Introduction
In traditional theory, neurons of the central
nervous system (CNS) are considered to be non-renewable and any
injury to these cells is considered irreversible. If these neurons
are damaged, they cannot produce new neurons to repair injury. This
theory results in inhibition and distress in the prevention and
treatment of injury, and degenerative diseases of the central
nervous system (1). The discovery
of neural stem cells (NSCs) contradicted this hypothesis (2,3).
NSCs have the capacity to self-renew, proliferate, migrate and
differentiate, and can differentiate into all cell types contained
within the brain and spinal cord tissue, including neurons,
astrocytes and oligodendrocytes (4). NSCs in the brain can be observed in
two regions: The subventricle zone (SVZ) of lateral ventricles and
subgranular zone of the hippocampal dentate gyrus (5,6).
NSCs are also observed in the cortex (7), hippocampus (8), striatum (9) and other parts of the brain (10–12).
Under normal conditions, NSCs are in a resting state. Injury
stimulates these cells to replicate, proliferate and differentiate
into new neural cells and replace necrotic cells. NSCs are
essential for the formation of new neural circuits, and promote the
repair of the structure and function of the brain following damage
(13,14).
There are two intervention strategies involving NSCs
for the treatment of injury and degenerative diseases of the CNS.
The first type involves the promotion of endogenous NSC
proliferation and differentiation (15,16).
A recent study on this type of treatment has indicated that the
number of endogenous NSCs was not sufficient to repair the lesion
sites (17). The second type of
intervention involves the exogenous transplantation of NSCs to the
lesion sites (18,19). NSCs from the transplantation can
survive, replicate and differentiate into local nerve cells. As a
result, NSCs can repair the sites of injury (20,21).
One of the aims of the future research in the field of structural
repair of brain damage is the identification of drugs and methods
that can stimulate proliferation, directional migration and
differentiation of NSCs, through the precise isolation, culturing
and identification of NSCs.
Renshen (Panax ginseng C. A. Mey) comes from
the roots of Araliaceae Panax, first recorded in the ‘Shen
Nong's Herbal Classic’, one of the important ancient books of
traditional Chinese Medicine (22). Ginsenoside-Rg1 is the main active
component of a plant species Panax notoginseng.
Ginsenoside-Rg1 has a broad range of pharmacological functions.
Ginsenoside-Rg1 has been demonstrated to increase the proliferating
ability of neural progenitor cells, thus promoting the process of
neurogenesis (23,24). Ginsenoside-Rg1 induces a
neuroprotective effect against brain ischemia by inhibiting
Ca2+ influx into primary cultured hippocampal neurons
(25). In addition,
ginsenoside-Rg1 promotes neuroprotective effects following
intracerebral hemorrhage through anti-oxidation (26), reduces nerve cell damage, promotes
protein synthesis in the brain, increases the number of synapses,
improves memory and promotes recovery of brain function following
injury (27–30). The authors previously demonstrated
that ginsenoside-Rg1 can promote expression of proliferating cell
nuclear antigen (PCNA), nestin, bromo-2-deoxyuridine (BrdU)
incorporation, and the survival and self-renewal of cells in the
SVZ of the lateral ventricle in the adult rat brain following
cortical devascularization (31).
In the present study, the cortical NSCs of embryonic rat brain were
isolated, cultured and identified, to study the effect of
ginsenoside-Rg1 on the survival, self-renewal and
glial-like-directional differentiation of NSCs in vitro.
Materials and methods
Materials
A total of four female Sprague Dawley (SD) rats
(weighing 280±10 g) at day 17 of gestation from the Genetic
Institute of the Chinese Academy of Medical Sciences (Beijing,
China; certificate of conformity, SCXK 2011-0004) were used for all
experiments. All procedures in the animal experiments followed the
instructions for the care and use of animals provided by the
Beijing University of Chinese Medicine (Beijing, China). All
procedures in the present study were carried out in accordance with
institutional guidelines and approved by the Ethics Committee of
Beijing University of Chinese Medicine; all surgeries were
performed under anesthesia, and all efforts were made to minimize
suffering. Ginsenoside-Rg1 (purity >99%, purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products, Beijing, China) was used as a stock solution for
subsequent dilutions.
Antibodies: anti-nestin (cat. no. ab11306; Abcam,
Cambridge, CB, UK), anti-BrdU (cat. no. ab8152; Abcam),
anti-vimentin (cat. no. ab8978; Abcam), anti-Cy3-conjugated
Affinipure goat anti-rabbit immunoglobulin (Ig) G (cat. no.
SA00009-2; ProteinTech, Wuhan, Hubei, P.R.C), fluorescein
isothiocyanate (FITC)-conjugated Affinipure goat anti-mouse IgG
(cat. no. SA00003-1; ProteinTech).
Primary culture and passage of
cortical NSCs of embryonic rat brain
Cortical tissue of E17 embryonic rats was harvested
under sterile conditions, the meninges were removed, and the cortex
was washed in D-Hanks' solution (HyClone; GE Healthcare, Logan, UT,
USA) under an anatomical microscope. The cortex was subsequently
cut into 1-mm3 pieces in Dulbecco's modified Eagle's
medium (DMEM)/F12 and then incubated at 37°C for 25 min in a 0.125%
trypsin solution to digest the cortical tissue. The solution was
then neutralized by the addition of 10% fetal bovine serum
(HyClone; GE Healthcare). Single cell suspensions were prepared by
repeated pipetting. Viable cells stained by trypan blue assay for 3
min at 37°C were then counted with light microscope (magnification,
×40) and the cell number was adjusted to 1×106 cells/ml
culture medium. The cell suspensions were placed in
25-cm2 flasks coated with poly-lysine (5 ml/flask) and
placed in a cell incubator at 37°C containing 5%
CO2.
Following the formation of spheres in primary
culture, clones were mechanically isolated and passaged in culture
to form a single cell suspension at a density of 1×105
cells/ml. Thereafter, clones were isolated mechanically and
passaged every 5–7 days, using the above method and medium.
Screening for the optimal dose of
ginsenoside-Rg1 for maximized proliferation of NSCs using the MTS
method
NSCs were collected by centrifugation at 4°C (800 ×
g for 10 min), and single cell suspensions were prepared by
mechanical pipetting and separating. NSC solutions were adjusted to
densities of 1×105 cells/ml and 100 µl/well was seeded
into a 96-well culture plate, using serum-free medium [DMEM/F12,
basic fibroblast growth factor (bFGF); Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA] and B27 (Invitrogen; Thermo
Fisher Scientific, Inc). Groups, including the control group and
ginsenoside-Rg1 groups (concentrations used were as follows: 0.001,
0.004, 0.016, 0.064, 0.32, 0.4, 2, 4, 8 and 16 µg/ml), were
cultured for 3 days and 8 repeats were used for each group. A total
of 3 h prior to the termination of culture experiment, 20 µl
MTS/phenazine methosulfate (PMS) solution was added to each well
and the solutions were cultured at 37°C for another 3 h. The
optical density (OD) value was measured at a wavelength of 570
nm.
Screening for the optimum in vitro
incubation time of NSCs in the ginsenoside-Rg1 solution using the
MTS method
NSCs were collected by centrifugation at 4°C (800 ×
g for 10 min), and single cell suspensions were prepared by
pipetting and separating. Using the optimum dose of ginsenoside-Rg1
identified in the preceding experiment, NSCs were seeded at
1×105 cells/ml serum-free medium (DMEM/F12 and B12) in a
96-well culture plate (100 µl/well). The time points tested
included 1, 3, 6, 12, 24, 48 and 72 h. NSC proliferation was
verified at each time point and compared between the control and
experimental groups. NSCs were cultured for different time points
and 20 µl/well MTS/PMS solution was added 3 h prior to the end of
each experiment. The OD value was measured at a wavelength of 570
nm.
BrdU labeling and immunofluorescence
staining
BrdU was added into cell culture medium at a final
concentration of 6 µg/ml. The cells were cultured for 4 days prior
to the immunofluorescent staining. After 4 days of incubation under
the described conditions, immunofluorescent staining using nestin,
BrdU and vimentin was performed in order to analyze cell
proliferation and differentiation. Antibodies against nestin, BrdU
and vimentin were used to identify NSCs or NSC precursors and
immature glial cells, respectively.
NSCs (1×105/ml, 500 µl) were plated onto
coverslips coated with poly-D-lysine and immunofluorescent labeling
for nestin/BrdU or nestin/vimentin was carried out for 6 h. The
primary antibodies were nestin (1:500), nestin/BrdU (1:500 and
1:400 respectively), and nestin/vimentin (1:500 and 1:400,
respectively), and incubated at 4°C overnight. Secondary antibodies
were Cy3-conjugated affinipure goat anti-rabbit IgG (1:30) and
FITC-conjugated affinipure goat anti-mouse IgG (1:30), and
incubated in dark place at 4°C for 30 min.
Oxygen glucose deprivation (OGD)
Cells were washed twice with D-Hanks' solution
following culture for 9 or 10 days, and then the medium was
replaced with Earle's balanced salt solution (HyClone; GE
Healthcare) with or without glucose. The cells were randomly
divided into the following three groups: The control group
incubated in Earle's solution containing glucose under normoxic
conditions; and the OGD group in which medium was replaced with
glucose-free Earle's solution. These cells were placed in a hypoxic
chamber, and treated with a gas mixture consisting of 7%
CO2 and 93% N2 for 30 min to achieve an
equilibrium, and then cultured in hypoxia for another 6 h. OGD was
terminated by replacing the medium with one containing glucose and
returning the cells back to a normoxic incubator. In the final
group, the cells were treated as the OGD group above and
additionally, ginsenoside-Rg1 was added at a dose of 0.32 µg/ml.
Ginsenoside-Rg1 was maintained following OGD for 6 h at 37°C in an
incubator containing 5% CO2.
Image capture and statistical
analysis
A total of three sections were randomly taken from
each sample and three visual fields were randomly selected from
each section. Area density, optical density and the number of
positive cells were analyzed using Image Pro Proplus (version 6.0;
Media Cybernetics, Inc., Rockville, MD, US). All results are
presented as the mean ± standard deviation. The significance of
variables was determined using one-way analysis of variance
followed by the Bonferonni correction. Statistical analysis was
performed using SPSS software (version 11.5; IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Growth of cortical NSCs of the
embryonic rat brain
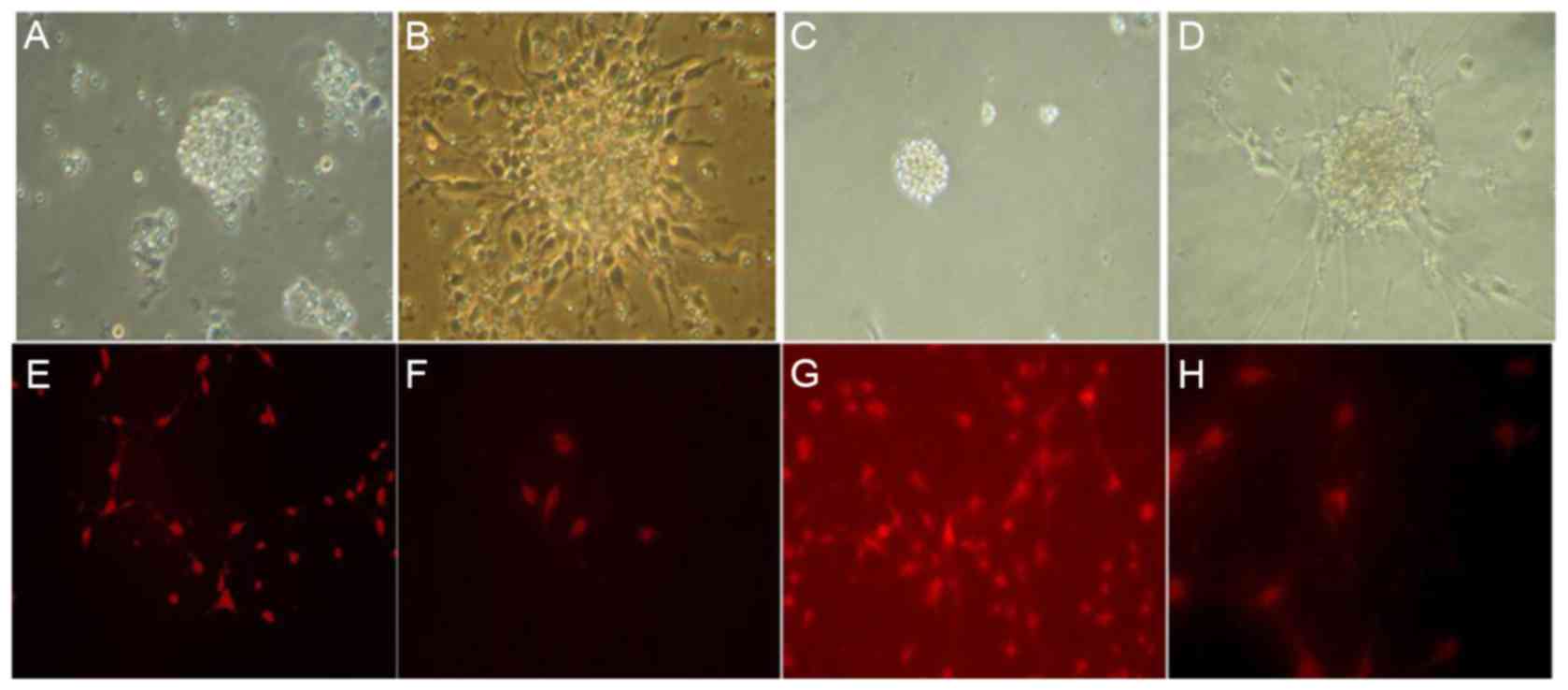
NSCs proliferated to form spheres (primary cloning)
24 h following the primary culture with neural stem cell culture
medium (DMEM/F12, bFGF and B27). An increase in the number of cells
was observed after 7 days. The NSCs remained in a suspension and
exhibited spherical shape and no neurite outgrowth (Fig. 1A and B). A large number of second
generation clones were formed in the passage culture and the
subcloning culture process is similar to the primary culture
(Fig. 1C and D).
Identification of cortical NSCs in the
embryonic rat tissue
Nestin was expressed in the cytoplasm of the
cultured NSC cells. Positive cells exhibited a round or oval shape,
certain cells exhibited symptoms of neuritis. Nuclear areas were
nestin-negative and frequently positioned to one side of the cell
body (Fig. 1E-H).
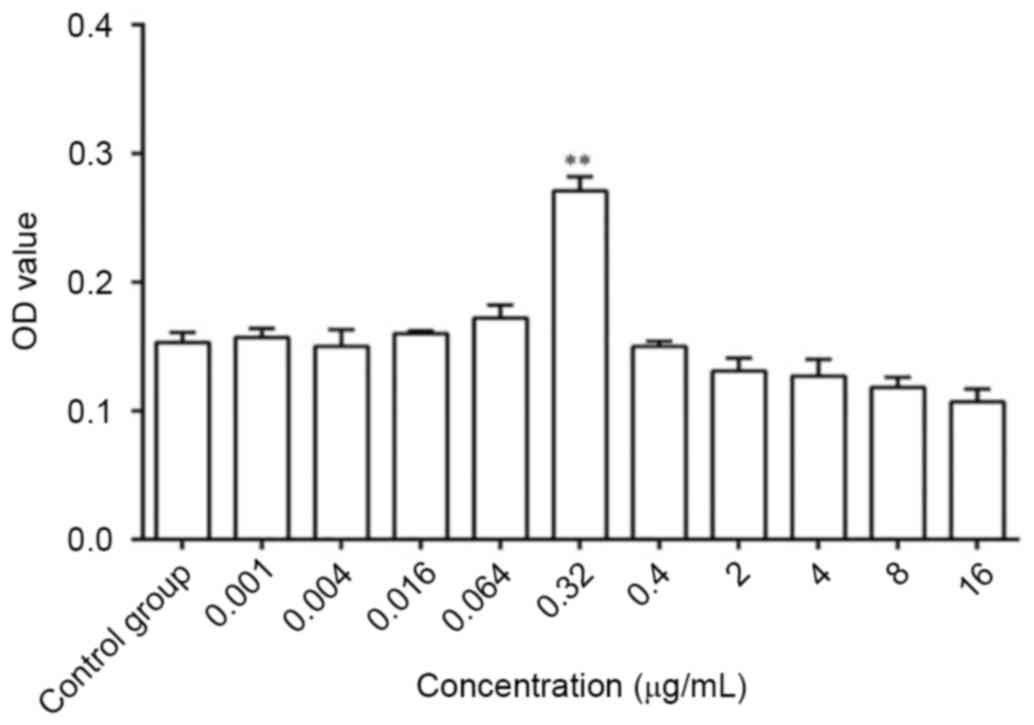
Optimum dose of ginsenoside-Rg1 on the
proliferation of NSCs cultured for 3 days
The OD value increased significantly following
treatment with 0.32 µg/ml ginsenoside-Rg1 measured by MTS compared
with the control group (P<0.01; Fig. 2). Therefore, the dose of 0.32 µg/ml
ginsenoside-Rg1 was the optimum dose for the proliferation of NSCs
cultured for 3 days.
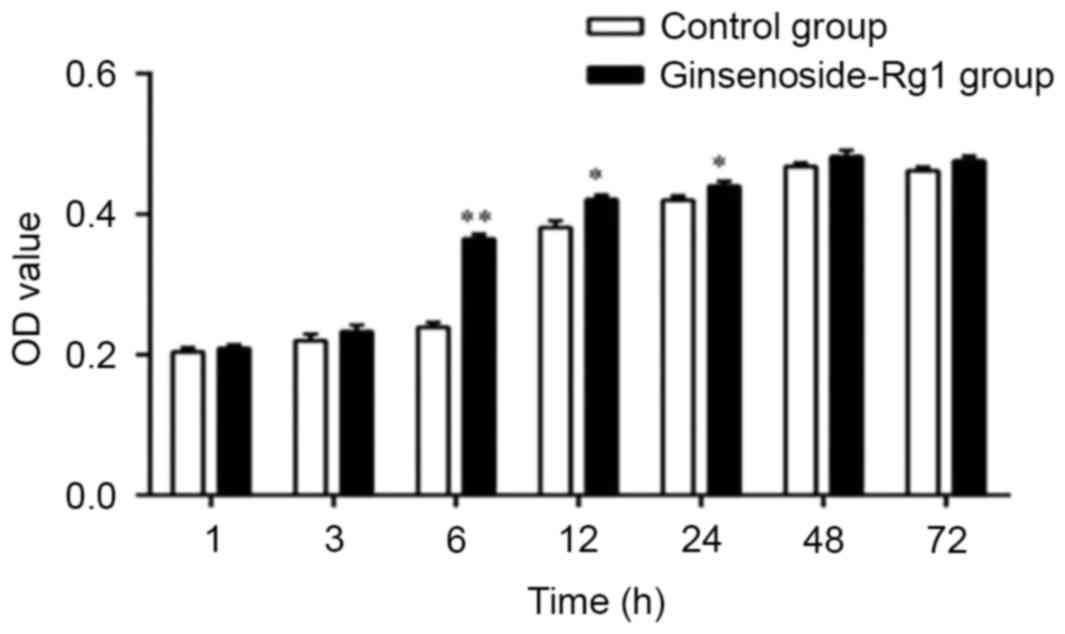
Optimal duration for treatment with
0.32 µg/ml ginsenoside-Rg1 for the proliferation of NSCs
Compared with the control groups, the OD value of
NSCs treated with 0.32 µg ml−1 of ginsenoside-Rg1
increased significantly 6 h post initial treatment (P<0.01;
Fig. 3). Therefore, the optimal
duration of incubation with 0.32 µg/ml ginsenoside-Rg1 for the
proliferation of NSCs was 6 h.
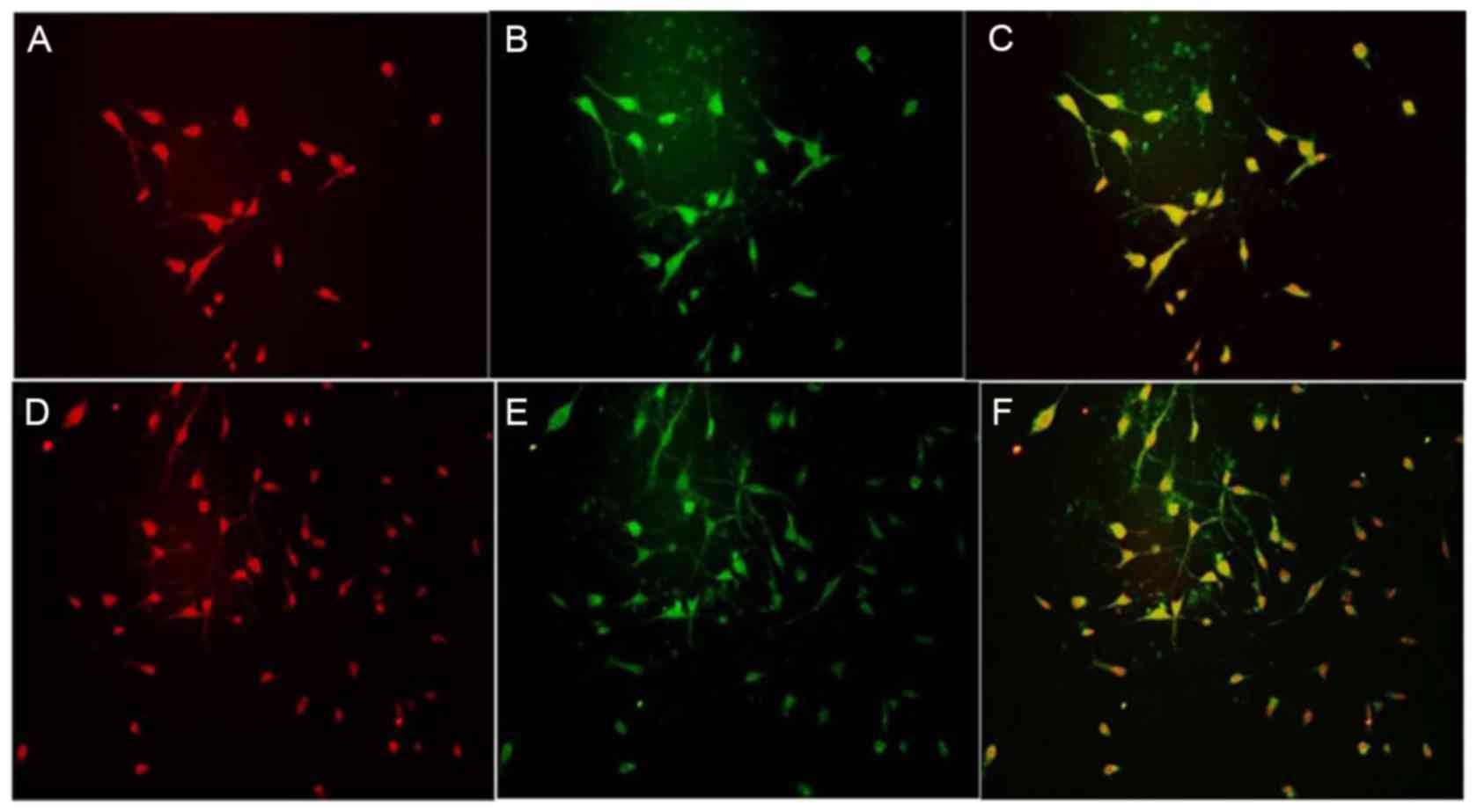
Effect of ginsenoside-Rg1 on the
proliferation of cortical NSCs from embryonic rats
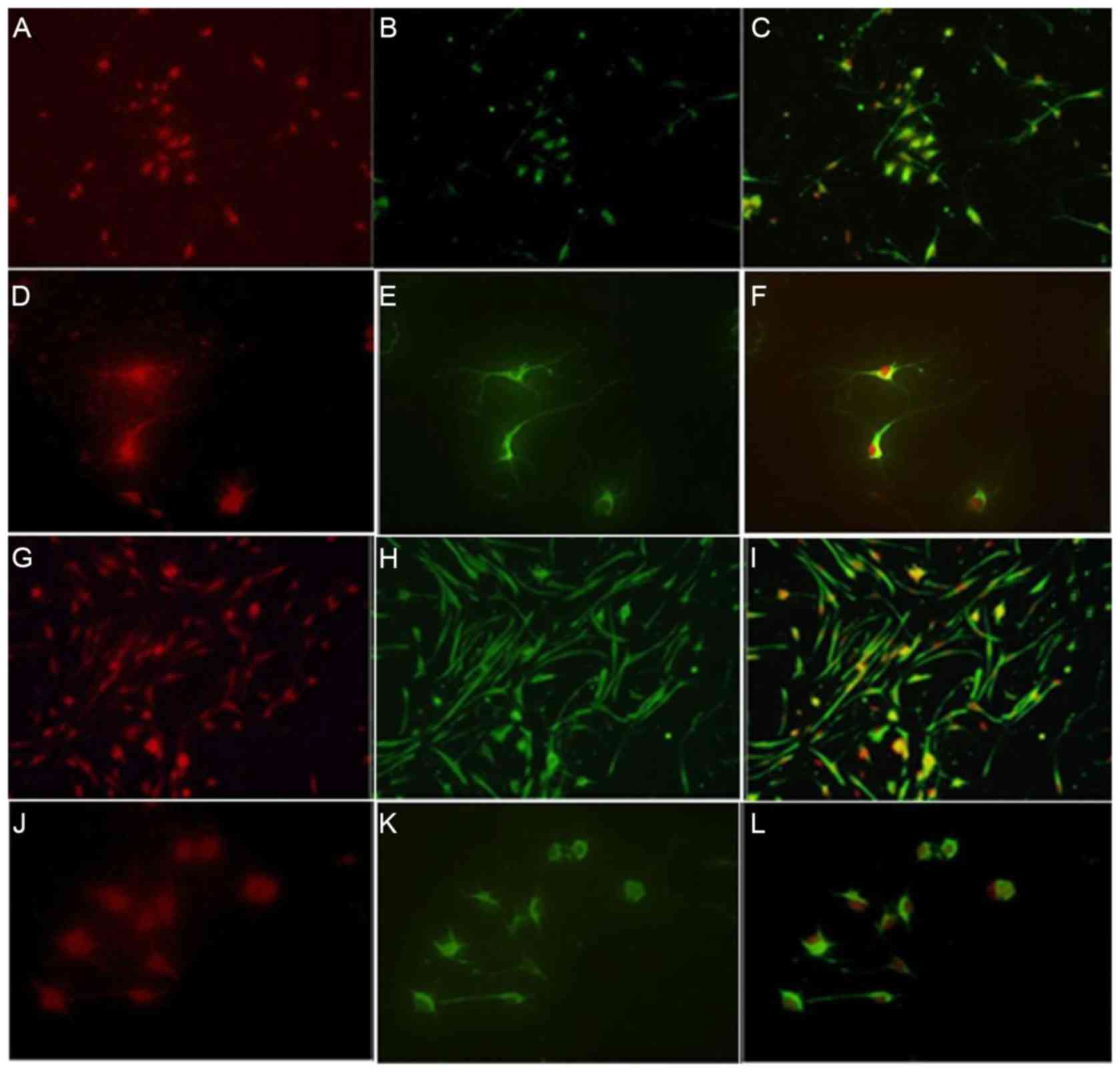
BrdU and nestin were co-expressed in the cortical
NSCs of the control, OGD and ginsenoside-Rg1 groups.
Nestin-positive cells were round or oval shaped and could be
further divided into two kinds of cells: Those with and without
neuritis. Nuclear areas were negative and frequently positioned to
one side of the cell body. BrdU fluorescence in the nucleus of
cultured cells and the cell body of positive cells was low.
Nestin/BrdU co-expressing cells exhibited different sizes and
shapes with extensions (Fig. 4).
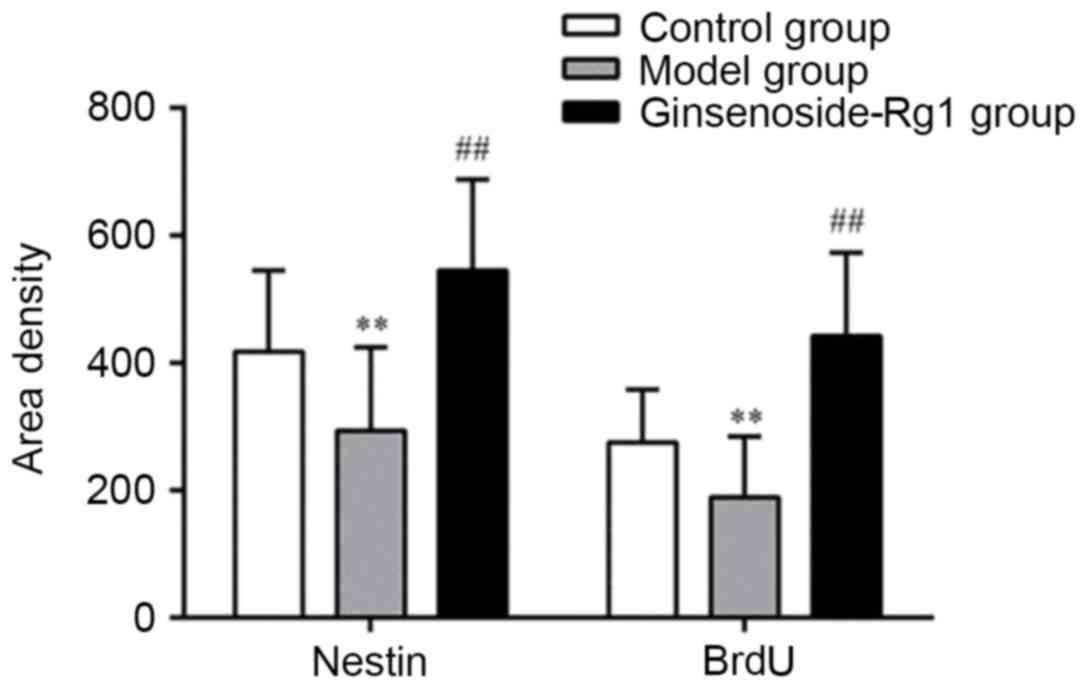
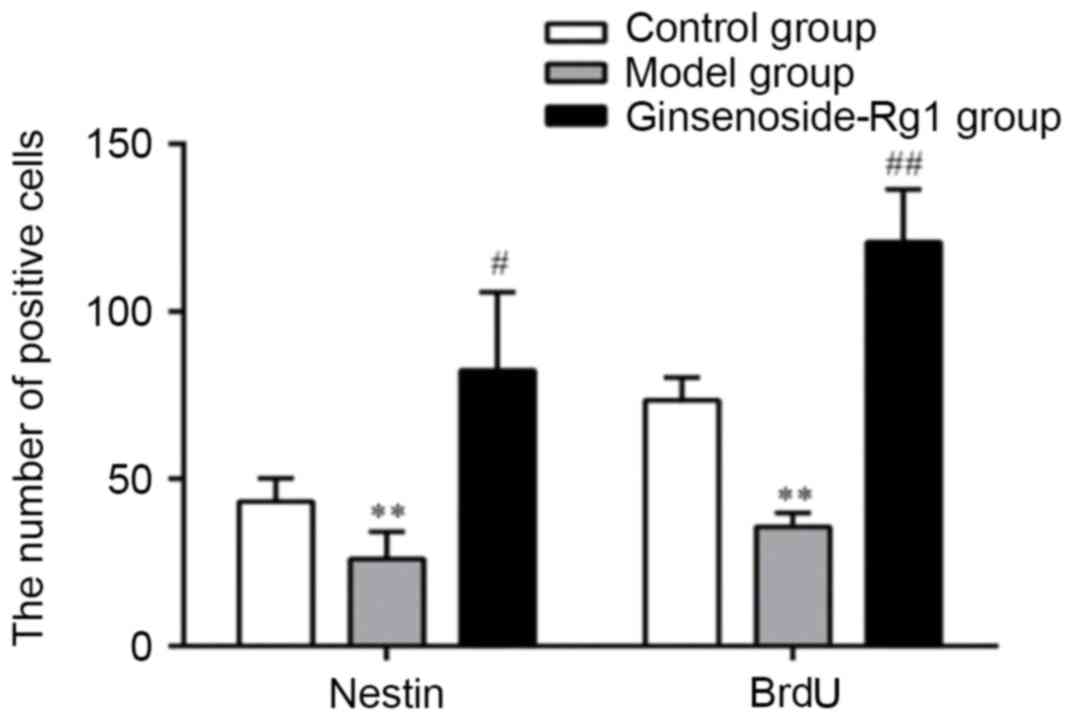
All types of positive cells decreased visibly in number in the OGD
group and increased in the ginsenoside-Rg1 group. Statistical
analysis of the data revealed that, compared with the OGD group,
the area density, optical density, and the number of nestin- or
BrdU- positive cells and nestin/BrdU double-positive cells,
increased significantly in the ginsenoside-Rg1 group (all
P<0.05; Figs. 5–7).
Effect of ginsenoside-Rg1 on
glial-like-directed differentiation of cortical NSCs in embryonic
rats
Vimentin and nestin were expressed and co-expressed
in the control, OGD and treatment groups. Vimentin was expressed in
the cytoplasm of NSCs towards one side of the cell but not in the
nucleus. Cell bodies of vimentin-positive cells in the
ginsenoside-Rg1-treatment group were round or oval-shaped, and
could be further divided into large, medium and small. Cells
exhibited single and multiple processes, which were woven into a
mesh or parallel arrangement. Nuclear areas of cytoplasmic
vimentin-positive cells were negative and certain cells were
binucleated. When nestin and vimentin were co-expressed in cultured
cells, sizes of double-stained cells were different and cell bodies
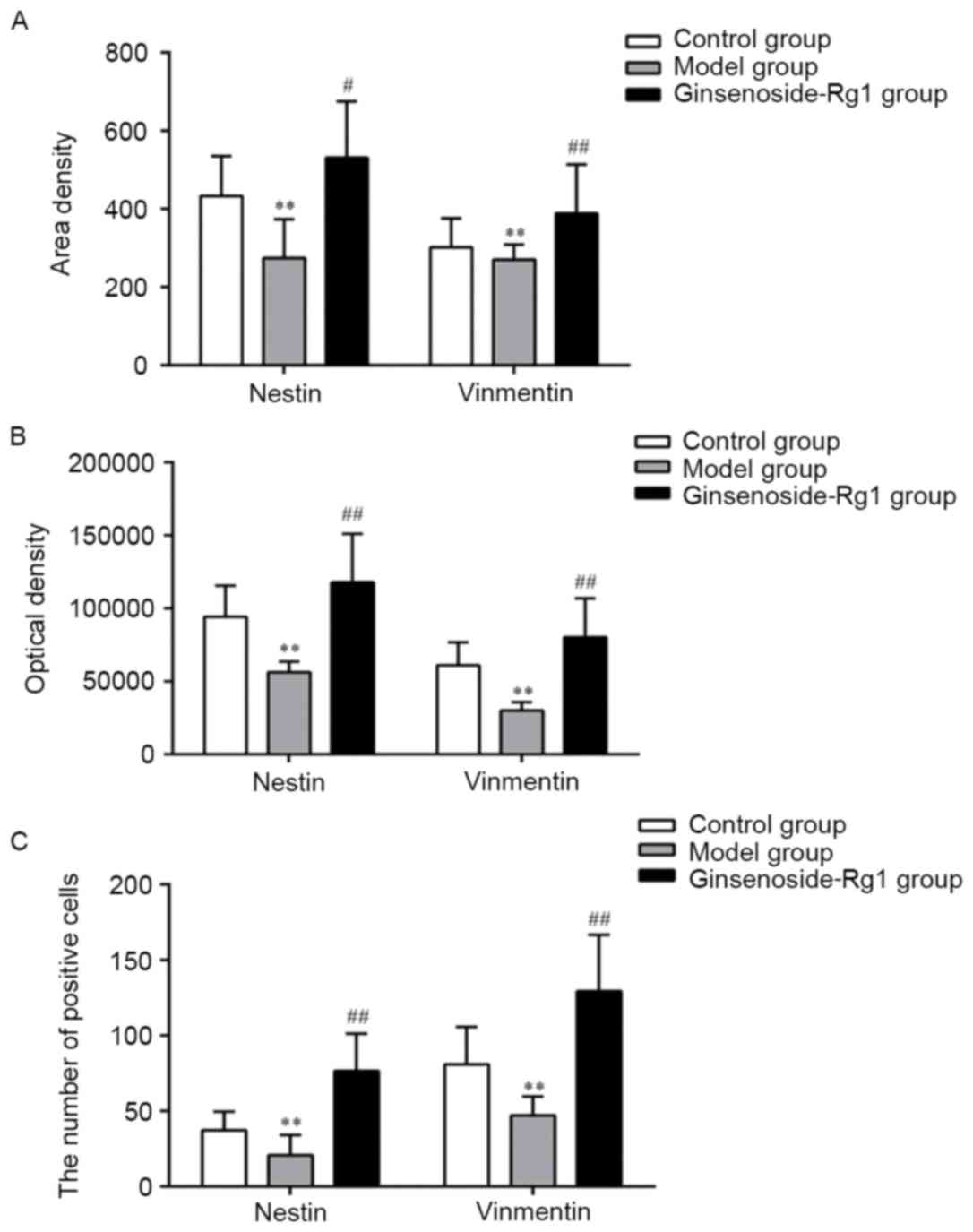
with processes were round or oval (Fig. 8). All positive cells visibly
decreased in number in the OGD group and increased in the
ginsenoside-Rg1 treatment group. Statistical analysis revealed
that, compared with the OGD group, the area density, optical
density, and the number of nestin and vimentin positive cells and
nestin/vimentin double staining cells, increased significantly in
the ginsenoside-Rg1 group (all P<0.05; Fig. 9).
Discussion
In previous studies NSCs have been successfully
isolated from the striatum, hippocampus and cortex of the rat
embryo (7–10). These cells have been demonstrated
to proliferate and differentiate into neurons and glial cells, when
stimulated with neurotrophic factors including the bFGF or
epidermal growth factor (32,33).
NSCs are normally cultured in serum-free culture medium. The main
purpose of using serum-free medium is to remove confounding
factors, including growth factors, which may induce the
differentiation of NSCs. NSC culture medium is sometimes enriched
with growth factor bFGF, as it promotes the survival and
self-renewal of NSCs (34). In the
present study, growth factor bFGF was added to the culture medium
used for the early isolation of NSCs, and the primary and passaged
culture of cortical NSCs; however, a serum-free medium was used
during experiments using ginsenoside-Rg1 and OGD.
Nestin is an intermediate filament protein that is
commonly used as a neural stem/progenitor cell marker and it can be
defined as a class VI intermediate filament protein (35). Nestin expression begins during
neurulation, decreases when the migration of nerve cells is
completed and stops with the completion of the differentiation of
NSCs (36,37). Therefore, nestin is a specific
marker protein for neural stem cells. Embryonic rat cortical cells
that were isolated and cultured in the present study expressed
nestin. Sizes and morphology of nestin-positive cells were
different, certain nuclei were offset, or were in possession of one
or no cellular process, which suggested that NSCs were in an
undifferentiated state. Therefore, the isolated cells in the
present study exhibited the biological properties of NSCs.
Nestin-positive NSCs increased in size and number following
treatment with ginsenoside-Rg1. These results indicated that
ginsenoside-Rg1 had effects on the survival, proliferation and
self-renewal of cortical NSCs of embryonic rats.
Ginsenoside-Rg1 promoted the proliferation of
cortical NSCs of embryonic rats at a concentration of 0.32 µg/ml.
Increasing the concentration of ginsenoside-Rg1 above this
threshold gradually decreased the NSC proliferative effect. The OD
values of cells treated with ginsenoside-Rg1 decreased compared
with the control group when the ginsenoside-Rg1 concentration
reached 2 µg/ml. Therefore, 0.32 µg/ml was the optimum dose of
ginsenoside-Rg1 for the proliferation of cortical NSCs of embryonic
rats. During the screening for the time window of ginsenoside-Rg1
administration at 0.32 µg ml−1 concentration, there was
no difference in OD values between the ginsenoside-Rg1 and control
groups when the chemical was added for 1–3 h. The efficacy of
ginsenoside-Rg1-induced cell proliferation was higher after 6 h of
treatment. Therefore 6 h was the optimum duration of incubation
with ginsenoside-Rg1 at 0.32 µg ml−1 for the
proliferation of NSCs.
BrdU is an analogue of thymidine, which can be
incorporated into the DNA of differentiated cells in the S phase of
the cell cycle (38). BrdU
antibody does not cross-react with thymine, therefore BrdU
incorporation can be observed by immunohistochemical staining in
the cell, so that <1% being synthetic BrdU cells can be reliably
detected (39). BrdU is a specific
marker for proliferation of NSCs. BrdU and nestin/BrdU
double-labeled positive cells increased in number following
treatment with ginsenoside-Rg1. Compared with the control group,
there was a difference in the number, area and optical density of
BrdU and nestin/BrdU double-positive cells in the ginsenoside-Rg1
group. These results indicated that ginsenoside-Rg1 had a
significant, positive effect on the proliferation of NSCs.
NSCs are pluripotent and can differentiate into
neurons and glial cells. Ginsenoside-Rg1 promoted the proliferation
of NSCs and differentiation into vimentin-positive glial cells. The
number of vimentin-positive cells in the ginsenoside-Rg1 group was
increased compared with the control group, and certain cells were
woven together or gathered into a group. The cell sizes also
differed between vimentin-positive cells suggesting that they might
not have been mature NSCs. Nestin/vimentin double staining was
frequently observed. Since vimentin is a marker for naive glial
cells and nestin is a marker of neural stem cells, it was concluded
that ginsenoside-Rg1 promoted the differentiation of NSCs into
glial-like cells.
The proliferation and differentiation of NSCs
requires the presence of neurotrophic factors, and different growth
factors affect NSC differentiation. As secretory cells, adult NSCs
can promote certain growth factors to maintain neurogenesis in a
paracrine and autocrine manner. Ginsenoside-Rg1 served a role in
promoting the proliferation and glial-like-directed differentiation
of cortical NSCs. A plausible explanation for these observations is
that ginsenoside-Rg1 exhibits effects similar to growth factors,
stimulating NSCs in an autocrine or paracrine manner to promote
proliferation and differentiation of NSCs; however, the mechanism
underlying these observations remain to be elucidated.
In conclusion, ginsenoside-Rg1 could maintain the
survival and self-replication of embryonic rat cortical NSCs, and
ginsenoside-Rg1 exhibited significant effects on proliferation and
glial-like-directed differentiation. The mechanism of the
proliferation and differentiation of NSCs remains unknown and can
be studied further in the future.
Acknowledgements
The present study was funded by grants from the
National Natural Science Foundation of China (grant no.
81373830).
References
|
1
|
McKay R: Stem cells in the central nervous
system. Science. 276:66–71. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okano H and Sawamoto K: Neural stem cells:
Involvement in adult neurogenesis and CNS repair. Philos Trans R
Soc Lond B Biol Sci. 363:2111–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergström T and Forsberg-Nilsson K: Neural
stem cells: Brain building blocks and beyond. Ups J Med Sci.
117:132–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ming GL and Song H: Adult neurogenesis in
the mammalian central nervous system. Annu Rev Neurosci.
28:223–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin X, Li L, Zhang X, Yang Y, Chai Y, Han
X and Feng Z: Development of neural stem cells at different sites
of fetus brain of different gestational age. Int J Clin Exp Pathol.
6:2757–2764. 2013.PubMed/NCBI
|
|
8
|
Li G, Fang L, Fernández G and Pleasure SJ:
The ventral hippocampus is the embryonic origin for adult neural
stem cells in the dentate gyrus. Neuron. 78:658–672. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pencea V, Bingaman KD, Wiegand SJ and
Luskin MB: Infusion of brain-derived neurotrophic factor into the
lateral ventricle of the adult rat leads to new neurons in the
parenchyma of the striatum, septum, thalamus, and hypothalamus. J
Neurosci. 21:6706–6717. 2001.PubMed/NCBI
|
|
10
|
Gould E: How widespread is adult
neurogenesis in mammals? Nat Rev Neurosci. 8:481–488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Si YC, Li Q, Xie CE, Niu X, Xia XH and Yu
CY: Chinese herbs and their active ingredients for activating xue
(blood) promote the proliferation and differentiation of neural
stem cells and mesenchymal stem cells. Chin Med. 9:132014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Si YC, Zhang JP, Xie CE, Zhang LJ and
Jiang XN: Effects of Panax noto-ginseng saponins on proliferation
and differentiation of rat hippocampal neural stem cells. Am J Chin
Med. 39:999–1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng Y, Zhang J, Deng L, Johnson NR, Yu
X, Zhang N, Lou T, Zhang Y, Wei X, Chen Z, et al: Intravenously
delivered neural stem cells migrate into ischemic brain,
differentiate and improve functional recovery after transient
ischemic stroke in adult rats. Int J Clin Exp Pathol. 8:2928–2936.
2015.PubMed/NCBI
|
|
14
|
Jie F, Shi-Dou Z, Hui-Juan L, Yuan QH, Liu
SM, Zhang YM, Ling EA and Hao AJ: Melatonin promotes proliferation
and differentiation of neural stem cells subjected to hypoxia in
vitro. J Pineal Res. 51:104–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Feng Z, Zhang X, Gao Z and Cao Y:
Ipsilateral versus bilateral limb-training in promoting the
proliferation and differentiation of endogenous neural stem cells
following cerebral infarction in rats. Neural Regen Res.
7:2698–2704. 2012.PubMed/NCBI
|
|
16
|
Chen L, Qiu R, Li L, He D, Lv H, Wu X and
Gu N: The role of exogenous neural stem cells transplantation in
cerebral ischemic stroke. J Biomed Nanotechnol. 10:3219–3230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Tan B, Wang L, Long Z, Li Y, Liao W
and Wu Y: Endogenous neural stem cells in central canal of adult
rats acquired limited ability to differentiate into neurons
following mild spinal cord injury. Int J Clin Exp Pathol.
8:3835–3842. 2015.PubMed/NCBI
|
|
18
|
Li X, Liu X, Zhang N and Wen X:
Engineering in situ cross-linkable and neurocompatible hydrogels. J
Neurotrauma. 31:1431–1438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou B, Ma J, Guo X, Ju F, Gao J, Wang D,
Liu J, Li X, Zhang S and Ren H: Exogenous neural stem cells
transplantation as a potential therapy for photothrombotic ischemia
stroke in kunming mice model. Mol Neurobiol. 54:1254–1262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishino H and Borlongan CV: Restoration of
function by neural transplantation in the ischemic brain. Prog
Brain Res. 127:461–476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park KI: Transplantation of neural stem
cells: Cellular & gene therapy for hypoxic-ischemic brain
injury. Yonsei Med J. 41:825–835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuang P, Wang G, Yuan Q and Liang H:
Separation and purification of ginsenoside Re from ginseng bud by
selective adsorption of active carbon and preparative
high-performance liquid chromatography. Nat Prod Res. 26:286–290.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen L and Zhang J: NMDA receptor and iNOS
are involved in the effects of ginsenoside Rg1 on hippocampal
neurogenesis in ischemic gerbils. Neurol Res. 29:270–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YF, Fan XJ, Li X, Peng LL, Wang GH,
Ke KF and Jiang ZL: Ginsenoside Rg1 protects neurons from
hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through
NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur J
Pharmacol. 586:90–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Li M, Li Y, Quan Q and Wang J:
Cellular and molecular mechanisms underlying the action of
ginsenoside Rg1 against Alzheimer's disease. Neural Regen Res.
7:2860–2866. 2012.PubMed/NCBI
|
|
27
|
Zhou Y, Jiang R, Yang B, Yao X, Wang P,
Liu D and Wang Y: Changes of telomere and telomerase in effect of
ginsenoside Rg1 to delay hematopoietic stem cell senescence.
Zhongguo Zhong Yao Za Zhi. 36:3172–3175. 2011.(In Chinese).
PubMed/NCBI
|
|
28
|
Wu J, Pan Z, Wang Z, Zhu W, Shen Y, Cui R,
Lin J, Yu H, Wang Q, Qian J, et al: Ginsenoside Rg1 protection
against β-amyloid peptide-induced neuronal apoptosis via estrogen
receptor α and glucocorticoid receptor-dependent anti-protein
nitration pathway. Neuropharmacology. 63:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang B, Xiong Z, Yang J, Wang W, Wang Y,
Hu ZL, Wang F and Chen JG: Antidepressant-like effects of
ginsenoside Rg1 are due to activation of the BDNF signaling pathway
and neurogenesis in the hippocampus. Br J Pharmacol. 166:1872–1887.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang T, Fang F, Chen L, Zhu Y, Zhang J,
Chen X and Yan SS: Ginsenoside Rg1 attenuates oligomeric
Aβ(1–42)-induced mitochondrial dysfunction. Curr Alzheimer Res.
9:388–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Si Y and Zhu P: Experimental
study of ginsenoside Rg1 on the differentiation of rat hippocampus
neural stem cells. Chin J Neuroanat. 25:335–338. 2009.
|
|
32
|
Yang F, Liu Y, Tu J, Wan J, Zhang J, Wu B,
Chen S, Zhou J, Mu Y and Wang L: Activated astrocytes enhance the
dopaminergic differentiation of stem cells and promote brain repair
through bFGF. Nat Commun. 5:56272014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y and Dong MM: Effects of different
combinations of growth factors on the differentiation of neural
stem cells into hair-like cells. Ear Nose Throat J. 94:E23–E28.
2015.PubMed/NCBI
|
|
34
|
Yang ZJ, Wang HY and Wang LM: Serum-free
culture and identification of neural stem cells from hippocampus of
neonatal rats. J Appl Clin Pediatr. 22:1090–1093. 2007.
|
|
35
|
Lendahl U, Zimmerman LB and Mckay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frederiksen K and Mckay RD: Proliferation
and differentiation of rat neuroepithelial precursor cells in vivo.
J Neurosci. 8:1144–1151. 1988.PubMed/NCBI
|
|
37
|
Dahlstrand J, Lardelli M and Lendahl U:
Nestin mRNA expression correlates with the central nervous system
progenitor cell state in many, but not all, regions of developing
central nervous system. Brain Res Dev Brain Res. 84:109–129. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taupin P: BrdU immunohistochemistry for
studying adult neurogenesis: Paradigms, pitfalls, limitations, and
validation. Brain Res Rev. 53:198–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cooper-Kuhn CM and Kuhn HG: Is it all DNA
repair? Methodological considerations for detecting neurogenesis in
the adult brain. Brain Res Dev Brain Res. 134:13–21. 2002.
View Article : Google Scholar : PubMed/NCBI
|