Introduction
Colorectal carcinoma is one of the most common
malignant tumors (1) with
obviously increased metastasis and mortality in its advanced stage
(2). Insulin-like growth factor II
mRNA binding protein 3 (IMP3) is a kind of binding protein, which
can specifically recognize mRNA sequence. It is also an oncofetal
protein and expressed in a number of malignant tumors, related to
the advances in tumor invasion. Invasion and metastasis of tumor
cells might be modulated by the expression of IMP3 (3–5).
Indeed, the members of IMPs family-IMP3 and IMP1 are applied as
vital biological invasion markers for pancreatic cancer (6). Moreover, IMP3 expression in different
stages of colorectal carcinoma was discrepant and IMP3 was expected
to be a new target in the treatment of colorectal carcinoma
(7).
As a pro-oncogene, DEK not only regulates cell
senescence and apoptosis (8) but
also the transcriptional regulator of retinoblastoma tumor
suppressor protein (Rb)/E2F, which is often disturbed in colorectal
carcinoma (9). DEK protein is
highly-expressed in tumors and can be easily detected by commercial
antibodies. Therefore, it holds great promise to be utilized as a
tumor diagnostic marker. Benign and malignant melanoma could be
easily identified according to the expression level of DEK
(10). Moreover, DEK expression in
undifferentiated cells was much higher than that in mature cells
(11), which could be used to
assess the differentiative capacity of tumor cells. In the present
study, colorectal carcinoma cell lines SW620 and SW480 were
selected. The cells were transfected with DEK interfering
lentivirus and empty carrier, respectively. Consequently, effects
of DEK silence on the cell viability, apoptosis, invasion and
epithelial-mesenchymal transition (EMT) were detected.
Materials and methods
Cell culture and transfection
Colorectal carcinoma cell lines SW620 and SW480
(Shanghai Cell Bank of Chinese Academy of Science, China) were
cultured in dulbecco minimum essential medium (DMEM) (Gibco, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (FBS)
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 100 U/ml
penicillin-streptomycin (Sigma, Ronkonkoma, NY, USA) in 5%
CO2 at 37°C.
The experiment was divided into three groups: blank
control, lentivirus encoding NC-siRNA, and lentivirus encoding DEK
siRNA. The cells at 70% confluence were transfected with empty
carrier and DEK interfering lentivirus (GenePharma, Shanghai,
China), respectively. After 6 h, the medium were replaced with
fresh DMEM medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% FBS. The cells were incubated in
CO2 incubator at 37°C for 48 h. Finally, the expression
levels of DEK and IMP3 in mRNA and protein levels were detected by
quantitative real-time polymerase chain reaction (QRT-PCR) and
western blot, respectively.
MTT assay
Colorectal carcinoma SW620 cells were seeded in
96-well plates. After transfection of 24, 48, 72 and 96 h, 20 µl
medium with MTT (final concentration: 5 mg/ml) (Gibco; Thermo
Fisher Scientific, Inc.) was added. After additional 4-h incubation
in CO2 incubator at 37°C, the medium were discarded.
DMSO (150 µl) (Sigma, USA) was added into each well and the plates
were shaken to dissolve formazan sufficiently. The absorbance at
560 nm was recorded by a microplate reader (Thermo Fisher
Scientific, Inc.). The optical density (OD) values represented cell
viability.
Flow cytometry
Colorectal carcinoma SW620 cells were seeded in
6-well plates. After transfection of 48 h, the cells were collected
after digestion by trypsin (Gibco; Thermo Fisher Scientific, Inc.).
Under the protection from light, the cells were incubated with
Annexin V-FITC/PI (C1062, Beyotime, Ningbo, China) for 30 min.
Next, apoptosis was detected by flow cytometry (BD Company,
Franklin Lakes, NJ, USA) within 1 h.
Transwell assay
After 48 h transfection, the cells were digested and
seeded into the upper chamber of transwell. The lower chamber only
contained DMEM medium. After 48 h incubation in CO2
incubator at 37°C, the down chamber was taken out and fixed with
polyformaldehyde. After staining with crystal violet, inverted
microscopy was used to count the cell numbers in five fields. The
average number represented the capacity of cell invasion.
Quantitative real-time polymerase
chain reaction (QRT-PCR)
After viral transfection, mRNA in different groups
was extracted using a TRIzol assay kit (Baosheng Science &
Technology Innovation Co, Ltd., Shanghai, China). mRNA was
transcribed into cDNA according to the reverse transcription kit
(639522, Takara Biotechnology Co., Ltd., Dalian, China) and
fluorescence quantitative PCR was utilized to detect the expression
level of the targeted genes using cDNA as template. The relevant
expression level of E-cadherin, Vimentin and MMP-9 were normalized
to β-actin. The primer sequences were listed as follows:
DEK-F: AAA CCT AGC CAG CTT CAC GA; DEK-R: AGG CCC
AAC TGC AGA GAA AC; E-cadherin-F: CGC CGA GAG CTA CAC GTT CA;
E-cadherin-R: TGT CGA CCG GTG CAA TCT TC; Vimentin-F: TGC GTG AAA
TGG AAG AGA ACT; Vimentin-R: TGC GTG AAA TGG AAG AGA ACT; IMP3-F:
GCG CTG CTG GAC AAG CTG TAT G; IMP3-R: AGG ACG AGG CCG TGA CGA A;
MMP-9-F: CAT CTT CCA AGG CCA ATC C; MMP-9-R: CCA TCA CCG TCG AGT
CAG C; β-actin-F: ACT CTT CCA GCC TTC CTT C, β-actin-R: ATC TCC TTC
TGC ATC CTG TC.
Western blot analysis
Cell lysis solution was added into each group of
cells. After 30 min dissociation at 4°C, the solutions were
centrifuged at 11,670 g for 10 min. The supernatant was collected,
which contained total protein. The protein concentration was
measured by BCA kit (Beyotime). 20 µg protein in each group was
loaded for gel electrophoresis and the membrane was transferred by
wet methods. The antibodies, including anti-DEK (1:1,000, catalogue
no. ab221545), anti-IMP3 (1:1,000, catalogue no. ab177477),
anti-E-cadherin (1:1,000, catalogue no. ab1416), anti-Vimentin
(1:1,000, catalogue no. ab8978; Abcam, Cambridge, UK), anti-MMP-9
(catalogue no. ab73734; 1:1,000; Abcam) and β-actin (1:1,000;
catalogue no. AF0003, Beyotime Institute of Biotechnology,
Shanghai, China) were incubated with nitrocellulose membranes
overnight at 4°C. Next, the secondary antibody (1:100; catalogue
nos. ab131368; Abcam) was added and co-incubated for 1–2 h at room
temperature. ECL exposure liquid droplet (catalogue no. RPN2133; GE
Healthcare Life Sciences, Chalfont, UK) was added on the membrane.
In the end, the membrane was used for exposure utilizing gel
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Grey density was analyzed using Quantity One analysis software
v1.4.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
The data were presented as mean ± standard deviation
(SD), and analyzed using SPPSS 17.0. Significant differences were
calculated by t-test, where P<0.05 was considered to
indicate a statistically significant difference.
Results
DEK silence down-regulates IMP3
expression and influences EMT
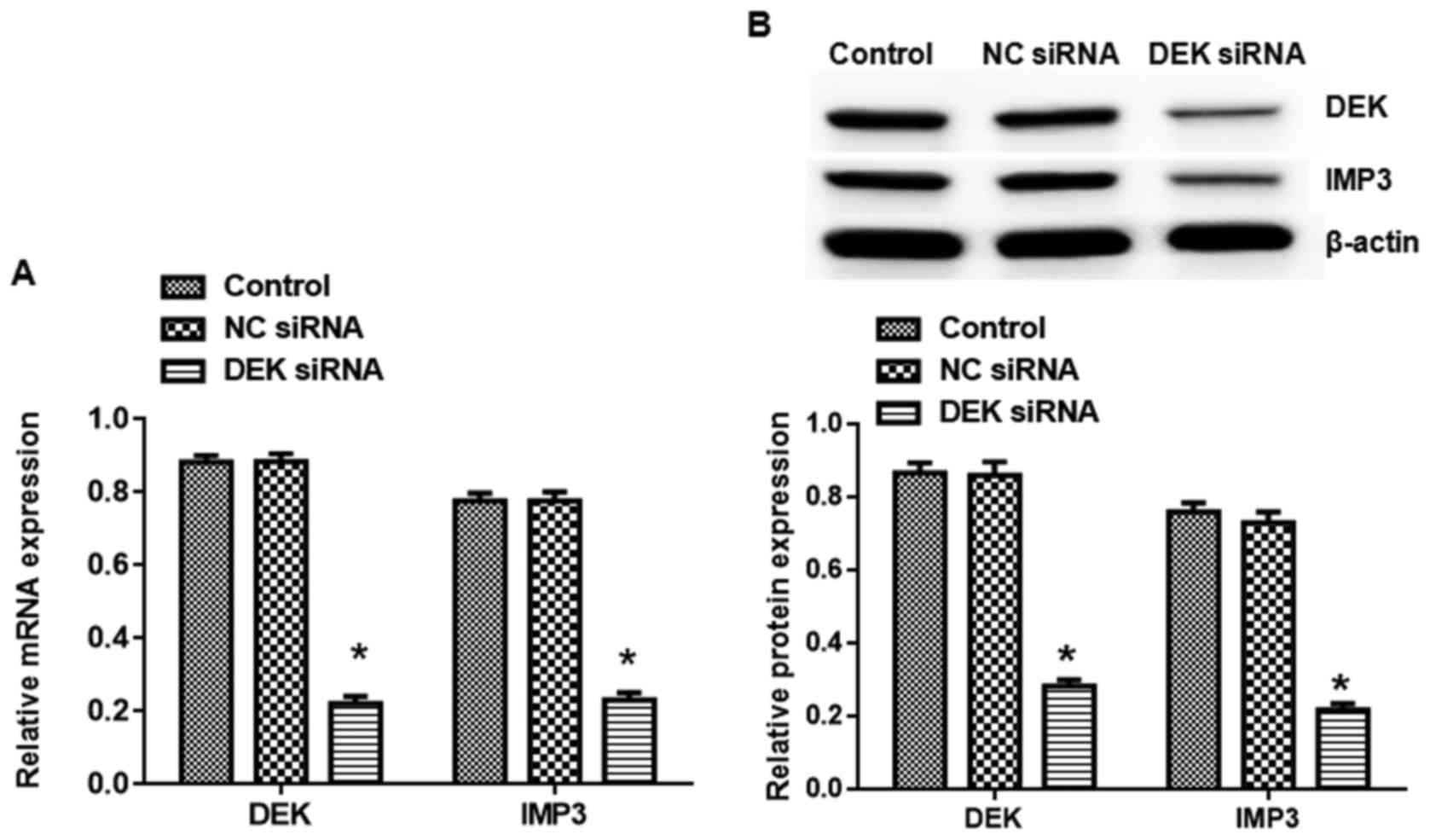
As shown in Fig. 1,
the expression of DEK and IMP3 in mRNA and protein levels in DEK
interfering lentivirus group was reduced significantly compared to
the blank control group (P<0.05). By contrast, empty virus did
not affect DEK and IMP3 expression in SW620 cells. Later, we
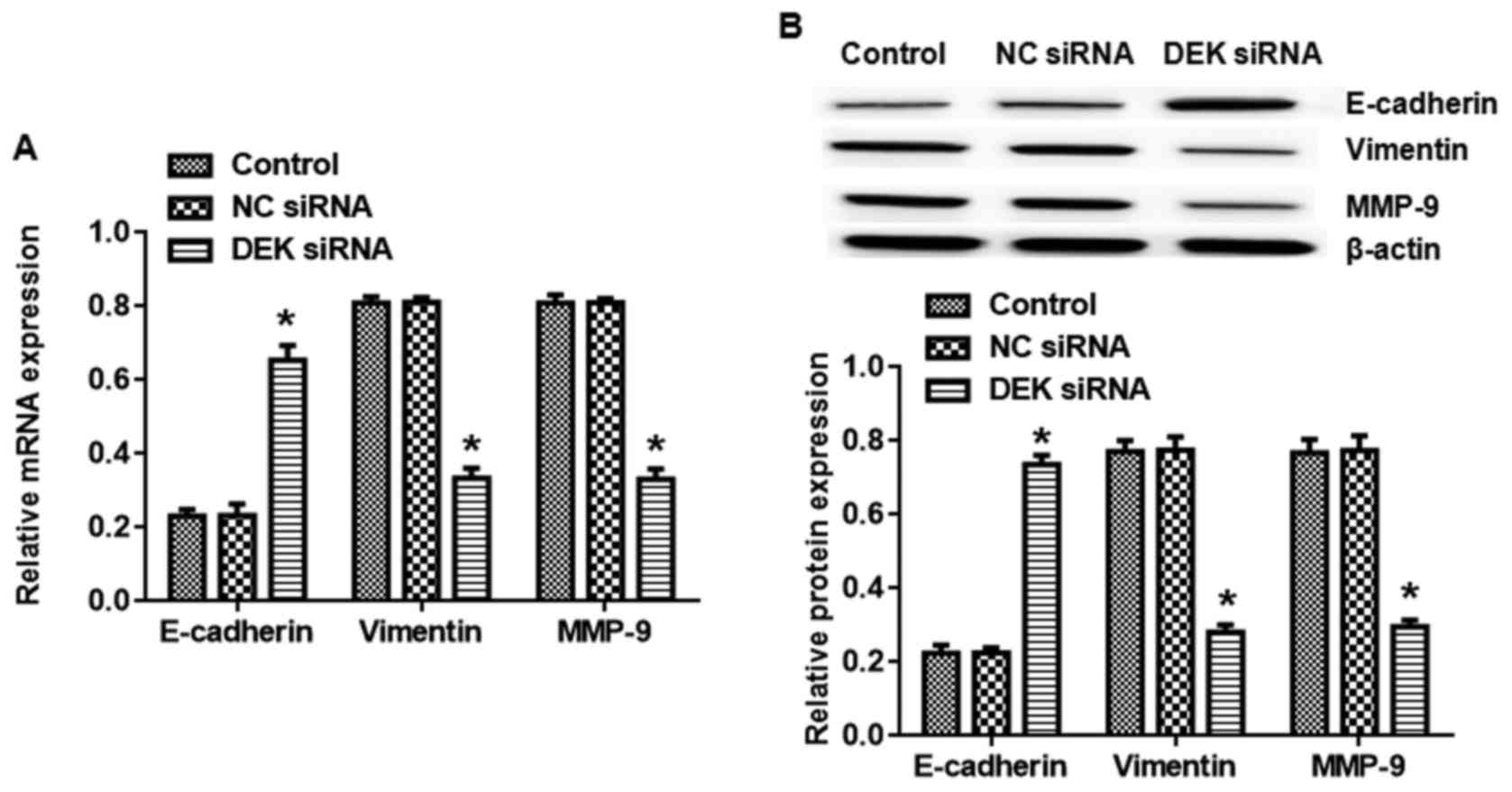
assessed the effects of DEK silence on EMT. As shown in Fig. 2A, there was a significant
enhancement in the mRNA level of E-cadherin and decrease in the
mRNA level of Vimentin and MMP-9 when SW620 cells were treated with
DEK interfering lentivirus (vs. blank control, P<0.05).
Consistently, DEK silence also affected E-cadherin, Vimentin and
MMP-9 protein expression in the similar trend (Fig. 2B). We also confirmed the effects of
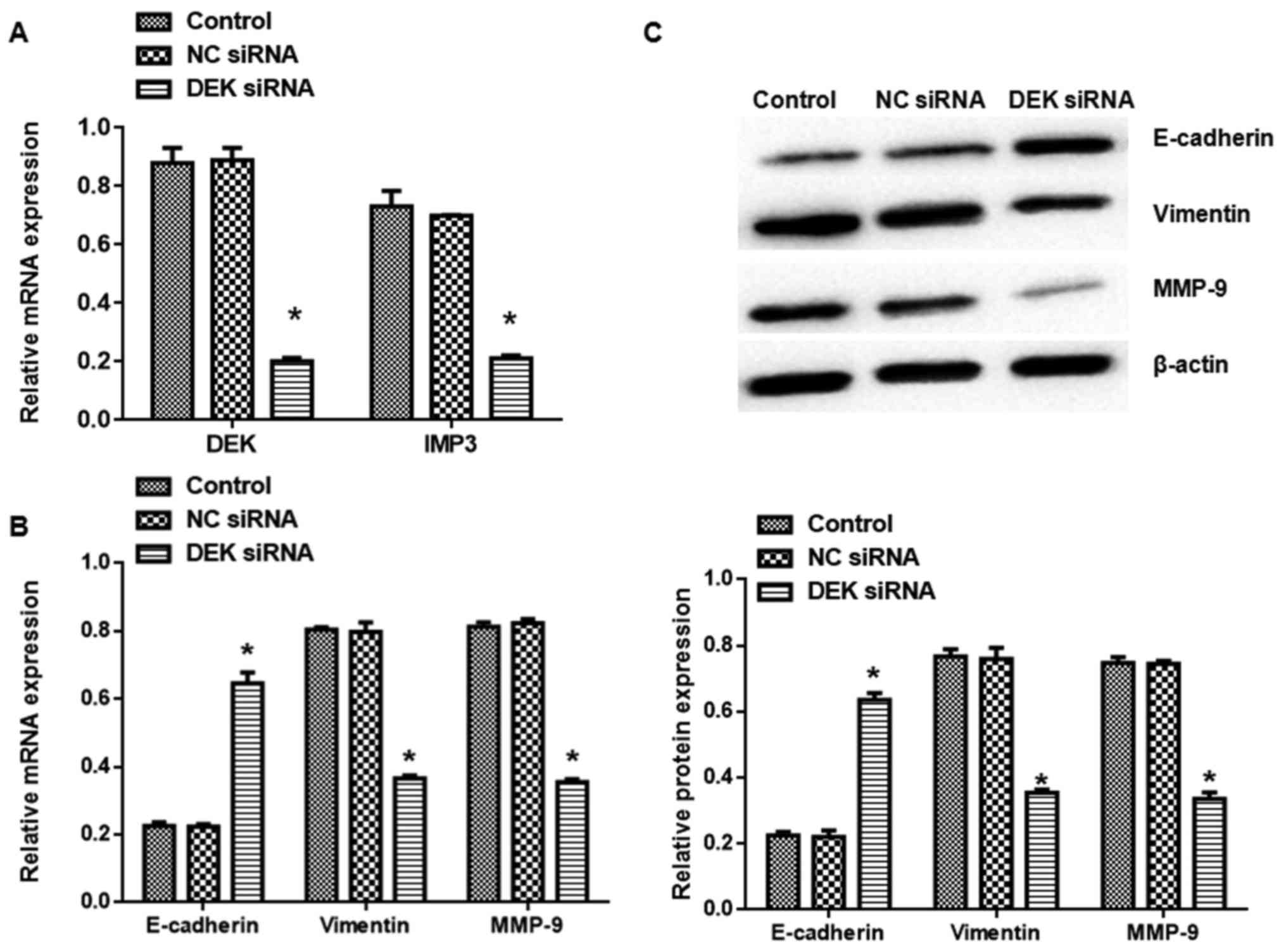
DEK silence on EMT in SW480 cells. DEK interfering lentivirus also
decreased DEK and IMP3 mRNA expression (Fig. 3A). Interestingly, DEK silence also
elevated E-cadherin and mitigated Vimentin and MMP-9 expression in
both mRNA and protein levels (Fig. 3B
and C).
DEK silence inhibits proliferation of
colorectal carcinoma cells
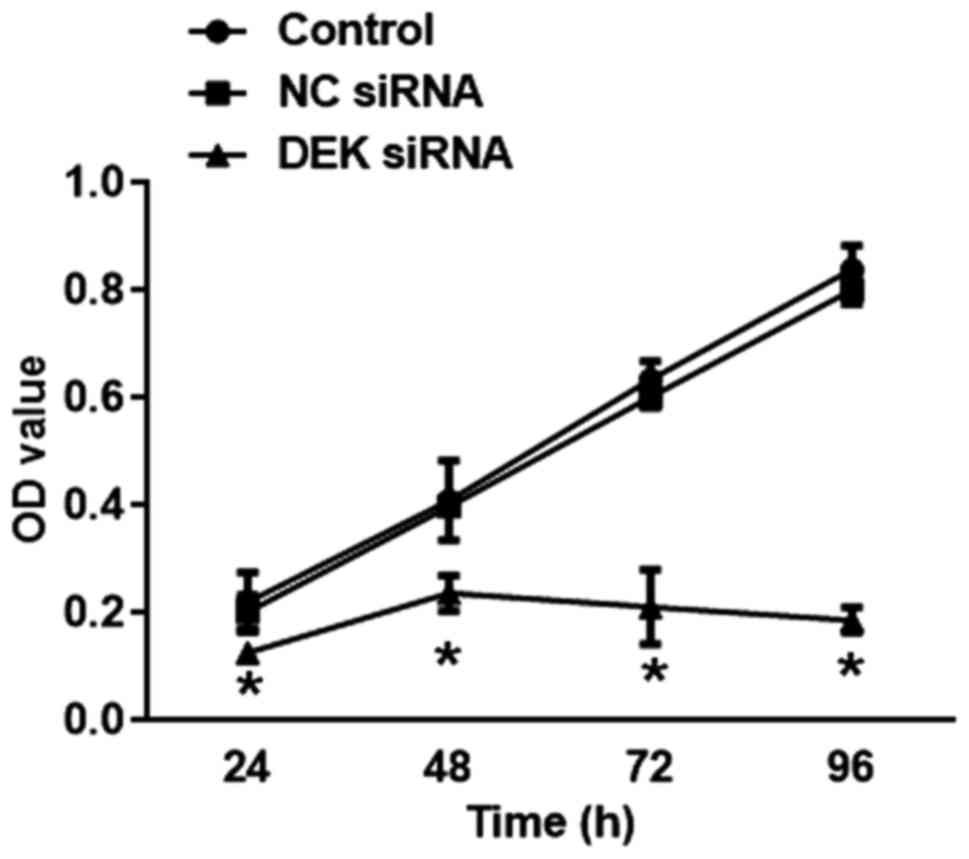
As shown in Fig. 4,
transfection of DEK interfering virus for 24, 48, 72, 96 h caused a
drastic decrease in the percentage of the cell viability of SW620
cells in DEK interfering lentivirus group (vs. blank control,
P<0.05).
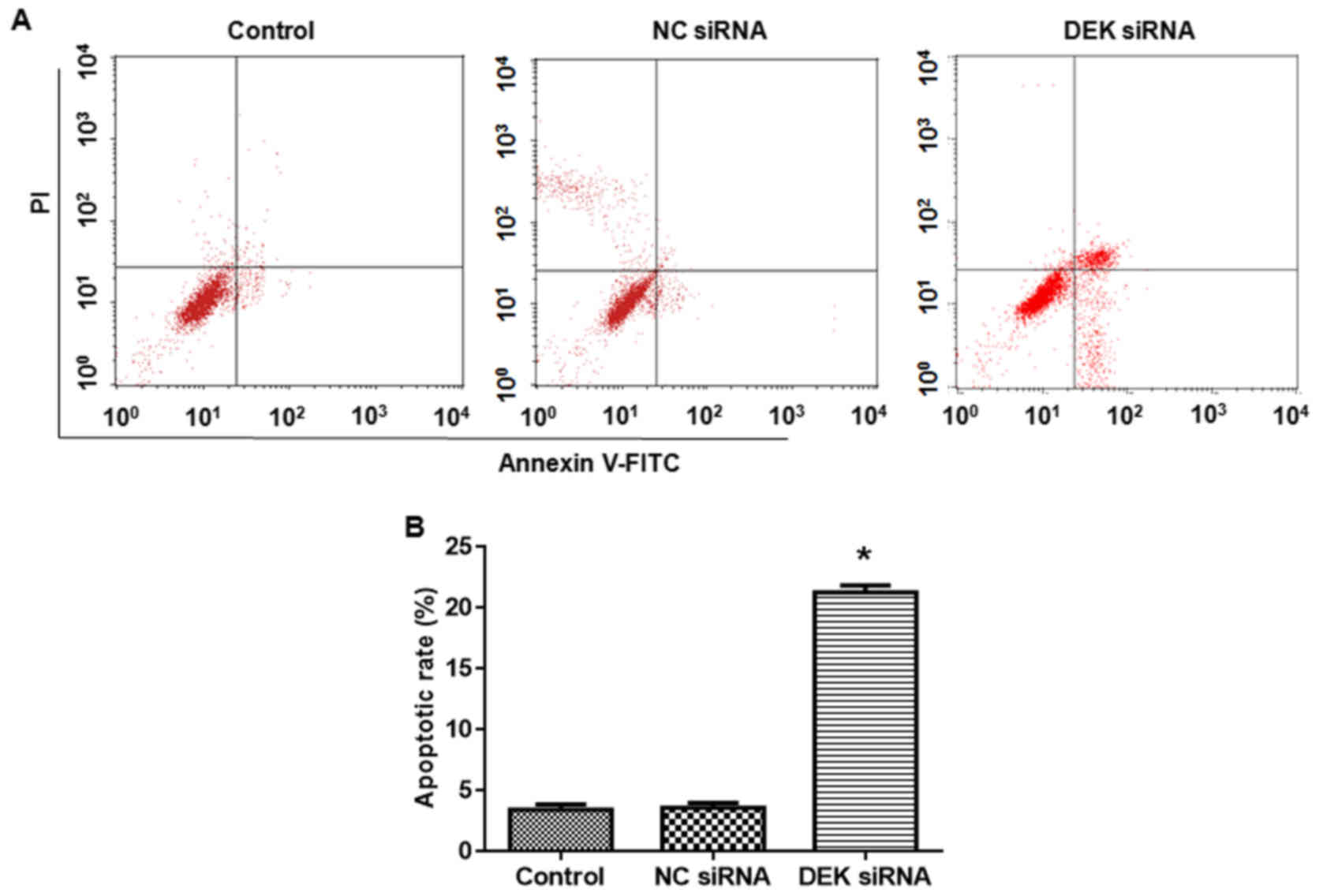
DEK silence promotes apoptosis of
colorectal carcinoma cells
Compared with the blank control group, the apoptotic
rate of cells in DEK interfering lentivirus group was significantly
elevated (vs. blank control, P<0.05, Fig. 5).
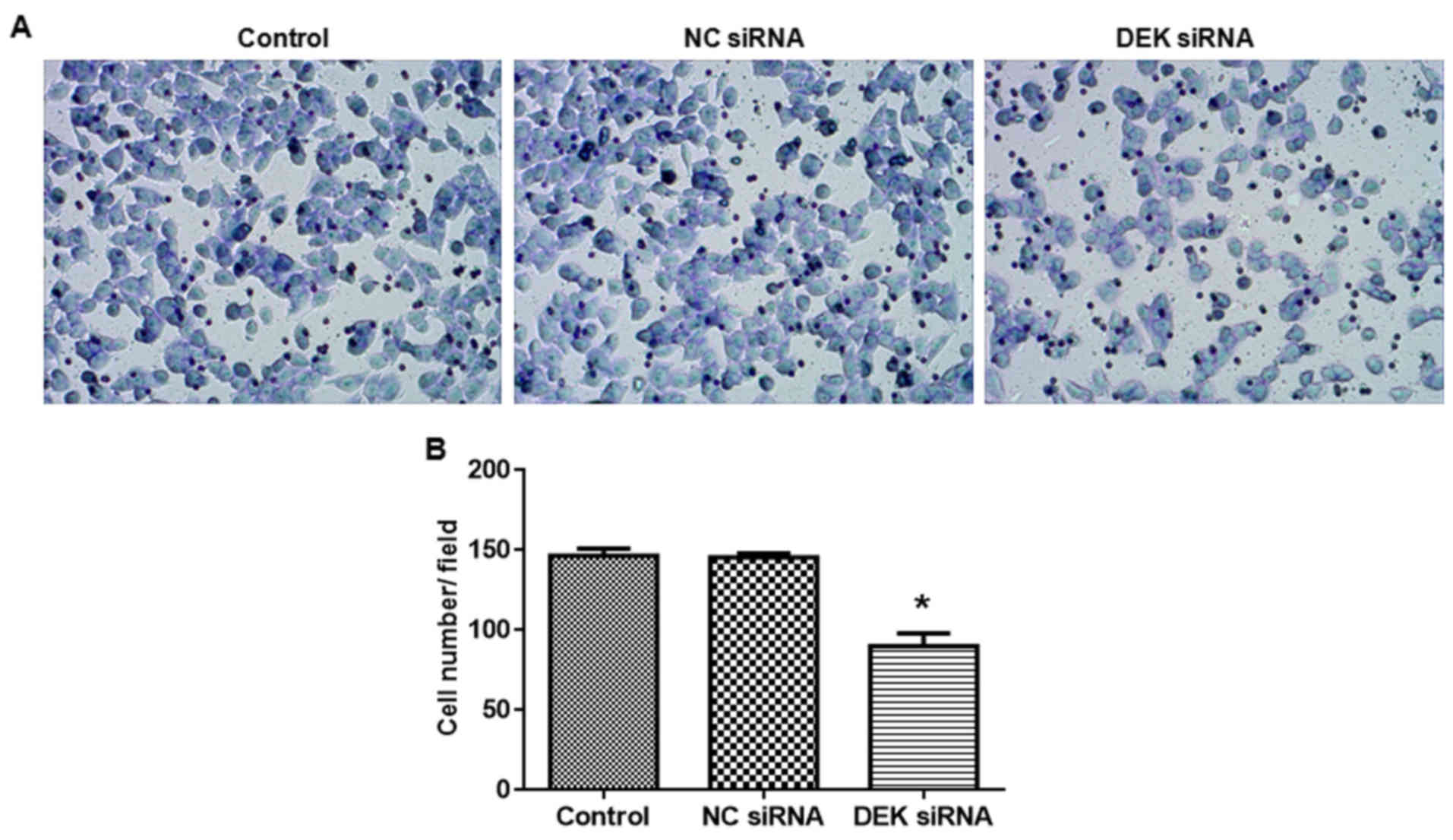
DEK silence inhibits cell invasion of
colorectal carcinoma cells
As shown in Fig. 6,
transfection of DEK interfering lentivirus significantly decreased
the cell invasion ability (vs. blank control, P<0.05).
Discussion
IMP3 is a major component involved in
epithelial-mesenchymal transition (7). Since IMP3 is a highly-conserved
protein with the potential to bind to mRNA sequence. It is a main
contributor in promoting embryonic development as a member of IMP
family. Moreover, the expression level of IMP3 is obviously higher
in embryonic period (6). In
pathological conditions, the expression of IMP3 is elevated in
various malignant tumors (7). In
addition, there were researches demonstrating that the possibility
of distant carcinoma metastasis was associated with unfavorable
prognosis (12–15). DEK is a new member of DNA topology
adjustment factors (10). The
expression level of DEK is not only apparently related to cell
proliferation and apoptosis but also affects the migration and
invasion of colorectal carcinoma cells (16,17).
In this study, we applied colorectal carcinoma SW620
and SW480 cells as the cell models and constructed DEK interfering
lentivirus. Once DEK was silenced, IMP3 expression was also reduced
remarkably. These data indicated that DEK and IMP3 were associated
in colorectal carcinoma. EMT is featured by the decrease of
E-cadherin and increase of Vimentin (18). The up-regulation of E-cadherin
expression can inhibit the invasion and metastasis of tumors, while
down-regulation of E-cadherin is related to enhancement of invasion
and metastasis (19,20). The invasion ability of tumor cells
was inhibited after vimentin silence (21). Considering its high expression in
various epithelial tumors, vimentin was thought to affect malignant
degree of tumor cells (22). The
aim of our study was to explore the regulatory effect of DEK/IMP3
pathway on EMT. The expression level of DEK protein was decreased
by transfecting with DEK interfering lentivirus. Our data showed
that cell invasion of colorectal carcinoma cells could be inhibited
as a result of transfection of DEK interfering lentivirus. Cell
morphology changed from interstitial-like spindle cells to
epithelioid cells. Compared to the blank control group, the
expression level of EMT-related markers E-cadherin was enhanced
obviously, while the expression level of vimentin and MMP-9 were
apparently down-regulated. It is possible that the expression of
DEK was positively associated with vimentin and MMP-9 expression,
while it was negatively correlated with E-cadherin expression. This
result indicated that DEK played important roles in the regulation
of epithelial mesenchymal transition in colorectal carcinoma.
Consistently, interference of IMP3 could also significantly
up-regulate the expression of E-cadherin in hepatocellular
carcinoma (23).
Silence of DEK also decreased the invasion ability,
reduced the cell viability and triggered apoptosis. Therefore, DEK
played a vital role in regulating the invasion, viability and
apoptosis of colorectal carcinoma cells. As demonstrated
previously, DEK knockout could also cause apoptosis of cervical
cancer cells. The possible mechanism was through p53 dependent
apoptosis (24). In addition to
the regulation of anti-apoptotic protein myeloid cell leukemia 1
(Mcl-1), DEK also regulates apoptosis (10). Liu et al demonstrated that
silence of DEK contributed to apoptosis and senescence of cervical
cancer cells (18).
In conclusion, our research demonstrated a
regulatory mechanism of DEK on EMT of colorectal carcinoma cells
which would finally influence the invasion of colorectal carcinoma
cells. Our research provided theoretical guidance for the treatment
of colorectal carcinoma.
References
|
1
|
Bordonaro M and Lazarova DL: CREB-binding
protein, p300, butyrate, and Wnt signaling in colorectal cancer.
Word J Gastroenterol. 21:8238–8248. 2015. View Article : Google Scholar
|
|
2
|
Wang J, Wang X, Lin S, Chen C, Wang C, Ma
Q and Jiang B: Identification of kininogen-1 as a serum biomarker
for the early detection of advanced colorectal adenoma and
colorectal cancer. PLoS One. 8:e705192013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okada K, Fujiwara Y, Nakamura Y, Takiguchi
S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Takahashi T, Mori
M and Doki Y: Oncofetal protein, IMP3, a potential marker for
prediction of postoperative peritoneal dissemination in gastric
adenocarcinoma. J Surg Oncol. 105:780–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Yan D, Tang H, Zhou C, Fan J, Li S,
Wang X, Xia J, Huang F, Qiu G and Peng Z: IMP3 is a novel
prognostic marker that correlates with colon cancer progression and
pathogenesis. Ann Surg Oncol. 16:3499–3506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perak Beljan R, Durdov MG, Capkun V,
Ivcevic V, Pavlovic A, Soljic V and Peric M: IMP3 can predict
aggressive behaviour of lung adenocarcinoma. Diagn Pathol.
7:1652012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yantiss RK, Woda BA, Fanger GR, Kalos M,
Whalen GF, Tada H, Andersen DK, Rock KL and Dresser K: KOC (K
honlology domain containing protein overexpressed in cancer): A
novel molecular maker that distinguishes between benign and
malignant lesions of the pancreas. Am J Surg Pathol. 29:188–195.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumara Shantha H, Kirchoff D, Caballero
OL, Su T, Ahmed A, Herath SA, Njoh L, Cekic V, Simpson AJ,
Cordon-Cardo C and Whelan RL: Expression of the cancer testis
antigen IGF2BP3 in colorectal cancers; IGF2BP3 holds promise as a
specific immunotherapy target. Oncoscience. 2:607–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riveiro-Falkenbach E and Soengas MS:
Control of tumorigenesis and chenoresistance by the DEK oncogene.
Clin Cancer Res. 16:2932–2938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang L, Xu Y, Cai G, Guan Z and Cai S:
Downregulation of S100A4 expression by RNA interference suppresses
cell growth and invasion in human colorectal cancer cells. Oncol
Rep. 27:917–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khodadoust MS, Verhaegen M, Kappes F,
Riveiro-Falkenbach E, Cigudosa JC, Kim DS, Chinnaiyan AM, Markovitz
DM and Soengas MS: Melanoma proliferation and chemoresistance
controlled by the DEK oncogene. Cancer Res. 69:6405–6413. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oyarzo MP, Lin P, Glassman A, Bueso-Ramos
CE, Luthra R and Medeiros LJ: Acute myeloid leukemia with
t(6;9)(p23;q34) is associated with dysplasia and a high frequency
of flt3 gene mutations. Am J Clin Pathol. 122:348–358. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lederer M, Bley N, Schleifer C and
Hüttelmaier S: The role of the oncofetal IGF2 mRNA-binding protein
3 (IGF2BP3) in cancer. Semin Cancer Biol. 29:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell JL, Wächter K, Mühleck B, Pazaitis N,
Köhn M, Lederer M and Hüttelmaier S: Insulin-like growth factor 2
mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of
cancer progression? Cell Mol Life Sci. 70:2657–2675. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JY, Choe M, Kang Y and Lee SS: IMP3,
a promising prognostic marker in clear cell renal cell carcinoma.
Korean J Pathol. 48:108–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Li HG, Xia ZS, Lü J and Peng TS:
IMP3 is a novel biomarker to predict metastasis and prognosis of
gastric adenocarcinoma: A retrospective study. Chin Med J (Engl).
123:3554–3558. 2010.PubMed/NCBI
|
|
16
|
Lin L, Piao J, Ma Y, Jin T, Quan C, Kong
J, Li Y and Lin Z: Mechanisms underlying cancer growth and
apoptosis by DEK overexpression in colorcetal cancer. PLoS One.
9:e1112602014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin L, Piao J, Gao W, Piao Y, Jin G, Ma Y,
Li J and Lin Z: DEK over expression as an independent biomarker for
poor prognosis in colorectal cancer. BMC Cancer. 13:3662013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu K, Feng T, Liu J, Zhong M and Zhang S:
Silencing of the DEK gene induces apoptosis and senescence in CaSki
cervical carcinoma cells via the up-regulation of NF-κB p65. Biosci
Rep. 32:323–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oliveras-Ferraros C, Corominas-Faja B,
Cufí S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM,
López-Bonet E, Martin ÁG and Menendez JA: Epithelial-to-mesenchymal
transition (EMT) confers primary resistance to trastuzumab
(Herceptin). Cell Cycle. 11:4020–4032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkar FH, Li Y, Wang Z and Kong D:
Pancreatic cancer stem cells and EMT in drug resistance and
metastasis. Minerva Chir. 64:489–500. 2009.PubMed/NCBI
|
|
22
|
Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL,
Wang TH and Hsu HC: RNA-binding protein insulin-like growth factor
II mRNA-binding protein 3 expression promotes tumor invasion and
predicts early recurrence and poor prognosis in hepatocellular
carcinoma. Hepatology. 48:1118–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen C, Wang FH, An X, Luo HY, Wang ZQ,
Liang Y, Zhang L and Li YH: Triplet combination with paclitaxel,
cisplatin and 5-FU is effective in metastatic and/or recurrent
nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 71:371–378.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wise-Draper TM, Allen HV, Jones EE, Habash
KB, Matsuo H and Wells SI: Apoptosis inhibition by the human DEK
oncoprotein involves interfer-ence with p53 functions. Mol Cell
Biol. 26:7506–7519. 2006. View Article : Google Scholar : PubMed/NCBI
|