Introduction
Although the incidence of osteosarcoma is low,
accounting for 1% of all cancer diagnoses (1), osteosarcoma remains the most
prevalent primary malignant bone tumor in children and young adults
globally. It is predominant among children and adolescents and
represents 5–10% of malignancies in this age group (2). Despite advances in surgery and
multiagent chemotherapy, the 5-year survival rate is 60–70% for
patients with localized disease, and as low as 20–30% for patients
with metastasis (3). Therefore,
identifying novel, highly effective antitumor agents is urgently
required.
Elemene
(1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane) is extracted from
the traditional Chinese medicinal herb Rhizomazedoariae and
is considered as a novel anticancer drug (4). Elemene has been evaluated in clinical
trials and is used for the effective treatment of a number of
cancer types, including leukemia, glioma, non-small cell lung
cancer, and cervical, breast and liver cancers (5–8).
These previous studies have indicated that elemene may be a
broad-spectrum anticancer agent, and therefore presents a
potentially important chemotherapeutic agent. A previous study
demonstrated that elemene inhibited the viability and induced
apoptosis of human osteosarcoma cells in a dose- and time-dependent
manner, and the anticancer effects of elemene were reduced by the
hypoxia-inducible factor 1 protein (9). An additional study revealed that all
essential components of the renin-angiotensin system (RAS) axis,
including angiotensin-(1–7) [Ang-(1–7)]
generating proteases and the putative Ang-(1–7)
receptor (Mas) are expressed in U2OS and MNNG-HOS osteosarcoma cell
lines (10). Therefore, it is
possible that the mechanism underlying the effects of elemene on
osteosarcoma may be associated with the RAS signaling pathway.
The aim of the present study was to investigate the
precise mechanisms underlying the effects of elemene on
osteosarcoma development, and to determine whether this mechanism
may be associated with the RAS signaling pathway. The results
revealed that elemene inhibited the growth of the osteosarcoma cell
lines MG-63 and U2OS potentially via the RAS singling pathway.
Therefore, the present study provides a potential therapeutic
target for the treatment of osteosarcoma.
Materials and methods
Cell culture
The human osteosarcoma cell lines MG-63 and U2OS
were obtained from Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin G
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 100 U/ml
streptomycin (Sigma-Aldrich; Merck KGaA), and maintained at 37°C
and 5% CO2. MG-63 and U2OS cells were then treated with
0, 10, 40, 80 and 160 µg/ml elemene (Dalian Jingang Pharmaceutical
Co., Ltd., Dalian, China) for 48 or 72 h, respectively.
Cell viability assay
MG-63 and U2OS cells were treated with 0, 10, 40, 80
and 160 µg/ml elemene for 48 or 72 h, respectively. Cells were
seeded in 96-well plates at a density of 1×104
cells/well and incubated for 24 h. Cell viability was evaluated
using an MTT assay kit (Sigma-Aldrich; Merck KGaA) as previously
described (11). Briefly, 10 µl
tetrazolium salt (MTT, 3 mg/ml) was added to the culture medium for
a 2 h incubation at 37°C. Culture media was removed and cells were
lysed in 100 µl DMSO (Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min. Microplates were read using a multiwell
scanning spectrophotometer (Titertek-Berthold; Berthold Detection
Systems GmbH, Pforzheim, Germany) at 540 nm.
Determination of caspase-3
activity
The assay performed was based on the ability of the
active enzyme to cleave the chromophore from the enzyme substrates
of caspase-3 and acetyl-Asp-Glu-Val-Asp-p-nitroanilide
(Ac-DEVD-pNA) (12). The
hydrolysis of the peptide substrate Ac-DEVD-pNA by caspase-3
results in the release of the p-nitroaniline (pNA) moiety (12). pNA moiety is yellow, and the
concentration may be detected by measuring the absorbance at a
wavelength of 405 nm; with its capacity, this can infer the
activity of caspase-3 (12). MG-63
and U2OS cells (1×106/well) were seeded into 6-well
plates, incubated at 37°C overnight, and then exposed to 0, 10, 40,
80 and 160 µg/ml elemene at room temperature for 72 h. Caspase-3
activity was measured using a caspase-3 activity assay kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions; the release of pNA was monitored
at 405 nm using a standard curve of defined pNA solutions.
Migration assay
Cell migration assays were performed using 24-well
Transwell plates (8-µm pore size; BD Biosciences, Franklin Lakes,
NJ, USA), according to the manufacturer's instructions. Briefly,
MG-63 and U2OS cells were treated with 0, 10, 40, 80 and 160 µg/ml
elemene for 72 h. Then, cells were trypsinized with 0.05% trypsin
and washed 3 times in DMEM without FBS. A total of 5×104
cells were then suspended in 500 µl DMEM without FBS and added to
the upper chamber, while 750 µl DMEM containing 10% FBS was placed
in the lower chamber. The cells were incubated for 24–48 h at 37°C
in a 5% CO2 humidified incubator. The non-migrating
cells were removed with cotton swabs and the migrated cells were
fixed in 4% paraformaldehyde for 15 min at 4°C and stained with
0.5% crystal violet at room temperature for 1 h. Cells in at least
5 random microscopic fields (magnification, ×100) were counted by
light microscopy (LeicaDM4000; Leica Microsystems GmbH, Wetzlar,
Germany), images were captured using a Nikon Eclipse 80i (Nikon
Corporation, Tokyo, Japan) and analyzed with Image-Pro Plus
computer software (version 4.0; Media Cybernetics, Inc., Rockville,
MD, USA). All experiments were performed in duplicate and repeated
three times.
Invasion assay
Transwell filters (Corning Incorporated, Corning,
NY, USA) were coated with 3.9 µg/µl Matrigel (60–80 µl). MG-63 and
U2OS cells that treated with 0, 10, 40, 80 and 160 µg/ml elemene
for 72 h, were used for cell invasion determination. The cells
(2×104 cells/well) were resuspended in 100 µl serum-free
RPMI-1640 medium and added into the upper compartment of the
chambers. Following 24 h of incubation at 37°C, the cells migrating
from the Matrigel into the pores of the inserted filter were fixed
with 100% methanol (Sigma-Aldrich; Merck KGaA) for 30 min at room
temperature, and stained with 50% hematoxylin (Sigma-Aldrich; Merck
KGaA) at room temperature for 15 min. The positively-stained cells
were counted under three randomly selected visual fields at ×400
magnification with a fluorescence microscope (SMZ1000; Nikon
Corporation,), and analyzed with Image-Pro Plus computer software
(version 4.0, Media Cybernetics, Inc.).
Analysis of cell apoptosis by Annexin
V/propidium iodide (PI) staining
MG63 and U2OS cells were stained with Annexin
V-fluorescent isothiocyanate (FITC) conjugate and PI to analyze
cell apoptosis. MG63 and U2OS cells (1×105) were
cultured in 24-well plates. Following overnight incubation, cells
were treated with various concentrations of elemene (0, 10, 40, 80
and 160 µg/ml) for 72 h and collected by trypsinization with 0.05%
trypsin. Cells were washed twice with 4°C PBS following
centrifugation at 2,000 × g for 5 min at 4°C, the cell pellets were
suspended in 195 µl ice-cold 1X binding buffer at a density of
~1×106 cells/ml, and then incubated with 5 µl Annexin
V-FITC (Beyotime Institute of Biotechnology) for 10 min at room
temperature in the dark. Following cell centrifugation at 1,000 × g
for 5 min at 4°C, 200 µl ice-cold 1X binding buffer containing 10
µl PI (BD Biosciences, San Jose, CA, USA) was added for flow
cytometric analysis using the FACSCalibur flow fluorocytometer (BD
Biosciences) for 1 h. The data obtained were analyzed using
CellQuest software (version 3.1; BD Biosciences).
Xenograft tumor model
Experiments involving mice were approved by the
Institutional Animal Care and Use Committee of The Cancer Hospital
of The Chinese Academy of Medical Sciences (Beijing, China). A
total of 24 female BALB/c nude mice (4–6 weeks old, weighting 18–20
g, n=6 in each group) were purchased from The Shanghai Laboratory
Animal Center, Chinese Academy of Sciences (Shanghai, China). Mice
were housed in polystyrene cages (Two mice/cage) with free access
to food and water, a 12-h light-darkness cycle, and an ambient
temperature of 20–25°C. MG-63 or U2OS cells (5×106)
resuspended in 0.1 ml DMEM were subcutaneously inoculated into the
lower right flank of nude mice. When the developing tumors reached
100 mm3 in size, treatment was initiated by
intraperitoneal injection of elemene (50 mg/kg) or normal saline (1
mg/kg) every other day for 21 days. Tumor size was measured at 10,
12, 16, 19 and 21 days, and the tumor volume (V) was calculated
using the following formula: V=(length × width × height) × 0.5236
(13). Following the last
treatment of elemene, all mice were sacrificed and the tumors were
weighed.
Immunohistochemical analysis
Following the last treatment of elemene, all mice
were sacrificed and after weighing the tumors, all tissues were
fixed in formalin for immunohistochemical analysis in order to
detect the expression of angiotensin II (AngII), which was
performed as described previously (14). Briefly, samples were fixed in 10%
neutral formalin for 48 h at 4°C, embedded in paraffin and cut into
4-µm sections for immunohistochemical staining. Samples were
incubated overnight at 4°C with anti-AngII antibody (1:50; cat. no.
ab47831; Abcam, Cambridge, UK). The samples were then incubated for
1 h at room temperature with a biotinylated secondary antibody
(1:200; Vector Laboratories, Burlingame, CA, USA; cat. no.
BA-9200). The bound secondary antibody was then amplified using the
Elite ABC kit (Vector Laboratories, Inc.), according to the
manufacturer's instructions. The antibody-biotin-avidin-peroxidase
complex was visualized using 0.02% 3,3′diaminobenzidene staining
for 10 min at room temperature. The sections were mounted onto
gelatin-coated slides that were air-dried overnight at room
temperature; the coverslips were then mounted using Permount medium
(Thermo Fisher Scientific, Inc.) and imaged using a light optical
microscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
MG-63 and U2OS cells that treated with different
concentrations of elemene (0, 10, 40, 80 and 160 µg/ml) for 72 h,
were used for western blot analysis. Protein was collected from
MG-63 and U2OS cellsusing radioimmunoprecipitation buffer (Santa
Cruz Biotechnology, Inc., Dallas, CA, USA) containing protease
inhibitors at 4°C for 30 min. Protein concentrations were
quantified using a Bio-Rad protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Proteins (30 µg) were separated by 8%
SDS-PAGE and transferred to polyvinylidene difluoride membranes
(Amersham; GE Healthcare, Chicago, IL, USA). The membranes were
blocked in 5% non-fat milk (Merck KGaA) overnight at 4°C.
Transferred membranes were then stained with the following primary
antibodies: Anti-B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no.
ab37899; Abcam), anti-Bcl-2-like protein 4 (Bax; 1:1,000; cat. no.
ab32503; Abcam), anti-cleaved caspase-3 (1:500; cat. no. ab13847;
Abcam), anti-procaspase-3 (1:1,000; cat. no. ab32150; Abcam),
anti-renin (1:500; cat. no. ab180608; Abcam), anti-rennin receptor
(1:1,000; cat. no. GTX114169; GeneTex, Inc., Irvine, CA, USA),
anti-AngII (1:200; cat. no. EPR2931, Abcam), anti-ACE (1:200; cat.
no. PB0089; Boster Biological Technology, Pleasanton, CA, USA) and
anti-β-actin (1:200; cat. no. ab8227; Abcam) overnight at 4°C.
Subsequently, protein bands were detected by incubation with a
horseradish peroxidase-conjugated secondary antibody (1:1,000;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc., Beijing, China; cat. no. A50-106P) at room
temperature for 1 h. Signals were detected using an enhanced
chemiluminescence kit (Wuhan Booute Biotechnology Co., Ltd, Wuhan,
China; cat. no. orb90504) and exposed to Kodak X-OMAT film (Kodak,
Rochester, NY, USA). Each experiment was performed at least three
times and the results were analyzed using Alpha View Analysis Tools
(Alpha View SA software version 3.2.2; Protein Simple, San Jose,
CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation of at least three
experiments. Statistical differences between two independent groups
were analyzed using a Student's t-test, and differences among
multiple groups were analyzed using one-way analysis of variance
and post-hoc Tukey tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Elemene inhibits the viability,
migration and invasion of MG-63 and U2OS cells
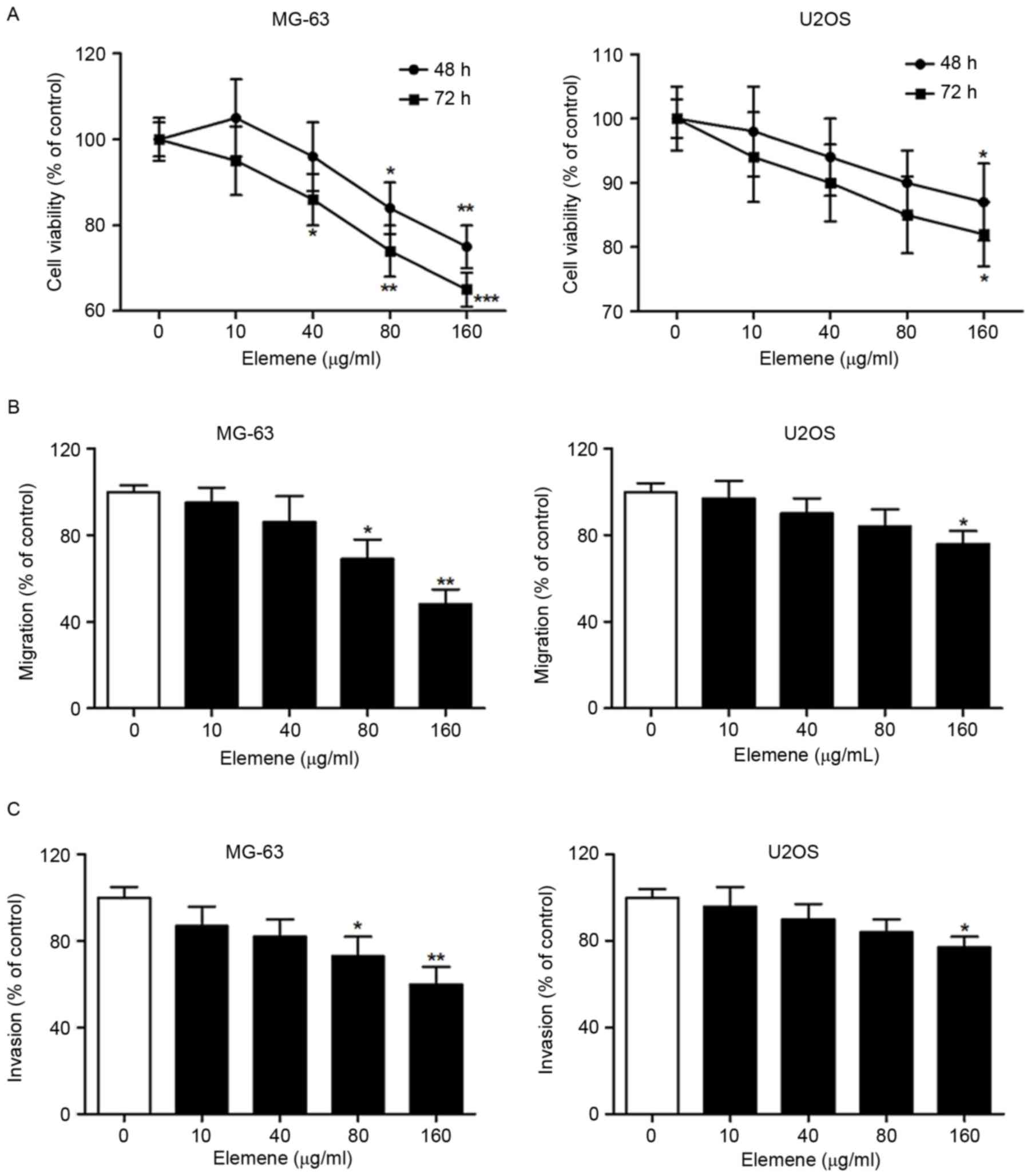
Human osteosarcoma MG-63 and U2OS cells were treated
with 0, 10, 40, 80 and 160 µg/ml elemene for 48 or 72 h. The
results demonstrated that elemene inhibited the viability of MG-63
and U2OS cells in dose- and time-dependent manners (Fig. 1A). The Transwell migration assay
also revealed that elemene inhibited the migration of MG-63 and
U2OS cells in a dose-dependent manner (Fig. 1B). In addition, a significantly
reduced level of invasion in MG-63 cells treated with 80 and 160
µg/ml elemene, and U2OS cells treated with 160 µg/ml elemene was
observed (Fig. 1C). MG-63 cells
were more sensitive to elemene when compared with U2OS cells, as
MG-63 cells exhibited a greater repression of cell viability,
migration and invasion following elemene treatment (Fig. 1). These results indicated that
elemene demonstrated a significant antitumor effect on MG-63 and
U2OS cells by inhibiting cell viability, migration and
invasion.
Elemene induces apoptosis in MG-63 and
U2OS cells
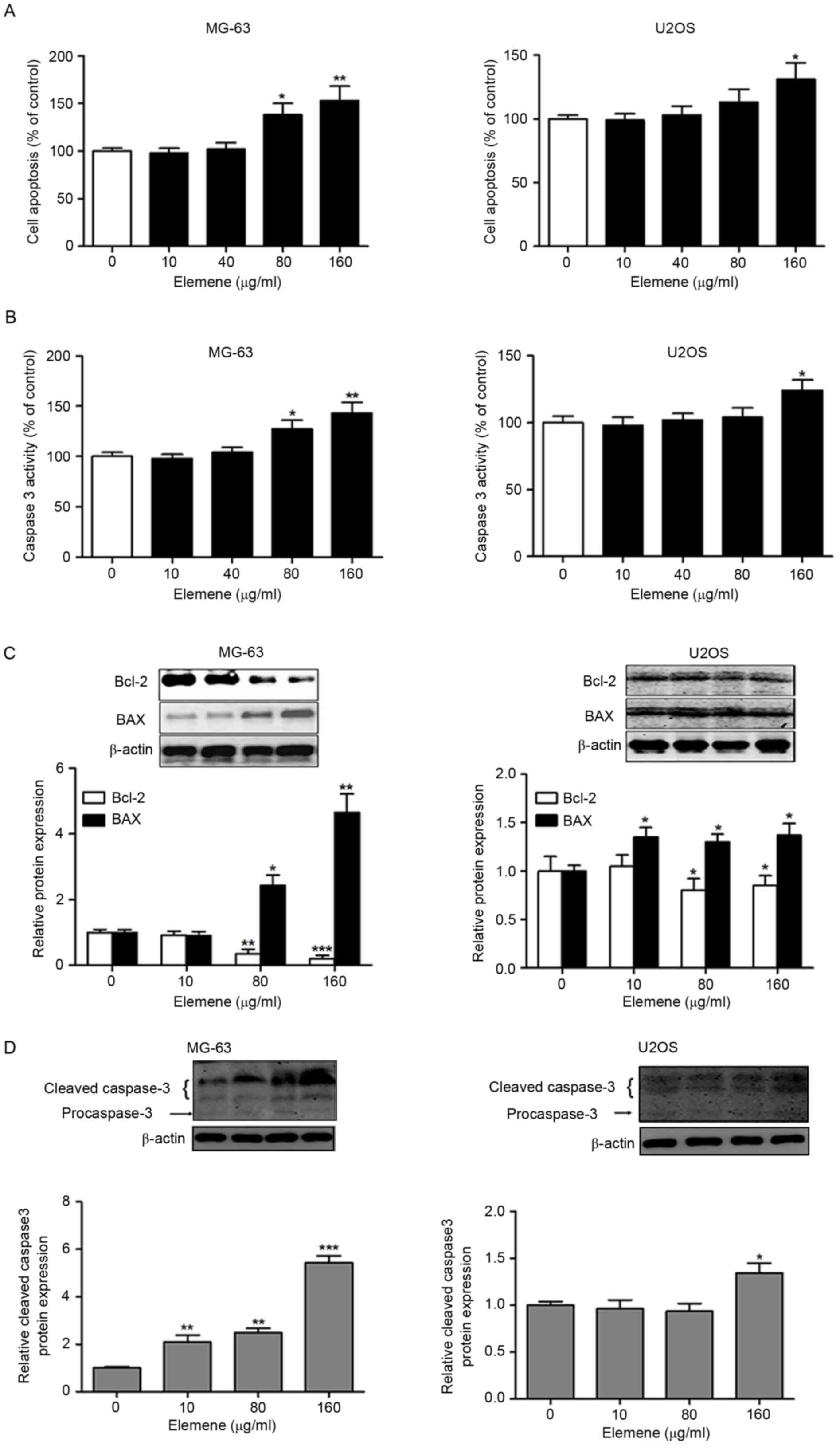
In order to investigate whether elemene effectively
inhibits osteosarcoma cell growth via apoptosis induction, MG-63
and U2OS cells were treated with 0, 10, 40, 80 and 160 µg/ml
elemene and cell apoptosis was measured. A significant increase in
the number of apoptotic MG-63 cells was observed following
treatment with 80 and 160 µg/ml elemene, and in U2OS cells
following treatment with 160 µg/ml elemene (Fig. 2A). In addition, a dose-dependent
increase in caspase 3 activity was observed in MG-63 and U2OS cells
treated with elemene, which reached statistical significance at 80
and 160 µg/ml elemene in MG-63 cells and 160 µg/ml in U2OS cells
(Fig. 2B). To further confirm that
elemene induced apoptosis, western blotting was performed to detect
the expression levels of apoptosis-associated proteins. Elemene
significantly downregulated the level of Bcl-2 expression; however,
elemene treatment upregulated the levels of BAX and cleaved
caspase-3 in MG-63 and U2OS cells (Fig. 2C and D). Notably, MG-63 was more
sensitive to elemene treatment when compared with U2OS cells. These
results indicated that elemene inhibits cell viability potentially
via apoptosis induction of human osteosarcoma cells.
Elemene inhibits the RAS signaling
pathway in MG-63 and U2OS cells
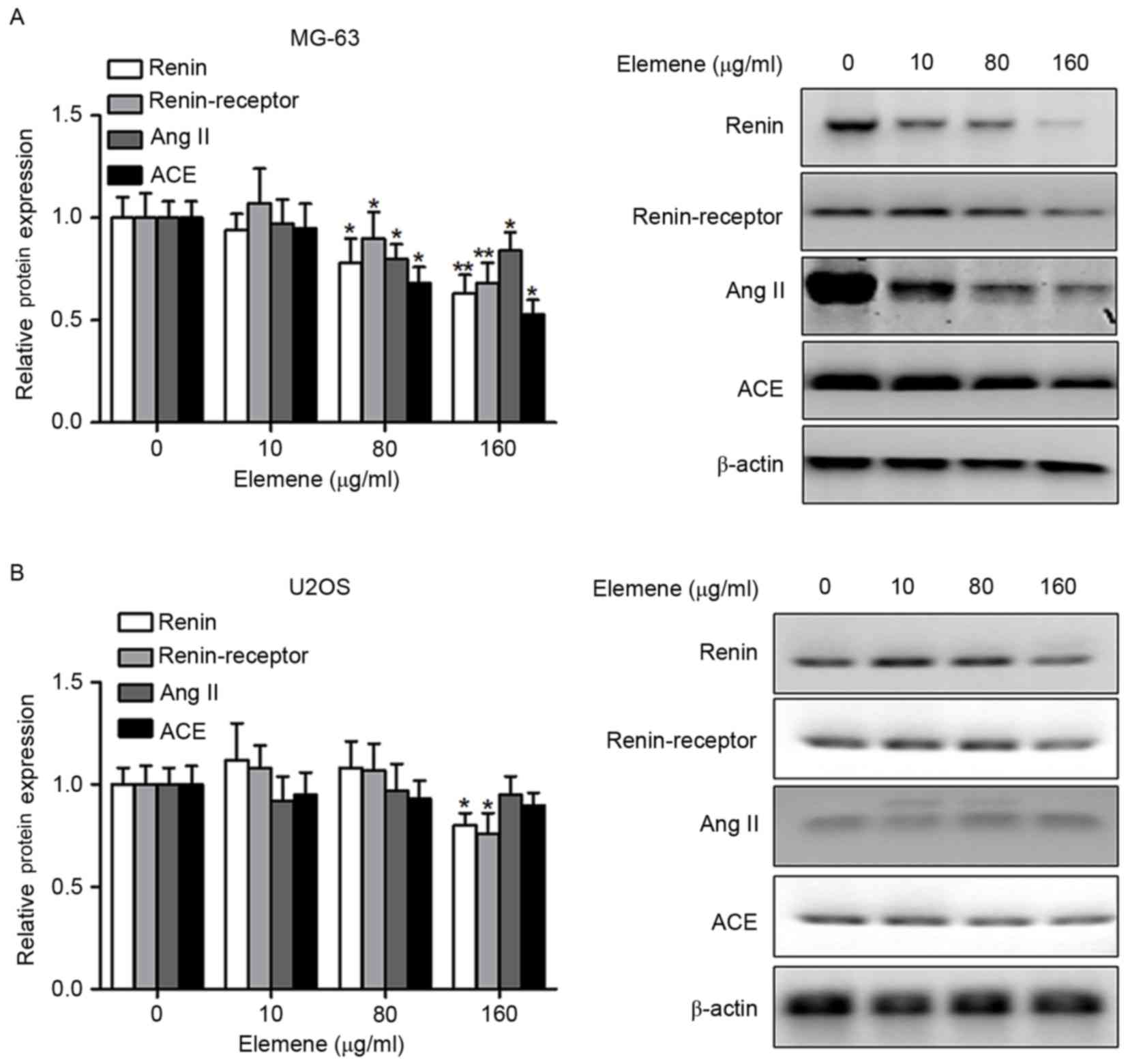
It has been well documented that the RAS signaling
pathway is implicated in tumorigenesis (15). Therefore, the effects of elemene on
the activity of this signaling pathway were investigated in the
present study. MG-63 and U2OS cells were treated with 0, 10, 80 or
160 µg/ml elemene, and the levels of renin, renin-receptor, AngII
and ACE were detected by western blotting. The levels of renin,
renin-receptor, AngII and ACE were downregulated by elemene
treatment in a dose-dependent manner (Fig. 3A). In U2OS cells, renin and the
renin-receptor were significantly downregulated in a dose-dependent
manner following elemene treatment; however, AngII and ACE
expression was not significantly altered (Fig. 3B). Elemene demonstrated a greater
inhibitory effect on MG-63 cells when compared with U2OS cells, as
the levels of renin, renin-receptor, AngII and ACE were
significantly downregulated following treatment with 80 µg/ml
elemene, whereas treatment with 160 µg/ml elemene significantly
decreased renin and renin-receptor expression in U2OS cells
(Fig. 3).
Elemene inhibits osteosarcoma
development by suppressing the RAS signaling pathway
inmouseosteosarcoma xenograft tumors
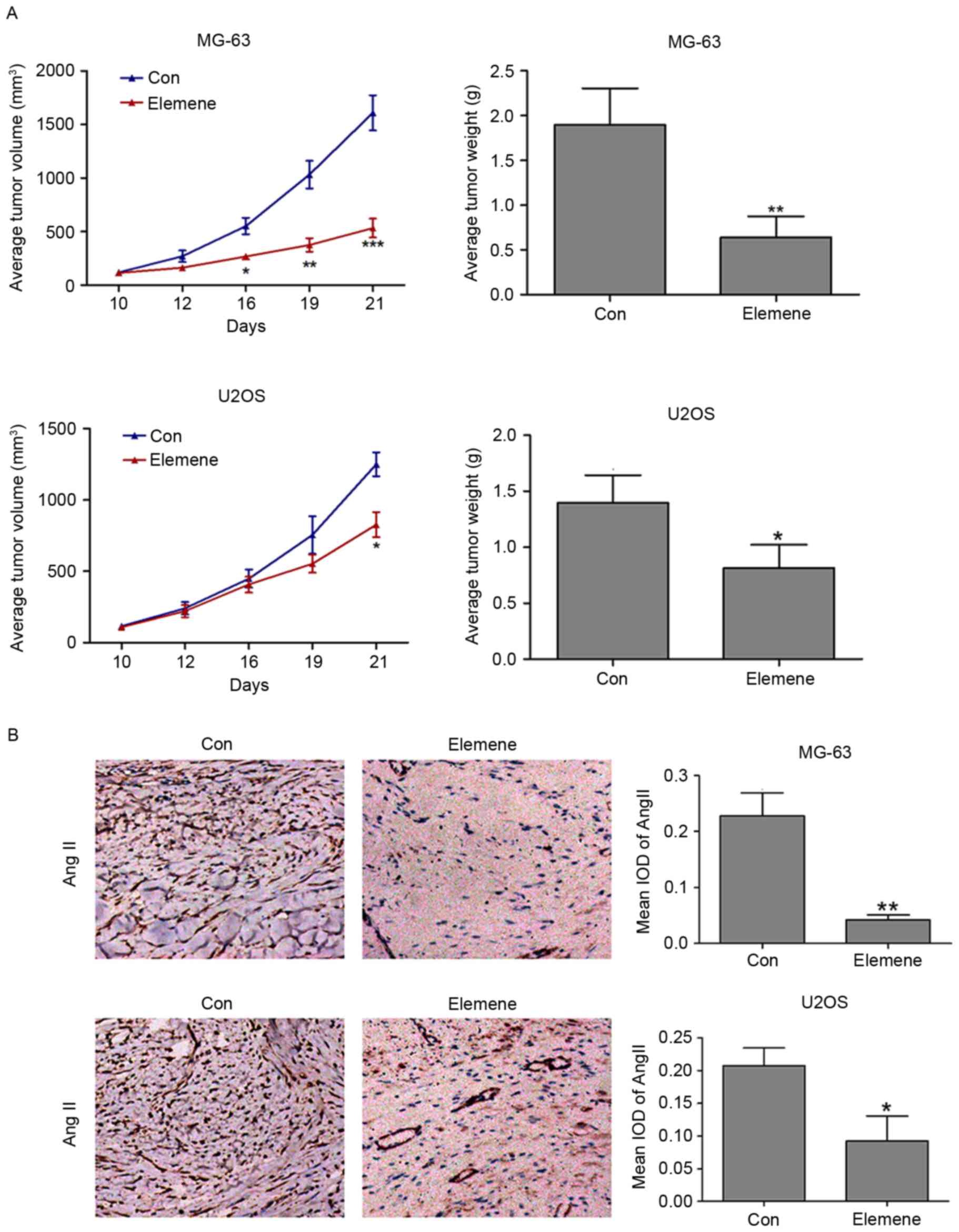
To provide direct evidence that the RAS signaling
pathway is involved in the inhibitory effects of elemene on tumor
development, MG-63 and U2OS cells were injected into the flank of
nude mice, and then treated with NaCl or elemene. The role of
elemene in osteosarcoma development was first evaluated. As shown
in Fig. 4A, tumor volume was
significantly reduced in elemene-treated mice from days 16 to 21
following injection with MG-63 cells when compared with the
controls (P<0.05, day 16; P<0.01, day 19; P<0.001, day
21). At 21 days post-injection, the tumor weight of the
elemene-injected group was significantly lower when compared with
the control group (P<0.01; Fig.
4A). In mice injected with U2OS cells and treated with elemene,
tumor volume was significantly reduced at 21 days following
treatment (P<0.05), and the tumor weight of the elemene-treated
group was significantly reduced when compared with the control
group (P<0.05; Fig. 4A).
Immunohistochemistry was used to detect the
expression of AngII in MG-63 and U2OS xenograft tumors. Ang II is
known to be an important component of the RAS signaling pathway
(16). As exhibited in Fig. 4B, a significant reduction in the
expression of AngII in MG-63 and U2OS cells was observed when
compared with the control groups.
Discussion
The results of the present study demonstrate that
elemene inhibits the viability, migration and invasion of human
MG-63 and U2OS osteosarcoma cell lines, as well as induces
apoptosis in these cell lines. In addition, the expression of
pro-apoptotic proteins, cleaved caspase-3 and BAX were upregulated,
whereas the expression of the anti-apoptotic protein, Bcl-2, was
downregulated following elemene treatment of these cells. Further
investigation revealed the potential involvement of the RAS
signaling pathway in the inhibitory effect of elemene on
osteosarcoma development, as renin, the renin-receptor, AngII and
ACE were downregulated following elemene treatment. In addition,
elemene was observed to downregulate the weight and volume of MG-63
and U2OS xenograft tumors, and reduce AngII expression. These
results indicate that elemene may demonstrate the ability to
inhibit osteosarcoma cell viability, migration and invasion, as
well as induce cell apoptosis, partially via downregulation of the
RAS signaling pathway.
Elemene is a novel plant-derived anticancer agent
that exhibits a broad spectrum of antitumor activities in different
cancers (17). In the present
study, elemene was observed to exert its antitumor effects in
osteosarcoma by inhibiting the viability, migration, invasion of
osteosarcoma cells in vitro, as well as tumor volume and
weight in vivo, potentially via induction of apoptosis.
These results are consistent with previous studies demonstrating
that elemene induces tumor cell apoptosis and cell cycle arrest in
the G2/M-phase, as determined by morphological
alterations, DNA fragmentation and flow cytometry analysis
(7,18,19).
In addition, these previous studies have suggested that apoptosis
and G2/M-phase arrest induced by elemene may contribute
to the inhibition of cell proliferation (7,18,19).
Furthermore, it has been demonstrated that elemene decreases the
level of Bcl-2 protein, increases BAX and cleaved-caspase-3 protein
levels, and activates caspase-3 activity (18,20,21).
The primary focus of previous investigations has
been the intrinsic mechanisms underlying the antitumor activities
of elemene. A previous study reported that elemene inhibited tumor
growth in glioblastoma cells depending on the activation of the p38
mitogen-activated protein kinase pathway (22). In addition, elemene inhibited
melanoma growth and metastasis by suppressing vascular endothelial
growth factor (VEGF)-mediated angiogenesis (23). Furthermore, cell apoptosis induced
by Δ-elemene in colorectal adenocarcinoma cells was demonstrated to
involve the mitochondrial-mediated pathway (24). The results of the present study
suggest that the antitumor activity of elemene may be associated
with the RAS signaling pathway, as elemene treatment downregulated
renin, renin-receptor, AngII and ACE protein expression levels.
This is consistent with a previous report, which revealed the role
of the ACE2/Ang 1–7)/Mas axis in RAS-mediated effects on
osteosarcoma cell proliferation (10).
RAS is mitogenic and angiogenic, and contributes to
neoplastic growth in ovarian, prostate, lung, breast and pancreatic
cancers (25). A number of RAS
components, including renin, renin-receptor, ACE and AngII, are
locally upregulated in tumors (26). In the present study, these
molecules were significantly reduced by elemene treatment,
suggesting that its antitumor effects may involve downregulation of
the RAS signaling pathway. The implication of RAS in tumorigenesis
is partially due to its robust angiogenic activity, which is
mediated by the AngII/angiotensin type 1 receptor-dependent
induction of proangiogenic factors, including angiopoietin 2, VEGF
and platelet-derived growth factor (27–30),
which affect key processes, including fibrosis, inflammation,
proliferation and apoptosis (31).
In the present study, AngII, the major effector peptide of the
classical RAS signaling pathway, was downregulated by elemene
treatment in human osteosarcoma cell lines and cell-transplanted
tumors in nude mice.
In conclusion, the results of the present study
indicated that elemene inhibits the growth of osteosarcoma cells
potentially via the RAS signaling pathway. In addition, elemene
suppressed tumor growth and AngII expression in MG-63 and U2OS
cell-transplanted tumors in nude mice. The results therefore reveal
a novel mechanism by which elemene may inhibit osteosarcoma cell
growth.
Acknowledgements
The present study was financially supported by the
Integrated Traditional Chinese and Western Medicine Project of
Beijing Municipal Administration of Traditional Chinese Medicine
(grant no. 2014-ZYJ03), and the China Postdoctoral Science
Foundation Project (grant no. 2014M551001).
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van den Berg H, Kroon HM, Slaar A and
Hogendoorn P: Incidence of biopsy-proven bone tumors in children: A
report based on the Dutch pathology registration ‘PALGA’. J Pediatr
Orthop. 28:29–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
PosthumaDeBoer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF,
Liu YP and Wu B: β-Elemene induces apoptosis in human renal-cell
carcinoma 786-0 cells through inhibition of MAPK/ERK and
PI3K/Akt/mTOR signalling pathways. Asian Pac J Cancer Prev.
13:2739–2744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Li X, Huang F, et al: Elemene, a
novel anticancer drug, triggers cell death and enhances cisplatin
sensitivity via induction of apoptosis and cell cycle arrest in
human non-small cell lung cancer cells. Cancer Res. 64:689.
2004.PubMed/NCBI
|
|
6
|
Kai LI, Cong X and Zhao X: The inhibitory
effect of Xiaoaiping combined with elemene on cell proliferation
and expression of Bcl-2 protein in cervix cancer HeLa cells. Chin J
Clin Oncol. 35:705–561. 2008.
|
|
7
|
Li QQ, Wang G, Zhang M, Cuff CF, Huang L
and Reed E: beta-Elemene, a novel plant-derived antineoplastic
agent, increases cisplatin chemosensitivity of lung tumor cells by
triggering apoptosis. Oncol Rep. 22:161–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou HY, Shen JK, Hou JS, Qiu YM and Luo
QZ: Experimental study on apoptosis induced by elemene in glioma
cells. Ai Zheng. 22:959–963. 2003.(In Chinese). PubMed/NCBI
|
|
9
|
Liang D, Yang M, Guo B, Yang L, Cao J and
Zhang X: HIF-1α induced by β-elemene protects human osteosarcoma
cells from undergoing apoptosis. J Cancer Res Clin Oncol.
138:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ender SA, Dallmer A, Lässig F, Lendeckel U
and Wolke C: Expression and function of the
ACE2/angiotensin(1–7)/Mas axis in osteosarcoma cell lines U-2 OS
and MNNG-HOS. Mol Med Rep. 10:804–810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fromigué O, Haÿ E, Modrowski D, Bouvet S,
Jacquel A, Auberger P and Marie PJ: RhoA GTPase inactivation by
statins induces osteosarcoma cell apoptosis by inhibiting
p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell
differentiation. Cell Death Differ. 13:1845–1856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gatti L, Cossa G, Tinelli S, et al:
Improved apoptotic cell death in drug-resistant non small cell lung
cancer cells by TRAIL-based treatment. J Pharmacol Exp Ther.
194–201. 2013.
|
|
13
|
Ball DW, Jin N, Rosen DM, Dackiw A,
Sidransky D, Xing M and Nelkin BD: Selective growth inhibition in
BRAF mutant thyroid cancer by the mitogen-activated protein kinase
kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab.
92:4712–4718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krajewska M, Krajewski S, Epstein JI,
Shabaik A, Sauvageot J, Song K, Kitada S and Reed JC:
Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1
expression in prostate cancer. Am J Pathol. 148:1567–1576.
1996.PubMed/NCBI
|
|
15
|
Bangalore S: Renin-angiotensin system
inhibitors and risk of cancer. Trends Mol Med. 17:176–177. 2011.
View Article : Google Scholar
|
|
16
|
Wang SR, Sang WK and Kim CJ: Overview of
the Renin-Angiotensin system. Korean Circ J. 37:91–96. 2007.
View Article : Google Scholar
|
|
17
|
Li X, Wang G, Zhao J, Ding H, Cunningham
C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of
beta-elemene in chemoresistant ovarian carcinoma cells is mediated
through arrest of the cell cycle at the G2-M phase. Cell Mol Life
Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng S, Yang H, Zhang S, Wang X, Yu L, Lu
J and Li J: Initial study on naturally occurring products from
traditional Chinese herbs and vegetables for chemoprevention. J
Cell Biochem Suppl. 27:106–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumour cells. J Pharm Pharmacol.
62:1018–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: β-elemene induces
caspase-dependent apoptosis in human glioma cells in vitro through
the upregulation of Bax and Fas/FasL and downregulation of Bcl-2.
Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen W, Lu Y, Wu J, Gao M, Wang A and Xu
B: Beta-elemene inhibits melanoma growth and metastasis via
suppressing vascular endothelial growth factor-mediated
angiogenesis. Cancer Chemother Pharmacol. 67:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie CY, Yang W, Li M, Ying J, Tao SJ, Li
K, Dong JH and Wang XS: Cell apoptosis induced by delta-elemene in
colorectal adenocarcinoma cells via a mitochondrial-mediated
pathway. Yakugaku Zasshi. 129:1403–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
George AJ, Thomas WG and Hannan RD: The
renin-angiotensin system and cancer: Old dog, new tricks. Nat Rev
Cancer. 10:745–759. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dougherty U, Mustafi R, Sadiq F,
Almoghrabi A, Mustafi D, Kreisheh M, Sundaramurthy S, Liu W, Konda
VJ, Pekow J, et al: The renin-angiotensin system mediates EGF
receptor-vitamin d receptor cross-talk in colitis-associated colon
cancer. Clin Cancer Res. 20:5848–5859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamarat R, Silvestre JS, Durie M and Levy
BI: Angiotensin II angiogenic effect in vivo involves vascular
endothelial growth factor- and inflammation-related pathways. Lab
Invest. 82:747–756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pupilli C, Lasagni L, Romagnani P, Bellini
F, Mannelli M, Misciglia N, Mavilia C, Vellei U, Villari D and
Serio M: Angiotensin II stimulates the synthesis and secretion of
vascular permeability factor/vascular endothelial growth factor in
human mesangial cells. J Am Soc Nephrol. 10:245–255.
1999.PubMed/NCBI
|
|
29
|
Otani A, Takagi H, Oh H, Koyama S and
Honda Y: Angiotensin II induces expression of the Tie2 receptor
ligand, angiopoietin-2, in bovine retinal endothelial cells.
Diabetes. 50:867–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita M, Hayashi I, Yamashina S, Itoman M
and Majima M: Blockade of angiotensin AT1a receptor signaling
reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys
Res Commun. 294:441–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deshayes F and Nahmias C: Angiotensin
receptors: A new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|