
associated resistance to cisplatin in NSCLC. PLoS One.
6:e284062011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Y, Peng Z, Shi M, Ji M, Guo H and Shi
H: Metformin combined with p38 MAPK inhibitor improves cisplatin
sensitivity in cisplatin-resistant ovarian cancer. Mol Med Rep.
10:2346–2350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pal HC, Baxter RD, Hunt KM, Agarwal J,
Elmets CA, Athar M and Afaq F: Fisetin, a phytochemical,
potentiates sorafenib-induced apoptosis and abrogates tumor growth
in athymic nude mice implanted with BRAF-mutated melanoma cells.
Oncotarget. 6:28296–28311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pereira L, Igea A, Canovas B, Dolado L and
Nebreda AR: Inhibition of p38 MAPK sensitizes tumour cells to
cisplatin-induced apoptosis mediated by reactive oxygen species and
JNK. EMBO Mol Med. 5:1759–1774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Y, Nikulenkov F, Zawacka-Pankau J, Li
H, Gabdoulline R, Xu J, Eriksson S, Hedström E, Issaeva N, Kel A,
et al: ROS-dependent activation of JNK converts p53 into an
efficient inhibitor of oncogenes leading to robust apoptosis. Cell
Death Differ. 21:612–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou J, Zou P, Lou Y, Xiao Y, Wang J and
Liu L: The cross-talk between ROS and p38MAPKα in the ex vivo
expanded human umbilical cord blood CD133(+) cells. J Huazhong Univ
Sci Technolog Med Sci. 31:591–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Jiang H, Wan Y, Bi C and Yuan Y:
H(2)O(2)-induced secretion of tumor necrosis factor-α evokes
apoptosis of cardiac myocytes through reactive oxygen
species-dependent activation of p38 MAPK. Cytotechnology. 64:65–73.
2012. View Article : Google Scholar : PubMed/NCBI
|