Introduction
Aplastic anemia (AA) is defined as pancytopenia
which hypocellular bone marrow, no abnormal infiltrate, and no
increase in reticulin. The etiology of the disease remains unclear,
but most experts have posited that ‘AA is an immune-mediated
disease with active destruction of hematopoietic cells by T
lymphocytes’ (1–3). Activated suppressor T lymphocytes
producing interferon (IFN) participate in the pathogenesis of bone
marrow failure (4,5).
CD4+CD25+FOXP3+ regulatory T cells
decrease in patients with AA, which may explain the increased
autoreactive T cells and the development of AA phenotype (6). The disequilibrium of T cells has an
important function in the pathogenesis of AA.
Telomeres are heterochromatic structures with tandem
DNA repeats of 5′-TTAGGG-3′ at the chromosomal ends. With each cell
division, telomeres became shorter and shorter because of
‘end-replication problem’. Therefore, telomere shortening is an
important suppressive mechanism by limiting cellular proliferative
capacity through regulating senescence checkpoint activation.
Critically short telomeres are likely to form telomeres fusions and
lead to genomic instability (7–10).
Abnormal telomere shortening has been reported in patients with
acquired hematologic disorders; extremely short telomeres suggest
of increased hematopoietic stress. Telomeres are also markers of
replication and/or oxidative stress in many disease pathways.
Our team has detected decreased telomere length in
SAA, which was associated with expression of sheltering component
POT1 (11). In order to understand
the abnormalities of telomere more deeply, we measured the relative
telomere length (RTL) of different T lymphocyte subsets in SAA
patients and the CD28, CD70, CD158 expression level on
CD8+T lymphocytes, apoptosis rate of primary
CD8+T lymphocytes, type 1 cytokines including IFN-γ,
tumor necrosis factor-α (TNF-α) secretion and cell cycles of
CD8+T lymphocytes to explore that the relationship
between the telomere and the T lymphocytes activate state in the
pathogenesis of this disease.
Materials and methods
Patients and normal controls
All the patients with SAA were collected in the
Hematology Department of General Hospital Tianjin Medical
University from July 2015 to July 2016 and were diagnosed according
to International AA Study Group Criteria (2). SAA was defined as bone marrow
cellularity of <25% and severe pancytopenia with at least two of
the following peripheral blood count criteria: i) Absoulute
neutrophil counts are <0.5×109/l; ii) absolute
platelet counts are <20×109/l; iii) absoulute
reticulocyte counts are <15×109/l. If the neutrophil
count was <0.2×109/l, SAA was considered very severe.
Patients were excluded if they had congenital AA or other diseases,
such as paroxysmal noctunal hemoglobinuria (PNH), myelodysplastic
syndrome (MDS), iron deficiency anemia (IDA), megaloblastic anemia
(MA), anemia of chronic disease (ACD), autoimmune hemolytic anemia
(AIHA). Thirty SAA patients (15 males, 15 females) were enrolled in
our study with a median age of 29 years (range, 5–63 years),
including 22 untreated SAA (10 males, 12 females), 8 recover SAA (5
males, 3 females), all recover SAA patients have received the
treatment of cyclosporine (CsA; 3–5
mg.kg−1.day−1), and anti-human thymocyte
globulin (ATG; 2.5–5 mg.kg−1.day−1, 5 days).
Twenty-five healthy volunteer as health controls whose race, living
area, sex and age were same as those of SAA patietns were also
enrolled in this study with a median age of 25 years (range, 15–64
years). Sufficient samples such as peripheral blood which were
taken from their peripheral veins were available for testing.
Cell separation
Peripheral blood mononuclear cells (PBMCs) were
isolated from heparin anti-coagulant venous blood of SAA and normal
controls using Ficoll-Hypaque density gradient centrifugation,
CD4+, CD8+T lymphocytes were purified using
the respective anti-CD4 and anti-CD8 mAb-conjugated microbeads
(Miltenyi Biotec, Bergisch Gladbach, Germany) according to the
manufacturer's instructions.
Non-adherent cells culture of
MOLT-4
As inner control cells, MOLT-4s were plated in
RPMI-1640 culture medium (containing 10% FBS and 1% mycillin;
Gibco-BRL, Grand Island, NY, USA), at 37°C in an atmosphere
containing 5% CO2, 2–3 days in liquid. MOLT-4 was human
acute 1ymphoblastic leukemia cell 1ine, which has longer telomere
length. Some scholars have veritied that there are no significant
difference among the different passages, so we makes it be a
feasible control cells in measurement of telomere length by
Flow-FISH (12).
Telomere length measurement by
Flow-FISH
According to Telomere PNA kit/FITC for Flow
Cytometry (Dako, Carpinteria, CA, USA), 1–2×106 sorted
sample cells and control cells (MOLT-4) were diluted with PBS 3 ml,
divided into A, B tubes, and centrifugated to get rid of
supernatant. DNA is denatured at 82°C for 10 min in an eppendorf
tube in the presence of hybridization solution with or without
fluorescein-conjugated PNA telomere probe. Then, hyvridization
takes place in the dark at room temperature overnight. The
hyvridization is followed by 2 washes in wash solution at 40°C for
10 min each. Finally the cells are resuspended in DNA-staining
solution and stored in the dark at 2–8°C for 2 to 3 h before
analysis by flow cytometry. The specific fluorescence from telomere
staining will be observed in FL1, and fluorescence from DNA
staining will be observed in FL3. Finally, at least 20,000 cells
were acquired and analysed by fluorescence-activated cell sorting
(FACS) Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). DNA index of the cells determined as the following: RTL =
(mean FL1 sample cells with probe – mean FL1 sample cells without
probe) × DNA index of control cells × 100/(mean FL1 control cells
with probe – mean FL1 control cells without probe) × DNA index of
sample cells.
CD28, CD158 and CD70 expression level
on CD8+T lymphocytes by flow cytometry
Heparin anticoagulant venous blood of SAA and normal
controls were prepared. Firstly, 20 µl whole blood were incubated
with for 15 min 5 µl PerCP-conjugated anti-human CD3, 5 µl antigen
presenting cell (APC)-conjugated anti-human CD8, the separately
mixing with 5 ul PE-conjugated anti human CD28, 20 µl PE-conjugated
anti human CD158 and 20 µl PE-conjugated anti human CD70 (all
antibody except for CD158 from BD Biosciences; PE-conjugated
anti-human CD158 from R&D Systems, Minneapolis, MN, USA) in the
dark at 4°C. Subsequently, erythrocyte were lysed for 10 min by
hemolysin (BD Biosciences) in the dark at 4°C, and washed by PBS.
Finally the CD28, CD158 and CD70 expression level on
CD8+T were detected using FACSC alibur flow cytometry
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The apoptosis rate the primary
CD8+T lymphocytes by flow cytometry
Apoptosis is normal physiologic processes, which is
characterized by certain morphologic features, including plasma
membrane asymmetry and attachment, condensation of the cytoplasm
and nucleus, and inter nucleosomal cleavage of DNA (13). In apoptotic cells, the membrane
phosphorlipid phosphatedylserine (PS) is translocated from inner to
the outer leaflet of the plasma membrane, thereby exposing PS to
the external celluar environment. Annexin V a has high affinity for
PS in the earlier stages of apoptosis, so FITC Annexin V can stain
cells from the earliest stages to necrotic processes. However,
viable cells with intact membranes exclude propidium iodide (PI),
wheras the membranes of dead and damaged cells are permeable to
PI.
In our experiments, the 1×106 sorted
CD8+T lymphocytes were stained using FITC Annexin V and
PI (FITC Annexin V Apoptosis Detection kit I; BD Biosciences) for
15 min in the dark at room temperature, then added 400 µl 1X
binding buffer and the stained cells were analyzed by FACSCalibur
flow cytometry (Bio-Rad Laboratories, Inc.).
IFN-γ, TNF-α of the post-stimulate
CD8+T lymphocytes by ELISA
The sorted cells (1×106) were collected
and cultured in RPMI-1640 culture medium (containing 10% FBS and 1%
mycillin; Gibco-BRL), then fixed with 10 ng/ml PMA (Sigma) and 0.4
ug/ml ionomycin calcium salt (sigma) at 37°C in an atmosphere
containing 5% CO2 for 6 h and then centrifuge supernate
for 20 min to remove insoluble impurity and cell debris at 1,000 ×
g at 2–8°C. Collect the clear supernate and IFN-γ, TNF-α were
respectively measured in the method of sandwich-ELISA using human
IFN-γ ELISA kit and human TNF-α ELISA kit (Elabscience
Biotechnology Co., Ltd., Wuhan China) by the microplate reader
(Bio-Rad Laboratories, Inc.).
Cell cycle analyzed by flow
cytometry
Cell cycle of lymphocytes in SAA was detected using
DNA analyzing agent (BD Biosciences). The sorted cells
(1×106) were collected and prepared in a suspended
solution, then fixed with 125 µl solution A at room temperature for
10 min. Futhermore, the cells were incubated with 200 µl solution B
at room temperature for 10 min, followed by solution C for 10 min
at 4°C in the dark. The cell cycle was analyzed using FACSC alibur
flow cytometry (Bio-Rad Laboratories, Inc.).
Statistical analysis
Date were calculated and displayed as mean ±
standard and analyzed with SPSS 16.0 statistical software. For
comparison of disease parameters, a t-test and correlation analysis
was used. P-values <0.05 were considered to indicate a
statistically significant difference.
Results
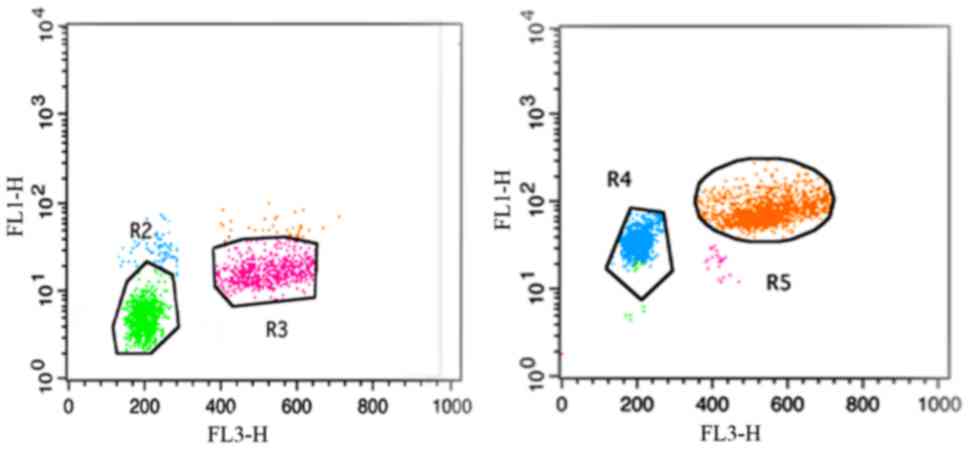
The purity of lymphocytes
The purity of enriched CD4+,
CD8+T lymphocytes were evaluated by flow cytometry and
was generally >90% (Fig.
1).
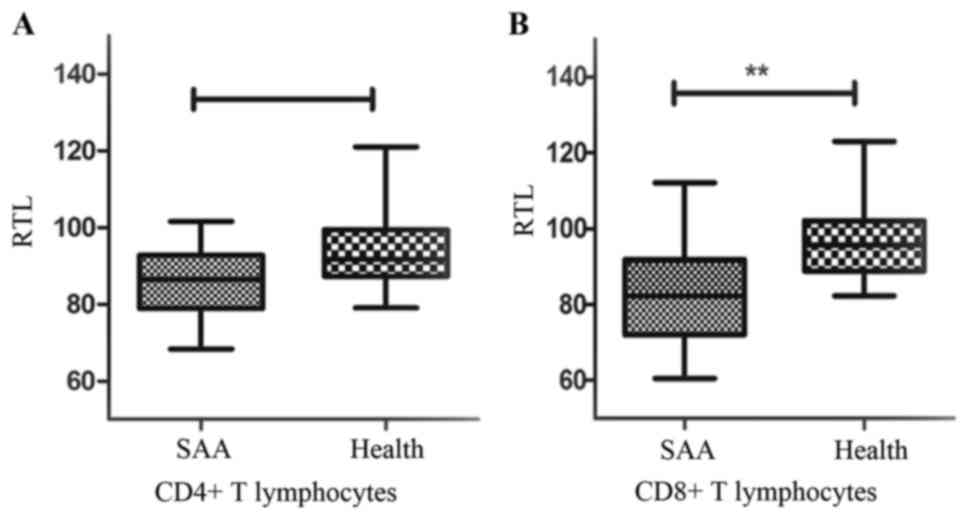
RTLs of CD4+ and
CD8+T lymphocytes
RTLs were measured by Flow-FISH (Fig. 2). RTLs of CD4+T
lymphocytes were (86.16±11.91%) in SAA, which was no statistical
difference those of healthy controls (91.65±7.25%) (P=0.09)
(Fig. 3A). RTLs of
CD8+T lymphocytes were (82.17±12.17%), which was
significant shorter than the healthy controls (95.71±9.11%)
(P<0.01) (Fig. 3B).
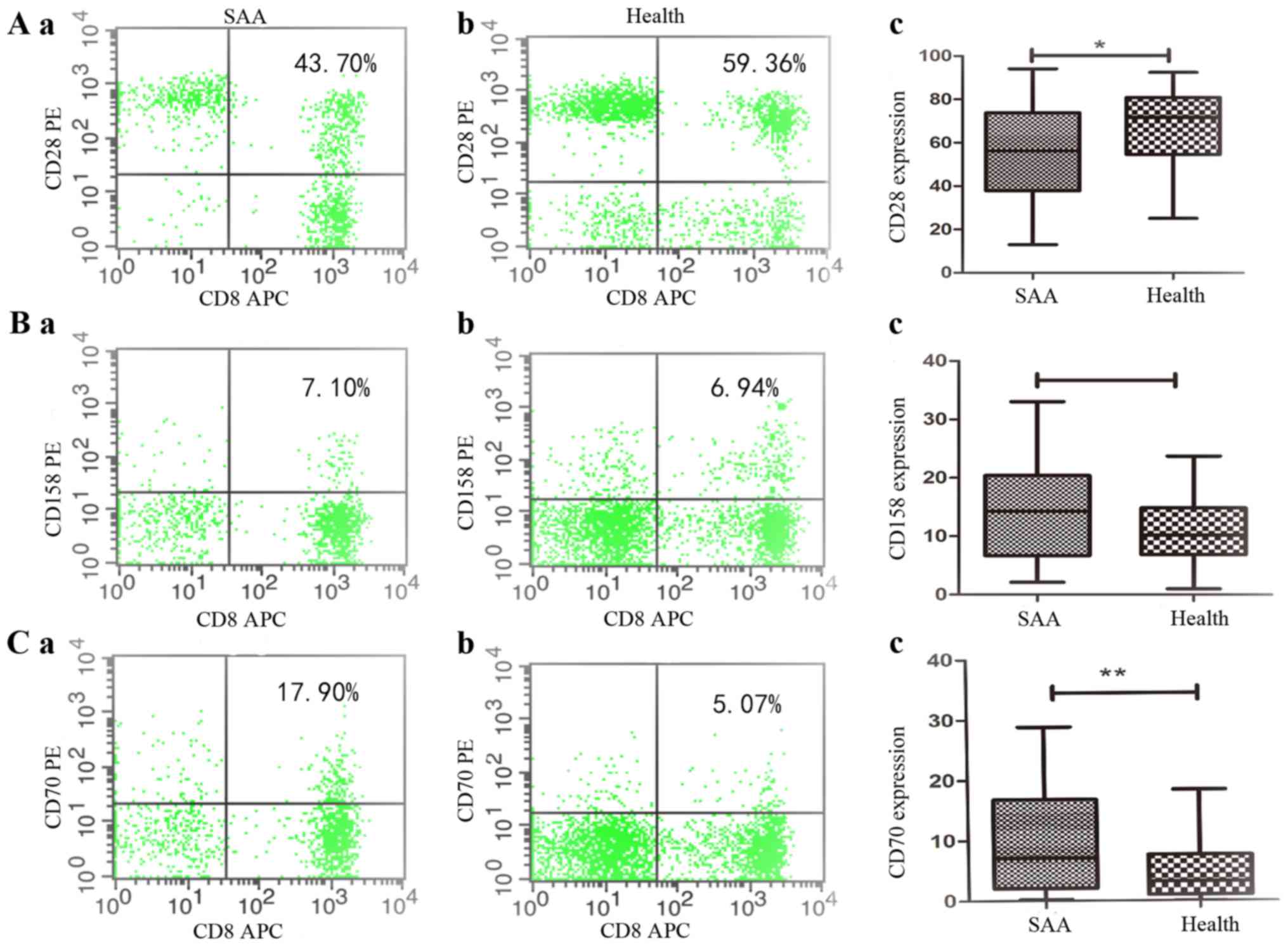
Persistent CD28, CD158, CD70
expression on CD8+T lymphocytes
Flow cytometric analysis revealed the co-stimulatory
signals CD28 expression level on CD8+T lymphocytes in
SAA was (56.56+20.89%), which were significantly lower than those
in health controls (66.53+17.82%) [P=0.033, 95% CI (−19.11, −0.84)]
(Fig. 4A). In SAA patients,
expression of CD158 on CD8+T lymphocytes was
(14.0030+8.1719%), that was no significantly with the health
controls (11.35+5.92%) [P=0.146, 95% CI (−0.95, 6.26)] (Fig. 4B), and expression of CD70 on
CD8+T lymphocytes was (9.82+8.80%), which were
significantly higher than those in health controls (4.77+4.67%)
[P=0.006, 95% CI (1.55,8.55)] (Fig.
4C). These data were verified at the protein level by
multicolor flow cytometry using CD8+T cell.
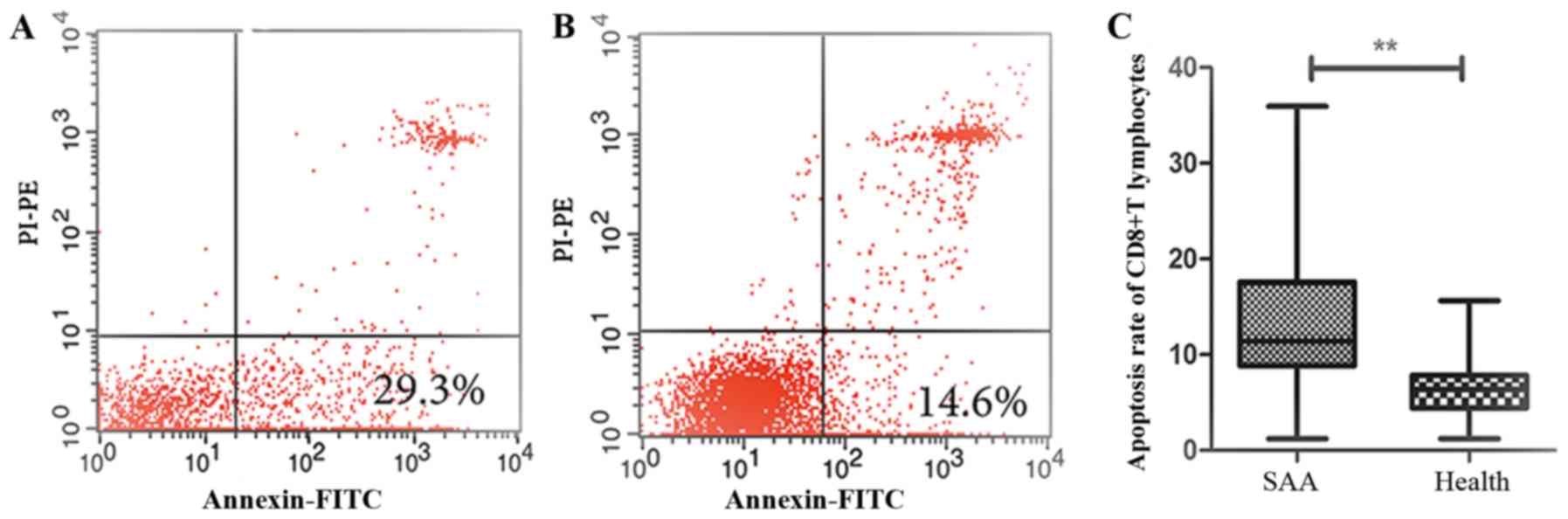
The apoptosis rate of the primary
CD8+T lymphocytes
Flow cytometry assay revealed that CD8+T
lymphocytes in SAA were vulnerable to apoptosis (13.20+7.48%),
which were significantly higher than those in normal controls
(6.75+3.50%) [P<0.01, 95% CI (3.35, 9.55)] (Fig. 5).
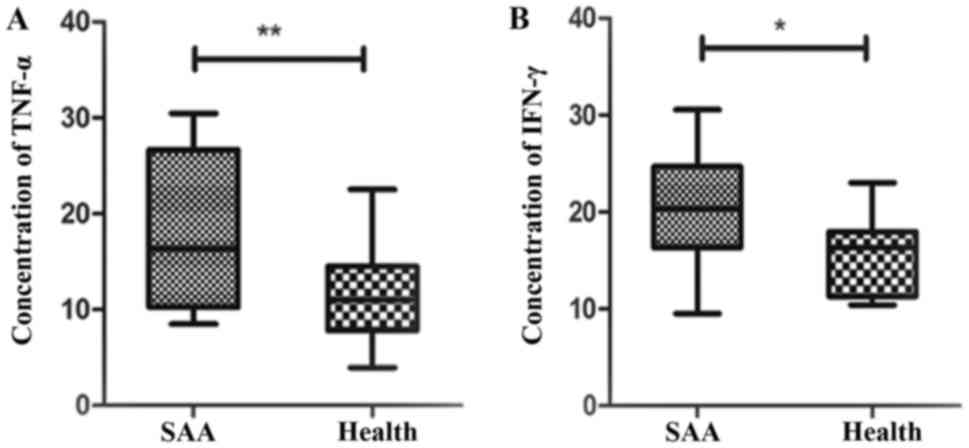
IFN-γ, TNF-α of the post-stimulate
CD8+T lymphocytes
The microplate reader show that CD8+T
lymphocytes secreted IFN-γ in SAA were apparently higher than the
health controls (20.59+6.05 vs. 15.82+4.21 U/ml) [P=0.018, 95% CI
(0.90, 8.65)]. The concentration of TNF-α increased in SAA
(18.12+7.96 U/ml), which were significantly higher than those in
normal controls (11.49+4.72 U/ml) [P=0.002, 95% CI (2.52, 10.74)]
(Fig. 6).
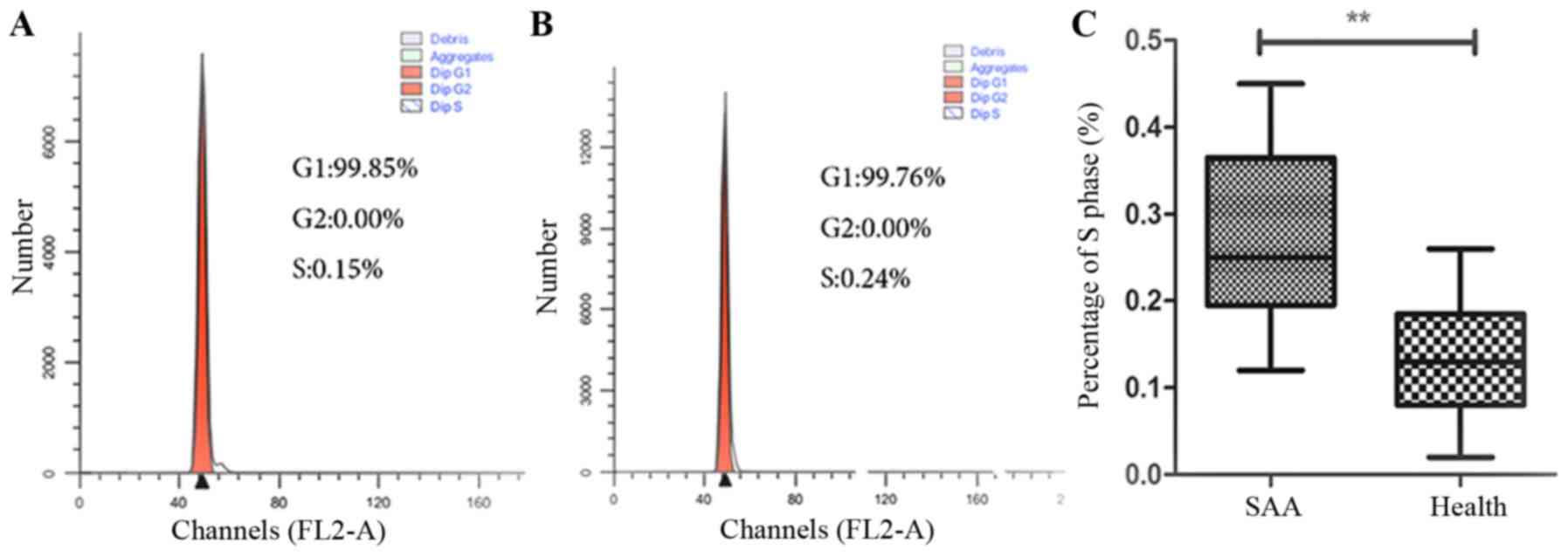
Cell cycle progression of
CD8+T lymphocytes
Flow cytometry assay revealed that CD8+T
lymphocytes in SAA were stimulated to enter the S phase
(0.21±0.08%), which were significantly higher than those in normal
controls (0.05±0.03%) (P<0.01) (Fig. 7). CD8+T lymphocytes of
untreated AA were promoted to S phase, which were significantly
higher than those in normal controls.
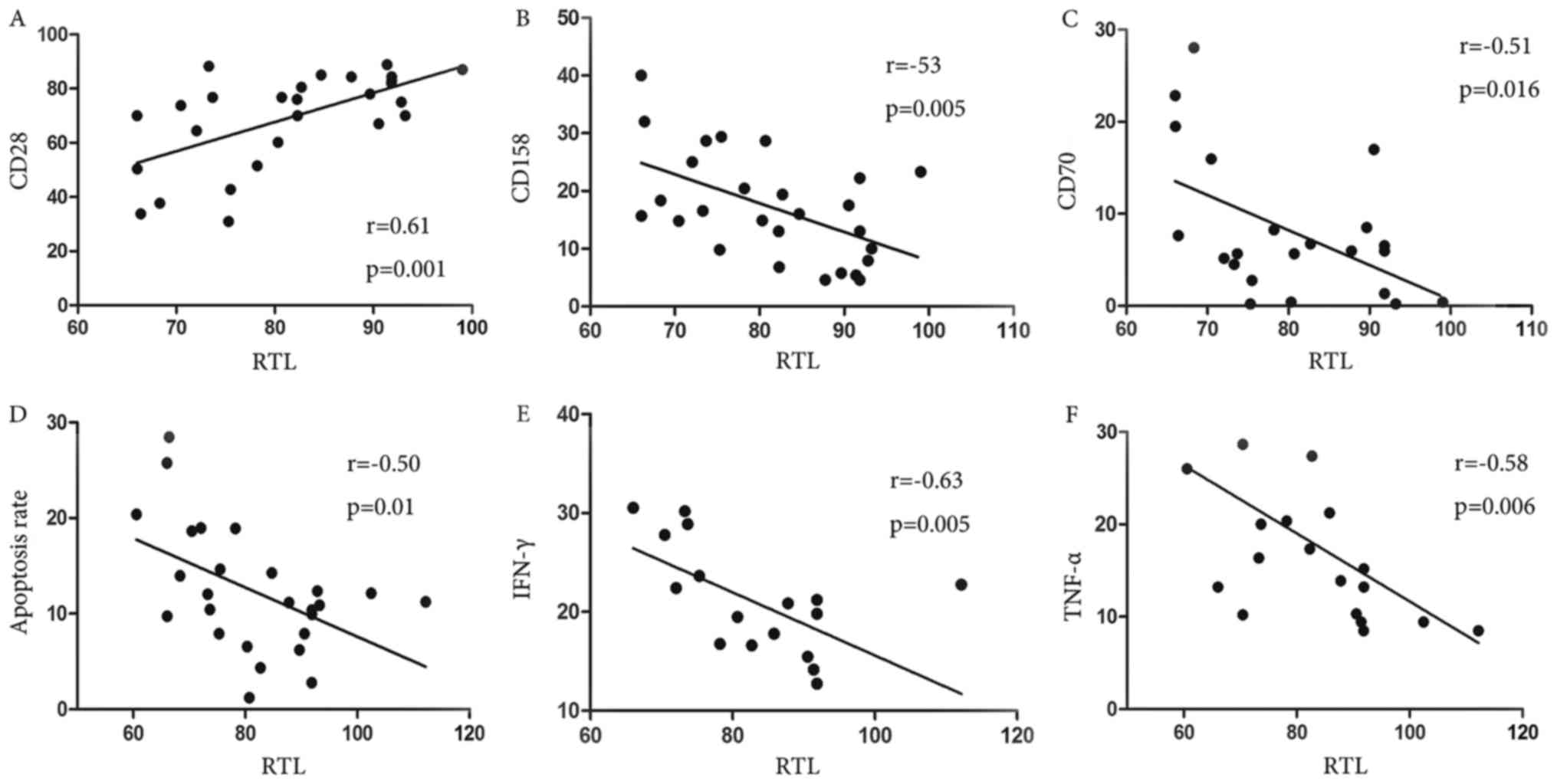
Correlation RTL and the function of
the CD8+T lymphocytes
We next addressed the question of whether the
shorted telomere would affect the function of the CD8+T
cell. Using linear regression analysis, we made the correlation
between telomere length and those, such as expression of CD28,
CD158 or CD70 on CD8+T lymphocytes, apoptosis rate of
primary CD8+T lymphocytes, the level of secretion IFN-γ,
TNF-α after stimulating CD8+T lymphocytes and cell cycle
progression of CD8+T lymphocytes. We found significant
positive correlations with RTL for CD28 (r=0.61, P=0.001) (Fig. 8A). However, there have been
significant negative correlations with RTL for CD158 (r=−53,
P=0.005) (Fig. 8B), CD70 (r=−0.51,
P=0.016) (Fig. 8C), apoptosis
(r=−0.50, P=0.01) (Fig. 8D), IFN-γ
(r=−0.63, P=0.005) (Fig. 8E),
TNF-α (r=−0.58, P=0.006) (Fig. 8F)
of CD8+T lymphocytes (Fig.
8).
Discussion
Telomeres play an important role in maintaining
chromosomes construction integrity and protecting genetic
information integrity (14), the
shortening of which mainly depends on antigen irritated and many
stimulatory factors (15). On one
hand, telomere shortening in lymphocytes is considered to present
the immune system aging and may be relative to autoimmune responses
(16,17). On the other hand, it presents a
marker for replicative history of lymphocyte (18). Shortened telomere may ultimately
trigger replicative senescence leading to cellular aging (19,20).
Scheinberg et al (21)
found that telomere length of mononuclear cells shortened, which
had no response to drug reaction undergoing immunosuppressive
therapy in AA patients. Also, disease relapse, clonal evolution,
and overall survival rates were closely related with telomere
length. So telomeres shorten generated chromosome instability,
which was the important reason of bone marrow failure. Calado et
al (22) found that the
telomere lengths of AA were inversely correlated with the
developing of cytogenetically abnormal clone. Wang et al
(23) found shorter telomere
length in CD3+T lymphocytes of bone marrow with
untreated AA patients, implying that telomere length change maybe
the reason of bone marrow failure.
In most normal human somatic cells, telomere repeats
are gradually lost with replication and age, owing to the inability
of conventional DNA polymerase to fully replicate the 3′-end of
DNA, then when it shorten to a certain degree, the cell will be
tend to death (21). Several
experimental findings suggest that the effectiveness of the immune
response declines with age particular in the latter stages of life
(24), organism will be lost CD28,
which is a co-stimulatory signaling molecule and is believed to set
the threshold for T cell activation. The co-stimulatory signaling
molecule is found on T cells must bind B7-1 and B7-2, which are
expressed on APCs, to trigger T cell activation (25). In a word, CD28 has multiple roles
during T cell activation, proliferation and survival (26). Simultaneously, these cells will
acquire many new effector factors, such as killer-cell
immunoglobulin-like receptors (KIRs), leukocyte function receptor
(LFA-1), CD70, perforin and profound altered expression of several
chemokines and cytokines (27).
CD158 is KIR, which function in T cell activation is complicated.
CD70 is the immunoglobulin superfamily member, which CD70 is
similar with TNF family members and can regard as the
co-stimulatory signaling molecule of T leukomonocytes regulating
the activation of B leukomonocytes (28,29).
In normal, T cells hardly express CD70 and CD158, but when the
organism undergone a series of allergic reaction or autoimmune
response, the activated T lymphocytes increased expression of CD70
and CD158, which indicates that they play a important role in
pathological damage caused by immune disorders (30,31).
The high level of effector molecules has the additional effect of
lowering the T cell activation threshold, enhanced cytotoxicity and
display suppressive functions, finally, it would progress into an
autoimmune disease, e.g., rheumatoid arthritis (RA), multiple
sclerosis, Wegener's granulomatosis, Graves' disease, or Ankylosing
spondylitis (32,33).
SAA is a primary disorder of severe bone marrow
failure, pathogenesis of which is known to be closely related to
autoimmune T cell hyper-function, especially CD8+T
cells. Patients who suffered from SAA have a significant increase
in CD8+ suppressor T lymphocytes (34). Our study demonstrated for the first
time that telomere length of CD8+T lymphocytes shorted
significantly in SAA patients, while telomere length of
CD4+T lymphocytes in SAA patients was no significant
changes compared with normal controls, indicating that cellular
immunity plays the dominant role, especially CD8+T
lymphocytes in AA. The results suggesting telomere length
shortening is an important role in cellular immunity in the
pathogenesis of AA. Our present data are consistent with findings
in other autoimmune diseases, such as SLE, RA, systemic sclerosis
and Type I diabetes, above all there are telomere shortening
(35–38).
A further study indicated that CD8+T
lymphocytes was sustained activated in SAA. With sustained
CD8+T cell stimulation, CD28 expression decreases, CD70
expression increases, apoptosis rate increases, type 1 cytokines
including IFN-γ, TNF-α secretion increases and the percentage of S
phase will be high in CD8+T lymphocytes. Above all
results suggesting CD8+T lymphocytes has lower
activation threshold and hyper-function in SAA. Furthermore, we
analyze the relationship between the RTLs and the function of
CD8+T lymphocytes. We found that there have been
significant positive correlations with RTL for CD28. However, there
have been significant negative correlations with RTL for CD158,
CD70, apoptosis, IFN-γ, TNF-α. This phenomenon show that the
shorten telomere of CD8+T lymphocytes may be change
their function in SAA.
In conclusion, we reported the short telomere length
of different lymphocytes function subsets in SAA for the first time
and primary explore the role of the shorten telomere cells.
Telomere attrition is not only simply a biomarker; but also, a
plausible mechanism for destabilization of the genome has been
inferred from basic telomere biology. Furthermore, many studies
should be done to explore the function of shortening telomere
length of T cells in pathogenesis of AA.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81570816, 81370607,
81570111, 81400085 and 81400088), Tianjin Municipal Natural Science
Foundation (grant nos. 15JCYBJC24300, 14JCYBJC25400), Tianjin City
High School Science and Technology Fund Planning Project (grant no.
20140109).
References
|
1
|
Bacigalupo A: Aplastic anemia:
Pathogenesis and treatment. Hematology Am Soc Hematol Educ Program.
23–28. 2007.PubMed/NCBI
|
|
2
|
Marsh JC, Ball SE, Cavenagh J, Darbyshire
P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca
J, et al: Guidelines for the diagnosis and management of aplastic
anaemia. Br J Haematol. 147:43–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young NS, Calado RT and Scheinberg P:
Current concepts in the pathophysiology and treatment of aplastic
anemia. Blood. 108:2509–2519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zoumbos NC, Gascon P, Djeu JY and Young
NS: Interferon is a mediator of hematopoietic suppression in
aplastic anemia in vitro and possibly in vivo. Proc Natl Acad Sci
USA. 82:188–192. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Fu R, Wang HQ, Liu CY, Ruan EB, Qu
W, Liang Y, Wang GJ, Wang XM, Liu H, et al: Research of regulative
factors on CD8(+)HLA-DR(+) effector T cells in severe aplastic
anemia. Zhonghua Yi Xue Za Zhi. 92:1665–1668. 2012.(In Chinese).
PubMed/NCBI
|
|
6
|
Solomou EE, Rezvani K, Mielke S, Malide D,
Keyvanfar K, Visconte V, Kajigaya S, Barrett AJ and Young NS:
Deficient CD4+CD25+FOXP3+ T
regulatory cells in acquired aplastic anemia. Blood. 110:1603–1606.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longhese MP: DNA damage response at
functional and dysfunctional telomeres. Genes Dev. 22:125–140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffith JD, Comeau L, Rosenfield S,
Stansel RM, Bianchi A, Moss H and de Lange T: Mammalian telomeres
end in a large duplex loop. Cell. 97:503–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayflick L: The limited in vitro lifetime
of human diploid cell strains. Exp Cell Res. 37:614–636. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Mei SC, Fu R, Wang HQ and Shao ZH:
Expression of Shelterin component POT1 is associated with decreased
telomere length and immunity condition in humans with severe
aplastic anemia. J Immunol Res. 2014:4395302014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma XC, Liu CY, Sun XJ, He JJ, Wan SG and
Sun WL: Genetic characteristics of human acute lymphoblastic
leukemia cell line Molt-4. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
22:280–284. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Jing Yan, Kang Min, Liu Jin, Li Jingyu and
Tang Anzhou: Mechanism of apoptosis of nasopharyngeal carcinoma
cells induced by polysaccharides extracts from Hedyotic
diffusa. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
29:641–644. 2015.(In Chinese). PubMed/NCBI
|
|
14
|
Calado RT and Dumitriu B: Telomere
dynamics in mice and humans. Semin Hematol. 50:165–174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son NH, Murray S, Yanovski J, Hodes RJ and
Weng N: Lineage-specific telomere shortening and unaltered capacity
for telomerase expression in human T and B lymphocytes with age. J
Immunol. 165:1191–1196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goronzy JJ, Fujii H and Weyand CM:
Telomeres, immune aging and autoimmunity. Exp Gerontol. 41:246–251.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klapper W, Moosig F, Sotnikova A, Qian W,
Schröder JO and Parwaresch R: Telomerase activity in B and T
lymphocytes of patients with systemic lupus erythematosus. Ann
Rheum Dis. 63:1681–1683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blasco MA: Immunosenescence phenotypes in
the telomerase knockout mouse. Springer Semin Immunopathol.
24:75–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kipling D: Telomeres, replicative
senescence and human ageing. Maturitas. 38:25–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klapper W, Parwaresch R and Krupp G:
Telomere biology in human aging and aging syndromes. Mech Ageing
Dev. 122:695–712. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scheinberg P, Cooper JN, Sloand EM, Wu CO,
Calado RT and Young NS: Association of telomere length of
peripheral blood leukocytes with hematopoietic relapse, malignant
transformation, and survival in severe aplastic anemia. JAMA.
304:1358–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calado RT, Cooper JN, Padilla-Nash HM,
Sloand EM, Wu CO, Scheinberg P, Ried T and Young NS: Short
telomeres result in chromosomal instability in hematopoietic cells
and precede malignant evolution in human aplastic anemia. Leukemia.
26:700–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Fu R, Wang HQ, Qu W, Ruan EB, Wang
GJ, Liu H, Wu YH, Wang XM, Song J, et al: Telomere length and gene
expression of shelterin in CD3(+) T cell of severe aplastic anemia
patients. Zhonghua Yi Xue Za Zhi. 93:1533–1536. 2013.(In Chinese).
PubMed/NCBI
|
|
24
|
Brümmendorf TH, Maciejewski JP, Mak J,
Young NS and Lansdorp PM: Telomere length in leukocyte
subpopulations of patients with aplastic anemia. Blood. 97:895–900.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fagnoni FF, Vescovini R, Mazzola M,
Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M and
Sansoni P: Expansion of cytotoxic CD8+CD28− T
cells in healthy ageing people, including centenarians. Immunology.
88:501–507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Gál I, Vermes C, Alegre ML, Chong
AS, Chen L, Shao Q, Adarichev V, Xu X, Koreny T, et al: Cutting
edge: Cbl-b: One of the key molecules tuning CD28- and
CTLA-4-mediated T cell costimulation. J Immunol. 173:7135–7139.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riley JL and June CH: The CD28 family: A
T-cell rheostat for therapeutic control of T-cell activation.
Blood. 105:13–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weng NP, Akbar AN and Goronzy J: CD28(−) T
cells: Their role in the age-associated decline of immune function.
Trends Immunol. 30:306–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borst J, Hendriks J and Xiao Y: CD27 and
CD70 in T cell and B cell activation. Curr Opin Immunol.
17:275–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coquet JM, Middendorp S, van der Horst G,
Kind J, Veraar EA, Xiao Y, Jacobs H and Borst J: The CD27 and CD70
costimulatory pathway inhibits effector function of T helper 17
cells and attenuates associated autoimmunity. Immunity. 38:53–65.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nazari M, Mahmoudi M, Rahmani F, Akhlaghi
M, Beigy M, Azarian M, Shamsian E, Akhtari M and Mansouri R:
Association of killer cell immunoglobulin-like receptor genes in
iranian patients with rheumatoid arthritis. PLoS One.
10:e1437572015. View Article : Google Scholar
|
|
32
|
Schirmer M, Goldberger C, Würzner R,
Duftner C, Pfeiffer KP, Clausen J, Neumayr G and Falkenbach A:
Circulating cytotoxic CD8(+) CD28(−) T cells in ankylosing
spondylitis. Arthritis Res. 4:71–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Z, Yi L, Tao H, Huang J, Jin Z, Xiao
Y, Feng C and Sun J: Enhancement of soluble CD28 levels in the
serum of Graves' disease. Cent Eur J Immunol. 39:216–222. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Young NS, Bacigalupo A and Marsh JC:
Aplastic anemia: Pathophysiology and treatment. Biol Blood Marrow
Transplant. 16 Suppl 1:S119–S125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Georgin-Lavialle S, Aouba A, Mouthon L,
Londono-Vallejo JA, Lepelletier Y, Gabet AS and Hermine O: The
telomere/telomerase system in autoimmune and systemic
immune-mediated diseases. Autoimmun Rev. 9:646–651. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeanclos E, Krolewski A, Skurnick J,
Kimura M, Aviv H, Warram JH and Aviv A: Shortened telomere length
in white blood cells of patients with IDDM. Diabetes. 47:482–486.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurosaka D, Yasuda J, Yoshida K, Yoneda A,
Yasuda C, Kingetsu I, Toyokawa Y, Yokoyama T, Saito S and Yamada A:
Abnormal telomerase activity and telomere length in T and B cells
from patients with systemic lupus erythematosus. J Rheumatol.
33:1102–1107. 2006.PubMed/NCBI
|
|
38
|
Wu CH, Hsieh SC, Li KJ, Lu MC and Yu C:
Premature telomere shortening in polymorphonuclear neutrophils from
patients with systemic lupus erythematosus is related to the lupus
disease activity. Lupus. 16:265–272. 2007. View Article : Google Scholar : PubMed/NCBI
|