Introduction
Obesity has been identified as a kind of chronic
low-grade inflammatory condition and is closely associated with the
development of insulin resistance, type 2 diabetes, cardiovascular
disease, and cancer (1–3). Adipose tissue, which contains diverse
types of cells including pre-adipocytes, adipocytes, endothelial
cells, and immune cells, has recently been identified as a pivotal
endocrine tissue (4,5). A number of recent studies have shown
that adipocytes synthesize and secrete a large amount of hormones,
and inflammatory cytokines into systemic circulation, including
adiponectin, leptin, tumor necrosis factor-α (TNF-α), monocyte
chemoattractant protein 1 (MCP-1), interleukin-6 (IL-6) and
plasminogen activator inhibitor-1 (6–8). In
the obese state, most free fatty acids (FFAs) are derived from
adipose tissue, which stimulate adipocytes to release
pro-inflammatory cytokines and contribute to development of the
inflammatory state and oxidative stress (9,10).
FFAs mediate these responses in part through activation of the
nuclear factor-κB (NF-κB) pathway, which activate abundant
secretion of inflammatory cytokines and inhibit insulin signaling
(11). In addition, FFAs are
implicated in the activation of oxidative stress partly by
impairment of endogenous antioxidant defenses.
Asian ginseng, the root of Panax ginseng C.A. Meyer
(Araliaceae), is a widely used herbal medicine in East Asia.
Ginsenosides, the major pharmacologically active ingredients of
ginseng, appear to provide an effective therapy for
neurodegenerative diseases (12)
and inhibit inflammation, redox stress (13), and cellular senescence.
Ginsenosides are generally divided into two groups, panaxadiols and
panaxatriols, based on their chemical structure. Panaxadiols
include compounds such as the ginsenoside Rb1, the most abundant
among more than 40 ginsenosides. Rb1 has been extensively studied
and found to have multiple biological functions including
anti-inflammation, anti-apoptosis, anti-oxidation, increasing
nitric oxide production in endothelial cells, and inhibiting
angiogenesis.
Recent studies have found that Rb1 improves insulin
sensitivity in obese and diabetic db/db mice by reducing hepatic
fat accumulation and suppressing adipocyte lipolysis via
up-regulation of perilipin expression in adipocytes (14). Another important finding is that
Rb1 has anti-obesity and anti-hyperglycemic effects in diet-induced
obese rats (15). Our recent study
demonstrated that Rb1 pretreatment prevents human umbilical vein
endothelial cell (HUVEC) senescence through modulation of the redox
status and protects HUVECs from hydrogen peroxide
(H2O2)-induced senescence through stimulation
of the Sirtuin 1 pathway (16).
However, limited data have been reported concerning the effect of
Rb1 on FFA-induced inflammation in adipocytes. In this study, we
investigated whether Rb1 inhibits inflammatory responses induced by
FFAs in 3T3-L1 adipocytes and the underlying mechanism.
Materials and methods
Cell culture and treatments
Mouse embryonic 3T3-L1 pre-adipocytes were purchased
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in high-glucose Dulbecco's modified Eagle's medium
(DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% bovine
calf serum (Hyclone, Logan, UT, USA) at 37°C in a 5% CO2
incubator until confluency and then induced to differentiate as
described previously (17,18). Briefly, at 2 days post-confluency
(defined as day 0), the cells were exposed to differentiation
medium containing 0.5 mM isobutylmethylxanthine, 1 M dexamethasone,
10 µg/ml insulin (Sigma, St. Louis, MO, USA), and 10% fetal bovine
serum (FBS) for 3 days. Then, the cells were transferred to DMEM
with 10 µg/ml insulin and 10% FBS. The medium was changed every two
days. Maturation of adipocytes was confirmed by Oil red staining.
Differentiated adipocytes were serum starved for 16 h in DMEM
supplemented with 2% FBS before treatment and then exposed to FFAs
for 4 h. For Rb1 treatments, 3T3-L1 adipocytes were treated with
various concentrations of Rb1 (10–40 µmol/l) for 4 h, followed by
treatment with 0.5 mM FFAs for 4 h. At the end of experiments, the
culture supernatants and monolayered cells were harvested for
analysis.
Preparation of fatty acid-albumin
complexes
Saturated palmitic acid was used in this study as
FFA. Lipid-containing media were prepared by conjugation of
palmitic acids with bovine serum albumin (BSA) using a modified
method described by Svedberg et al (19). Briefly, palmitic acids were first
dissolved in ethanol at 200 mmol/l and then combined with 10%
FFA-free low endotoxin BSA to concentrations of 1–10 mmol/l. The pH
of all solutions was adjusted to 7.5, and the stock solutions were
filter sterilized and stored at −20°C. A control solution
containing ethanol and BSA was prepared similarly. Fresh working
solutions were prepared by diluting stock solutions (1:10) in 2%
FBS/endothelial cell basic medium (EBM) or 0.5% FCS/EBM as
appropriate. The final 1% BSA was consistent in all FFA media,
while the FFA-to-BSA ratio varied with the FFA concentration.
Cell viability assay
MTT assay was performed to test viability of 3T3-L1
adipocytes. 3T3-L1 preadipocytes were seeded at a density of
1×104 cells per well in a gelatin-coated 96-well plate.
After differentiated to mature adipocytes, the cells were then
treated with various doses of palmitate (0, 0.125, 0.25, 0.5, 1.0
mM) for 4 h or 24 h. The cells were used for determination of
viability, using the MTT Cell Proliferation Assay kit (Beyotime).
Briefly, at indicated time point, the cells were incubated with an
MTT solution for 4 h at 37°C in the dark. After supernatants were
aspirated, DMSO was added and the plates were agitated to dissolve
the formazan crystal product. Absorbance was then measured at 570
nm in a Victor microplate reader. The percentage of viable cells
was calculated by defining the cell viability of control group as
100%.
Measurement of MCP-1 and IL-6
secretion by enzyme-linked immunosorbent assays (ELISAs)
Culture supernatants were diluted 2-fold to
determine MCP-1 and IL-6 levels. ELISAs were performed according to
the manufacturer's instructions (R&D Systems,
Wiesbaden-Nordenstadt, Germany). Briefly, the culture supernatant
was collected after treatment and centrifuged to remove any debris.
Assay Diluent (50 µl) were add to each well, and then standards or
sample (50 µl/well) were added to the antibody pre-coated
microtiter plates, followed by incubation for 2 h at room
temperature. Then, 100 µl MCP-1/IL-6 conjugate were added to each
well, followed by incubation for 2 h at room temperature. After
four washes, 100 µl Substrate Solution were added to each well,
followed by incubation for 30 min while protected from light at
room temperature. Stop Solution (100 µl) were then added, followed
by incubation for less than 30 min. The plate was read immediately
at 450 nm with 540 or 570 nm as reference wavelengths in a Victor
microplate reader. MCP-1 and IL-6 concentrations were calculated
according to the standard curve and normalized to the cell
numbers.
Western blot analysis
3T3-L1 preadipocytes were grown and differentiated
into adipocytes in six-well plates. After serum starvation in 2%
FBS/DMEM overnight, the cells were incubated in 2% FBS/DMEM
containing 10, 20, or 40 mmol/l Rb1 for 4 h, Then, 0.5 mM palmitic
acid was added to treated groups for 4 h. Cells were washed twice
with precooled PBS and then lysed in RIPA buffer with a protease
inhibitor cocktail, PMSF, and sodium orthovanadate (Santa Cruz
Biotechnology, Santa Cruz, CA). The protein concentration was
measured by the Bradford method. Thirty micrograms of protein in 30
µl reducing sample buffer was boiled for 5 min at 100°C and then
resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis for 2 h at 100 V. Then, the proteins were
transferred onto a polyvinylidene difluoride membrane for 90 min at
100 V. After transfer, the membrane was incubated in 25 ml blocking
buffer (1xTBS, 0.1% Tween-20 with 5% non-fat dry milk) for 1 h at
room temperature. Then, the membrane was incubated with primary
antibodies against TNF-α, endothelial nitric oxide synthase (eNOS),
superoxide dismutase 2 (SOD2), phospho-NF-κB (Ser536), NF-κB, or
β-actin (Cell Signaling Technologies, Danvers, MA, USA) in 10 ml
primary antibody dilution buffer with gentle agitation overnight at
4°C. After washing three times for 10 min each with 15 ml 10X
TBS/0.1% TBS/T, the membrane was incubated with a horseradish
peroxidase-conjugated secondary antibody (1:3,000; Cell Signaling
Technologies, Danvers, MA) in 10 ml blocking buffer with gentle
agitation for 1 h at room temperature, followed by three washes for
10 min each.
Nitric oxide (NO) production
measurement
NO production was evaluated by measuring the
accumulation of nitrites, a stable oxidative end product of NO
metabolism, in the cell lysate of cultured 3T3-L1 adipocytes using
the Greiss reagent kit (Beyotime) following the manufacturer's
instructions. Briefly, 50 µl samples were incubated with 50 µl
Greiss reagent I and 50 µl Greiss reagent II in a 96-well
microplate at room temperature for 30 min. The optical density was
measured with the Victor microplate reader at 540 nm. Nitrite
concentrations in the cell lysates were calculated according to the
standard curve.
Determination of reactive oxygen
species (ROS)
Differentiated 3T3-L1 adipocytes were exposed to 0.5
mM BSA or FFA with or without Rb1 for 4 h after serum starvation
for 16 h. The generation of intracellular ROS was detected by the
DCF method using a ROS assay kit (Beyotime). Briefly, treated cells
were washed in PBS and then incubated with 10 µM 2′,
7′-dichlorodihydrofluorescein diacetate in PBS at 37°C for 20 min.
Fluorescence was measured with excitation/emission wavelengths of
493/538 nm using a fluorescence microscope (DM4000B, Leica, Solms,
Germany).
Statistical analysis
Data were calculated and expressed as group means ±
standard deviation. Statistical analyses were performed using the
Student's t-test, analysis of variance (ANOVA), and Bonferroni's
multiple comparison test. Statistical differences were considered
significant at P<0.05.
Results
FFA induces IL-6 and MCP-1 secretion
as well as TNF-α expression in 3T3-L1 adipocytes, and Rb1 inhibits
these effects
FFAs are a major inducer of the pro-inflammatory
response in 3T3-L1 adipocytes and play a central role in obesity.
However, the effect of Rb1 on FFA-induced MCP-1 and IL-6 secretion,
as well as TNF-α expression is unknown. In the present study, we
determined MCP-1 and IL-6 secretion induced by FFAs using ELISAs
and detected the TNF-α protein abundance with an anti-TNF-α
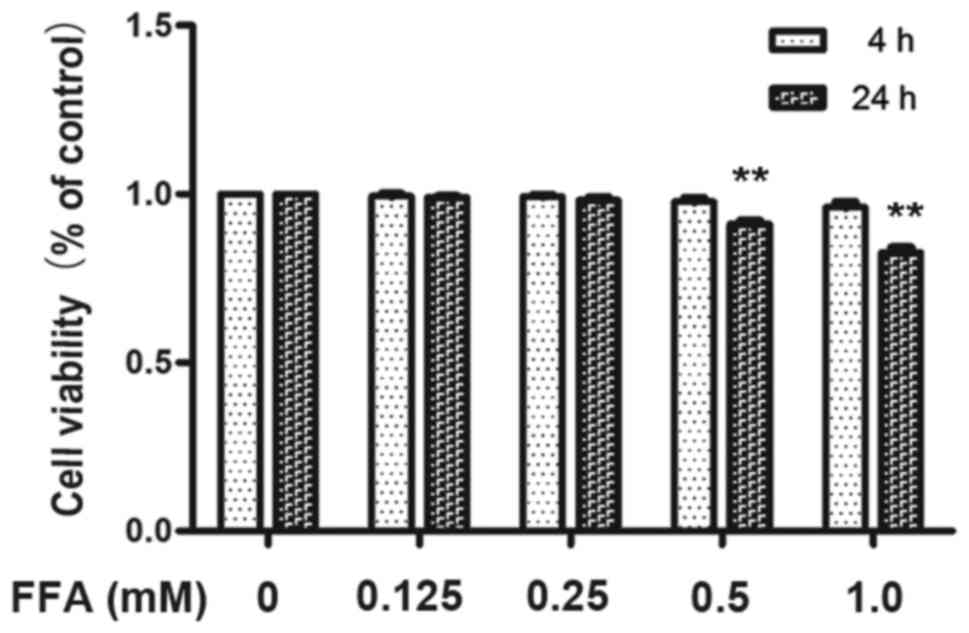
antibody by immunoblotting in 3T3-L1 adipocytes. Preliminary
experiments using the MTT assay confirmed that incubating 3T3-L1
adipocytes with 1 mM FFAs for 4 h as well as 24 h resulted in
excessive toxicity whereas 0.5 mM FFA for 4 h did not affect 3T3-L1
adipocytes viability (Fig. 1).
Therefore, the maximum concentration of FFA used in all subsequent
experiments was 0.5 mM for 4 h. Differentiated 3T3-L1 adipocytes
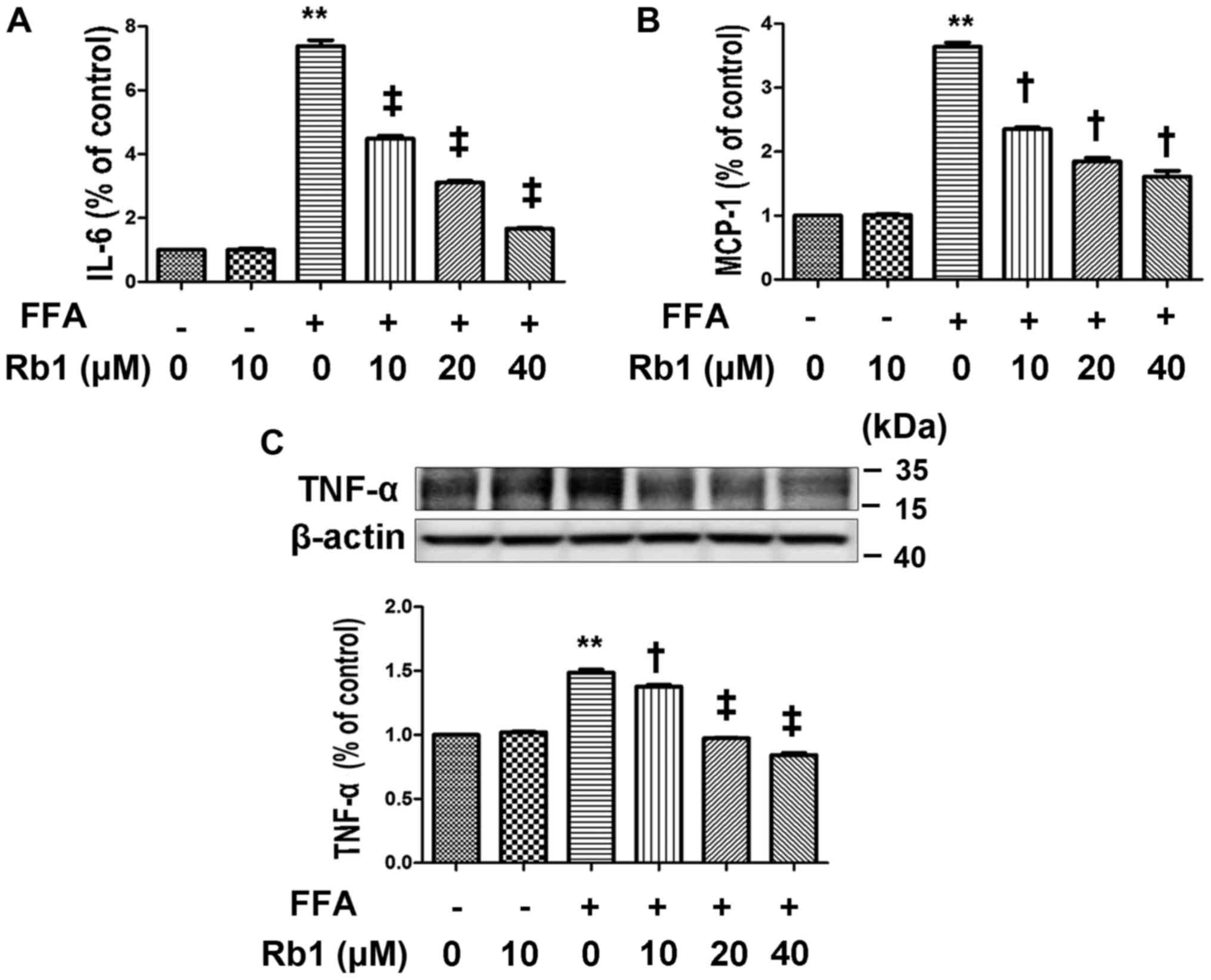
were exposed to 0.5 mM BSA or 0.5 mM FFAs for 4 h. The results
showed that no significant difference was observed between cells
exposed to BSA and the normal control, whereas IL-6 (Fig. 2A) and MCP-1 (Fig. 2B) in the medium and TNF-α protein
expression (Fig. 2C) exposed to
FFAs were significantly elevated compared with those exposed to BSA
or the normal control (Fig. 2,
P<0.01, ANOVA). To determine the effect of Rb1 on FFA-induced
MCP-1 and IL-6 production and the TNF-α protein level in 3T3-L1
adipocytes, we exposed cultured 3T3-L1 adipocytes to 0.5 mM FFAs
with or without Rb1 at 10, 20, and 40 µM for 4 h. The results
showed that Rb1 significantly decreased IL-6 (Fig. 2A) and MCP-1 (Fig. 2B) production as well as TNF-α
expression (Fig. 2C) in a
dose-dependent manner (Fig. 2,
P<0.05, ANOVA).
FFA decreases eNOS expression and NO
production in 3T3-L1 adipocytes, and Rb1 blocks this effect
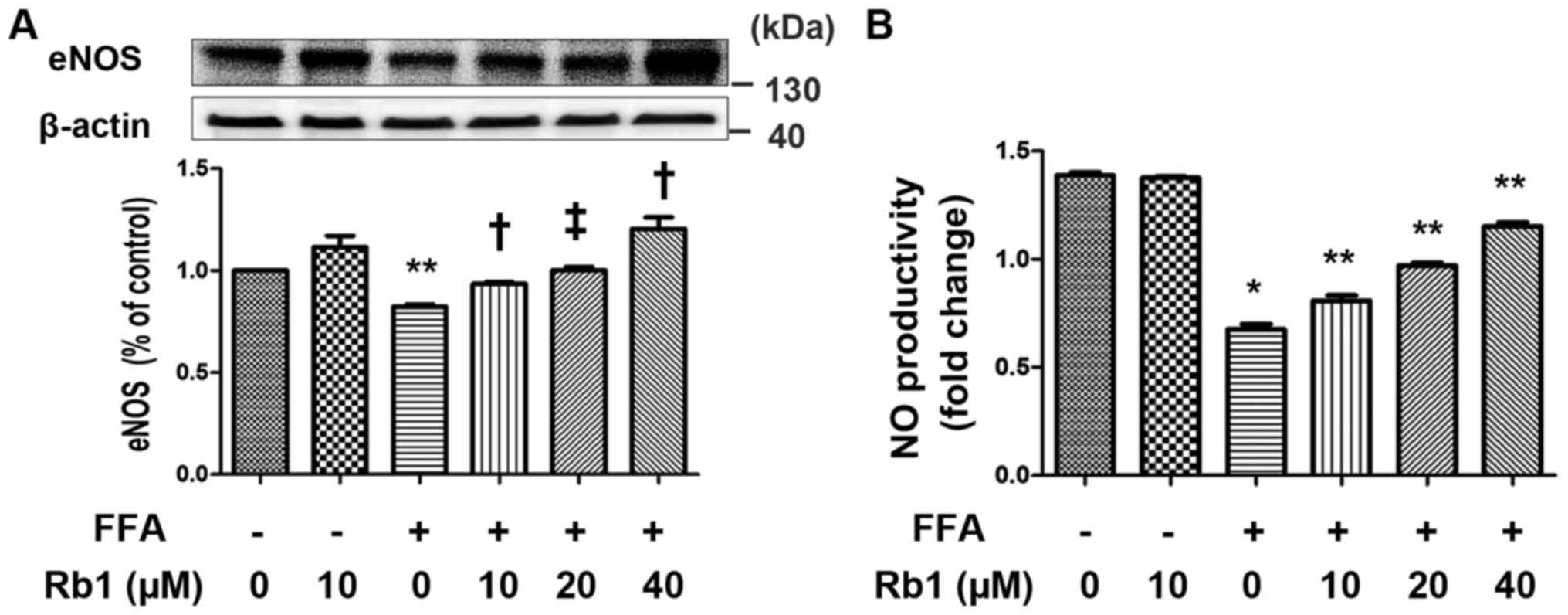
NO has been recognized as a potential mediator of
inflammation-induced insulin resistance and plays an important role
in energy metabolism (20). Among
the known NO synthases, eNOS was originally identified as playing
an important role in the regulation of vascular tone and blood
pressure. However, eNOS expression is not restricted to vascular
endothelium and has been shown to be more ubiquitous. eNOS has a
major role in adiponectin synthesis of adipocytes (21). In present study, we determined the
effects of FFAs on NO production and eNOS expression of 3T3-L1
adipocytes in the presence or absence of Rb1. The results showed
that the levels of eNOS expression (Fig. 3A) and NO production (Fig. 3B) were very low in control cells.
FFA significantly decreased eNOS expression and the corresponding
NO production that were significantly restored by 40 µM Rb1
(Fig. 3).
FFA decreases SOD2 expression and
increases ROS generation in 3T3-L1 adipocytes, and these effects
are reversed by Rb1
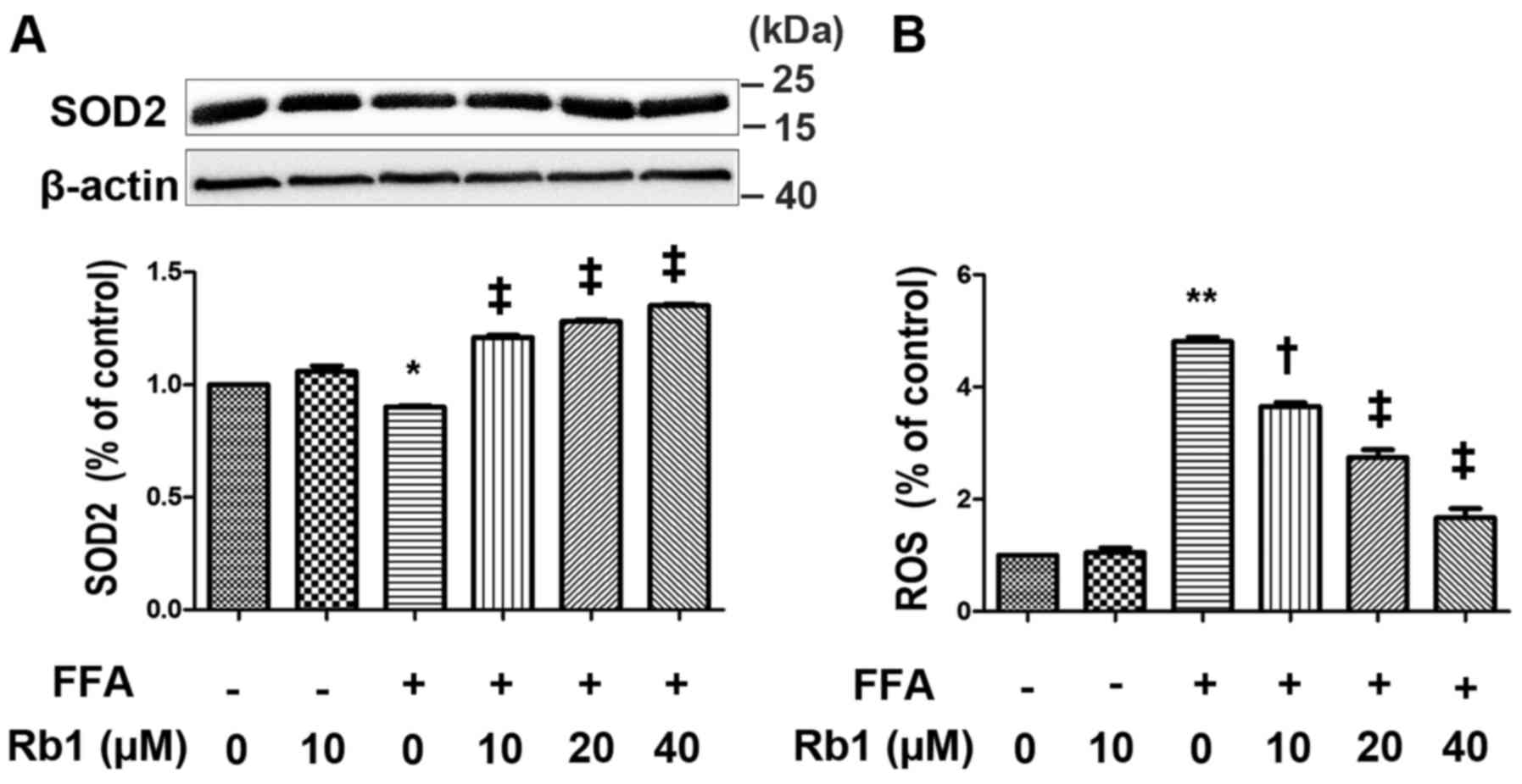
SOD2 is a major anti-oxidant enzyme in mitochondria,
which catalyzes the dismutation of O2 into
H2O2, and is one of the Nrf2-regulated SODs.
To compensate for the lack of NO bioavailability and reduce
O2-mediated damage, SOD increases
H2O2 levels by dismutation of superoxide
anions (22). In this study,
cultured 3T3-L1 adipocytes were exposed to 0.5 mM FFAs in the
absence or presence of Rb1 at 10, 20 and 40 µm for 4 h. ROS
generation as well as SOD2 expression were then measured. The
results showed that FFA treatment for 4 h drastically decreased
SOD2 expression (Fig. 4A), and
increased ROS levels (Fig. 4B) in
3T3-L1 adipocytes, which were restored by Rb1 in a dose-dependent
manner.
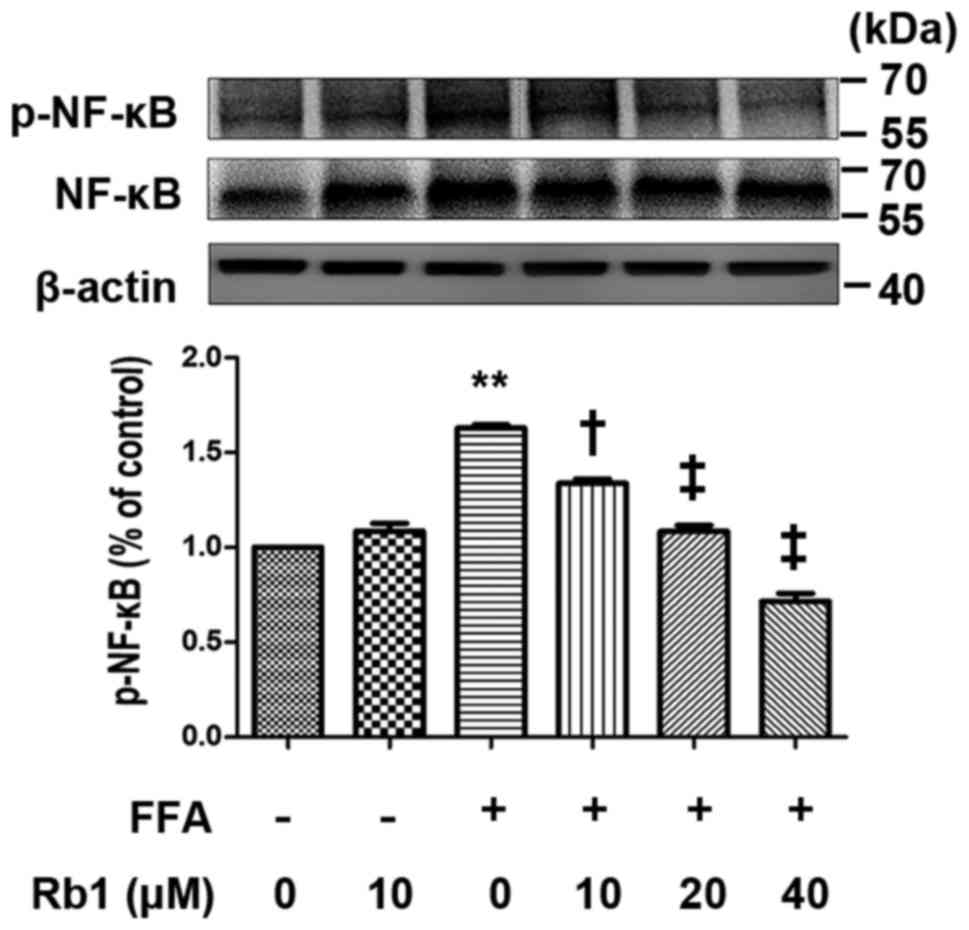
NF-κB activation in 3T3-L1 adipocytes
is induced by FFA, and the effect is inhibited by Rb1
NF-κB-dependent pathways are important to regulate
inflammatory gene expression in adipocytes. In the inactive state,
NF-κB/Rel transcription factors are present in the cytosol.
However, when cells are stimulated by stress factors, the NF-κB p65
subunit is phosphorylated and initiates the inflammatory response.
In the present study, we examined the effect of Rb1 on
phosphorylation of the NF-κB p65 subunit at Ser536. The results
showed that FFAs (0.5 mmol/l, 4 h) induced phosphorylation of the
NF-κB p65 subunit at Ser536, and Rb1 pretreatment (10, 20, 40 µM)
reduced phosphorylation of the NF-κB p65 subunit at Ser536 in a
dose-dependent manner (P<0.01) (Fig. 5). However, total NF-κB p65 in
3T3-L1 adipocytes was unchanged (Fig.
5).
Discussion
Past extensive studies have identified that Rb1
protects various cell types from injuries by anti-inflammatory and
anti-oxidant functions. Cheng et al confirmed that Rb1
suppresses the IL-1β-induced inflammatory response and apoptosis in
human articular chondrocytes (23). Xia et al demonstrated that
Rb1 inhibits myocardial ischemia/reperfusion injury in diabetic
rats by enhancing eNOS expression (24). Moreover, Rb1 functions as an
anti-diabetic factor by improving central leptin sensitivity
(25), increasing basal glucose
uptake, and promoting browning by improving PPAR-γ activity
(26). Interestingly, direct
evidence concerning the role of Rb1 in FFA-induced oxidative stress
and inflammatory responses in 3T3-L1 adipocytes has not been fully
elucidated. Our previous study showed that Rb1 protects HUVECs from
senescence by modulation of eNOS activity (27) and stimulation of the Sirtuin-1
pathway (16). In the present
study, we demonstrated that 0.5 mM FFAs decreased NO production and
increased ROS generation that subsequently activated the NF-κB
pathway, leading to overproduction of IL-6, MCP-1, and TNF-α in
3T3-L1 adipocytes. Rb1 ameliorates oxidative stress by increasing
SOD2 and eNOS expression and suppressing NF-κB activation, and thus
reduces IL-6, MCP-1, and TNF-α production. The inhibitory effect of
Rb1 on pro-inflammatory cytokine production contributes to its
beneficial anti-obesity and anti-diabetic effects.
Circulating pro-inflammatory cytokines from
adipocytes to their downstream sensors in liver, muscle, etc. lead
to insulin resistance. Moreover, previous studies have demonstrated
that excessive circulating levels of FFAs are released from ectopic
fat deposits, which activate TNF-α, protein kinase C, c-Jun
NH2-terminal kinase, JNK1, and IL-6 expression (28–30)
in the state of obesity. The concentrations of FFAs in vivo
range from 0.1 to 1 mM (31,32).
Therefore, we chose an FFA concentration of 0.5 mM for our studies
to reflect a conservative estimate of the in vivo
conditions. We also confirm that the saturated FFA palmitate is an
effective inducer of the inflammatory response in 3T3-L1
adipocytes, which is consistent with the study by Kolapo and
associates (33).
The NF-κB pathway is a classical inflammation
signaling pathway. NF-κB is activated by a variety of stimuli and
plays a critical role in the regulation of multiple cytokines such
as TNF-α, MCP-1, and IL-6 (34,35).
Previous studies have demonstrated that FFA is a potent inducer of
NF-κB activation in monocyte/macrophages (36,37).
In our study, we demonstrated that FFAs upregulated phosphorylation
of the NF-κB p65 subunit at Ser536, which is in consistent with the
result of previous studies (33,38,39).
In addition, our findings were in agreement with those showing that
palmitate induced secretions of MCP-1 and IL-6 as well as TNF-α
expressions in 3T3-L1 adipocytes (39,40).
Furthermore, we showed that Rb1 inhibited palmitate-induced NF-κB
p65 phosphorylation and release of pro-inflammatory cytokines. Few
literatures have reported the effect of Rb1 on FFA treated 3T3-L1
adipocytes and mechanisms. Wang et al (41) demonstrated that Rb1 attenuated
intestinal injury by inhibiting the NF-κB activation and induced
inflammatory cytokines in the lung tissues. Cheng and associates
showed that Rb1 inhibited osteoclast genesis by modulating NF-κB
pathway (42). These results are
consistent with our findings and highlight possible protective
mechanism of anti-inflammatory effects of Rb1. However,
phosphorylation of NF-κB does not represent direct evidence of
subsequent DNA binding, phosphorylation of NF-κB is an essential
step for subsequent DNA binding. Further experiments investigating
the mechanism of Rb1 in phosphorylation at cellular or animal
levels should be conducted in the future.
Oxidative stress is also closely associated with the
development of obesity and diabetes (43,44),
which is characterized by decreased expression of anti-oxidant
genes, such as eNOS and SOD2, overproduction of ROS, and less NO
generation. There are many conflicting studies on NO production
during oxidative stress. Previous reports have shown that NO is
associated with the initiation and maintenance of inflammation
through the generation of peroxynitrite in human inflammatory bowel
disease (45), which can result in
decreased cellular insulin sensitivity by causing inhibitory
nitrosylation of Akt (46).
Conversely, Kashyap et al showed that impaired NO activity
might play an important role in the insulin resistance of type 2
diabetic individuals, indicating that an NO-dependent increase is
an important mediator of insulin-stimulated glucose disposal in
insulin target tissues (47).
These conflicting results may be caused by different cell types and
different time courses. In the present study, we found that FFAs
decreased NO production in 3T3-L1 adipocytes, whereas Rb1 treatment
attenuated this effect. eNOS plays a major role in adipocytes
metabolism. Nisoli et al demonstrated eNOS−/−
mice showed features of insulin resistance (48). Koh and associates showed that
plasma adiponectin concentrations were reduced in adult
eNOS−/− mice compared with age-matched wild-type mice
(21). In our study, expression of
eNOS was upregulated by Rb1 in FFA-treated 3T3-L1 adipocytes in a
dose-dependent manner followed by increased NO production in 3T3-L1
adipocytes.
Mitochondria metabolize oxygen and is a major source
of ROS. One outcome of excessive levels of ROS is modification of
the structure and function of adipocytes and lipids, leading to
adipocyte dysfunction including altered cell signaling, impaired
energy metabolism, and inflammation (42). SODs are considered as antioxidant
defense enzymes that catalyze the conversion of two superoxides
into hydrogen peroxide and oxygen. Yeop et al demonstrated
that treatment with the antioxidants, N-acetyl cysteine, catalase
and SOD repressed ROS generation and NF-κB translocation stimulated
by excess glucose and palmitate, and decreased inflammatory gene
expression (40). A decreased
level and activity of SODs can result in the accumulation of
superoxide anion radicals in cell and induction of SOD2 is
suggested to protect against excess ROS (49). To study the role of SOD2 in
obesity, Krautbauer and associates treated 3T3-L1 preadipocytes or
mature adipocytes with increasing concentrations of palmitate (PA),
oleate (OA) or linoleate (LA) (from 0 to 200 µM). They demonstrated
that SOD2 is induced not only in visceral adipose tissues of
rodents fed a high diet but also induced by increased
concentrations of FFA in mature 3T3-L1 adipocyte in contrast to
premature adipocytes. In addition, they showed that OA (200 µM)
upregulated SOD2 during day 6 and day 9 in mature 3T3-L1 adipocytes
(50). Wang and associates
identified that SOD2 expression had been markedly increased as well
as mitochondrial DNA content in visceral fat (VF) of C57BL/6J mice
fed a high-fat and high-sucrose diet (HFHSD) at 6th month, while a
further extension of HFHSD diet intervention resulted in a decrease
of mitochondrial biogenesis and SOD2 expression in the VF until to
the 12th month (51). The findings
of a recent study by Kang and colleagues suggested that
heterozygous SOD2 deletion impaired glucose-stimulated insulin
secretion in high-fat-fed (HF) mice (52). Moreover, the study of Bauer et
al showed that elevated free fatty acids and impaired
adiponectin bioactivity contribute to reduced SOD2 protein in
monocytes of type 2 diabetes patients (53). These findings argue that whether
increased concentrations of FFA decrease SOD2 expression, and
whether SOD2 has beneficial or deleterious effects on obesity and
insulin sensitivity. However, the effect of palmitate to SOD2 in
mature adipocyte have not been reported in their paper. Our result
showed that palmitate at the concentration of 0.5 mM suppressed
SOD2 protein expression in mature 3T3-L1 adipocytes. Rb1 has been
shown to up-regulate the activity of SODs and enhance expression of
hypoxia-inducible factor-1α in hepatic tissues in previous studies
(54). Furthermore, recent studies
showed that pretreatment with Rb1 significantly protects various
cell types against oxidative injury and upregulates Nrf2 and its
downstream antioxidant-responsive genes including SOD2 (55,56).
In our study, we found that Rb1 increased the expression of SOD2,
which was consistent with the diminished production of ROS. It has
been clearly indicated that Rb1 protects 3T3-L1 adipocytes from
FFA-induced redox stress, which is in line with previous
investigations (57).
Taken together, our study demonstrates that
pretreatment with Rb1 ameliorates pro-inflammatory cytokine
expression through suppressed NF-κB translocation and blockade of
its activation. The inhibitory effect of Rb1 on oxidative stress is
attributed to its anti-inflammatory activity and anti-oxidative
functions, and thus may contribute to the anti-obesity effect of
Rb1 in insulin resistance and diabetes. The lack of animal and
clinical data is a limitation of our study, but it provides an
important basis for future research.
Acknowledgements
This work was supported by the grants from the
National Natural Science Foundation of China (grant number:
81300707, to Min Wang; 81370447, to Xiaoxian Qian); The funders had
no role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript. We thank Mitchell Arico
for critical proofreading and editing of the manuscript.
References
|
1
|
Lau DC, Dhillon B, Yan H, Szmitko PE and
Verma S: Adipokines: Molecular links between obesity and
atheroslcerosis. Am J Physiol Heart Circ Physiol. 288:H2031–H2041.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz B and Yehuda-Shnaidman E:
Putative role of adipose tissue in growth and metabolism of colon
cancer cells. Front Oncol. 4:1642014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grant RW and Dixit VD: Adipose tissue as
an immunological organ. Obesity (Silver Spring). 23:512–518. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harwood HJ Jr: The adipocyte as an
endocrine organ in the regulation of metabolic homeostasis.
Neuropharmacology. 63:57–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mraz M and Haluzik M: The role of adipose
tissue immune cells in obesity and low-grade inflammation. J
Endocrinol. 222:R113–R127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wellen KE and Hotamisligil GS:
Obesity-induced inflammatory changes in adipose tissue. J Clin
Invest. 112:1785–1788. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Mottillo EP, Zhao J, Gartung A,
VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, et al:
Adipocyte lipolysis-stimulated interleukin-6 production requires
sphingosine kinase 1 activity. J Biol Chem. 289:32178–32185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boden G: Obesity and free fatty acids.
Endocrinol Metab Clin North Am. 37:635–646, viii-ix. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto
M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, et al: High
glucose level and free fatty acid stimulate reactive oxygen species
production through protein kinase C-dependent activation of NAD
(P)H oxidase in cultured vascular cells. Diabetes. 49:1939–1945.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasgupta S and Bhattacharya S, Biswas A,
Majumdar SS, Mukhopadhyay S, Ray S and Bhattacharya S: NF-kappaB
mediates lipid-induced fetuin-A expression in hepatocytes that
impairs adipocyte function effecting insulin resistance. Biochem J.
429:451–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong X, Zheng L, Lu S and Yang Y:
Neuroprotective effects of pretreatment of ginsenoside Rb1 on
severe cerebral ischemia-induced injuries in aged mice: Involvement
of anti-oxidant signaling. Geriatr Gerontol Int. 17:338–345. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y and Ji LL: Chronic ginseng
consumption attenuates age-associated oxidative stress in rats. J
Nutr. 133:3603–3609. 2003.PubMed/NCBI
|
|
14
|
Yu X, Ye L, Zhang H, Zhao J, Wang G, Guo C
and Shang W: Ginsenoside Rb1 ameliorates liver fat accumulation by
upregulating perilipin expression in adipose tissue of db/db obese
mice. J Ginseng Res. 39:199–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong Y, Shen L, Liu KJ, Tso P, Xiong Y,
Wang G, Woods SC and Liu M: Antiobesity and antihyperglycemic
effects of ginsenoside Rb1 in rats. Diabetes. 59:2505–2512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Z, Liu Y, Hao B, Yu S, Zhang H, Liu
D, Zhou B, Wu L, Wang M, Xiong Z, et al: Ginsenoside Rb1 prevents
H2O2-induced HUVEC senescence by stimulating sirtuin-1 pathway.
PLoS One. 9:e1126992014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kratchmarova I, Kalume DE, Blagoev B,
Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS,
Fernandez MM, Bunkenborg J, et al: A proteomic approach for
identification of secreted proteins during the differentiation of
3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 1:213–222.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Wang JJ, Li J, Park K, Qian X, Ma
JX and Zhang SX: Pigment epithelium-derived factor suppresses
adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1
preadipocytes. Am J Physiol Endocrinol Metab. 297:E1378–E1387.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Svedberg J, Björntorp P, Smith U and
Lonnroth P: Free-fatty acid inhibition of insulin binding,
degradation and action in isolated rat hepatocytes. Diabetes.
39:570–574. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugita H, Kaneki M, Tokunaga E, Sugita M,
Koike C, Yasuhara S, Tompkins RG and Martyn JA: Inducible nitric
oxide synthase plays a role in LPS-induced hyperglycemia and
insulin resistance. Am J Physiol Endocrinol Metab. 282:E386–E394.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koh EH, Kim M, Ranjan KC, Kim HS, Park HS,
Oh KS, Park IS, Lee WJ, Kim MS, Park JY, et al: eNOS plays a major
role in adiponectin synthesis in adipocytes. Am J Physiol
Endocrinol Metab. 298:E846–E853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas SR, Chen K and Keaney JF Jr:
Hydrogen peroxide activates endothelial nitric-oxide synthase
through coordinated phosphorylation and dephosphorylation via a
phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem.
277:6017–6024. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng W, Wu D, Zuo Q, Wang Z and Fan W:
Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation
and apoptosis in human articular chondrocytes. Int Orthop.
37:2065–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia R, Zhao B, Wu Y, Hou JB, Zhang L, Xu
JJ and Xia ZY: Ginsenoside Rb1 preconditioning enhances eNOS
expression and attenuates myocardial ischemia/reperfusion injury in
diabetic rats. J Biomed Biotechnol. 2011:7679302011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Yu Y, Szabo A, Han M and Huang XF:
Central inflammation and leptin resistance are attenuated by
ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS
One. 9:e926182014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mu Q, Fang X, Li X, Zhao D, Mo F, Jiang G,
Yu N, Zhang Y, Guo Y, Fu M, et al: Ginsenoside Rb1 promotes
browning through regulation of PPARgamma in 3T3-L1 adipocytes.
Biochem Biophys Res Commun. 466:530–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu DH, Chen YM, Liu Y, Hao BS, Zhou B, Wu
L, Wang M, Chen L, Wu WK and Qian XX: Ginsenoside Rb1 reverses
H2O2-induced senescence in human umbilical endothelial cells:
Involvement of eNOS pathway. J Cardiovasc Pharmacol. 59:222–230.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neacsu O, Cleveland K, Xu H, Tchkonia TT,
Kirkland JL and Boney CM: IGF-I attenuates FFA-induced activation
of JNK1 phosphorylation and TNFalpha expression in human
subcutaneous preadipocytes. Obesity (Silver Spring). 21:1843–1849.
2013.PubMed/NCBI
|
|
29
|
Chiadak JD, Arsenijevic T, Verstrepen K,
Gregoire F, Bolaky N, Delforge V, Flamand V, Perret J and Delporte
C: Forskolin inhibits lipopolysaccharide-induced modulation of
MCP-1 and GPR120 in 3T3-L1 adipocytes through an Inhibition of
NFκB. Mediators Inflamm. 2016:14317892016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiao P, Chen Q, Shah S, Du J, Tao B,
Tzameli I, Yan W and Xu H: Obesity-related upregulation of monocyte
chemotactic factors in adipocytes: Involvement of nuclear
factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes.
58:104–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laine PS, Schwartz EA, Wang Y, Zhang WY,
Karnik SK, Musi N and Reaven PD: Palmitic acid induces IP-10
expression in human macrophages via NF-kappaB activation. Biochem
Biophys Res Commun. 358:150–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiao P, Ma J, Feng B, Zhang H, Diehl JA,
Chin YE, Yan W and Xu H: FFA-induced adipocyte inflammation and
insulin resistance: Involvement of ER stress and IKKβ pathways.
Obesity (Silver Spring). 19:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ajuwon KM and Spurlock ME: Palmitate
activates the NF-kappaB transcription factor and induces IL-6 and
TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 135:1841–1846.
2005.PubMed/NCBI
|
|
34
|
Zhang WJ and Frei B: Astragaloside IV
inhibits NF-κB activation and inflammatory gene expression in
LPS-treated mice. Mediators Inflamm. 2015:2743142015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye J and Keller JN: Regulation of energy
metabolism by inflammation: a feedback response in obesity and
calorie restriction. Aging (Albany NY). 2:361–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty
acid-induced insulin resistance. J Clin Invest. 116:3015–3025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun J, Luo J, Ruan Y, Xiu L, Fang B, Zhang
H, Wang M and Chen H: Free fatty acids activate renin-angiotensin
system in 3T3-L1 adipocytes through nuclear factor-kappa B pathway.
J Diabetes Res. 2016:15875942016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McCall KD, Holliday D, Dickerson E,
Wallace B, Schwartz AL, Schwartz C, Lewis CJ, Kohn LD and Schwartz
FL: Phenylmethimazole blocks palmitate-mediated induction of
inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7
macrophages. J Endocrinol. 207:343–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han C Yeop, Kargi AY, Omer M, Chan CK,
Wabitsch M, O'Brien KD, Wight TN and Chait A: Differential effect
of saturated and unsaturated free fatty acids on the generation of
monocyte adhesion and chemotactic factors by adipocytes:
Dissociation of adipocyte hypertrophy from inflammation. Diabetes.
59:386–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Qiao L, Li S and Yang G:
Protective effect of ginsenoside Rb1 against lung injury induced by
intestinal ischemia-reperfusion in rats. Molecules. 18:1214–1226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng B, Li J, Du J, Lv X, Weng L and Ling
C: Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB
and MAPKs pathways. Food Chem Toxicol. 50:1610–1615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gerber PA and Rutter GA: The role of
oxidative stress and hypoxia in pancreatic beta-cell dysfunction in
diabetes mellitus. Antioxid Redox Signal. 26:501–518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang YC and Chuang LM: The role of
oxidative stress in the pathogenesis of type 2 diabetes: From
molecular mechanism to clinical implication. Am J Transl Res.
2:316–331. 2010.PubMed/NCBI
|
|
45
|
Kolios G, Valatas V and Ward SG: Nitric
oxide in inflammatory bowel disease: A universal messenger in an
unsolved puzzle. Immunology. 113:427–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yasukawa T, Tokunaga E, Ota H, Sugita H,
Martyn JA and Kaneki M: S-nitrosylation-dependent inactivation of
Akt/protein kinase B in insulin resistance. J Biol Chem.
280:7511–7518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kashyap SR, Roman LJ, Lamont J, Masters
BS, Bajaj M, Suraamornkul S, Belfort R, Berria R, Kellogg DL Jr,
Liu Y and DeFronzo RA: Insulin resistance is associated with
impaired nitric oxide synthase activity in skeletal muscle of type
2 diabetic subjects. J Clin Endocrinol Metab. 90:1100–1105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nisoli E, Clementi E, Paolucci C, Cozzi V,
Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada
S and Carruba MO: Mitochondrial biogenesis in mammals: The role of
endogenous nitric oxide. Science. 299:896–899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ikegami Y, Inukai K, Imai K, Sakamoto Y,
Katagiri H, Kurihara S, Awata T and Katayama S: Adiponectin
upregulates ferritin heavy chain in skeletal muscle cells.
Diabetes. 58:61–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krautbauer S, Eisinger K, Neumeier M,
Hader Y, Buettner R, Schmid PM, Aslanidis C and Buechler C: Free
fatty acids, lipopolysaccharide and IL-1alpha induce adipocyte
manganese superoxide dismutase which is increased in visceral
adipose tissues of obese rodents. PLoS One. 9:e868662014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang PW, Kuo HM, Huang HT, Chang AY, Weng
SW, Tai MH, Chuang JH, Chen IY, Huang SC, Lin TK and Liou CW:
Biphasic response of mitochondrial biogenesis to oxidative stress
in visceral fat of diet-induced obesity mice. Antioxid Redox
Signal. 20:2572–2588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang L, Dai C, Lustig ME, Bonner JS, Mayes
WH, Mokshagundam S, James FD, Thompson CS, Lin CT, Perry CG, et al:
Heterozygous SOD2 deletion impairs glucose-stimulated insulin
secretion, but not insulin action, in high-fat-fed mice. Diabetes.
63:3699–3710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bauer S, Wanninger J, Neumeier M, Wurm S,
Weigert J, Kopp A, Bala M, Schäffler A, Aslanidis C and Buechler C:
Elevated free fatty acids and impaired adiponectin bioactivity
contribute to reduced SOD2 protein in monocytes of type 2 diabetes
patients. Exp Mol Pathol. 90:101–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo Y, Yang T, Lu J, Li S, Wan L, Long D,
Li Q, Feng L and Li Y: Rb1 postconditioning attenuates liver warm
ischemia-reperfusion injury through ROS-NO-HIF pathway. Life Sci.
88:598–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ye J, Yao JP, Wang X, Zheng M, Li P, He C,
Wan JB, Yao X and Su H: Neuroprotective effects of ginsenosides on
neural progenitor cells against oxidative injury. Mol Med Rep.
13:3083–3091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan J, Liu D, He C, Li X and He F:
Inhibiting adhesion events by Panax notoginseng saponins and
Ginsenoside Rb1 protecting arteries via activation of Nrf2 and
suppression of p38-VCAM-1 signal pathway. J Ethnopharmacol.
192:423–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu DH, Chen YM, Liu Y, Hao BS, Zhou B, Wu
L, Wang M, Chen L, Wu WK and Qian XX: Rb1 protects endothelial
cells from hydrogen peroxide-induced cell senescence by modulating
redox status. Biol Pharm Bull. 34:1072–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|