Introduction
Nanotechnology is increasingly used in the
biomedical field, as the materials generated have notable
physicochemical properties on the nanometer scale (1–3).
Advances in nanotechnology, and our understanding of cellular and
molecular biology have provided various biomedical imaging
modalities and nanosized imaging agents (4). The surface of nanomaterials is
important in crossing the cell membrane: Nano-cores and functional
molecules are often incorporated to enhance cellular penetration
(5–7). In order to improve anticancer
therapeutic effects, nanosized particulate systems based on the
merging of nanotechnology with modern biology techniques have been
created (4,8).
Radiotherapy is a major treatment modality for
malignant tumours, particularly in the case of nasopharyngeal
carcinoma, which originates from the epithelium and is one of the
most common malignant tumours in southern China (9). Nasopharyngeal carcinoma is
particularly radiosensitive; therefore radiotherapy has been a
principal front line treatment. However, this approach has certain
limitations; in order to avoid toxicity to healthy tissues, a high
single irradiation dose is fractionated into several lower dose
treatments. Unfortunately, this strategy fails during the later
stages of treatment, as the rate of tumour cell proliferation
increases and outpaces the cytotoxic effects of irradiation
(10). Therefore, an aim of
current investigations in the field is to identify additional
agents, which can act as radiosensitizers.
The advent of targeted cancer therapies has led to
the development of potent tumour-specific agents, which show
reduced toxicity towards normal tissues. Epidermal growth factor
receptor (EGFR) is the member of a family of four ErbB receptor
tyrosine kinases. The activation of EGFR triggers the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway (11). In several human malignancies,
including colorectal cancer and non-small cell lung cancer, the
overexpression of EGFR correlates with tumour cell growth, altered
cell metabolism, and increased proliferation and angiogenesis;
these perturbations lead to disease progression, including local
invasion and metastasis (12). In
previous years, several inhibitors have been developed, which are
designed to treat malignant tumours by disrupting PI3 K/Akt
signalling cascades and preventing the development of metastasis
(13). Different approaches have
been used to target EGFR, including the use of small molecules,
including gefitinib (Iressa) or lapatinib (Tyverb), and humanized
monoclonal antibodies targeting EGFR, including cetuximab (Erbitux;
C225). C225 can exert effective locoregional control of tumour
growth and reduce mortality rates, without increasing the common
toxic effects associated with radiotherapy to the head and neck
(14–16). However, resistance to these drugs,
possibly due to the build-up of hypoxia in solid tumours,
invariably develops. Hypoxic tumours are characterized by more
aggressive and metastatic phenotypes, with lower sensitivity to
treatments, and are associated with a poor prognosis.
In our previous study, silver (Ag)-based
nanoparticles (AgNPs) were found to have potential utility as
radiosensitizers. For example, AgNPs with diameters of 20–50 nm
significantly sensitize glioma cells to irradiation, and this
radiosensitization effect of AgNPs has important implications in
the design of nanotechnology-based radiosensitizers to improve the
outcomes of cancer radiotherapy (17). Therefore, in the present study,
AgNPs coupled to C225 were designed, and their use in radiotherapy
was evaluated. The Ag/C225 conjugates significantly inhibited the
growth of CNEs in a dose- and time-dependent manner. Ag/C225
enhanced the suppression of clonogenic cell growth, which was
induced by X-ray irradiation. The potency of the Ag/C225 and X-ray
combination was attributed, at least in part, to the suppression of
EGFR signalling. Therefore, Ag/C225 offers potential as either a
radiosensitizer or targeted radiotracer in clinical
radiotherapy.
Materials and methods
Preparation of AgNPs and Ag/C225
nanocomposites
The AgNP colloid was prepared by thermal reduction
of 10 mM AgNO3 aqueous solution in the presence of 15 mM
trisodium citrate at 90°C. Subsequent to the solution turning green
within 30 sec; it was rapidly cooled in an ice bath. The citrate
ions act as reductants and stabilizers of Ag particles in solution.
The synthesized sol-1 (20 ml) was reacted with 0.2 ml of 0.1 mM
hexadecyl trimethyl ammonium bromide (CTAB) aqueous solution at
room temperature for 15 min, in order to prevent the excess
aggregation of CTAB-modified Ag colloids. The AgNPs were
self-assembled on poly (diallyldimethylammonium chloride
(PDDA)-modified (1% w/w) glass slides by immersing them in the
solution for 24 h and withdrawing at a speed of 10 mm/min, followed
by extensive rinsing with water along the fixed direction (18). Finally, these films were treated at
100°C for 60 min in a stream of 20% H2/80% N2
in order to partially remove organic agents. The films were then
stored in a vacuum for 15 days.
AgNO3 and PDDA were purchased from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). CTAB (99%)
and mercaptoundecanoic acid were obtained from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). All other reagents were from
Sigma-Aldrich; Merck KGaA and were distributed by Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China). Ultrapure deionized water
(Barnstead Nanopure H2O purification system; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used throughout the
experiments. C225 was purchased from Merck KGaA.
Cell culture
The human nasopharyngeal carcinoma CNE cells,
originally isolated from a 58-year-old patient with nasopharyngeal
carcinoma, were purchased from Procell Life Science and Technology
Co., Ltd. (Wuhan, China; cat. no. CL-0063). The cells were cultured
in a humidified atmosphere at 37°C containing 5% CO2 in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with heat-inactivated foetal bovine serum (15% by
volume, Bovogen Biologicals Pty Ltd., Melbourne, Australia),
penicillin G (50 U/ml) and streptomycin (50 µg/ml). The cell line
was maintained in the exponential growth phase and the medium was
replaced with fresh medium every 2–3 days.
Enzyme-linked immunosorbent assay
(ELISA)
A double-antibody sandwich was used to quantify the
level of C225 in the Ag/C225 nanocomposites. The CNEs were seeded
in a 96-well plate in 300 µl medium at an average density
(1×104/ml) and were grown for 2 days, followed by
washing three times with PBS, drying and fixing in 0.125% pentyl
glycol for 30 min at 4°C. The cells in the wells were blocked with
1% BSA (Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 1 h and
washed twice. C225 and Ag/C225 were serially diluted (final
concentrations: 72.5, 125, 250 and 500 µg/ml), with six replicates
per dilution. Horseradish peroxidase (HRP) goat-anti-mouse IgG
(sc-2005, 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 2 h at 25°C and substrate working solution were then added. The
absorbance was measured at 450 nm using a microplate
spectrophotometer (SpectraMax; Molecular Devices LLC, Sunnyvale,
CA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay of cell proliferation
To examine the cytotoxicity of
Fe3O4/Ag/C225, the cells were seeded in
96-well plates at a density of 2.5×104 cells/well and
allowed to adhere for 24 h at 37°C. The cells were then cultured in
the presence of Fe3O4/Ag/C225 (final
concentrations: 0.083, 0.166, 0.332, 0.664, 1.328, 2.656, 5.313,
10.63, 21.250 and 42.500 µg/ml) for 48 h, following which MTT
(Sigma; Merck KGaA) was added to each well for 4 h at 37°C.
Dimethylsulphoxide (Sigma; Merck KGaA) was then added to each well
to dissolve the dark blue crystal product. The absorbance was
measured at a wavelength of 488 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Irradiation conditions
The cell culture plates and 5-cm thick water tanks
were set below a PRIMUS type Siemens linear accelerator (Siemen AG,
Munich, Germany). The source target distance was 100 cm, with a
10×10 cm portal to 6MV X-ray irradiation (dose rate 200 cGy/min).
According to the experimental requirements of different doses of
irradiation, the irradiated cells were cultured at 37°C and 5%
CO2 in a humidified incubator.
Analysis of apoptosis
The cells (5×105) were harvested, washed
with PBS and resuspended in binding buffer (Nanjing KeyGen Biotech
Co., Ltd.), followed by mixing with Annexin V-FITC and propidium
iodide (Nanjing KeyGen Biotech Co., Ltd). The cells were analysed
using a BD FACSCalibur flow cytometer provided with CellQuest
software (version 5.1; BD Biosciences, Franklin Lakes, NJ, USA).
Every experiment was repeated three times.
Cell cycle analysis
The cells (1×106) were harvested, washed
with PBS and fixed in 70% ethanol. The fixed cells were then washed
with PBS and resuspended in RNaseA (Nanjing KeyGen Biotech Co.,
Ltd.), followed by incubation at 37°C for 30 min. The cells were
stained with PI solution (Nanjing KeyGen Biotech Co., Ltd) and
analysed using a BD FACSCalibur flow cytometer provided with
CellQuest software (BD Biosciences). Every experiment was repeated
three times.
Clonogenic assays
The exponentially growing cells were irradiated
using an X-ray source at doses of 0, 2, 4, 6 or 8 Gy at room
temperature, and then incubated in the presence or absence of
Ag/C225 for 24 h at 37°C. Following incubation, the cells were
washed in PBS and trypsinized. The cells were then seeded into a
24-well plate with 5 ml medium at a density of 200 cells/well.
Colonies were grown for 10 days. The plates were then washed in PBS
and colonies were fixed with 95% ethanol. Staining was performed
with 0.1% crystal violet solution. Colonies of >50 cells were
counted under an inverted microscope for calculating the surviving
fraction. Six parallel samples were scored for each treatment
condition.
Western blot analysis
Proteins were extracted with cell lysis buffer
(Nanjing KeyGen Biotech Co., Ltd) and quantified via A280
absorption using a NanoDrop 2000C (Thermo Fisher Scientific, Inc.).
Equal quantities of proteins (100 µg/lane) were separated on 4–12%
NuPAGE Novex Bis-Tris Mini Gels (Invitrogen; Thermo Fisher
Scientific, Inc.) and the separated proteins were transferred onto
Immobilon-P PVDF membranes (Invitrogen; Thermo Fisher Scientific,
Inc.). The membranes were blotted using primary antibodies directed
against Ku-80 (rabbit polyclonal IgG, ABP51684, 1:500; Abbkine
Scientific Co., Ltd., Wuhan, China), Ku-70 (rabbit polyclonal IgG,
sc-9033; 1:300; Santa Cruz Biotechnology, Inc.), Rad51 (rabbit
polyclonal IgG, sc-8349, 1:400, Santa Cruz Biotechnology, Inc.) and
β-actin (mouse monoclonal IgG, A1978, 1:1000, Sigma-Aldrich; Merck
KGaA). Following incubation with the appropriate anti-rabbit
(sc-2004) or anti-mouse (Sc-2005) HRP-conjugated secondary antibody
(1:10,000; Santa Cruz Biotechnology, Inc.), the immunoreactive
bands were visualized using chemiluminescence reagents and exposed
to X-ray film. The protein expression of β-actin was used as a
normalization control for protein loading.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS version 19 (IBM SPSS, Armonk, NY, USA) statistical software
was used to perform one-way analysis of variance, followed by an
SNK test. P<0.05 was considered to indicate a statistically
difference.
Results
Characteristics of AgNPs and Ag/C225
nanocomposites
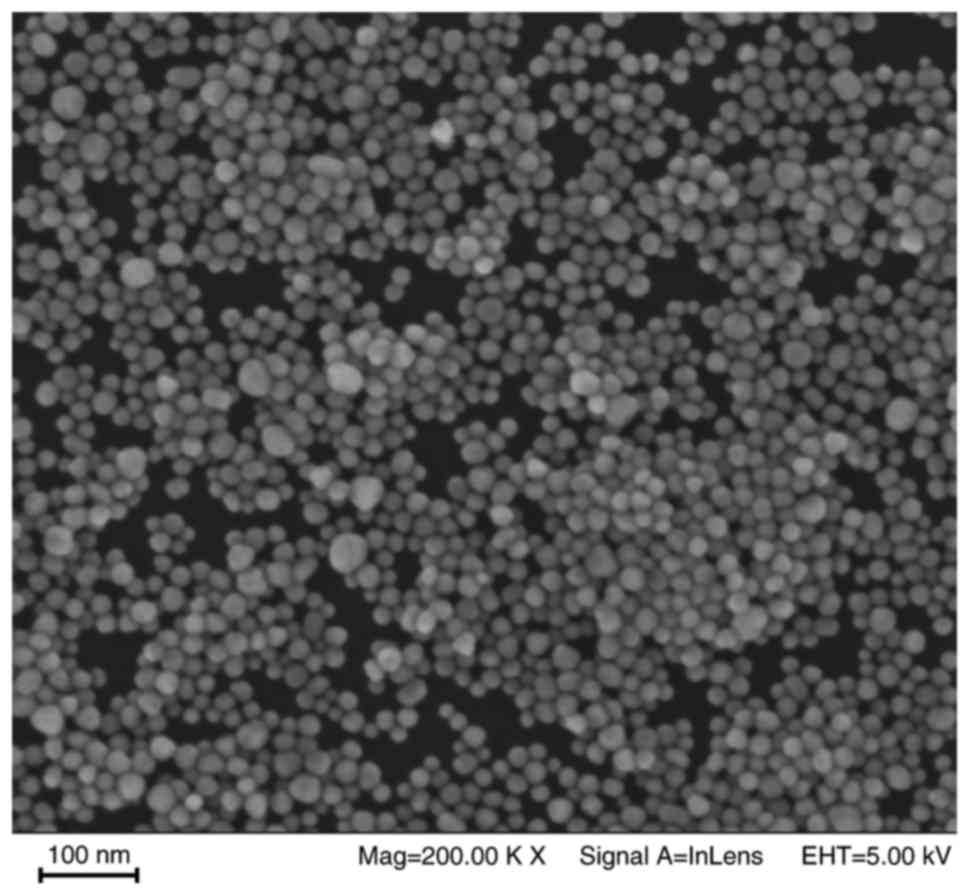
The morphology of the AgNPs, determined using TEM,
is shown in Fig. 1. The majority
of nanoparticles were spherical or almost spherical, with sizes
ranging between 15 and 45 nm. The ultraviolet spectra of the AgNPs
is shown in Fig. 2, indicating
that the absorption peak of Ag at 423 nm gradually increased
following binding to C225, and was red shifted from 21 to 42
nm.
Activity of the anti-EGFR antibody
C225 is preserved in Ag/C225
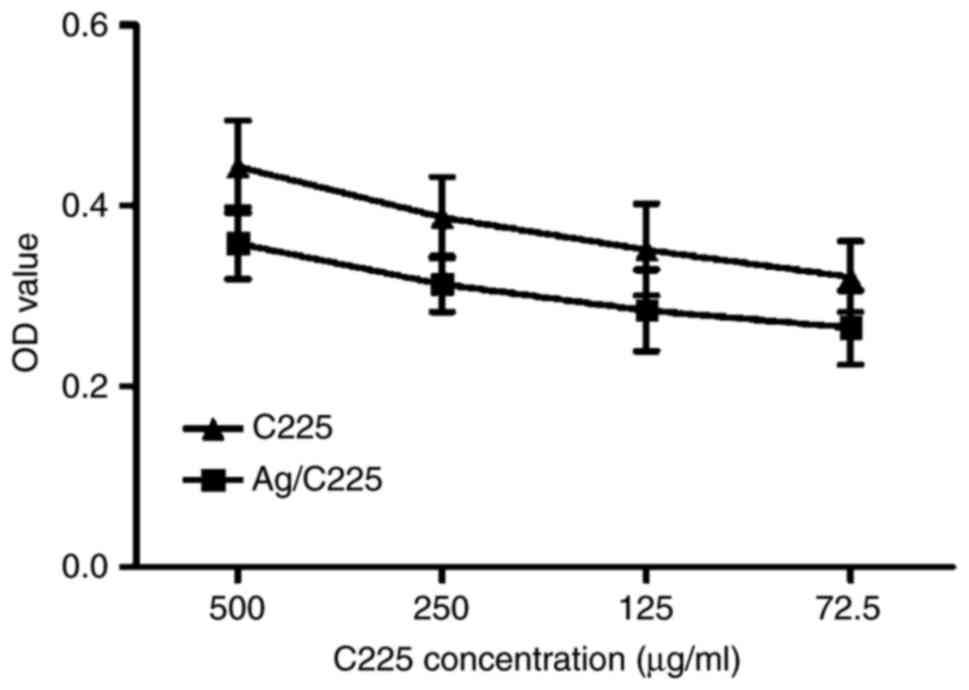
In order to determine whether the anti-EGFR antibody
C225 retained its activity in the Ag/C225 nanocomposites, ELISA was
performed. This revealed that the average preserved activity was
82.17±5.19% [optical density
(OD)g/C225/ODC225]. The level of preserved
activity was highest when the antibody concentration decreased.
Specifically, the activity of Ag/C225 at an absolute C225
concentration of 500 µg/ml was 81.18±4.53%, but was 82.17±5.19%
when the C225 concentration was 72.5 µg/ml. Therefore, the
anti-EGFR antibody activity was retained in the Ag/C225
nanocomposite (Fig. 3).
Ag/C225 causes irreversible growth
inhibition
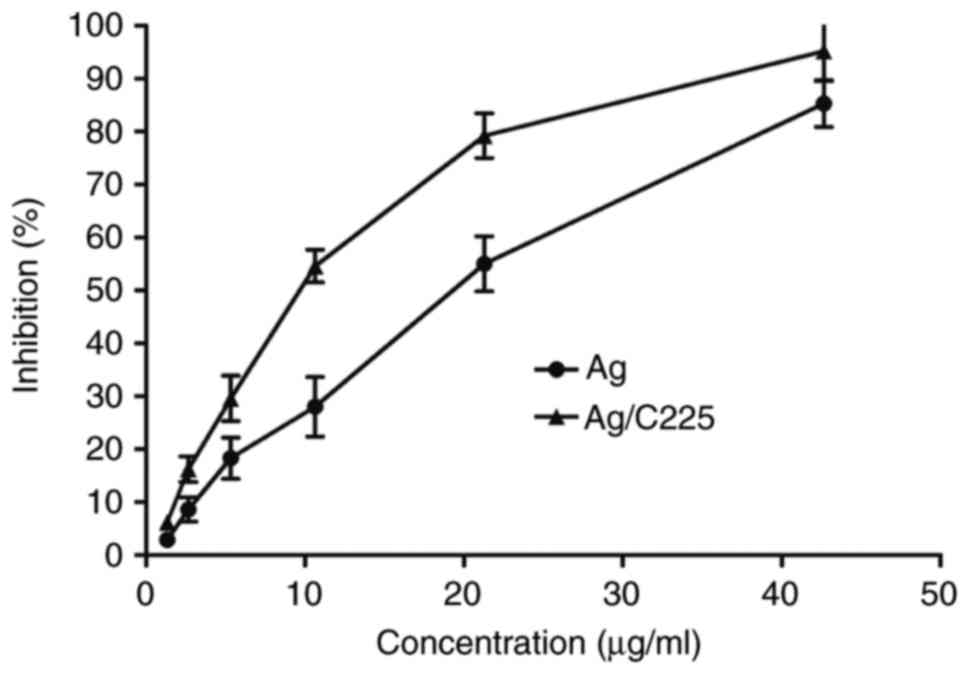
The antiproliferative effect of various
concentrations of Ag/C225 on CNE cells is shown in Fig. 4. The results of the MTT assays
showed that Ag/C225 inhibited cell growth in a
concentration-dependent manner, with half maximal inhibitory
concentration (IC50) values of 12.09±1.14 and 9.09±3.47
µg/ml for Ag and Ag/C225 exposure, respectively (P<0.05).
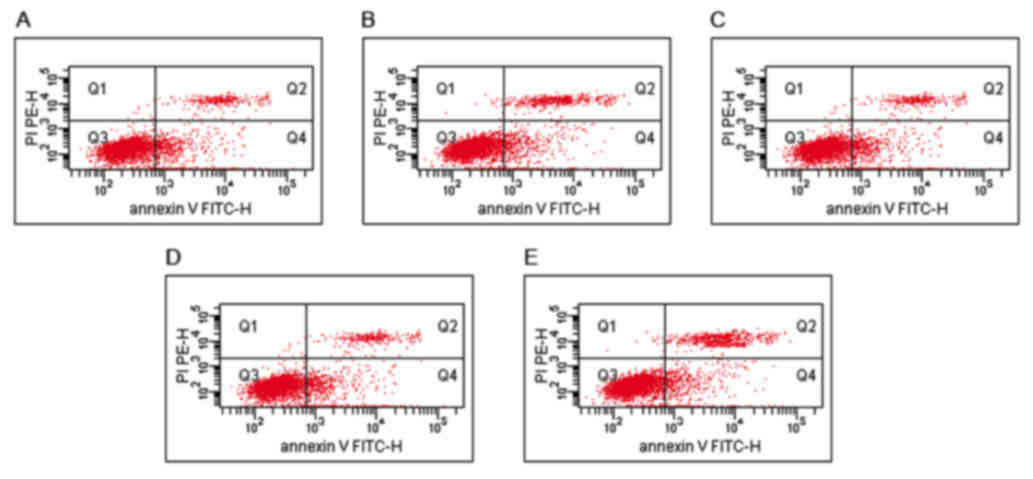
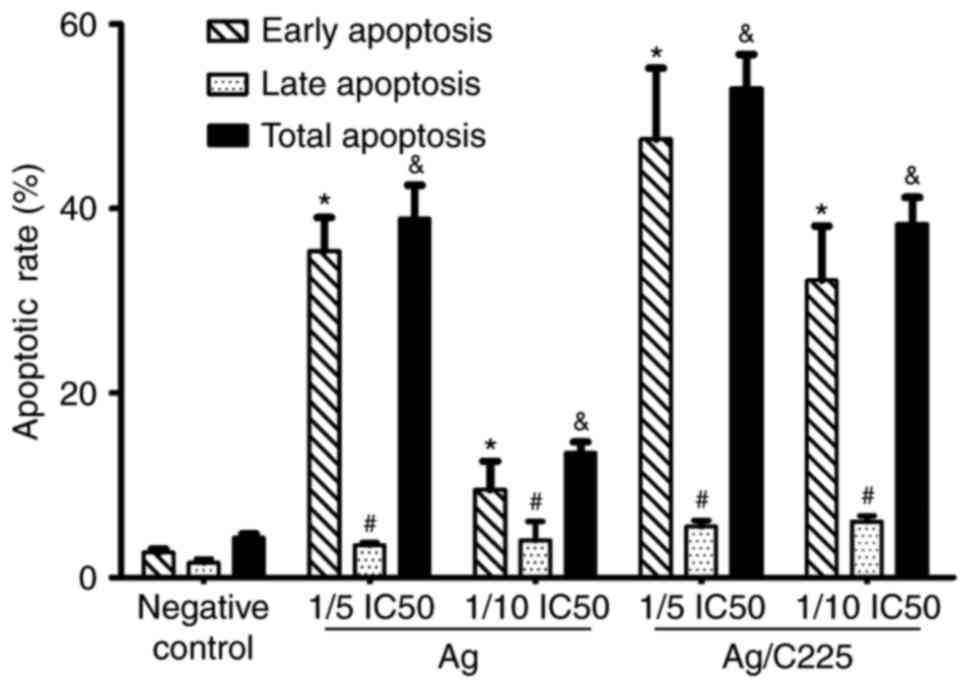
Following irradiation, the Annexin V/PI assay
revealed marked induction of apoptosis following treatment with
1.818 µg/ml (1/5 of the IC50 value) Ag/C225. The
percentage cell death was lower with either Ag/C225 or AgNPs at
1/10 of the IC50. In the control, the fraction of
apoptotic cells was 4.31±0.40%. The apoptotic rates of the Ag (1/10
IC50) and (1/5 IC50) treated-groups were
13.53±1.24 and 38.93±3.62%, respectively, whereas the apoptotic
rates in the Ag/C225 (1/10 IC50) and (1/5
IC50) treated-groups were 38.36±2.91 and 53.03±3.70%,
respectively (Figs. 5 and 6).
Ag/C225 enhances the cytotoxicity
induced by X-ray irradiation
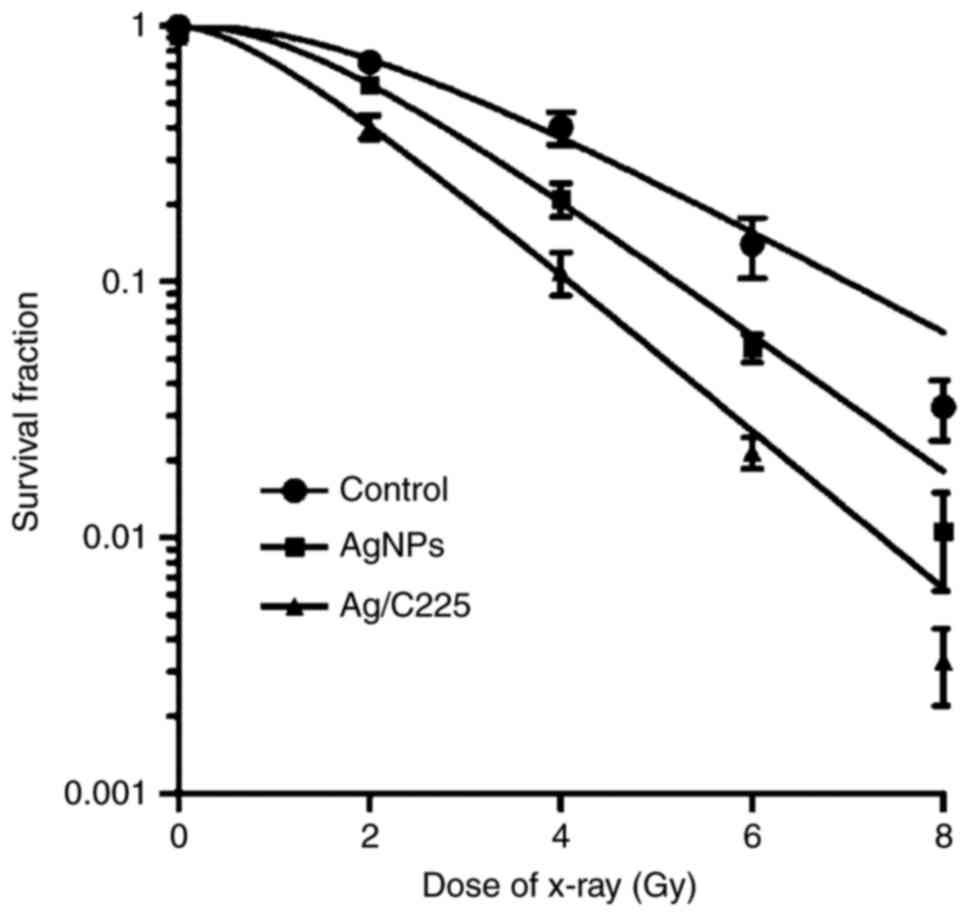
The effects of Ag/C225 on the cytotoxic effect of
X-ray irradiation in CNE cells were investigated by monitoring cell
survival using a clonogenic assay (Fig. 7). The cells were irradiated with
different X-ray doses, and then incubated with 2.418 µg/ml Ag and
1.818 µg/ml Ag/C225 (1/5 the IC50 value) for 24 h. These
concentrations of Ag and Ag/C225 were not cytotoxic alone at the
exposure duration used (data not shown). Cell survival curves were
plotted with a single-hit multitarget model in order to detect
differences between treatment conditions. The parameter
D0 was used to characterize the radiosensitivity in the
linear (high-dose) region, and the value of parameter Dq indicated
the ability of the cells to repair potentially lethal damage in the
shoulder (low-dose) region. In the CNE cells, which were irradiated
but not treated with NPs, the D0 and Dq values were
2.145±0.037 and 3.648±0.121 Gy, respectively. For the cells
irradiated and treated with Ag and Ag/C225, the values were
1.610±0.012 Gy (Ag for D0) and 1.405±0.033 Gy (Ag/C225
for D0) and 2.612±0.014 Gy (Ag for Dq) and 1.234±0.041
Gy (Ag/C225 for Dq), respectively. Therefore, Ag/C225 potentiated
the cytotoxicity of X-rays in the CNE cells, compared with exposure
to Ag alone, by ~1.142-fold (D0Ag
/D0Ag/C225). This effect was observed at 1.818 µg/ml
Ag/C225 (P<0.05). Ag/C225 enhanced the cytotoxic effect of
X-rays on the CNE cells by ~1.527-fold
(D0control/D0Ag/C225).
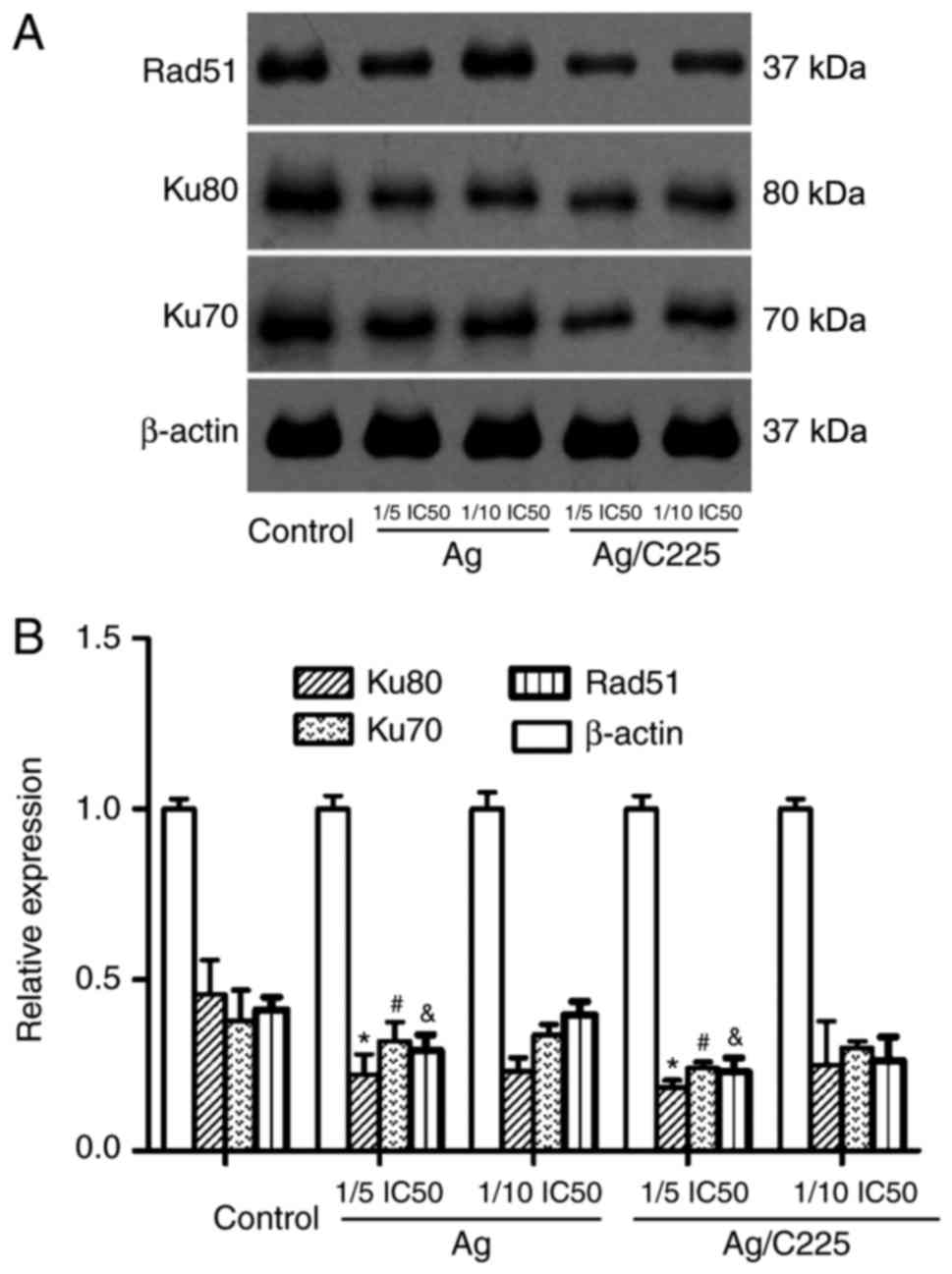
Signalling molecules associated with
mitosis arrest
As shown in Fig. 8,
Ag or Ag/C225 (at 1/5 the IC50 value) downregulated the
expression levels of Rad51, Ku-80 and Ku-70. These changes were
most marked at 4 h post-treatment with Ag or Ag/C225.
Discussion
Radiation therapy is the front line treatment for
~70% of patients with head and neck malignant tumours (18). However, the efficacy of this
treatment modality is compromised due to the need to balance
efficacy with unwanted side effects in normal tissues. This has
prompted investigations to identify radiosensitizing agents,
although clinically effective compounds of this type are difficult
to identify (19). Novel in
vivo tumour imaging agents are also required, as current
radionuclide imaging methods have low sensitivity and spatial
resolution, and are not without safety issues (20,21).
In the present study, a novel multi-functional
composite nanoparticle, Ag/C225, was synthesized, which showed
potential for use as a cancer theranostic agent. Unconjugated AgNPs
can enhance the inhibitory effect of radiation on tumour cells, and
exhibit radiosensitizing effects (22). C225 is an anti-EGFR monoclonal
antibody, which has been used clinically to inhibit tumour cell
growth; it also exhibits radiosensitizing properties. As the
expression of EGFR is high in the majority of solid tumours, the
present study hypothesized that the high-affinity C225 antibody in
the context of an Ag/C225 formulation may be an effective imaging
tracer in vivo and in vitro. The results of the
present study indicated that the activity of C225 was preserved in
Ag/C225 NPs, and that it exerted antiproliferative effects in a
human CNE cell line.
The Ku heterodimer (composed of Ku-70 and Ku-80)
contributes to genomic integrity through its ability to bind DNA
double-strand breaks and facilitate repair through the
non-homologous end-joining pathway (23,24).
In the present study, it was found that Ku-70, Ku-80 and Rad51 were
downregulated by AgNPs, and this was more marked upon Ag/C225
treatment.
Ag/C225 was an effective radiosensitizer, suggesting
that further pre-clinical and clinical trials with this compound
are warranted. Although the molecular mechanism by which Ag/C225
exerts its radiosensitization remains to be elucidated, the present
study observed the downregulation of several DNA damage/repair
proteins. Therefore, it was hypothesized that Ag/C225 compromises
the ability of cells to repair double strand breaks induced by
X-ray irradiation, leading to increased tumour cell death.
In conclusion, the results of the present study
demonstrated that the multifunctional Ag/C225 nanocomposite was a
promising radiosensitizing agent. Future investigations aim to
focus on the molecular mechanisms underlying the effects of this
compound, and on defining additional tumour types that may respond
well to this agent.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81301971).
References
|
1
|
Elnaggar YS: Multifaceted applications of
bile salts in pharmacy: An emphasis on nanomedicine. Int J
Nanomedicine. 10:3955–3971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SS, Harford JB, Pirollo KF and Chang
EH: Effective treatment of glioblastoma requires crossing the
blood-brain barrier and targeting tumors including cancer stem
cells: The promise of nanomedicine. Biochem Biophys Res Commun.
468:485–489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishihara M, Nguyen VQ, Mori Y, Nakamura S
and Hattori H: Adsorption of silver nanoparticles onto different
surface structures of chitin/chitosan and correlations with
antimicrobial activities. Int J Mol Sci. 16:13973–13988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saravanakumar G, Kim K, Park JH, Rhee K
and Kwon IC: Current status of nanoparticle-based imaging agents
for early diagnosis of cancer and atherosclerosis. J Biomed
Nanotechnol. 5:20–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verma A, Uzun O, Hu Y, Hu Y, Han HS,
Watson N, Chen S, Irvine DJ and Stellacci F:
Surface-structure-regulated cell-membrane penetration by
monolayer-protected nanoparticles. Nat Mater. 7:588–595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cedervall T, Lynch I, Lindman S, Berggård
T, Thulin E, Nilsson H, Dawson KA and Linse S: Understanding the
nanoparticle-protein corona using methods to quantify exchange
rates and affinities of proteins for nanoparticles. Proc Natl Acad
Sci USA. 104:pp. 2050–2055. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chithrani BD, Ghazani AA and Chan WC:
Determining the size and shape dependence of gold nanoparticle
uptake into mammalian cells. Nano Lett. 6:662–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buckle T, Chin PT and van Leeuwen FW:
(Non-targeted) radioactive/fluorescent nanoparticles and their
potential in combined pre- and intraoperative imaging during
sentinel lymph node resection. Nanotechnology. 21:4820012010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bailet JW, Mark RJ, Abemayor E, Lee SP,
Tran LM, Juillard G and Ward PH: Nasopharyngeal carcinoma:
Treatment results with primary radiation therapy. Laryngoscope.
102:965–972. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fandi A and Cvitkovic E: Biology and
treatment of nasopharyngeal cancer. Curr Opin Oncol. 7:255–263.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendelsohn J: Targeting the epidermal
growth factor receptor for cancer therapy. J Clin Oncol. 20(18
Suppl): 1S–13S. 2002.PubMed/NCBI
|
|
13
|
Gril B, Palmieri D, Bronder JL, Herring
JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino
MJ, Rubin SD and Steeg PS: Effect of lapatinib on the outgrowth of
metastatic breast cancer cells to the brain. J Natl Cancer Inst.
100:1092–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Unruh A, Ressel A, Mohamed HG, Johnson RS,
Nadrowitz R, Richter E, Katschinski DM and Wenger RH: The
hypoxia-inducible factor-1 alpha is a negative factor for tumor
therapy. Oncogene. 22:3213–3220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le QT, Denko NC and Giaccia AJ: Hypoxic
gene expression and metastasis. Cancer Metastasis Rev. 23:293–310.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu R, Ma J, Sun X, Chen Z, Jiang X, Guo Z,
Huang L, Li Y, Wang M, Wang C, et al: Ag nanoparticles sensitize
IR-induced killing of cancer cells. Cell Res. 19:1031–1034. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Shi J, Tanaka T and Nogami M:
Self-assembled silver nanochains for surface-enhanced Raman
scattering. Langmuir. 23:12042–12047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neklasova NY, Zharinov GM and Grebenyuk
AN: Modification of radiosensitivity in maignant and normal tissues
during radiotherapy of malignant neoplasms. Radiats Biol Radioecol.
54:597–605. 2014.(In Russian). PubMed/NCBI
|
|
21
|
Pelevina II, Aleshchenko AV, Antoshchina
MM, Birjukov VA, Reva EV and Minaeva NG: The radiosensitivity
change after low-dose irradiation, possible mechanisms and
regularities. Radiats Biol Radioecol. 55:57–62. 2015.(In Russian).
PubMed/NCBI
|
|
22
|
Zhao D, Sun X, Tong J, Ma J, Bu X, Xu R
and Fan R: A novel multifunctional nanocomposite C225-conjugated
Fe3O4/Ag enhances the sensitivity of nasopharyngeal carcinoma cells
to radiotherapy. Acta Biochim Biophys Sin (Shanghai). 44:678–684.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evans-Axelsson S, Timmermand O
Vilhelmsson, Welinder C, Borrebaeck CA, Strand SE, Tran TA and
Jansson B: Preclinical evaluation of (111)In-DTPA-INCA-X
anti-Ku70/Ku80 monoclonal antibody in prostate cancer. Am J Nucl
Med Mol Imaging. 4:311–323. 2014.PubMed/NCBI
|
|
24
|
O'Sullivan D, Henry M, Joyce H, Walsh N,
Mc Auley E, Dowling P, Swan N, Moriarty M, Barnham P, Clynes M and
Larkin A: 7B7: A novel antibody directed against the Ku70/Ku80
heterodimer blocks invasion in pancreatic and lung cancer cells.
Tumour Biol. 35:6983–6997. 2014. View Article : Google Scholar : PubMed/NCBI
|