Introduction
Asthma is a severe respiratory disease classified as
one of the hyper-responsive immune diseases. According to the 2013
report from the World Health Organization, asthma patients numbered
~235 million worldwide in 2013 (1). The United States Environmental
Protection Agency (EPA) estimated in 2013 that there were 25.9
million asthma patients in the USA alone. In addition, the EPA
stated that asthma was among the most serious respiratory diseases
that leads to hospitalization, especially in children under 15
years of age (2). Various
allergens can trigger asthma, such as dust mites, cockroaches, pet
dander, viral infections, pollen, mold, fungi, tobacco smoke and
pollutants (1). Asthma is an
incurable disease that is related to the pulmonary system, and
presents itself with various symptoms ranging from coughing to
constructive apnea, all resulting from mucous secretion, goblet
cell hyperplasia, epithelial cell shedding, basement membrane
thickening and eosinophil infiltration (3,4).
Asthma may be induced by an imbalance of T helper
(Th)1 and Th2 cells, as well as various other factors related to
disease occurrence. The transcription factor, T-bet, serves a role
as a Th1 cell transcription factor (5,6),
producing Th1-related cytokines including interferon (IFN)-γ, which
is stimulated by T-bet to produce more T-bet in a positive feedback
loop. Interleukin (IL)-12 is a key cytokine that regulates the
balance between Th1 and Th2 cells (7). It consists of a p35 subunit expressed
ubiquitously and a p40 subunit that is restricted to
IL-12-producing cells (8). IL-12
stimulates Th1-related immunoglobulins, including IgG2a, and it
influences Th cell trafficking (9).
GATA-3 is a Th2 cell transcription factor (10). In a manner similar to T-bet, GATA-3
regulates Th2-related cytokines and accelerates the differentiation
of Th2-related cytokines, including IL-4. Following IL-4
differentiation by GATA-3, IL-4 stimulates the production of more
GATA-3 in a positive feedback loop. IL-5 increases the eosinophil
population (11). IL-13 and IL-4
enhance B cell activation and Ig E production (12). IL-6 is a proinflammatory cytokine
(13) and, because it is a
regulatory factor for CD4+ cell balance (14), IL-6 is an important factor in
asthma regulation. TNF-α is a proinflammatory cytokine induced by
mast cells that has several regulatory functions that are related
to asthma, such as smooth muscle contraction (15), neutrophil and eosinophil
attractions (16) and T cell
activation, which involves cytokine release (17).
Symptomatic relievers have been investigated in the
past as asthma is a hyper-responsive disease and is difficult to
completely eradicate/cure (18).
For several decades, steroids were commonly prescribed for asthma,
but the drugs have many severe adverse effects such as inhibiting
the growth of children (19,20),
cataracts and glaucoma, hypertension, hyperlipidemia, peptic
ulcers, myopathy and immunological suppression (21). Consequently, there have been many
efforts to find more effective candidates from natural products
with fewer adverse effects (22,23).
M. atichisonii is a mushroom that has been consumed for a
long time in East Asia (24) and
has been reported to have several medicinal effects, such as the
synthesis of nerve growth factors (25), anti-obesity (26) and the prevention of cell death
(27).
In the present study, the authors attempted to
identify anti-asthmatic drug candidates from natural products to
reduce the adverse effects of existing therapies, and to develop a
drug that is more effective against asthma. Using
immunohistochemical studies, M. atichisonii was determined to
reduce the physiological and histopathological changes related to
asthma in the bronchoalveolar lavage fluid (BALF), including the
number of white blood cells, differential cell count and
immunoglobulin (Ig)E in the lungs, as well as the associated
morphological changes and asthma-inducing factors.
Materials and methods
Mycoleptodonoides (M.) aitchisonii
preparation
M. atichisonii used in the present study was
provided by the Jeollanam-do Wando Arboretum (Wando, Korea). The
fresh fruiting bodies were hot air-dried and ground into a powder.
M. atichisonii extract was prepared by boiling 1 kg of
mycelium powder in 10 l filtered sterile water for 8 h at 50°C. The
supernatant was saved and the pellet was re-boiled with 10 l water
for 8 h. Insoluble material was removed by filtration and a
two-fold volume of cold ethanol was added to the filtrate to
precipitate the polysaccharide. Following standing the mixture at
4°C overnight, the precipitate was collected by centrifugation
(4,000 × g, 30 min at room temperature). The precipitate was washed
with ethanol and resuspended in water. The resuspended solution was
then freeze-dried.
Ovalbumin-induced asthma mouse
model
Six-week-old female BALB/c mice (n=80; body weight,
22±2 g) were purchased from Samtako Bio Co. (Osan, Korea), fed with
an ad libitum diet and water, and housed in a controlled
environment (22±3°C, 12 h light/dark cycle). They were then divided
into six treatment groups: (i) Control, (ii) sterilized tap water
with ovalbumin (OVA) induction, (iii) 1 mg/kg/day dexamethasone
(DEX; Sam Nam Pharm, Chungcheongnam, Korea) with OVA induction,
(iv) 10 mg/kg/day M. atichisonii with OVA induction, (v) 100
mg/kg/day M. atichisonii with OVA induction and (vi) 1,000
mg/kg/day M. atichisonii with OVA induction. On days 1 and
8, all mice were sensitized via intraperitoneal injection of 20 µg
OVA (cat no. A5503-50G; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 1 mg aluminum hydroxide hydrate (Prod #77161; Thermo
Fisher Scientific, Inc. Waltham, MA, USA) in 500 µl saline. From
days 21 to 25, all mice, except for those used as control, were
challenged once daily with 5% OVA for 30 min using a nebulizer (3
ml/min, NE-U17; Omron Corporation, Kyoto, Japan). During the same 5
day period, the treatment groups were also administered once daily
with oral doses of sterilized tap water, DEX, 10, 100 or 1,000
mg/kg/day M. atichisonii 1 h prior to the OVA challenge.
Ethics statement
All experiments were approved by the Institutional
Animal Care and Use Committee at Dongshin University (Naju, Korea;
approval no. 2014-08-04).
BALF analysis
A total of one day following the final treatment,
the mice were anesthetized with intraperitoneal injections of 50
mg/kg Zoletil (Virbac, Carros, France), and their tracheas were
cannulated with disposable animal feeding needles. Lavages were
performed with three 0.4 ml aliquots of cold phosphate-buffered
saline (PBS; cat no. 17-516F; Lonza, Walkersville, MD, USA). BALF
samples were collected and immediately centrifuged at 900 × g for 5
min at room temperature (Sorvall Legend Micro 17R; Thermo Fisher
Scientific, Inc.). The cell pellets were resuspended in PBS for
total and differential cell counts. The numbers of total and
differential cells were counted using the Hemavet Multispecies
Hematology system (n=8 per group; Drew Scientific Inc., Miami
Lakes, FL, USA). Levels of IgE in the BALF were measured using a
specific mouse IgE enzyme-linked immunosorbent assay kit (cat no.
555248; BD Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol (n=8 per group).
Histopathological analysis
Lung tissue was fixed in 10% (v/v) formaldehyde
solution for 10 days at room temperature, dehydrated in a graded
ethanol series (99.9, 90, 80 and 70%), and embedded in paraffin.
Paraffin-embedded lung tissue was then sectioned (4 µm)
longitudinally and stained with hematoxylin and eosin. In addition,
the sections were stained with Periodic Acid-Schiff (PAS; periodic
acid; cat no. P7875; Sigma-Aldrich; Merck KGaA; Schiff's reagent;
cat no HX54780633; EMD Millipore, Billerica, MA, USA) for the
semi-quantitative analysis of glycoproteins.
Immunohistochemical analysis
Deparaffinized tissue sections were treated with 3%
hydrogen peroxide in methanol for 10 min to remove endogenous
peroxidase. Antigen retrieval was carried out with sodium citrate
buffer (0.1 M) using a microwave. The slides were incubated with
normal serum to block non-specific binding and then incubated
overnight at 4°C with the following primary antibodies (diluted
1:100 to 1:200): Rabbit anti-mouse CD3 polyclonal (cat no. ab5690;
1:100; Abcam, Cambridge, MA, USA), rat anti-mouse CD4 monoclonal
(cat no. 14-9766; 1:200; eBioscience, Inc., San Diego, CA, USA),
rat anti-mouse CD8 monoclonal (cat no. sc-18913; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-mouse CD19
polyclonal (cat no. 250585; 1:100; Abbiotec, San Diego, CA, USA),
rat anti-mouse MHC class II monoclonal (cat no. sc-59318; 1:100;
Santa Cruz Biotechnology, Inc.), rabbit anti-mouse Tbx21/T-bet
polyclonal (cat no. bs-3599R; 1:100; BIOSS, Beijing, China), goat
anti-mouse GATA-3 (cat no. TA305795; 1:100; OriGene Technologies,
Inc., Rockville, MD, USA), rat anti-mouse IFN-γ monoclonal (cat no.
sc-74104; 1:100), goat anti-mouse IL-12p35 polyclonal (cat no.
sc-9350; 1:100), rat anti-mouse IL-12p40 monoclonal (cat no.
sc-57258; 1:100) (all from Santa Cruz Biotechnology, Inc.), rabbit
anti-mouse TNF-α polyclonal (cat no. 3053R-100; 1:200; BioVision
Milpitas, CA, USA), rat anti-mouse IL-4 monoclonal (cat no.
sc-73318; 1:100), rabbit anti-mouse IL-5 polyclonal (cat no.
sc-7887; 1:100), goat anti-mouse IL-6 polyclonal (cat no. sc-1265;
1:100), and goat anti-mouse IL-13 polyclonal (cat no. sc-1776;
1:100) (all from Santa Cruz Biotechnology, Inc.). The slides were
incubated for 10 min with a biotinylated secondary antibody (cat
no. PK-7800; Vector Laboratories, Inc., Burlingame, CA, USA) and
horseradish-peroxidase conjugated streptavidin. Signals were
detected using a 3,3-diaminobenzidine tetrahydrochloride substrate
chromogen solution (cat no. SK-4105; Vector Laboratories), and
cells were counterstained with Mayer's hematoxylin. To determine
the number of positively stained cells, five random,
non-overlapping fields of view were selected, and cells were
counted (magnification, ×200) from three separately immunostained
lung sections per animal (n=8 per group).
Gas chromatography/mass spectrometry
(GC-MS) analysis
GC-MS (Agilent 5975C MSD and 7890A GC; Agilent
Technologies, Inc., Santa Clara, CA, USA) was tuned by
perfluorotribuylamine (cat no. 442747; Sigma-Aldrich; Merck KGaA)
using three mass fragments (m/z) of 69.0, 219.0 and 502.0 in the
condition of electron ionization. A 5MS GC column (DB-5
cross-linked 5% phenylmethyl silicone; Agilent Technologies, Inc.)
was used for the analysis as this column has low bleed that
improves sensitivity for constituent identification. The GC oven
was heated using the following program: Isothermal at 65°C for 10
min and increasing by 10°C every min to 300°C with He as the
carrier gas. The summarized operation parameters for the GC-MS are
presented in Table I. In order to
analyze the quality of extract in M. aitchisonii, the
certain amount of dried samples by dehydrofreezing procedure was
prepared. The samples were extracted using 10 min sonication at
room temperature, twice with dichloromethane, followed by
sonication twice with acetone using a sonicator
(Bransonic® CPXH; Branson Ultrasonics Corporation,
Dallas, TX, USA). The composited extract was evaporated by high
volume nitrogen blowdown (TurboVap II; Caliper Life Sciences,
Hopkinton, MA, USA) to reach 5–10 ml and was further evaporated to
a final volume of 100 µl using low volume nitrogen blowdown
(MGS-2200; Tokyo Rikakikai Co., Ltd., Tokyo, Japan). A final
extract volume of 100 µl was sialylated with
N,O-bis(trimethylsilyl) trifluoroacetamide (Supelco; Sigma-Aldrich;
Merck KGaA) to derivatize the constituents to their
trimethylsilyl-derivatives (TMS-derivatives) prior to GC-MS
analysis.
 | Table I.Operation parameters for the
GC/MS. |
Table I.
Operation parameters for the
GC/MS.
| Conditions |
| GC/MSa |
|
|---|
| Column |
| J&W Scientific,
DB-5 cross-linked 5% phenyl methyl silicone |
|
| Carrier |
| Helium |
|
|
Split/splitless |
| Split 10:1 |
|
| Injection
volume |
| 1µl |
|
| Detector |
| MS |
|
| MS source |
| 230°C |
|
| MS quad |
| 150°C |
|
|
|
|
|
| Conditions | Rate (°C/min) | Value (°C) | Hold time
(min) |
|
|
|
|
| Analytical
temperature |
|
Initial | – | 65 | – |
| Step
1 | – | 65 | 10 |
| Step
2 | 10 | 300 | 22 |
| Total |
| 55.5 min |
|
| Electron
ionization |
| 70 ev |
|
| Mass range |
| 50–550 amu |
|
| Scan method |
| Full scan |
|
Statistical analysis
Data are presented as the mean ± standard deviation.
Group differences were evaluated by one-way analysis of variance
followed by Dunnett's multiple comparison tests. Statistical
significance was set at P<0.05 or P<0.01.
Results
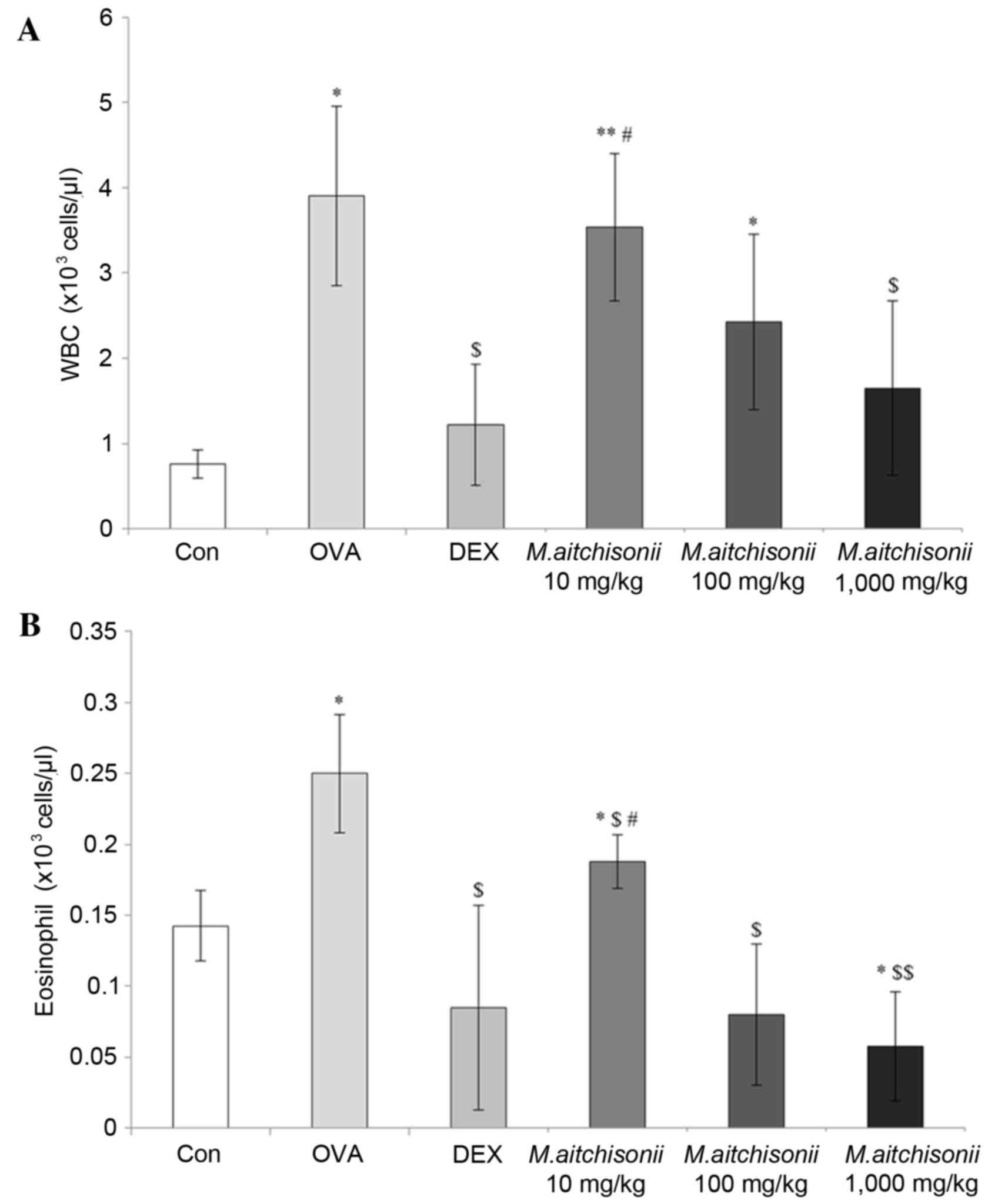
M. atichisonii dose-dependently
suppressed the populations of WBCs and eosinophils in BALF
In the OVA-induced asthma group, M.
atichisonii dramatically increased WBC count, when compared
with the level of WBCs in the control group. However, in the DEX
treated group, WBC number decreased by a similar amount in the
control group (Fig. 1A; P<0.05
or P<0.01). M. atichisonii suppressed WBC proliferation
induced by OVA in a dose-dependent manner. The number of WBCs in
the 1,000 mg/kg M. atichisonii treatment group was similar
to that in the DEX treatment.
Eosinophil number was dramatically was more affected
by M. atichisonii treatment than WBC number (Fig. 1B; P<0.05 or P<0.01). M.
atichisonii dose-dependently suppressed eosinophil
proliferation and the levels of eosinophils in the 1,000 mg/kg/day
M. atichisonii treatment group, and this was similar to that
in DEX treatment.
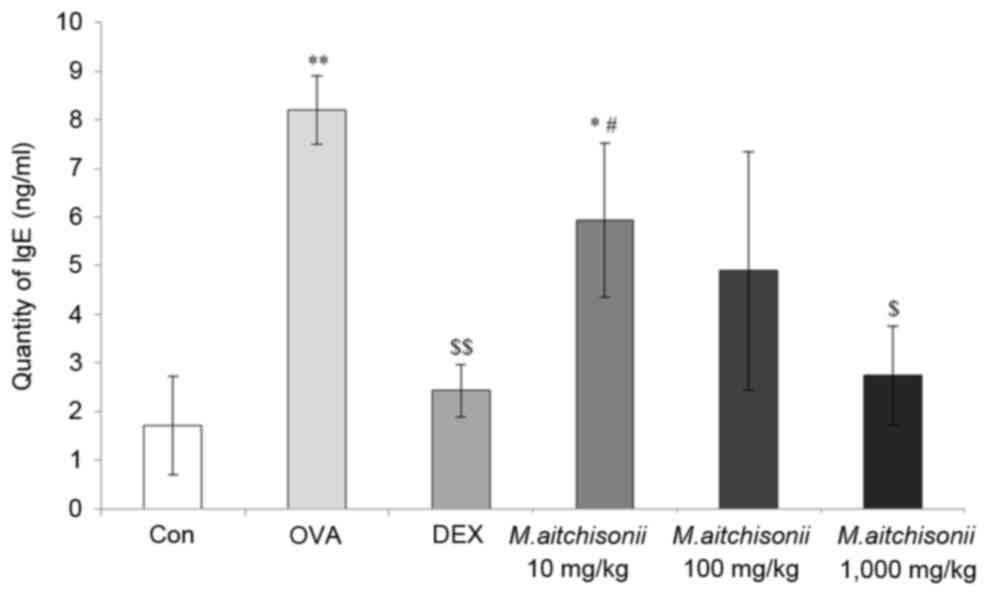
M. atichisonii effectively decreased
the quantity of IgE in the BALF of OVA-induced asthma mice
IgE is related to the hyperresponsiveness of asthma
and serves an important role in asthma occurrence (28). M. atichisonii effectively
decreased the level of IgE in a dose-dependent manner, which was
increased by OVA treatment, when compared with that the in OVA
treatment group (8.2±0.69 ng/ml). In addition, the quantity of IgE
in the 1,000 mg/kg treatment group (2.7±1.02 ng/ml) was similar to
that in the DEX treatment group (2.4±0.54 ng/ml; Fig. 2; P<0.05 or P<0.01).
Mycoleptodonoides atichisonii
recovered the typical asthmatic morphological changes in the
lung
To analyze the morphological changes in the lung
that were induced by OVA treatment, hematoxylin and eosin staining
was used, in addition to PAS staining (Fig. 3). In the lungs of mice with
OVA-induced asthma, typical histopathological changes were observed
such as airway remodeling, mucous hyper-secretion, epithelial
hyperplasia and eosinophil infiltration (Fig. 3A-b). In particular, PAS stained
mucous hyper-secretion in the bronchioles was clearly observed
using the specific staining method (Fig. 3B-b). DEX, which is typically used
as a representative anti-asthmatic drug, significantly suppressed
the morphological changes in the lung (Fig. 3A-c) and completely prevented mucous
secretion (Fig. 3B-c). M.
atichisonii not only recovered the asthmatic alterations
(Fig. 3A-d-f) but also suppressed
mucous secretion (Fig. 3B-d-f).
These results suggested that M. atichisonii may ameliorate
airway obstruction.
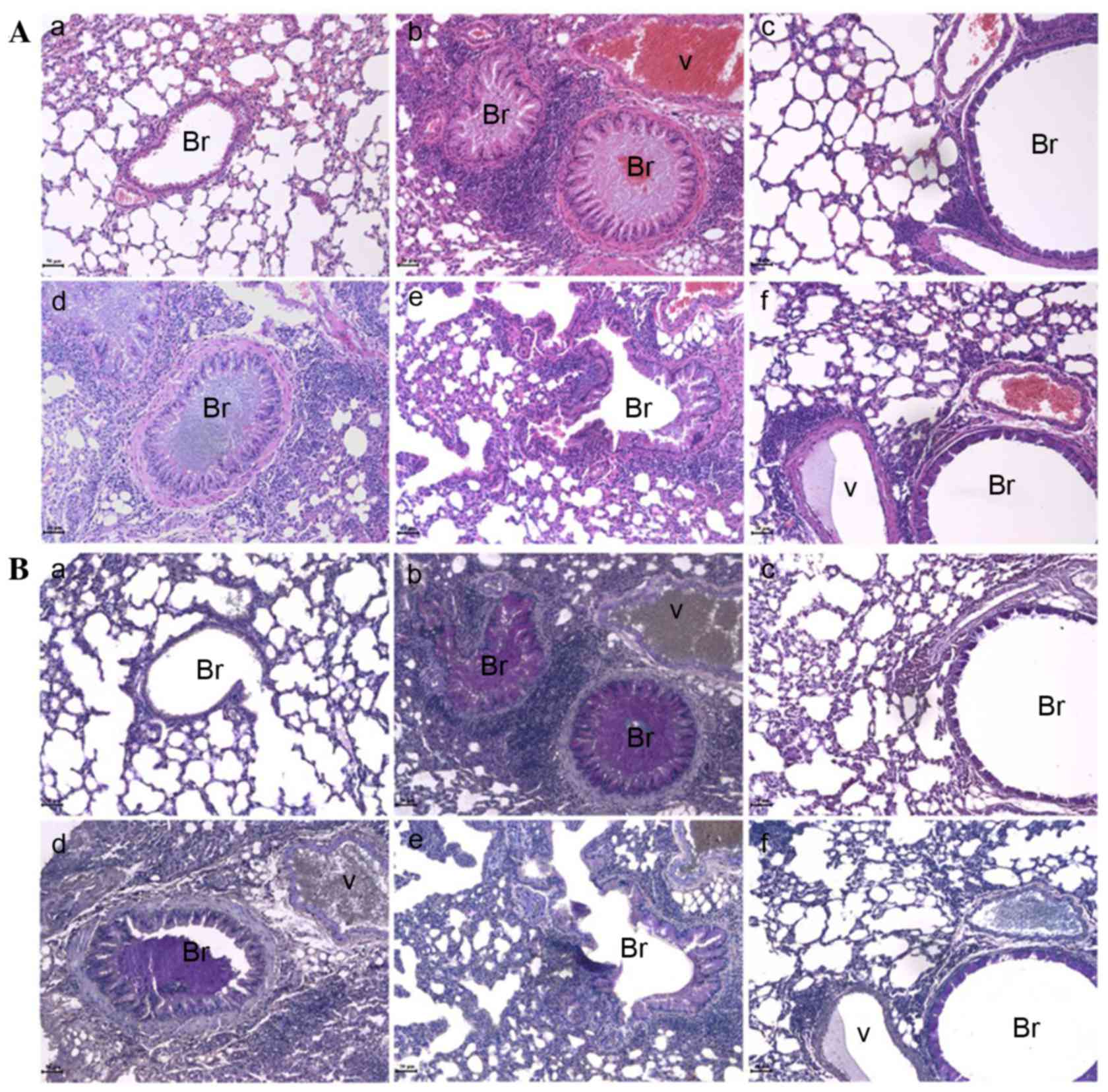 | Figure 3.M. atichisonii recovered the
asthmatic changes in the lung. (A) M. atichisonii
effectively and dose-dependently recovered the ovalbumin-induced
morphological changes such as mucous secretion in bronchioles,
epithelial hyperplasia and eosinophil infiltration. This is a
representative image of a lung stained by hematoxylin and eosin.
(B) M. atichisonii decreased ovalbumin-induced mucous
secretion similar to dexamethasone. This is a representative image
of a lung stained by Periodic Acid-Schiff. Scale bar (bottom-left
of each image)=50 µm. a, control; b, ovalbumin; c, dexamethasone;
d, 10 mg/kg/day M. atichisonii; e, 100 mg/kg/day M.
atichisonii; f, 1,000 mg/kg/day M. atichisonii. Br,
bronchiole; V, vessel. |
Mycoleptodonoides atichisonii
downregulated the expressions of MHC class II molecules and T
cells
In the 100 mg/kg/day M. atichisonii treatment
and 1,000 mg/kg/day M. atichisonii treatment, expression of
CD3+ total T cell (Fig. 4A;
P<0.05 or P<0.01) was almost fully diminished. The expression
of CD4+ helper T cells was suppressed in a dose-dependent manner
(Fig. 4B; P<0.05 or P<0.01).
A total of 1,000 mg/kg/day M. atichisonii treatment
effectively inhibited the expression of CD4+ helper T cells
(Fig. 4B-f; P<0.05 or
P<0.01) when compared with the DEX treatment group (Fig. 4B-c; P<0.05 or P<0.01). In the
100 mg/kg/day or more M. atichisonii treatment group, the
expression pattern of CD8+ cytotoxic T cells (Fig. 4C; P<0.05 or P<0.01) was the
same as that observed for the CD4+ helper T cells, which was that
they were almost fully diminished. Although M. atichisonii
downregulated the expression of CD19+ B cells, the decreased level
of CD19+ B cells was lower compared with other groups including
CD3+ total T cell, CD4+ helper T cell, and CD8+ cytotoxic T cell
(Fig. 4D; P<0.05 or P<0.01).
The expression of MHC class II+ molecule (Fig. 4E; P<0.05 or P<0.01) almost
disappeared in the 100 mg/kg/day M. atichisonii treatment
and in the 1,000 mg/kg/day M. atichisonii treatment groups.
The 1,000 mg/kg/day M. atichisonii treatment effectively
inhibited the expression of MHC class II+ molecules (Fig. 4E-f; P<0.05 or P<0.01) more,
when compared with the DEX treatment group (Fig. 4E-c; P<0.05 or P<0.01).
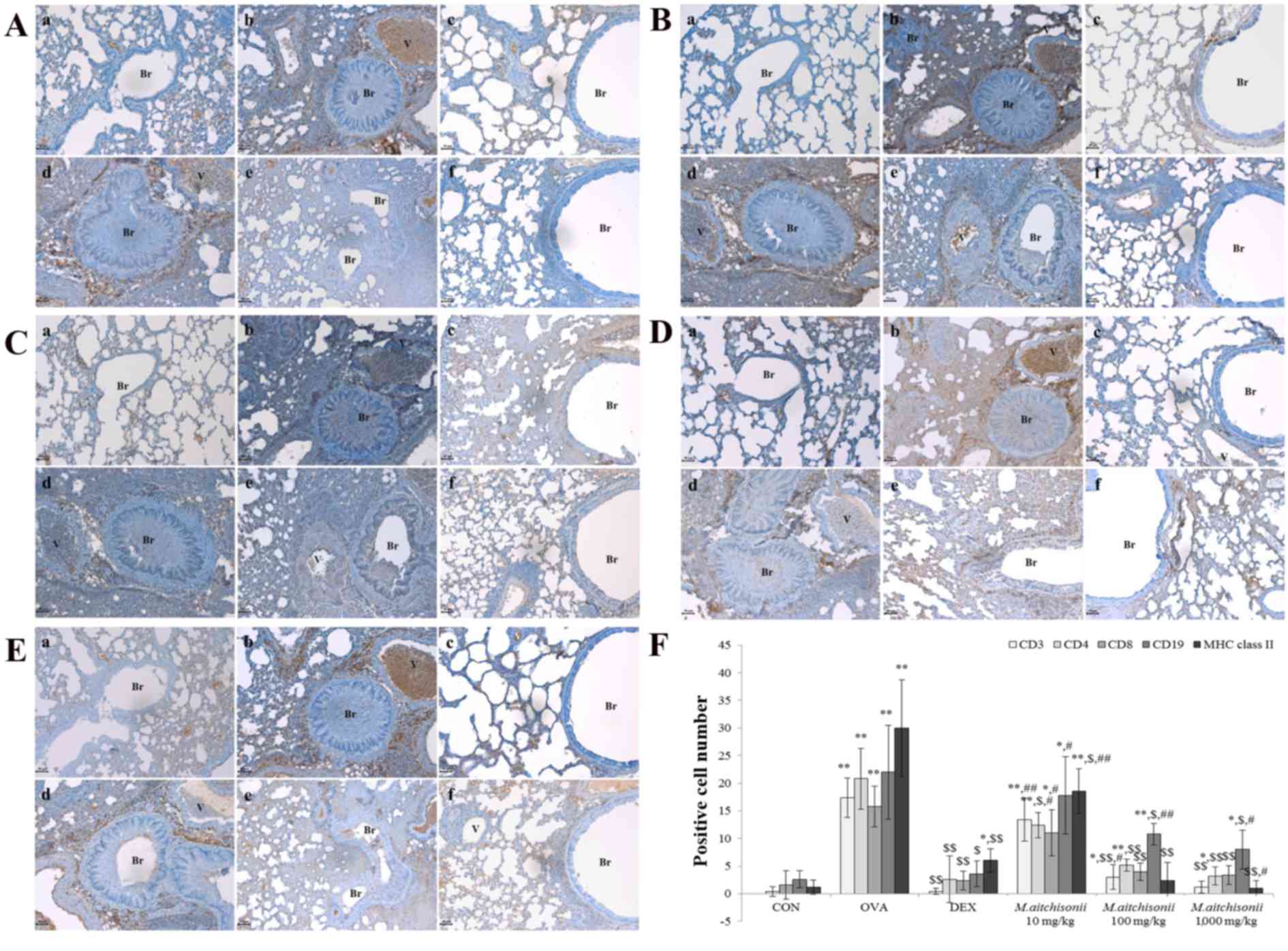 | Figure 4.M. atichisonii almost
completely inhibited the expressions of several cells related to
asthma occurrence. (A) M. atichisonii completely inhibited
the expression of CD3+ cell levels. (B) M. atichisonii
dose-dependently suppressed CD4+ cells. (C) M. atichisonii
almost fully diminished CD8+ cell levels. (D) M. atichisonii
downregulated the expression of the CD19+ cell, although the effect
of M. atichisonii was smaller than CD3+, CD+8 and MHC class
II. (E) The effect of 100 or 1,000 mg/kg M. atichisonii
treatment against MHC class II was greater than that of DEX
treatment. (F) Quantification of expression. Each bar represents
the mean ± standard deviation (n=6). a, Con; b, OVA; c, DEX; d, 10
mg/kg/day M. atichisonii; e, 100 mg/kg/day M.
atichisonii; f, 1,000 mg/kg/day M. atichisonii.
*P<0.05 vs. control; **P<0.001 vs. Con; $P<0.05
vs. OVA-induced asthma, $$P<0.001 vs. OVA-induced
asthma; #P<0.05 vs. DEX treatment,
##P<0.01 vs. DEX treatment. Br, bronchiole; V,
vessel; MHC, major histocompatibility complex; Con, control; OVA,
ovalbumin; DEX, dexamethasone. |
Although M. atichisonii appears to control
levels of both T cells (CD3+, CD4+, CD8+) and B cells (CD19+), the
potency to modulate B cell (CD19+) proliferation may be smaller
than that of T cell (CD3+, CD4+, CD8+) proliferation, however it
downregulated CD3+ positive cells (total T cell) and CD8+ positive
cells (cytotoxic T cell) and dose-dependently regulated CD4+
positive cell (helper T cell).
M. atichisonii controlled T-bet and
GATA-3
In order to compare the suppressive effects of the
Th1 cell transcription factor, T-bet, and the Th2 cell
transcription factor, GATA-3, the changes of expressions on T-bet
and GATA-3 were measured via immunohistochemical staining (Fig. 5). The expressions of T-bet and
GATA-3 in the OVA treatment group was significantly increased, when
compared with the control, however DEX decreased the expressions of
T-bet and GATA-3, which was induced by OVA, to a similar level as
the control groups (Fig. 5A and
B). Although M. atichisonii dose-dependently
downregulated the expression of T-bet, there was no significant
difference between 100 mg/kg M. atichisonii treatment and
1,000 mg/kg M. atichisonii treatment (Fig. 5C; P<0.05 or P<0.01).
Conversely, from treatment with 10 mg/kg M. atichisonii to
1,000 mg/kg, the changes in GATA-3 expression were dependent on the
dose-response relationship (Fig.
5C; P<0.05 or P<0.01).
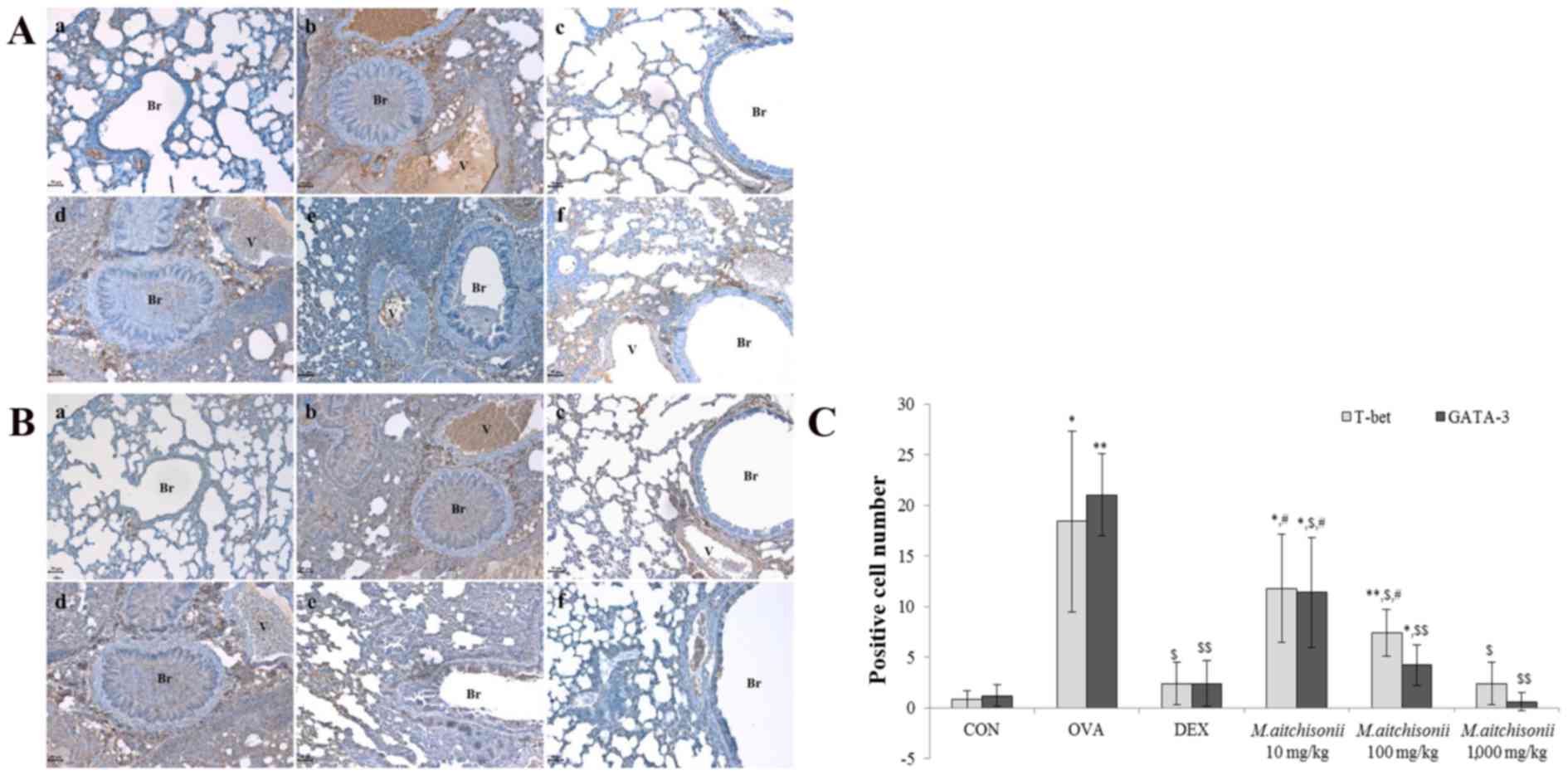 | Figure 5.M. atichisonii controlled
T-bet and GATA-3 expression levels. (A) M. atichisonii
dose-dependently suppressed the expression of T-bet. (B) M.
atichisonii downregulated GATA-3 more than T-bet, and the
effect of M. atichisonii was similar the effect of
dexamethasone. (C) Quantification of expression. Each bar
represents the mean ± standard deviation (n=6). a, Con; b, OVA; c,
DEX; d, 10 mg/kg/day M. atichisonii; e, 100 mg/kg/day M.
atichisonii; f, 1,000 mg/kg/day M. atichisonii.
*P<0.05 vs. Con; **P<0.001 vs. Con; $P<0.05 vs.
OVA-induced asthma, $$P<0.001 vs. OVA-induced asthma;
#P<0.05 vs. DEX. Br, bronchiole; V, vessel; Con,
control; OVA, ovalbumin; DEX, dexamethasone. |
M. atichisonii suppressed the
expression of Th1-related cytokines
M. atichisonii dose-dependently suppressed
the expression of IFN-γ and the effect of M. atichisonii on
INF-γ was less than DEX (Fig. 6A).
The results of IFN-γ by M. atichisonii were similar to that
of T-bet (Fig. 5A). M.
atichisonii inhibited the expression of IL-12p35 (Fig. 6B). The effect of 1,000 mg/kg M.
atichisonii treatment on IL-12p40 was similar to that of
dexamethasone. DEX and M. atichisonii effectively suppressed
the expression of IL-12p40 (Fig.
6C). Although there was no significant difference between 10
mg/kg M. atichisonii treatment and 100 mg/kg M.
atichisonii treatment (P>0.05), in the 1,000 mg/kg M.
atichisonii treatment group, IL-12p35 levels were almost fully
diminished (Fig. 6D).
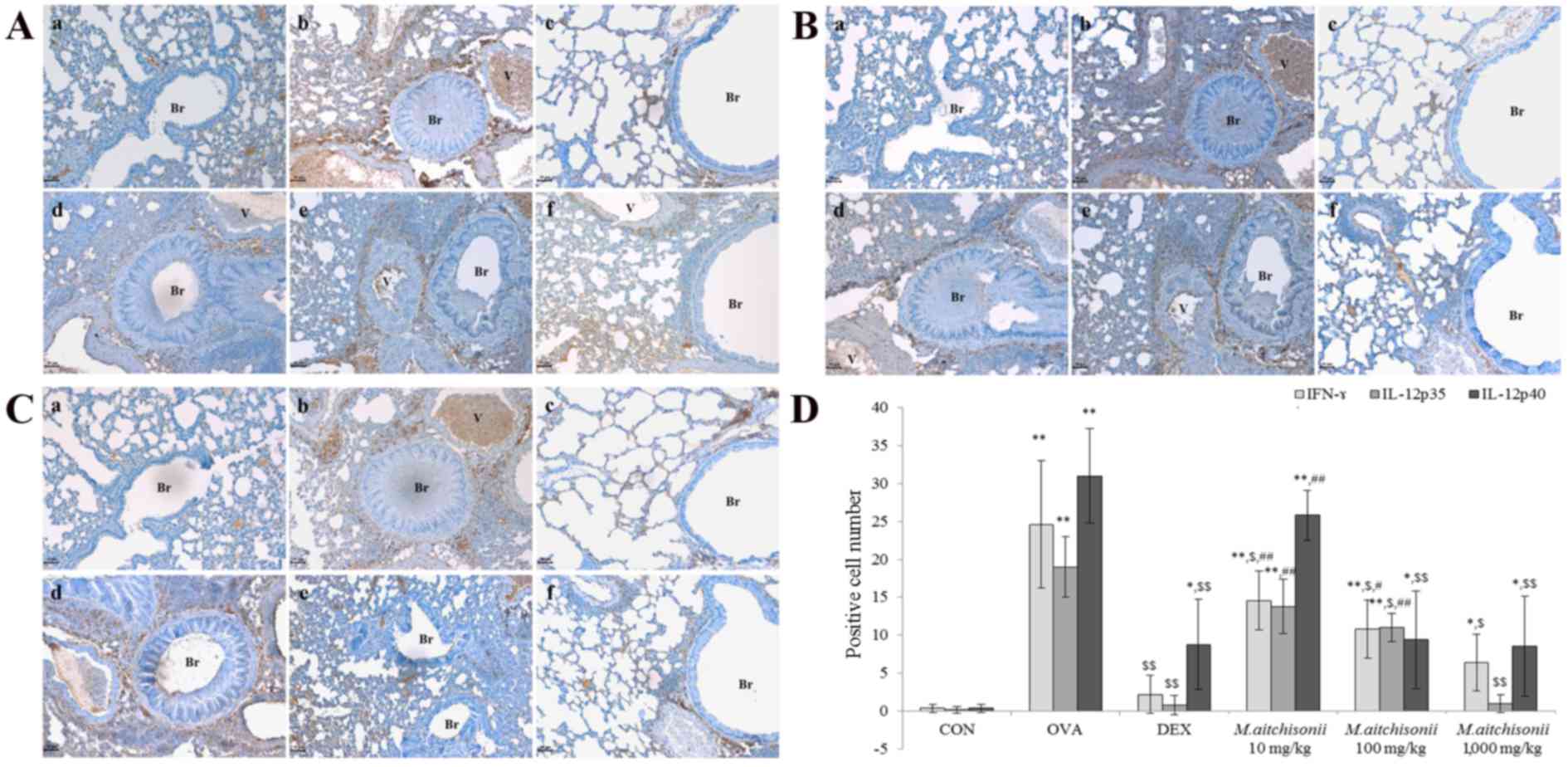 | Figure 6.M. atichisonii suppressed the
expressions of IFN-γ and IL-12p35. (A) In a dose-dependent manner,
M. atichisonii suppressed the expression of IFN-γ, however,
the effect of M. atichisonii is smaller than the effect of
DEX. (B) Although M. atichisonii inhibited the expression of
IL-12p35, the effect of M. atichisonii in 10 mg/kg treatment
and 100 mg/kg treatment groups was small. The 1,000 mg/kg M.
atichisonii treatment suppressed the expression of IL12-p35
similarly to DEX treatment. (C) IL-12p40 may be affected by DEX and
M. atichisonii as the changes of expression level were not
large following treatment. (D) Quantification of expression. Each
bar represents the mean ± standard deviation (n=6). a, Con; b, OVA;
c, DEX; d, 10 mg/kg/day M. atichisonii; e, 100 mg/kg/day
M. atichisonii; f, 1,000 mg/kg/day M. atichisonii.
*P<0.05 vs. Con; **P<0.001 vs. Con; $P<0.05 vs.
OVA-induced asthma, $$P<0.001 vs. OVA-induced asthma;
#P<0.05 vs. DEX treatment, ##P<0.01 vs.
DEX treatment. Br, bronchiole; V, vessel; IFN-γ; interferon-γ; IL,
interleukin; Con, control; OVA, ovalbumin; DEX, dexamethasone. |
M. atichisonii almost fully inhibited
the expression of Th2-related cytokines such as IL-5, IL-6, and
IL-13
In order to measure the downregulation effect of
M. atichisonii on Th2-related cytokines, such as TNF-α
(Fig. 7A), IL-4 (Fig. 7B), IL-5 (Fig. 7C), IL-6 (Fig. 7D) and IL-13 (Fig 7E), immunohistochemical analysis was
conducted. Although M. atichisonii did not completely
prevent the expression of TNF-α, it was similar to that of DEX
(Fig. 7A). M. atichisonii
dose-dependently suppressed the expression of IL-4, but the
suppression was less than DEX (Fig. 7B
and F). The expression of IL-5 was controlled by M.
atichisonii and the effect was similar to DEX. In particular,
IL-6 and IL-13 were dose-dependently suppressed by M.
atichisonii treatment more than by DEX (Fig. 7F).
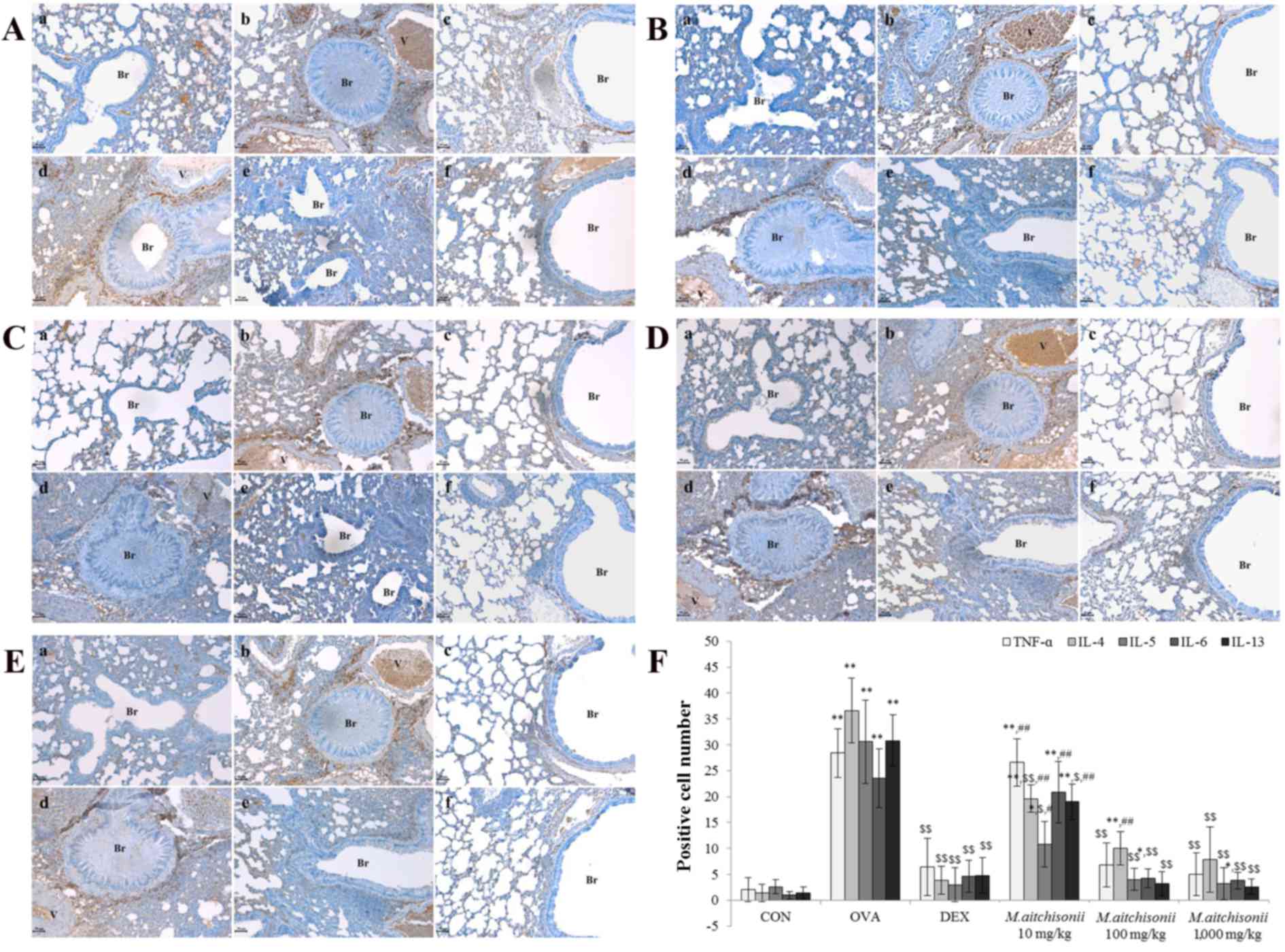 | Figure 7.M. atichisonii significantly
inhibited the expression of Th2-related cytokines in a
dose-dependent manner. (A) M. atichisonii suppressed the
expression of TNF-α similarly to dexamethasone treatment. (B) M.
atichisonii downregulated the expression of IL-4. (C) M.
atichisonii significantly inhibited the expression of IL-5,
similar to treatment with dexamethasone. (D) M. atichisonii
dramatically inhibited the expression of IL-6 more than treatment
with dexamethasone. (E) The expression of IL-13 dramatically
inhibited the expression of IL-6 more than treatment with
dexamethasone. (F) Quantification of expression. Each bar
represents the mean ± standard deviation (n=6). a, Con; b, OVA; c,
DEX; d, 10 mg/kg/day M. atichisonii; e, 100 mg/kg/day M.
atichisonii; f, 1,000 mg/kg/day M. atichisonii.
*P<0.05 vs. control; **P<0.001 vs. control;
$P<0.05 vs. OVA-induced asthma,
$$P<0.001 vs. OVA-induced asthma;
#P<0.05 vs. DEX treatment, ##P<0.01 vs.
DEX treatment. Th, T helper; TNF-α, tumor necrosis factor-α; IL,
interleukin; Br, bronchiole; V, vessel; Con, control; OVA,
ovalbumin; DEX, dexamethasone. |
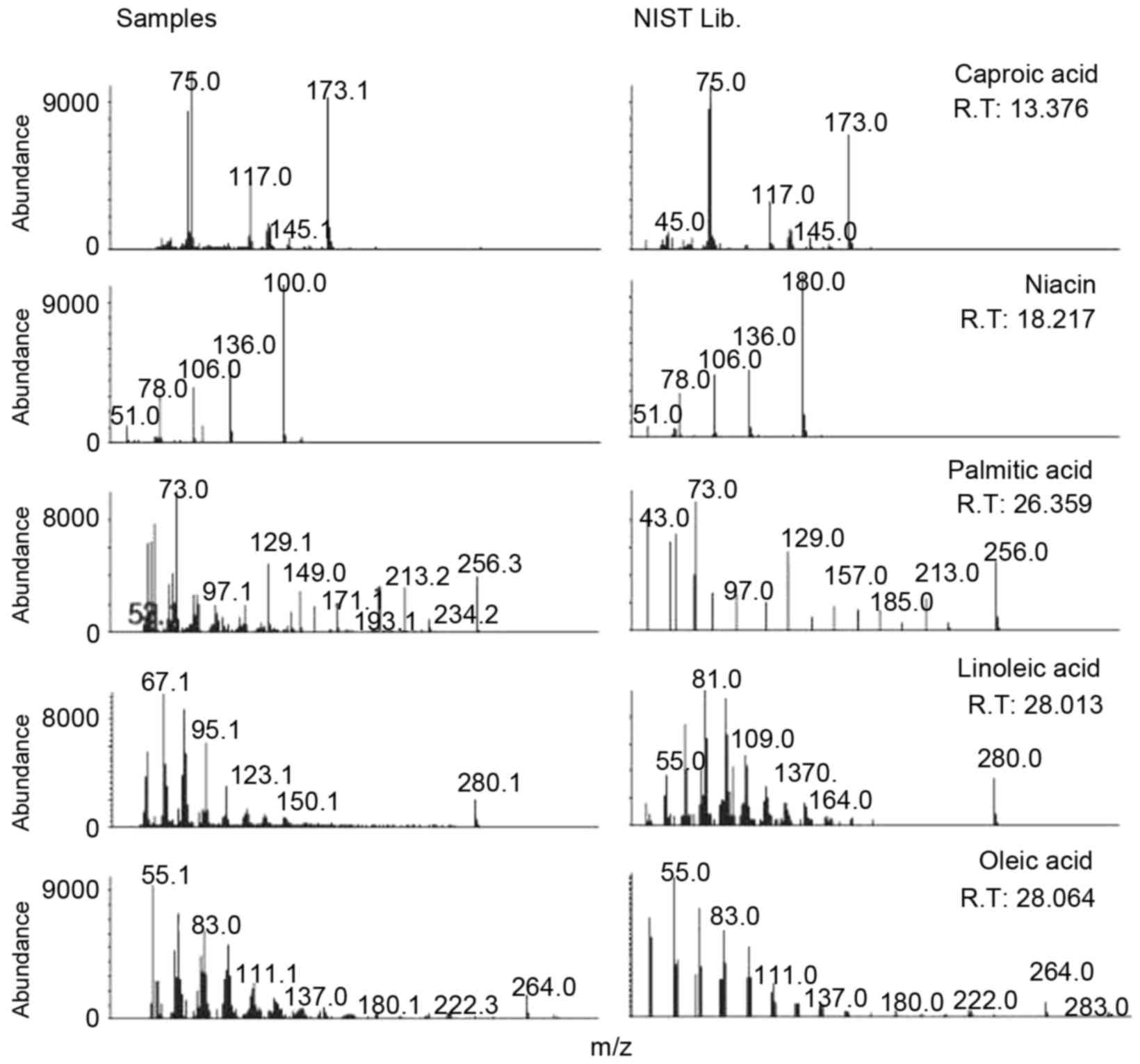
Nicotinic acid (niacin), oleic acid
and linoleic acid may act as anti-asthmatic compounds in M.
aitchisonii
To identify the compounds in M. aitchisonii
that may possess anti-asthmatic properties, GC-MS analysis was
conducted. Not all compounds in M. aitchisonii were
isolated, however nicotinic acid (niacin), oleic acid and linoleic
acid were identified as candidate compounds. Fig. 8 presents the identification of
silylated fatty acids. Of the identified fatty acids, niacin,
linoleic acid and linoleic acid were analyzed at retention times of
18.21, 28.01 and 28.06 min respectively.
Discussion
Eosinophils are a key mediator of innate and
adaptive immunity (29,30). The differentiation and activation
of eosinophils is strongly related to the IL-5 gene; if the
IL-5 gene is depleted, airway eosinophilia cannot occur
(31). Eosinophils are increased
in number in asthma, and this is induced by IL-5 (11). In allergic situations, eosinophils
remain for 8–12 h in circulatory blood, and for an additional 8–12
days in tissue following the removal of the stimuli (32). In the present study, although the
expression of IL-5 was more suppressed by M. atichisonii
than that of eosinophils, the downregulatory patterns were similar
for both, indicating that M. atichisonii may suppress
eosinophils through IL-5. As the level of IL-5 suppression by M.
atichisonii was larger than the other effects, IL-5 might be
one of the key mediators in the preventive mechanism of M.
atichisonii against asthma.
MHC class II molecules, which are made by antigen
presenting cells, have the function of presenting processed
antigens to helper T cells and triggering acquired immunity
(33). MHC class II+ expression
was almost completely eliminated by 100 mg/kg M. atichisonii
treatment, confirming that M. atichisonii may completely
control MHC class II molecule expression, which serves an important
role in asthma occurrence.
Based on the changes in IFN-γ levels, a
controversial hypothesis regarding the mechanism underlying asthma
was previously proposed that implicated the Th1/Th2 cell imbalance
and T cell priming induced by allergens (34). M. atichisonii slightly
decreased not only the expressions of Th2-related factors, but also
that of IFN-γ increased by OVA (Fig.
4D) in the present study and this result may support the T cell
priming theory. IL-12 is produced by cells including dendritic
cells, macrophages and monocytes (35) and is considered an IFN-γ-inducing
factor because it stimulates the production of IgM and IFN-γ
(8). Although M.
atichisonii suppresses or inhibits most asthma-induced factors,
the key factors for the anti-asthmatic effect may be IL-5, IL-6 and
IL-13 (Fig 7C-E). The ratio of
Th17 cells and Treg cells is related to the late phase asthma
induction and, in the case of late phase asthma, the analysis of
Th17 cells and Treg cells may be important (36).
In the current study, the authors determined that
M. atichisonii lowered the OVA-induced level of WBCs and
eosinophils in BALF and the level of IgE in serum, recovered
respiratory changes, such as mucous secretion, epithelial cell
hyperplasia and eosinophil infiltration and finally ameliorated
airway obstruction. This was controlled by T cell-related
molecules, such as CD3+, CD4+ and CD8+, as well as by MHC class II+
molecules that were increased following OVA treatment. Ultimately,
this led to downregulated T-bet and GATA-3 levels, and a
dose-dependent decreased to the level of Th1-related cytokines,
IFN-γ and IL-12p40, and Th2-related cytokines, TNF-α, IL-4, IL-5,
IL-6 and IL-13. In particular, the expression of IL-5, IL-6 and
IL-13 increased by OVA treatment were almost completely eliminated
by M. atichisonii application.
In this study, niacin, oleic acid and linoleic acid
in M. aitchisonii were identified and isolated. Melton
(37) previously reported that
nicotinic acid reduced the frequency of asthmatic paroxysm. In
addition, oleic acid has beneficial effects in inflammatory related
diseases (38) and linoleic acid
is one of the mediators that regulate inflammations and asthma
(39).
The present study is one of the ongoing efforts to
identify appropriate anti-asthmatic drug candidates. From the
results, it may be concluded that M. atichisonii has an
anti-asthmatic effect and that the pharmacological effect may be
based on the suppression or inhibition of various factors related
to Th1 and Th2. In particular, IL-5, IL-6 and IL-13 may be some of
the most important factors related to asthma occurrence, and they
are modulated by M. atichisonii. M. atichisonii is a
promising drug that is believe to be key in the control of
asthma.
Acknowledgements
The current study was conducted with a fund from the
Korea Forest Service (grant no. S121313L090100).
References
|
1
|
World Health Organization: Asthma Fact
Sheet No 307. November;2013.
|
|
2
|
United States Environmental Protection
Agency (EPA): Asthma Facts EPA-402-F-04-019. March;2013.
|
|
3
|
Kay AB: Allergy and allergic diseases.
First of two parts. N Engl J Med. 344:30–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Asthma Education and Prevention
Program: National asthma education and prevention program, . Expert
panel report: Guidelines for the diagnosis and management of asthma
update on selected topics-2002. J Allergy Clin Immunol. 110 5
Suppl:S141–S219. 2002.PubMed/NCBI
|
|
5
|
Lazarevic V and Glimcher LH: T-bet in
disease. Nature Immunol. 12:597–606. 2011. View Article : Google Scholar
|
|
6
|
Zhu J, Jankovic D, Oler AJ, Wei G, Sharma
S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, et al: The
transcription factor T-bet is induced by multiple pathways and
prevents an endogenous T helper-2 program during T helper-1
responses. Immunity. 37:660–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zedan MM, El-Chennawi FA and Fouda AE:
Interleukin-12 and peripheral blood invariant natural killer T
cells as an axis in childhood asthma pathogenesis. Iran J Allergy
Asthma Immunol. 9:43–48. 2010.PubMed/NCBI
|
|
8
|
Trinchieri G: Interleukin-12: A cytokine
at the interface of inflammation and immunity. Adv Immunol.
70:83–243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamza T, Barnett JB and Li B: Interleukin
12 a key immunoregulatory cytokine in infection applications. Int J
Mol Sci. 11:789–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yagi R, Zhu J and Paul WE: An updated view
on transcription factor GATA3-mediated regulation of Th1 and Th2
cell differentiation. Int Immunol. 23:415–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uhm TG, Kim BS and Chung IY: Eosinophil
development, regulation of eosinophil-specific genes and role of
eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol
Res. 4:68–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Platts-Mills TA: The role of
immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med.
164:S1–S5. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamimura D, Ishihara K and Hirano T: IL-6
signal transduction and its physiological roles: The signal
orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neveu WA, Allard JL, Raymond DM, Bourassa
LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA and Rincon M:
Elevation of IL-6 in the allergic asthmatic airway is independent
of inflammation but associates with loss of central airway
function. Respir Res. 11:282010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berry M, Brightling C, Pavord I and
Wardlaw A: TNF-alpha in asthma. Curr Opin Pharmacol. 7:279–282.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lukacs NW, Strieter RM, Chensue SW, Widmer
M and Kunkel SL: TNF-alpha mediates recruitment of neutrophils and
eosinophils during airway inflammation. J Immunol. 154:5411–5417.
1995.PubMed/NCBI
|
|
17
|
Scheurich P, Thoma B, Ucer U and
Pfizenmaier K: Immunoregulatory activity of recombinant human tumor
necrosis factor (TNF)-alpha: Induction of TNF receptors on human T
cells and TNF-alpha-mediated enhancement of T cell responses. J
Immunol. 138:1786–1790. 1987.PubMed/NCBI
|
|
18
|
Bosnjak B, Stelzmueller B, Erb KJ and
Epstein MM: Treatment of allergic asthma: Modulation of Th2 cells
and their responses. Respir Res. 12:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnes PJ: Current issues for establishing
inhaled corticosteroids as the anti-inflammatory agents of choice
in asthma. J Allergy Clin Immunol. 101:S427–S433. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wise J: Corticosteroids for asthma may
suppress growth in children in first year of treatment, researchers
say. BMJ. 349:g46232014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciriaco M, Ventrice P, Russo G,
Scicchitano M, Mazzitello G, Scicchitano F and Russo E:
Corticosteroid-related central nervous system side effects. J
Pharmacol Pharmacother. 4 Suppl 1:S94–S98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seo JW, Cho SC, Park SJ, Lee EJ, Lee JH,
Han SS, Pyo BS, Park DH and Kim BH: 1′-Acetoxychavicol acetate
isolated from Alpinia galangal ameliorates ovalbumin-induced asthma
in mice. PLoS One. 8:e564472013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bang MA, Seo JH, Seo JW, Jo GH, Jung SK,
Yu R, Park DH and Park SJ: Bacillus subtilis KCTC 11782BP-produced
alginate oligosaccharide effectively suppresses asthma via T-helper
cell type 2-related cytokines. PLoS One. 10:e01175242015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chandrasekaran G, Oh DS and Shin HJ:
Versatile applications of the culinary-medicinal mushroom
Mycoeptodonoides aitchisonii (Berk.) Maas G. (Higher
Basidiomycetes): A review. Int J Med Mushrooms. 14:395–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okuyama S, Lam NV, Hatakeyama T, Terashima
T, Yamagata K and Yokogoshi H: Mycoleptodonoides aitchisonii
affects brain nerve growth factor concentration in newborn rats.
Nutr Neurosci. 7:341–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MR, Begum S, Oh DS, Wee AJ, Yun BS and
Sung CK: Ameliorating effect of Mycoleptodonoides aitchisonii on
high-fat diet-induced obese mice. Prev Nutr Food Sci. 19:69–74.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi JH, Suzuki T, Okumura H, Noguchi K,
Kondo M, Nagai K, Hirai H and Kawagishi H: Endoplasmic reticulum
stress suppressive compounds from the edible mushroom
Mycoleptodonoides aitchisonii. J Nat Prod. 77:1729–1733. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burrows B, Martinez FD, Halonen M, Barbee
RA and Cline MG: Association of asthma with serum IgE levels and
skin-test reactivity to allergens. N Engl J Med. 320:271–277. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shamri R, Xenakis JJ and Spencer LA:
Eosinophils in innate immunity: An evolving story. Cell Tissue Res.
343:57–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galioto AM, Hess JA, Nolan TJ, Schad GA,
Lee JJ and Abraham D: Role of eosinophils and neutrophils in innate
and adaptive protective immunity to larval Strongyloides
stercoralis in mice. Infect Immun. 74:5730–5738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kouro T and Takatsu K: IL-5- and
eosinophil-mediated inflammation: From discovery to therapy. Int
Immunol. 21:1303–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Young B, Lowe JS, Stevens A, et al:
Wheater's functional histology; a text and colour atlas. 5th
edition. Edinburg: Elsevier; 2006, View Article : Google Scholar
|
|
33
|
Holling TM, Schooten E and van Den Elsen
PJ: Function and regulation of MHC class II molecules in
T-lymphocytes: Of mice and men. Human Immunol. 65:282–290. 2004.
View Article : Google Scholar
|
|
34
|
Kim YK: Th1/Th2 imbalance vs. T cell
priming in asthma. BioWave. 7:SubNo.12005.
|
|
35
|
Airoldi I, Guglielmino R, Carra G,
Corcione A, Gerosa F, Taborelli G, Trinchieri G and Pistoia V: The
interleukin-12 and interleukin-12 receptor system in normal and
transformed human B lymphocytes. Haematologica. 87:434–442.
2002.
|
|
36
|
Singh A, Yamamoto M, Ruan J, Choi JY,
Gauvreau GM, Olek S, Hoffmueller U, Carlsten C, FitzGerald JM,
Boulet LP, et al: Th17/Treg ratio derived using DNA methylation
analysis is associated with the late phase asthmatic response.
Allergy Asthma Clin Immunol. 10:322014. View Article : Google Scholar :
|
|
37
|
Melton G: Treatment of Asthma by Nicotinic
Acid. Br Med J. 1:600–601. 1943. View Article : Google Scholar :
|
|
38
|
Carrillo C, Mdel Cavia M and Alonso-Torre
S: Role of oleic acid in immune system; mechanism of action; a
review. Nutr Hosp. 27:978–990. 2012.
|
|
39
|
Wendell SG, Baffi C and Holguin F: Fatty
acids, inflammation, and asthma. J Allergy Clin Immunol.
133:1255–1264. 2014. View Article : Google Scholar :
|