Introduction
Periodontitis is a chronic inflammatory disease
which results in the loss of periodontal tissue, and is clinically
manifested with the loss of periodontal attachment, the formation
of periodontal pockets, alveolar bone osteopenia and ultimately
teeth exfoliation (1). Periodontal
ligament stem cells (PDLSCs), a newly recognized subpopulation of
mesenchymal stem cells (MSCs), have been suggested as potential
seed cells for periodontal tissue regeneration, for the formation
of cementum-periodontal ligament complexes and adjacent bone
tissues (2–4). However, effective strategies for the
regulation of their osteogenic differentiation potency have yet to
be developed. Therefore, the elucidation of the molecular
mechanisms underlying the osteogenic differentiation of PDLSCs is
critical prior to their use in tooth regeneration and osteogenic
tissue engineering applications.
Numerous regulatory pathways, including Wnt,
transforming growth factor-β and bone morphogenetic
protein-mediated signaling, are implicated in the progression of
osteogenesis (5–7). In particular, the canonical
Wnt/β-catenin signaling pathway has been identified as critical for
the differentiation of human MSCs into osteoblasts. Wnt/β-catenin
signaling has been demonstrated to be important in MSC fate
determination and differentiation, including osteogenic (8), chondrogenic (9), adipogenic (10) and myogenic differentiation
(11). However, the molecular
mechanisms underlying the role of Wnt/β-catenin signaling in PDLSC
differentiation, as well as its potential therapeutic applications
have yet to be explored.
Accumulating evidence suggests that osteoblastic
induction and differentiation are also regulated by
post-transcriptional mechanisms, partly through
temporarily-expressed microRNAs (miRNAs) (12). miRNAs are a class of endogenous
small noncoding RNA molecules, with a length of 18–22 nucleotides.
Mature miRNAs bind to the 3′-untranslated region (UTR) of target
mRNAs and repress their translation or induce their degradation
(13). Mizuno et al
(12) reported that miR-125b
inhibited the osteoblastic differentiation of stem cells through
the downregulation of cell proliferation. miR-27 has been reported
to regulate adipogenesis, myeloblast differentiation and skeletal
muscle development (14,15). However, to the best of our
knowledge, the putative roles of miRNAs in PDLSC differentiation
have only been investigated at a preliminary level (16), and the molecular mechanisms
underlying the involvement of miRNAs in PDLSC differentiation have
yet to be elucidated.
The aim of the present study was to investigate the
differential expression of miRNAs during the osteogenic
differentiation of PDLSCs and explore their biological actions. The
present results demonstrated that inhibition of miR-214 resulted in
the cellular accumulation of β-catenin and the activation of Wnt
signaling, by targeting β-catenin gene expression, and thus
potentiated stem cell differentiation.
Materials and methods
PDLSC isolation and cultivation
Human premolars were obtained from 5 men aged 18–24
years (mean age, 20.2) who underwent molar extraction at the
Department of Stomatology of the First Affiliated Hospital of Jinan
University (Guangzhou, China) from July 2015 to May 2016. Written
informed consent was obtained prior to study inclusion and the
present study was approved by the Ethics Committee of the First
Affiliated Hospital of Jinan University. Tissue from the
periodontal ligament was isolated as previously described (17). Briefly, following extraction, the
periodontal ligament was gently scraped from the middle portion of
the root surface, minced into 1 mm3 cubes and placed
into 6-well culture dishes. The explants were cultured in Minimum
Essential Medium Eagle-α modification (αMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 0.292
mg/ml glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
The cultures were incubated at 37°C in a 5% CO2
humidified atmosphere for 10–14 days. Stro-1-positive MSCs were
isolated using immunomagnetic Dynabeads (cat. no. 110.41 and
110.42; Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Following washing, bead-bound
cells were isolated after a sequential digestion of the
extracellular matrix with pronase (0.15%, w/v) for 2 h and
collagenase (0.2%, w/v) overnight at 37°C. The cells were
centrifuged with 200 × g for 5 min at 4°C and subsequently
suspended in αMEM with 10% FBS, and then seeded in 6-well culture
dishes at a density of ~1×105 cells/well. Cells were
cultured as monolayers until they reached confluence (10–12 days)
at 37°C in a humidified atmosphere containing 5% CO2,
with a change of culture medium every 3–4 days.
PDLSC differentiation
Differentiation was induced 3 days post confluence.
The culture media was exchanged for osteogenic medium, which
contained 10% FBS and DAG (100 nM dexamethasone, 50 µM ascorbic
acid, and 5 mM β-glycerophosphate) in α-MEM. The medium was changed
every 2–3 days during the course of incubation (0–21 days). Cells
cultured in basal media (αMEM with 10% FBS) served as a control.
These undifferentiated and differentiated cells were used in
subsequent experiments.
RNA extraction
Total RNA and miRNA were isolated from the
undifferentiated and differentiated PDLSCs with TRizol (Invitrogen;
Thermo Fisher Scientific, Inc.) or an miRNeasy Mini kit (Qiagen,
Inc., Valencia, CA, USA), respectively, according to the
manufacturers' instructions. Potential genomic DNA contamination
was removed from the samples by digestion with RNase-free DNase
(Qiagen, Inc.) for 15 min at room temperature. The RNA
concentration was measured using a NanoDrop 1000 Spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA),
and the quality of miRNA and mRNA was evaluated using an Agilent
2100 Bioanalyzer system (Agilent Technologies, Inc., Santa Clara,
CA, USA).
Polymerase chain reaction (PCR)
arrays
The expression levels of 84 miRNAs related to
osteoblastic differentiation in PDLSCs were profiled using miScript
miRNA PCR Array System (cat. no. 331221; Qiagen, Inc.). Briefly,
genomic DNA was eliminated from the RNA samples (1 µg) isolated
from undifferentiated and differentiated PDLSCs, using an miRNeasy
Mini kit (cat. no. 217004; Qiagen, Inc.), at 42°C for 5 min, and
then transferred on ice for ~1 min for the removal of any residual
DNA contamination. The purified RNA sample was used for cDNA
synthesis using the reverse transcription mixture provided in the
miScript II RT kit (cat. no. 218160; Qiagen, Inc.). The mixture was
incubated at 42°C for 15 min and then at 95°C for 5 min. cDNA was
subsequently used for quantitative PCR (qPCR) with the
RT2 SYBR-Green qPCR Mastermix (cat. no. 218073; Qiagen,
Inc.), according to the manufacturer's protocol. Data analysis was
performed using the manufacturer's integrated web-based software
package for the PCR Array System, using the 2−∆∆Cq
method (18). Hierarchical
clustering analysis and heatmap visualization were performed using
CIMminer (http://discover.nci.nih.gov/cimminer).
Reverse transcription qPCR
(RT-qPCR)
For detection of miRNAs and mRNAs, cDNA synthesis
was performed from total RNA (0.2–0.5 µg) using the PrimeScript
reverse transcription reagent kit (cat. no. DRR037A; Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's instructions. Quantitative analysis of the change in
expression levels was performed using SYBR Premix Ex Taq™ (DRR420A;
Takara Biotechnology Co., Ltd.) using an ABI 7300 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR cycling conditions
were as follows: Initial incubation at 95°C for 30 sec, followed by
40 cycles of denaturation at 95°C for 5 sec and annealing at 60°C
for 31 sec. Quantitative normalization was performed on the
expression of RNU6 small nucleolar RNA or GAPDH, for miRNA and
mRNA, respectively. The sequences of the primers are listed in
Table I. Relative expression
levels between samples were calculated using the 2−ΔΔCq
method (18). Experiments were
conducted in triplicate.
 | Table I.Primer sequences use for polymerase
chain reactions. |
Table I.
Primer sequences use for polymerase
chain reactions.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-214 |
AGCCGACAGCAGGCACAGACA |
AGCCGACAGCAGGCACAGACA |
| Let-7a |
GTCGTATCCAGTGCAGGGTCCGAG |
GTATTCGCACTGGATACGACAACT |
| miR-100 |
GCGGCAACCCGTAGATCCGAA | GTGCAGGGTCCGAGGT |
| miR-125a-5p |
TCCCTGAGACCCTTTAACCTGTGA |
ACAGGTGAGGTTCTTGGGAGCC |
| miR-98 |
ACACTCCCUAUACAACUUAC |
GGGAAAGUAGUGAGGCCTCAGA |
| miR-142-5p |
GGCCCATAAAGTAGAAAGC |
TTTGGCACTAGCACATT |
| miR-128a |
ATAGAATTCTTGAATACTGTGA |
CCGGGATCCTAAGCAATAGCTTTCA |
|
| AGTACACTGC | CAAATT |
| miR-184 |
CTGGTAGGTGGACGGAGAACTG |
TCAACTGGTGTCGTGGAGTCGGC |
| miR-19a |
GTCGTATCCAGTGCAGGGTCCGAG |
CTGGAGTGTGCAAATCTATGC |
|
|
GTATTCGCACTGGATACGACTCAGTTT |
|
| miR-27b |
CCGGCCTTCACAGTGGCTA |
CGGGTCGGTGGCAGAACTT |
| U6 |
CGCTTCACGAATTTGCGTGTCAT |
GCTTCGGCAGCACATATACTAAAAT |
| ALP |
CCACGTCTTCACATTTGGTG |
AGACTGCGCCTGGTAGTTGT |
| BSP |
AAAGTGAGAACGGGGAACCT |
GATGCAAAGCCAGAATGGAT |
| OCN |
GAGGGCAATAAGGTAGTGAA |
CATAGATGCGTTTGTAGGC |
| GAPDH |
GAAGATGGTGATGGGATTTC |
GAAGGTGAAGGTCGGAGT |
Transfection
The miR-214 mimic (sense, 5′-aca gca gg cac aga cag
gca gu-3′ and antisense, 5′-ugc cug ucu gug ccu gcu guu u-3′),
mimic negative control (NC; sense, 5′-uuc ucc gaa cgu guc acg
utt-3′ and antisense, 5′-acg uga cac guu cgg aga att-3′), miR-214
inhibitor (5′-ugc cug ucu gug ccu gcu guu u-3′) and inhibitor
negative control (5′-cag uac uuu ugu gua gua caa-3′) were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). PDLSCs
(1×106 cells/well) were plated in a six-well plate 1 day
prior to transfection. PDLSCs were then transfected with miR-214
mimic, mimic NC, miR-214 inhibitor or inhibitor NC, at a final
concentration of 25 nM, using siLentFect Lipid Reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Subsequently, cells were
cultured in serum-free Dulbecco's modified Eagle's medium: Nutrient
Mixture F-12 (Shanghai GeneChem Co., Ltd., Shanghai, China).
Following 4 h of incubation at 37°C, the medium was replaced with
DMEM/F12 supplemented with 10% FBS. The cells were harvested for
further experiments at 48 h after transfection.
Alizarin red staining
Cells were seeded into 24-well plates at a density
of ~1×105 cells/well. When the cells had reached ~80%
confluence, the culture medium was replaced with standard
osteogenic differentiation induction medium as aforementioned and
cultured for a further 3 weeks. The induction medium was replaced
every 3 days. Following 3 weeks of culture, cells were stained with
Alizarin red (pH 4.1) and analyzed under an inverted microscope
(TE2000U; Nikon Corporation, Tokyo, Japan). The staining was
quantified as previously described (19).
β-catenin/transcription factor (TCF)
transcription reporter assay
TOPflash and FOPflash reporters (EMD Millipore,
Billerica, MA, USA) contain wild type (WT) and mutated TCF-4
consensus binding sites, respectively, and are used to evaluate
β-catenin-dependent signaling events that drive the expression of
TCF. These reporters have been described previously (20). Briefly, 1×105 cells/well
were seeded into a 24-well plate followed by transfection with
TOPflash or FOPflash constructs, along with the miR-214 mimic, the
miR-214 inhibitor or the corresponding NC miRNAs. All transfections
were performed using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. At 24 h following transfection, the β-catenin
luciferase assay was performed using a Dual-Luciferase Assay System
kit (Promega Corporation, Madison, WI, USA).
miRNA target prediction
To identify potential targets of miR-214, two public
available algorithms, TargetScan (http://www.targetscan.org/) and PicTar (http://pictar.bio.nyu.edu/) were used. The interaction
between miR-214 and the 3′-UTR of the putative target was predicted
by RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/).
Luciferase assay
The 3′-UTR of the β-catenin mRNA CTNNB1 (accession
no. NM_001098209.1), with wild-type or mutant (Mut) binding sites
for miR-214, was amplified from human cDNA by PCR, and subcloned
into the pGL3 vector MCS cloning site (Promega Corporation) to
generate the pGL3-WT-CTNNB1-3′-UTR or pGL3-Mut-CTNNB1-3′-UTR
plasmids, respectively. CTNNB1 3′-UTR forward primer,
5′-TCTAGAATACAATGACTTTTTAGCTG-3′; CTNNB1 3′-UTR reverse primer,
5′-TCTAGATTAGCCAAG-3′. PDLSCs were seeded in duplicate in 24-well
plates and co-transfected with 1–2 µg/ml pGL3-WT-CTNNB1-3′-UTR or
pGL3-Mut-CTNNB1-3′-UTR plasmids and 25 nM miR-214 mimic or miR-214
inhibitor using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. Corresponding NCs (Shanghai GenePharma Co., Ltd.)
were transfected using the same procedure. Following incubation for
48 h, luciferase activity was analyzed using the Dual-Luciferase
Reporter Assay System (Promega Corporation). Renilla luciferase was
used for normalization.
Western blot analysis
Cells were lysed on ice using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and protein concentrations were
determined using a bicinchoninic acid assay. Proteins (40 µg) were
separated by 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane (EMD Millipore) and incubated with 5% fat-free
skim milk in TBS + 0.05% Tween 20, for 1 h at room temperature.
Membranes were subsequently incubated overnight at 4°C with the
following antibodies: Anti-CTNNB1 (rabbit monoclonal; 1:1,000;
ab16051), anti-β-catenin (rabbit monoclonal; 1:1,000; ab32572),
anti-TCF4 (rabbit polyclonal; 1:1,000; ab185736) or anti-β-actin
(rabbit polyclonal; 1:1,000; ab8227) (all from Abcam, Cambridge,
MA, USA). Secondary antibody incubations were performed with
horseradish peroxidase-conjugated mouse anti-rabbit antibody
(1:10,000; ab99702; Abcam) for 1 h at room temperature. Enhanced
chemiluminescence substrate was used to visualize signals (EMD
Millipore). β-actin was used as an endogenous protein for
normalization. Relative band intensities were determined by
densitometry using Quantity One 4.6.2 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ±
standard deviation of 3 independent experiments. Comparison of two
groups was achieved using an unpaired two-tailed t-test.
Differences between multiple groups were evaluated by one-way
analysis of variance followed by Tukey's multiple comparison test.
P<0.05 were considered statistically significant.
Results
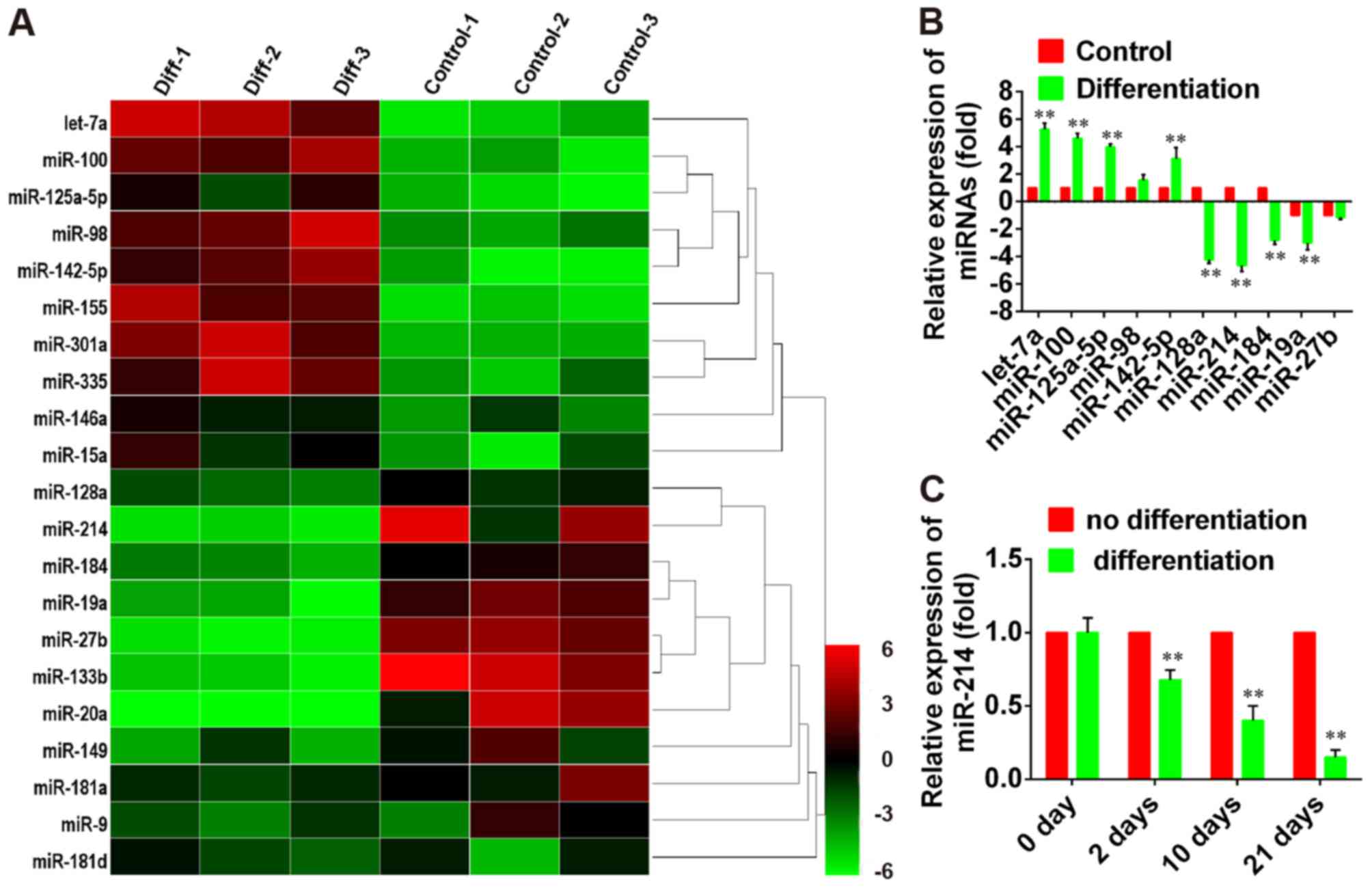
miRNA profiling during PDLSC
differentiation
Hierarchical clustering of PCR array results
demonstrated systematic variations in the expression of various
miRNAs between differentiated and non-differentiated PDLSCs
(Fig. 1A). To validate the miRNA
PCR array analysis findings, 5′upregulated and 5 downregulated
miRNAs with the largest fold-change in expression were selected and
their expression levels were assessed during PDLSC differentiation
by RT-qPCR. The results revealed that let-7a, miR-100, miR-125b-5p,
miR-98 and miR-142-5p were overexpressed during PDSLC
differentiation, whereas the expression of miR-128a, miR-214,
miR-184, miR-19a and miR-27b was suppressed (P<0.01; Fig. 1B); these results were in accordance
with the expression patterns detected by the PCR array. These
findings indicated that the expression of this set of miRNAs is
differentially regulated during PDLSC differentiation. Among them,
miR-214 was demonstrated to undergo the greatest change in
expression during differentiation. Therefore, miR-214 was further
investigated. The expression levels of miR-214 decrease in a
time-dependent manner following the induction of osteoblastic
differentiation of PDLSCs, compared with undifferentiated cells
(Fig. 1C). These results suggested
that miR-214 may be involved in the regulation of PDLSC
osteoblastic differentiation.
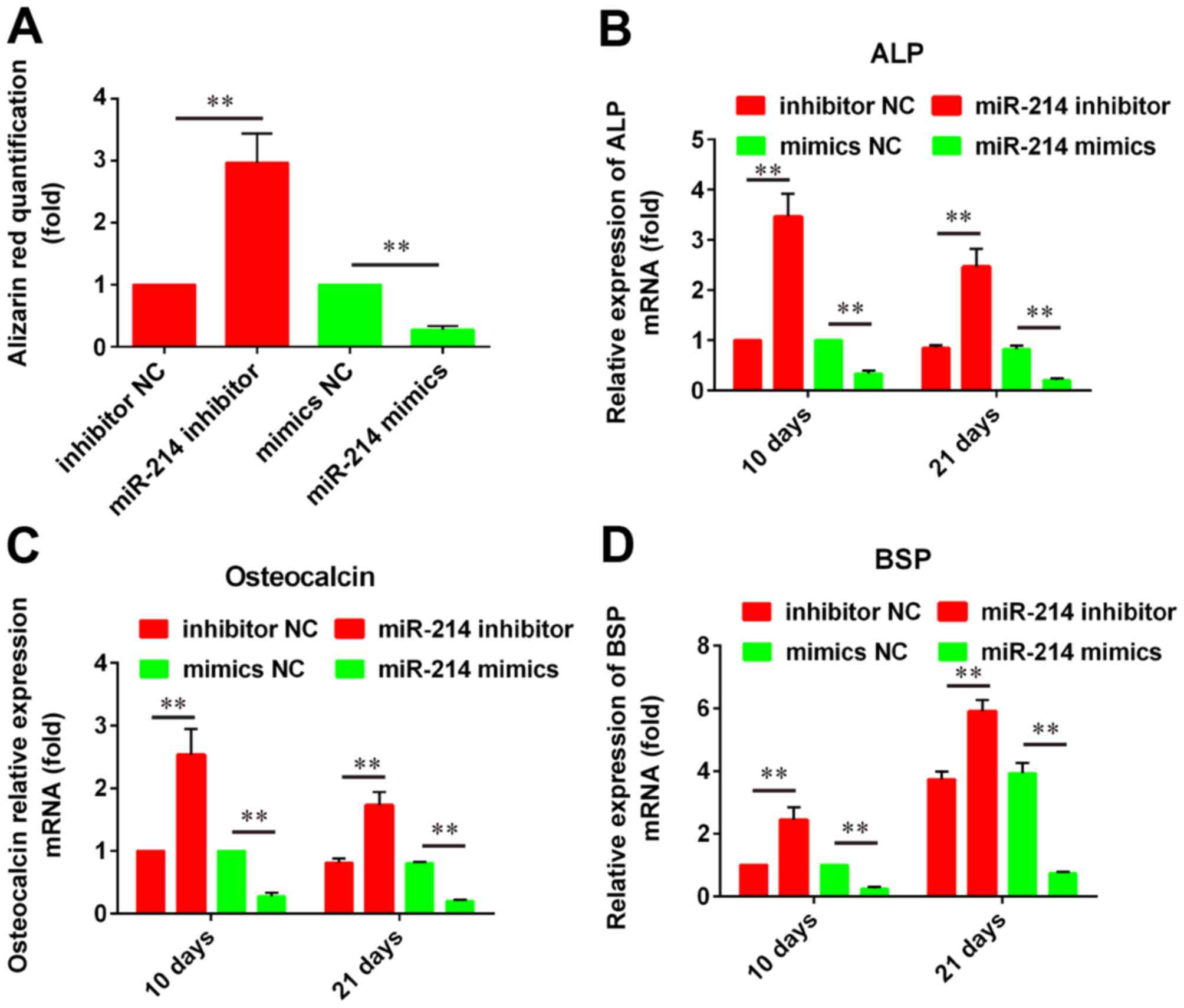
miR-214 inhibition promotes
osteoblastic differentiation of PDLSCs
To investigate the roles of miR-214 in the
osteoblastic differentiation of PDLSCs, cells were transfected with
a miR-214 mimic or a miR-214 inhibitor and stained with Alizarin
Red S. The results demonstrated that miR-214 knockdown
significantly promoted osteogenesis in PDLSCs compared with
control; conversely, miR-214 overexpression inhibited PDLSC
osteoblastic differentiation compared with control (Fig. 2A). In addition, RT-qPCR analysis
was performed in order to examine the mRNA expression levels of the
osteogenic marker gene ALP and the mineralization markers OCN and
BSP. The results demonstrated that miR-214 silencing significantly
upregulated ALP, OCN and BSP mRNA expression compared with control,
while miR-214 overexpression produced the opposite effects
(Fig. 2B-D). Since the expression
of these markers is indicative of osteoblastic differentiation
status, these results suggested that miR-214 may be important in
osteogenesis.
miR-214 inhibits β-catenin gene
expression
Bioinformatics analysis was used to predict
potential target genes for miR-214, and results identified a
putative miR-214 binding site in the 3′UTR of the β-catenin gene
CTNNB1 (Fig. 3A). A
luciferase reporter assay was used to investigate the putative
miR-214-dependent post-transcriptional regulation of CTNNB1.
The present results demonstrated that miR-214 knockdown
significantly increased the luciferase activity in cells
co-transfected with the plasmid containing wild-type CTNNB1
3′-UTR, whereas it produced no effect on mutant CTNNB1
3′-UTR-transfected cells (Fig.
3B).
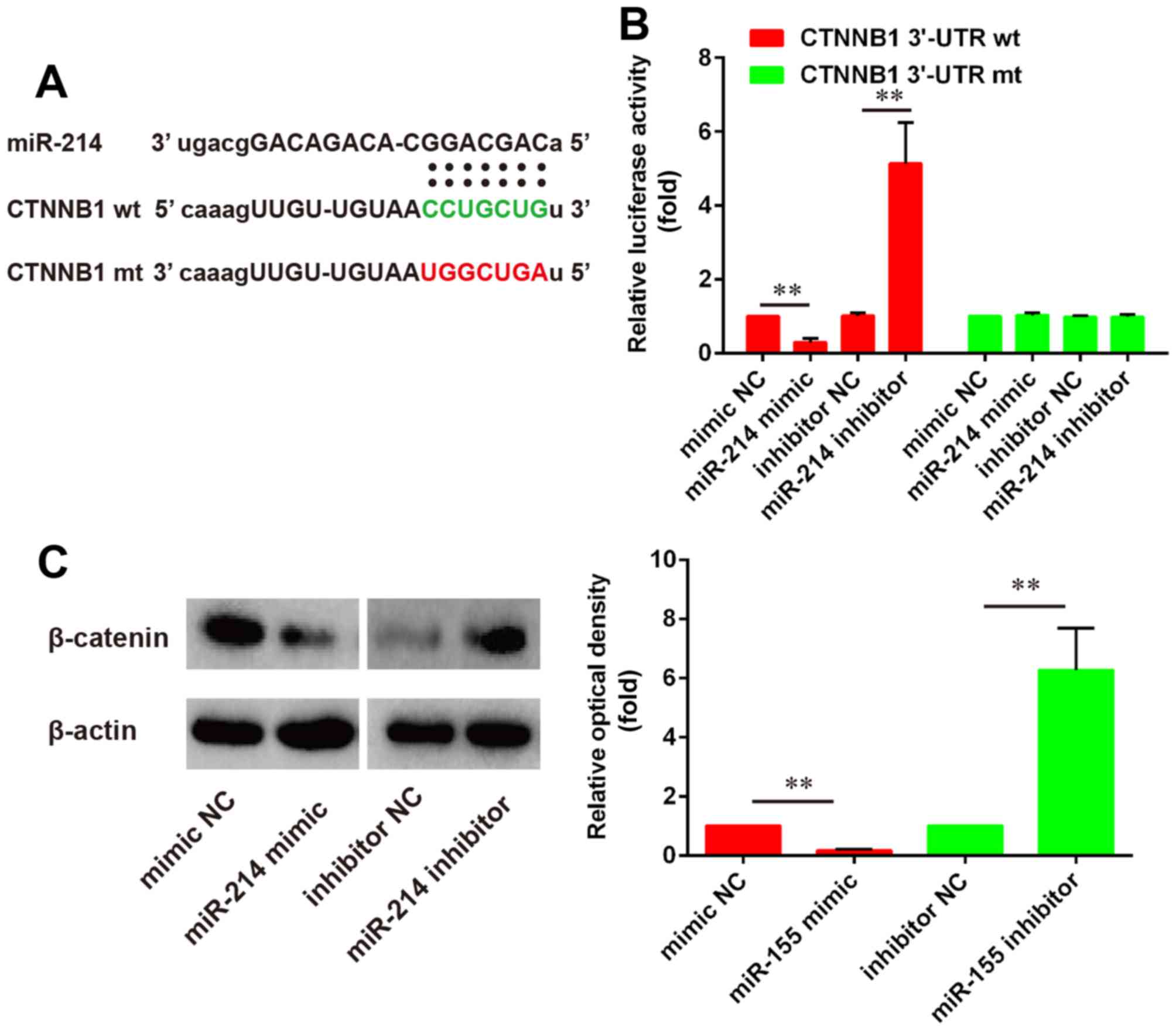 | Figure 3.miR-214 directly binds to and inhibits
the expression of the β-catenin CTNNB1 gene. (A) Schematic
representation of the firefly luciferase reporter constructs for
CTNNB1, indicating the putative interaction sites between
miR-214 and the 3′-UTR of CTNNB1. (B) PDLSCs were
co-transfected with luciferase reporter constructs containing wt or
mut CTNNB1 3′-UTR and miR-214 mimic, miR-214 inhibitor or NC
miRNA and luciferase activity was detected (n=3). (C) Western blot
analysis was used to assess the protein expression levels of
β-catenin in PDLSCs following transfection with miR-214 mimic,
miR-214 inhibitor or NC miRNA (n=3). Data are expressed as the mean
± standard deviation of 3 independent experiments. **P<0.01,
with comparisons indicated by lines. miR, microRNA; UTR,
untranslated region; PDLSC, periodontal ligament stem cell; wt,
wild-type; mt, mutant; NC, negative control. |
To further investigate whether miR-214 may regulate
the expression of β-catenin, the protein expression levels of
β-catenin in cells transfected with a miR-214 mimic or a miR-214
inhibitor were evaluated by western blotting. The results revealed
that miR-214 overexpression significantly downregulated the protein
expression levels of β-catenin compared with control, while miR-214
inhibition significantly increased β-catenin protein expression
compared with control (Fig.
3C).
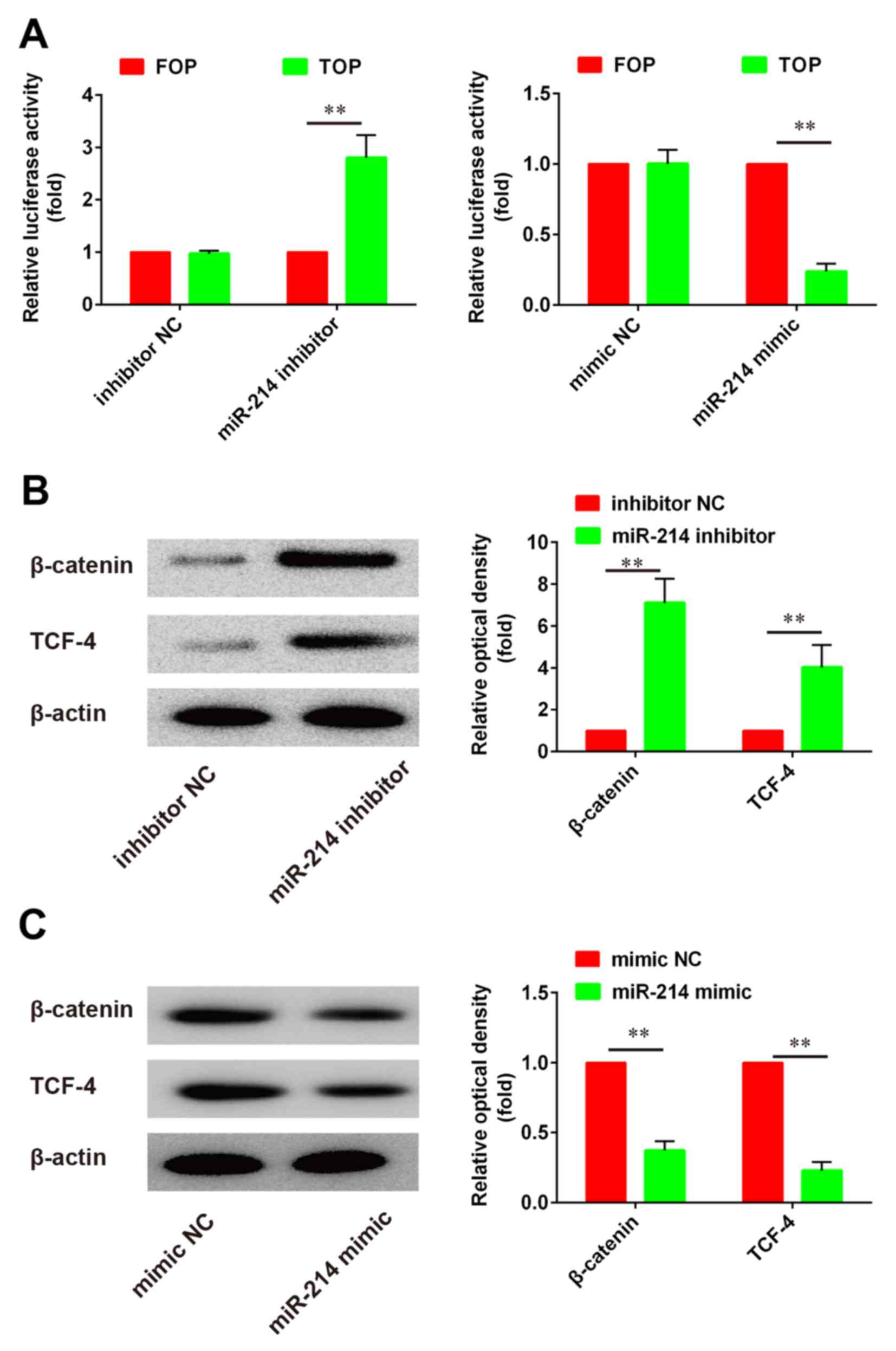
Relationship between miR-214
expression and activity of the β-catenin/Wnt pathway
The Wnt/β-catenin signaling pathway has been
reported to serve critical roles in the regulation of cell growth
and differentiation. To evaluate the effects of miR-214 on
Wnt/β-catenin signaling in PDLSCs, a luciferase assay based on a
TOPflash/FOPflash reporter plasmid system was used to evaluate
β-catenin-dependent transcriptional activity. The present results
demonstrated that Wnt/β-catenin signaling was activated following
miR-214 knockdown, whereas miR-214 overexpression produced the
opposite effects, as evidenced by the alterations in luciferase
activity (Fig. 4A). Western blot
analysis was used to investigate the protein expression levels of
β-catenin and TCF-4. The present results revealed that β-catenin
and TCF-4 protein expression was significantly upregulated
following miR-214 inhibition compared with control (Fig. 4B). By contrast, following miR-214
overexpression using a miR-214 mimic, β-catenin and TCF-4 protein
expression was significantly suppressed compared with control
(Fig. 4C). These findings
suggested that miR-214 inhibition may promote the osteoblastic
differentiation of PDLSCs, through the regulation of the
Wnt/β-catenin signaling pathway.
Discussion
In the present study a number of miRNAs were
identified that were differentially expressed between
differentiated and non-differentiated PDLSCs. Among them, miR-214
was further investigated by silencing and overexpression. The
results suggested that miR-214 may be implicated in the mechanisms
underlying osteoblastic differentiation of PDLSCs, as its
overexpression inhibited osteogenesis, whereas its knockdown had
the opposite effect. Furthermore, the β-catenin gene CTNNB1
was identified as a direct target of miR-214, and miR-214 was
demonstrated to promote osteoblastic differentiation by modulating
Wnt/β-catenin signaling.
miRNAs are small non-coding RNA molecules, with a
length of ~22 nucleotides, that bind to target mRNAs and prevent
their translation or promote their degradation (21). Through the post-transcriptional
regulation of gene expression, miRNAs have been demonstrated to
modulate numerous biological processes, including tumorigenesis,
cellular proliferation and differentiation. miR-214 has previously
been suggested to be implicated in osteogenic differentiation. Shi
et al (22) reported that
miR-214 suppressed osteogenic differentiation of C2C12 myoblasts by
targeting the transcription factor Osterix, whereas Yang et
al (23) demonstrated that
miR-214 attenuated osteogenesis by inhibiting the fibroblast growth
factor (FGF) receptor 1/FGF signaling pathway. These findings
suggested that targeting miR-214 may hold potential as a novel
therapeutic strategy for the treatment of patients with
postmenopausal osteoporosis. However, the exact roles of miR-214 in
the regulation of PDLSC differentiation, as well as the molecular
mechanisms underlying its actions have yet to be elucidated.
The Wnt/β-catenin signaling pathway has been
implicated in the mechanisms guiding cell fate decision in MSCs
(8), and miRNAs have been reported
to be involved in the regulation of Wnt/β-catenin signaling
(24). Su et al (25) demonstrated that miR-200a regulated
the epithelial-mesenchymal transition of cancer cells, via
regulating the activity of the Wnt/β-catenin signaling pathway. In
addition, Chen et al (26)
reported that miR-709 inhibited adipocyte differentiation, by
targeting glycogen synthase kinase 3β and subsequently activating
Wnt/β-catenin signaling. Similarly, miR-27 has been demonstrated to
promote odontoblastic differentiation in MDPC-23 cells by targeting
adenomatous polyposis coli and activating Wnt/β-catenin signaling,
through the accumulation of β-catenin (27). In the present study, the roles of
Wnt/β-catenin signaling during osteoblastic differentiation were
investigated in PDLSCs. The present results suggested that miR-214
may regulate the expression of β-catenin through the direct
interaction with the 3′-UTR of CTNNB1. Furthermore, a
TOPflash/FOPflash reporter luciferase assay was used to investigate
the Wnt/β-catenin-dependent transcriptional activity of the
TCF4 gene, and results suggested a significant association
between miR-214 expression and Wnt/β-catenin signaling
activity.
In conclusion, the present results suggested that
miR-214 may regulate the Wnt/β-catenin signaling pathway by
targeting CTNNB1, thus participating in the mechanisms
underlying PDLSC differentiation. The present findings provide
valuable insight into the molecular mechanisms underlying the
complex processes of PDLSC osteoblastic differentiation.
References
|
1
|
Armitage GC: Periodontal diagnoses and
classification of periodontal diseases. Periodontol. 34:9–21. 2000.
View Article : Google Scholar
|
|
2
|
Hiraga T, Ninomiya T, Hosoya A, Takahashi
M and Nakamura H: Formation of bone-like mineralized matrix by
periodontal ligament cells in vivo: A morphological study in rats.
J Bone Miner Metab. 27:149–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: Their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato T, Hattori K, Deguchi T, Katsube Y,
Matsumoto T, Ohgushi H and Numabe Y: Osteogenic potential of rat
stromal cells derived from periodontal ligament. J Tissue Eng Regen
Med. 5:798–805. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamplot JD, Qin J, Nan G, Wang J, Liu X,
Yin L, Tomal J, Li R, Shui W, Zhang H, et al: BMP9 signaling in
stem cell differentiation and osteogenesis. Am J Stem Cells.
2:1–21. 2013.PubMed/NCBI
|
|
6
|
Coleman DT, Gray AL, Stephens CA, Scott ML
and Cardelli JA: Repurposed drug screen identifies cardiac
glycosides as inhibitors of TGF-β-induced cancer-associated
fibroblast differentiation. Oncotarget. 7:32200–32209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishitani T, Kishida S, Hyodo-Miura J, Ueno
N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J and
Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade
functions in the Wnt-5a/Ca(2+) pathway to antagonize
Wnt/beta-catenin signaling. Mol Cell Biol. 23:131–139. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boland GM, Perkins G, Hall DJ and Tuan RS:
Wnt 3a promotes proliferation and suppresses osteogenic
differentiation of adult human mesenchymal stem cells. J Cell
Biochem. 93:1210–1230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartmann C and Tabin CJ: Dual roles of Wnt
signaling during chondrogenesis in the chicken limb. Development.
127:3141–3159. 2000.PubMed/NCBI
|
|
10
|
Ross SE, Hemati N, Longo KA, Bennett CN,
Lucas PC, Erickson RL and MacDougald OA: Inhibition of adipogenesis
by Wnt signaling. Science. 289:950–953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang Y, Zhang C, Wang S, Xiong F, Zhao C,
Peng F, Feng S, Yu M, Li M and Zhang Y: Activated beta-catenin
induces myogenesis and inhibits adipogenesis in BM-derived
mesenchymal stromal cells. Cytotherapy. 9:667–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizuno Y, Yagi K, Tokuzawa Y,
Kanesaki-Yatsuka Y, Suda T, Katagiri T, Fukuda T, Maruyama M, Okuda
A, Amemiya T, et al: miR-125b inhibits osteoblastic differentiation
by down-regulation of cell proliferation. Biochem Biophys Res
Commun. 368:267–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y,
Pan Z, Li T, Hu M, Cui H, et al: MicroRNAs contribute to
promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol
Biochem. 32:1818–1829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Iwama A, Satake M and Kohu K:
MicroRNA-27 enhances differentiation of myeloblasts into
granulocytes by post-transcriptionally downregulating Runx1. Br J
Haematol. 145:412–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McDaneld TG, Smith TP, Doumit ME, Miles
JR, Coutinho LL, Sonstegard TS, Matukumalli LK, Nonneman DJ and
Wiedmann RT: MicroRNA transcriptome profiles during swine skeletal
muscle development. BMC Genomics. 10:772009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Zhao ZN, Cheng JT, Zhang B, Xu J,
Huang F, Zhao RN and Chen YJ: Ibandronate promotes osteogenic
differentiation of periodontal ligament stem cells by regulating
the expression of microRNAs. Biochem Biophys Res Commun.
404:127–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Qu C, Chen C, Liu Y, Akiyama K, Yang
R, Chen F, Zhao Y and Shi S: Basic fibroblast growth factor
inhibits osteogenic differentiation of stem cells from human
exfoliated deciduous teeth through ERK signaling. Oral Dis.
18:285–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han L, Yang Y, Yue X, Huang K, Liu X, Pu
P, Jiang H, Yan W, Jiang T and Kang C: Inactivation of PI3K/AKT
signaling inhibits glioma cell growth through modulation of
β-catenin-mediated transcription. Brain Res. 1366:9–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones-Rhoades MW and Bartel DP:
Computational identification of plant microRNAs and their targets,
including a stress-induced miRNA. Mol Cell. 14:787–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B,
Li H and Ma C: MicroRNA-214 suppresses osteogenic differentiation
of C2C12 myoblast cells by targeting Osterix. Bone. 55:487–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Ge D, Cao X, Ge Y, Chen H, Wang W
and Zhang H: MiR-214 Attenuates osteogenic differentiation of
mesenchymal stem cells via targeting FGFR1. Cell Physiol Biochem.
38:809–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T,
Zhang J, Kang C and Zhang Q: MicroRNA-200a suppresses the
Wnt/β-catenin signaling pathway by interacting with β-catenin. Int
J Oncol. 40:1162–1170. 2012.PubMed/NCBI
|
|
26
|
Chen H, Mo D, Li M, Zhang Y, Chen L, Zhang
X, Li M, Zhou X and Chen Y: miR-709 inhibits 3T3-L1 cell
differentiation by targeting GSK3β of Wnt/β-catenin signaling. Cell
Signal. 26:2583–2589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang T and Xu Z: miR-27 promotes
osteoblast differentiation by modulating Wnt signaling. Biochem
Biophys Res Commun. 402:186–189. 2010. View Article : Google Scholar : PubMed/NCBI
|