Introduction
Ischemic stroke is a threat to human health with the
third leading cause of adult disability and mortality worldwide
(1). As the most common type of
stroke, cerebral focal ischemia is associated with neuronal
apoptosis or mortality, glial cell activation and proliferation,
inflammatory reaction and stress response, resulting in a series of
nonreversible neuronal loss and neurological deficits (2). Due to the fact that the central
nervous system of adults has limited self-repair and regeneration
abilities, survivors are likely to sustain lifelong impairments in
behavioral, communicative, cognitive, sensory and/or emotional
functionality, depending on the size and localization of the brain
injury (2). Although drug
therapies may prevent the loss of nerve cells after cerebral focal
ischemia, the therapeutic effect on restoring neural function in
patients remains limited (3).
In this context, neurorehabilitation exercise has
been developed to help stroke patients improve the impairments to
the nervous system and motor control, according to the neural
plasticity. Neuroplasticity refers to a range of adaptive changes
that occur in the structure and function of cells in the nervous
system in response to physiological or pathological perturbations
(4). These changes include the
sprouting and growth of axons or dendrites, synapse formation, the
strengthening of synapses in response to repeated activation and
the production of new neurons derived from differentiation of stem
cells (4,5). Motor training is able to enhance the
neuronal structural and functional synaptic plasticity in the motor
cortex; however, the effect varies depending on the
neurorehabilitation exercises used. Willed movement (WM) training
is defined as task-oriented training, which increases the
enthusiasm of patients to accomplish a specific task (6). In this training, patients are
actively participating in physical activities leading to profound
changes in neuroplasticity, which is likely to be associated to a
better therapeutic effect on the neurorehabilitation (6).
The extracellular signal-related kinase
(ERK)-mediated pathway is involved in neural plasticity and
neuroprotection following stroke. Endogenous neural regeneration in
the hippocampus represents a special type of neural plasticity.
Neural stem cells, located in the subgranular zone of hippocampal
dentate gyrus, are capable of proliferating, differentiating and
integrating into the existing neuronal circuits. These
self-renewing cells have the potential to replenish neural loss and
restore neurological function after stroke (7). Post-traumatic neurogenesis and
cognition recovery has been previously observed to be rescued by
the activation of mitogen-activated protein kinase (MEK)/ERK
(7). Conditional activation of ERK
enhances hippocampus neurogenesis in a rat model of traumatic brain
injury, improving the olfactory function under normal conditions
and after injury, and inhibition of MEK/ERK abolished the
neurogenesis improved by sphingosine-1-phosphate receptor 1
activation (7). The pivotal role
of ERK in endogenous neurogenesis is likely mediated through
regulating multiple factors, including cyclic adenosine
monophosphate response element-binding protein (CREB),
brain-derived neurotrophic factor (BDNF), nerve growth factor, and
synaptophysin (SYP) (8,9). Notably, it has been previously
suggested that there is a critical role of CREB activation in
plasticity of dendritic spines in central neurons (8,9). The
CREB-mediated pathway is implicated in enhanced structural
plasticity and the behavioral recovery that are promoted by
post-stroke forced limb-use in cerebral multi-infarction rats
(10). In addition,
resveratrol-mediated neuroprotection in a chronic cerebral
hypoperfusion rat model has been suggested to act via CREB
activation that restores functional and structural synaptic
plasticity (11). The
neuroprotection of CREB activation may also be associated with the
effect on DNA repair and anti-oxidation (12). It is known that reactive oxygen
species resulting from cerebral ischemia and reperfusion lead to
significant neuronal damage (12).
The critical role of glutamate receptor 2 (GluR2) in
activity-dependent forms of synaptic plasticity has been previously
reported (13). GluR2, known to be
one of the subunits of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
(AMPARs), negatively regulates calcium permeability, resulting in a
marked inward rectification in the current-voltage relationship
(14). It has been previously
reported that ischemia induces a delayed downregulation of GluR2
mRNA and protein expression, which enhances AMPAR-mediated
Ca2+ and Zn2+ influx into CA1 neurons and
subsequently activates the death pathway (14). This evidence indicates a crucial
role for Ca2+−permeable AMPARs via loss of GluR2 in
ischemic cell death. In addition, endocytosis and exocytosis
mediated by AMPAR are central to the necessary changes in the
synaptic receptor complement pathway (15). Protein interacting with C-kinase 1
(PICK1) is a calcium-sensing, PDZ domain-containing protein that
interacts with GluR2 AMPAR subunit and regulates AMPAR trafficking.
PICK1 over expression in CA1 pyramidal neurons causes a CaMK- and
PKC-dependent potentiation of AMPAR-mediated transmission (14). Glutamate receptor interacting
protein (GRIP) 1-associated protein 1 (GRASP-1) is a
GRIP-associated protein that affects GluR2/3-GRIP interaction and
regulates AMPAR trafficking to the synaptic membrane (16–18).
Increased delivery of AMPARs to the postsynaptic membrane leads to
long-term potentiation (LTP) and mice exhibit abnormal excitatory
synapse number, synaptic plasticity, and hippocampal-dependent
learning and memory due to a failure in learning-induced synaptic
AMPAR incorporation (19).
The present study aimed to elucidate the effect of
WM training on neurorehabilitation following focal cerebral
ischemia. The ERK/CREB pathway and GluR2/GRASP-1/PICK1 cascades
have been implicated in neuroplastic processes underlying recovery
from ischemia. The current study further investigated the influence
of WM training on these neural plasticity-associated signaling
pathways.
Materials and methods
Animals and treatment regimen
Male Sprague Dawley rats (n=160, 200±20 g, 2 months
old) were obtained from the Central Animal House Facility of
Xiangya Hospital (Changsha, China). The animal experiments were
approved by the Animal Care Committee of Animal Ethics Committee of
the Central South University. Animal care and all experimental
procedures were conducted according to the guidelines of the care
and use of laboratory animals (20). All animals were raised in plastic
cages (n=4/cage, 40×30×18 cm3) with soft bedding and
free access to food and water. Animals were housed at 23±2°C room
temperature with a 12-h light/dark cycle.
Temporary middle cerebral artery occlusion (tMCAO)
was performed according to a previously published protocol
(21) with minor modifications.
Prior to tMCAO surgery, animals were anesthetized deeply by an
intraperitoneal injection of 2% pentobarbital sodium (30 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A midline incision
was made on the ventral surface of neck to expose the right common
carotid artery. An intraluminal monofilament of filament (size 4.0,
length 30 mm and diameter 0.19 mm) with a silicon rubber coated tip
was purchased from Beijing Cinontech Co., Ltd. (Beijing, China). It
was inserted from the bifurcation of the common carotid artery into
the internal carotid artery, and then into the beginning of the
middle cerebral artery. Thread insertion was approximately 18.5±0.5
mm deep. Mild resistance indicated that the filament was properly
lodged in the anterior cerebral artery and thus blocked blood flow
to the middle cerebral artery. Reperfusion was obtained by
withdrawing the filament gently after 2 h.
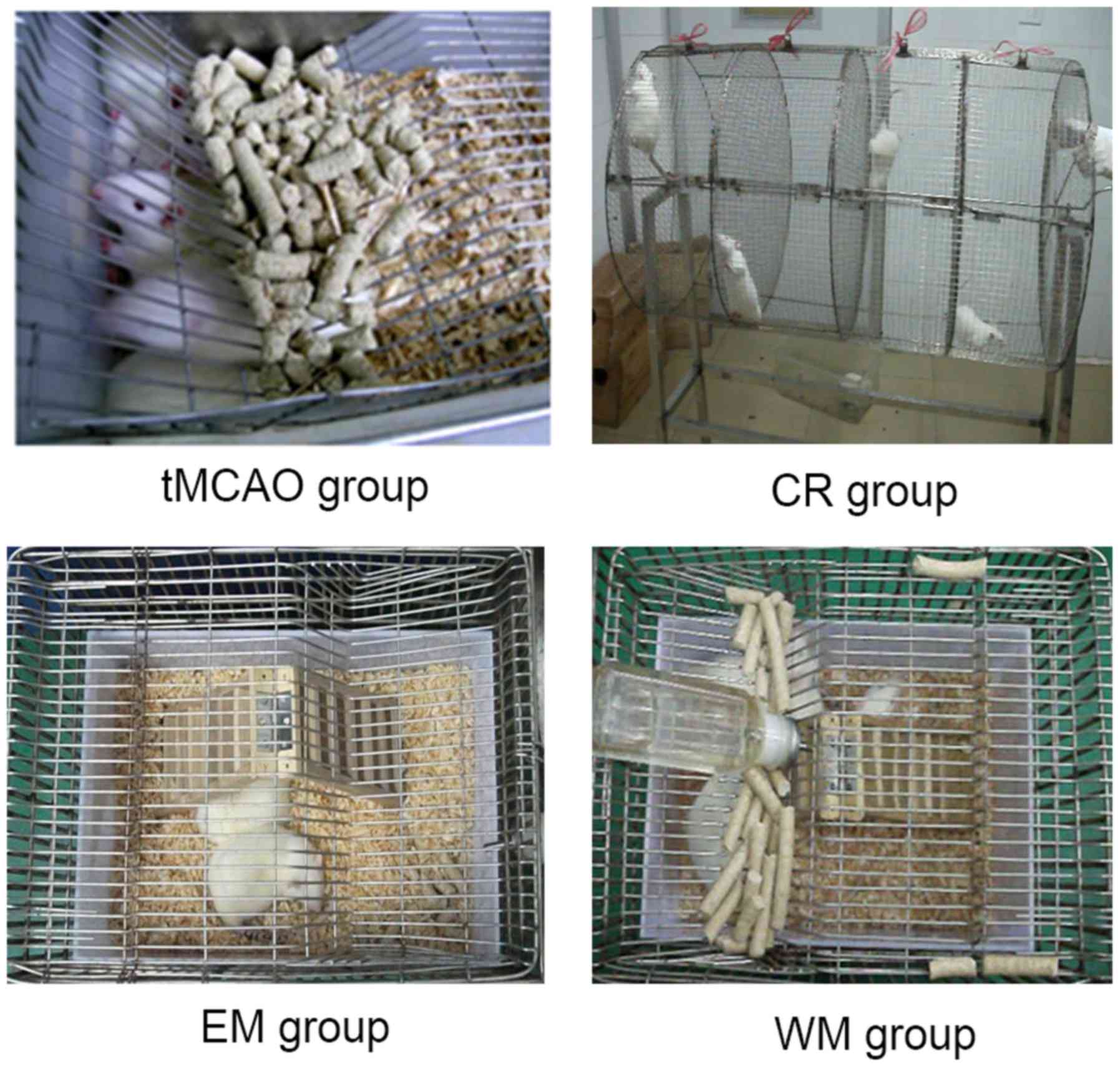
Subsequent to tMCAO, the rats were randomly divided
into 4 groups (n=18 rats/group): The tMCAO, control rehabilitation
(CR), environment modification (EM) and willed movement (WM)
groups, as presented in Fig. 1. In
order to accustom the rats to the training regime, rats in the EM
and WM groups were given 3 days of preliminary training for 10, 20
and 30 min/day on days 3, 2 and 1, respectively, prior to tMCAO
surgery. Rats in the tMCAO group were raised in plastic cages
without special rehabilitating training. Rats in the CR group were
forced to exercise (running) in a rotating wheel (100 cm long, 60
cm diameter) driven by hand. In the training, the running speed was
set as 5 revolutions/min. Rats were forced to train twice a day
respectively at 9:00 a.m. and 4:00 p.m. for 15 min per session.
Following training, rats were returned to their cages and allowed
free access to food and water.
A mini herringbone ladder was placed into the cages
in the CR, EM and WM groups. Rats in the CR and EM groups could
freely access food and water in cages independent of the
herringbone ladder, however rats in the WM group had to climb the
herringbone ladder to obtain food and water that were placed on the
top of the cages. To ensure that both EM and WM rats consumed
similar amounts of food and to ensure the attraction of the food
for the WM rats, EM and WM rats were deprived of food for 12 h from
9:00 p.m. to 9:00 a.m. of the next day.
Neurobehavioral and neurological
assessments: Frequency of rats climbing ladder
The behaviors of the rats climbing the herringbone
ladders were observed twice a day at the following time periods:
9:00-10:00 a.m. and 3:00-4:00 p.m. on days 3, 7, 15 and 30
post-surgery. The scoring method was: 1=climbing to the top of the
herringbone ladder; 0.5=climbing half of the herringbone ladder.
Investigators performing the outcome testing were kept blind to the
groups.
Assessment of grip strength
The grip strength of rats was assessed using a
string (~50 cm in length) as described by Andrabi (22). The string was pulled tight between
two vertical supports and elevated 40 cm from the flat surface. The
rats were put on the string at the midway point and scoring was
completed according to the following scoring scale: 0, fall off; 1,
hangs onto string by two forepaws; 2, hangs on string by two
forepaws and also attempts to climb on string; 3, hangs onto string
by two forepaws along with one or both hind paws; 4, hangs onto
string by all forepaws along with tail wrapped around the string;
and 5, escape.
Neurological deficit scoring
Neurological deficit scoring is a combination of
motor, sensory, reflex and balance tests. The neurological deficits
were assessed on a four-point scale, according to previous studies
(23,24) with minor modifications. 0 points,
no neurological damage, double forelimb symmetric stretching to the
ground; 1 point, contralateral forelimb sustained adduction; 2
points, contralateral forelimb grip strength decreased; 3 points,
slight stimulation of rat tails to the contralateral circling; 4
points, independent continuous circular motion. Motor functions
were evaluated by three researchers who were kept blind to the
groups. The animals in the model group with a score ≥1 were
selected for analysis in further experiments.
2,3,5-triphenyltetrazolium chloride
(TTC) staining
After the completion of motor function evaluation,
the animals were sacrificed and the brains were cut into 1.5~2 mm
thick coronal sections. The brain slices were immersed in a 2%
(w/v) solution of TTC in normal saline at 37°C for 15 min.
TTC-stained brain slices were scanned and analyzed by a
high-resolution scanner. The infarct volume was measured with Image
J software, version 1.37 (National Institutes of Health, Bethesda,
MD, USA).
Transmission election microscopy (TEM)
observation
The ischemic brain tissues were dissected and
post-fixed in 2.5% glutaraldehyde in phosphate-buffered saline
(PBS) overnight. Subsequent to washing with PBS, the brain tissues
were fixed in 0.1% osmium tetroxide for 2 h, dehydrated in gradient
ethanol and dimethyl ketone, and embedded in Epon 812 medium. The
brain tissues were further dissected to ultrathin sections prior to
observation using the transmission election microscope (Philips
Tecnai-10; Phillips Electronic Instruments, Mahway, NJ, USA).
Immunohistochemistry and
immunofluorescence
The cortical area and its adjacent corpus callosum
in the ischemic hemisphere were isolated from brain and fixed with
4% paraformaldehyde for 20 min at room temperature. The slices were
permeabilized with 0.3% Triton X-100 (Sigma-Aldrich; Merck KGaA)
for 10 min and subsequently blocked with 10% normal goat serum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) in 0.01 M PBS
for 1 h. Then, they were incubated overnight at 4°C with the
primary antibodies against phosphorylated (p)-ERK (1:500 dilution;
ab214036), pCREB (1:1,000 dilution; ab32096), GluR2 (1:800
dilution; ab133477), GRASP-1 (1:600 dilution; ab171940) and PICK1
(1:800 dilution; ab3420). These antibodies were purchased from
Abcam (Cambridge, MA, USA). For immunohistochemistry, the slices
were incubated with biotinylated horse-anti-rabbit IgG secondary
antibody (1:100; OriGene Technologies, Inc., Beijing, China) for 1
h, followed by incubation with avidin-biotin-peroxidase. For
immunofluorescence, the slices were incubated with Alexa Fluor 594
anti-rabbit IgG secondary antibody (1:2,000; Molecular Probes;
Thermo Fisher Scientific, Inc.) and observed with the Nikon ECLIPSE
Ti fluorescence microscope (Nikon Corporation, Tokyo, Japan) plus
NIS-Elements BR 3.0 software (Nikon Corporation, Tokyo, Japan).
Western blotting
The cortex adjacent to the injury brain were
isolated for protein harvest. These tissues were extracted with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) supplemented with phosphatase
inhibitors. The concentration in the tissue samples was determined
using the Bradford method (25),
using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Protein lysates were separated by 10% SDS-PAGE and
transferred to 0.45 µm polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
bovine serum albumin (Thermo Fisher Scientific, Inc.) overnight at
4°C, followed by incubation in primary antibodies from Abcam
against pERK (1:500 dilution; ab214036), CREB (1:800 dilution;
ab32096), GluR2 (1:800 dilution; ab133477), GRASP-1 (1:900
dilution; ab171940), and PICK1 (1:800 dilution; ab3420) at 4°C
overnight. Blots were washed (3×10 min) in Tris-buffered saline
containing 0.05% Tween-20 (TBS-T; Sigma-Aldrich; Merck KGaA),
following incubation with horseradish peroxidase-labeled donkey
secondary antibody (cat. no. SA1-100, anti-mouse, 1:2,000; cat. no.
SA1-200, anti-rabbit, 1:2,000; Invitrogen; Thermo Fisher
Scientific, Inc.) and washed in TBS-T (3×10 min), protein bands
were visualized using Amersham ECL Prime (GE Healthcare Life
Sciences, Piscataway, NJ, USA) on the GelDoc XR System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and quantified by ImageJ
software, version 9.0. The intensities of the protein bands were
all normalized to β-actin.
Statistical analysis
All data were analyzed using one-way analysis of
variance followed by Tukey's post hoc test. All data were analyzed
using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Rat mortality after tMCAO
According to the data in Table I, rat mortality was higher during
the first 24 h after tMCAO. In the second 24 h, rat mortality was
decreased in the CR, EM and WM groups, however not in the tMCAO
group. Rat mortality in all groups was progressively reduced during
the period of 48 h~30 days after tMCAO. The WM group exhibited the
lowest mortality in the first month after tMCAO among these
groups.
 | Table I.Rat mortality after tMCAO. |
Table I.
Rat mortality after tMCAO.
|
| Death count after
tMCAO |
|
|---|
|
|
|
|
|---|
| Groups | 0-24 h | 24-48 h | 48 h-3 days | 3-7 days | 7-15 days | 15-30 days | Mortality (%) |
|---|
| MCAO | 8 | 8 | 2 | 4 | 2 | 1 | 53 |
| CR | 6 | 3 | 1 | 2 | 1 | 0 | 33 |
| EM | 7 | 5 | 2 | 3 | 2 | 1 | 45 |
| WM | 7 | 3 | 0 | 1 | 1 | 0 | 30 |
Neurobehavioral analysis
In order to analyze the effect of WM training on
attenuation of the neurological deficits after tMCAO surgery,
several behavioral parameters that could to reflect motor ability
and coordination were studied. The number of times that rats
climbed the ladders was recorded at indicated times after surgery.
As presented in Table II, the
climbing frequencies of rats in the WM group were notably increased
7 days following surgery. In contrast, the climbing frequencies of
rats in the CR and EM groups were increased moderately 30 days
after surgery. The climbing frequencies of rats were significantly
higher in the WM group compared with that of the CR and EM groups
on days 7, 15 and 30 subsequent to surgery (P<0.05).
 | Table II.Frequency of rats climbing the
herringbone ladders. |
Table II.
Frequency of rats climbing the
herringbone ladders.
|
| Time after tMCAO
(days) |
|---|
|
|
|
|---|
| Groups | 3 | 7 | 15 | 30 |
|---|
| EM |
3.22±0.79 |
4.19±0.31 |
4.68±0.34 |
5.70±0.53 |
| CR |
2.73±0.96 |
4.06±0.14 |
4.30±0.17 |
5.58±0.25 |
| WM |
3.47±0.84 |
8.38±0.17a,b |
10.72±0.37a,b |
12.15±0.77a,b |
The grip strength of rats in the CR, EM and WM
groups was observed subsequent to surgery. The mean reading of
three successive trials for each rat was taken as a dependent
variable. The scoring reflected that there was a gradual increase
in the grip strength of rats in these groups 30 days after surgery.
Notably, the grip strength of WM rats was higher compared with CR
and EM rats on days 15 and 30 after surgery (P<0.05; Table III).
 | Table III.Grip strength of rats. |
Table III.
Grip strength of rats.
|
| Time after tMCAO
(days) |
|---|
|
|
|
|---|
| Groups | 3 | 7 | 15 | 30 |
|---|
| EM |
0.73±0.47 |
0.91±0.30 |
1.33±0.49 |
2.52±0.46 |
| CR |
0.55±0.52 |
0.83±0.39 |
1.25±0.45 |
2.46±0.38 |
| WM |
0.67±0.49 |
0.91±0.29 |
1.75±0.48a,b |
2.92±0.29a,b |
The neurological deficit scores did not differ
significantly among the four groups within 15 days after
recirculation. However, neurological deficit scores were
significantly reduced in the WM group compared with other groups 30
days after surgery (P<0.05; Table
IV).
 | Table IV.Neurological deficit scores. |
Table IV.
Neurological deficit scores.
|
| Time after
tMCAO |
|---|
|
|
|
|---|
| Groups | 1 h | 24 h | 3 days | 7 days | 15 days | 30 days |
|---|
| tMCAO |
3.08±0.29 |
2.08±0.29 |
1.92±0.29 |
1.75±0.45 |
1.50±0.52 |
1.33±0.49 |
| EM |
3.00±0.60 |
2.17±0.39 |
1.83±0.39 |
1.58±0.51 |
1.33±0.49 |
1.08±0.79 |
| CR |
3.08±0.29 |
2.08±0.29 |
1.83±0.39 |
1.67±0.49 |
1.41±0.51 |
1.17±0.72 |
| WM |
3.08±0.40 |
2.17±0.39 |
1.75±0.45 |
1.50±0.52 |
1.25±0.45 |
0.75±0.62a–c |
Infarct volume
TTC staining is a classical method to assess area of
cerebral infarctions caused by MCAO. Normal cerebral tissue was
stained red, and unstained areas were defined as the infarcted
tissue. As presented in Fig. 2 and
Table V, all groups exhibited a
gradual reduction in the infarct volume percentage during the first
month after the tMCAO. Infarct volume percentage was notably
decreased in the WM group on days 15 (P<0.05 vs. tMCAO group)
and 30 (P<0.05 vs. other groups) in addition to in the CR group
on days 7 and 15 (P<0.05 vs. tMCAO group).
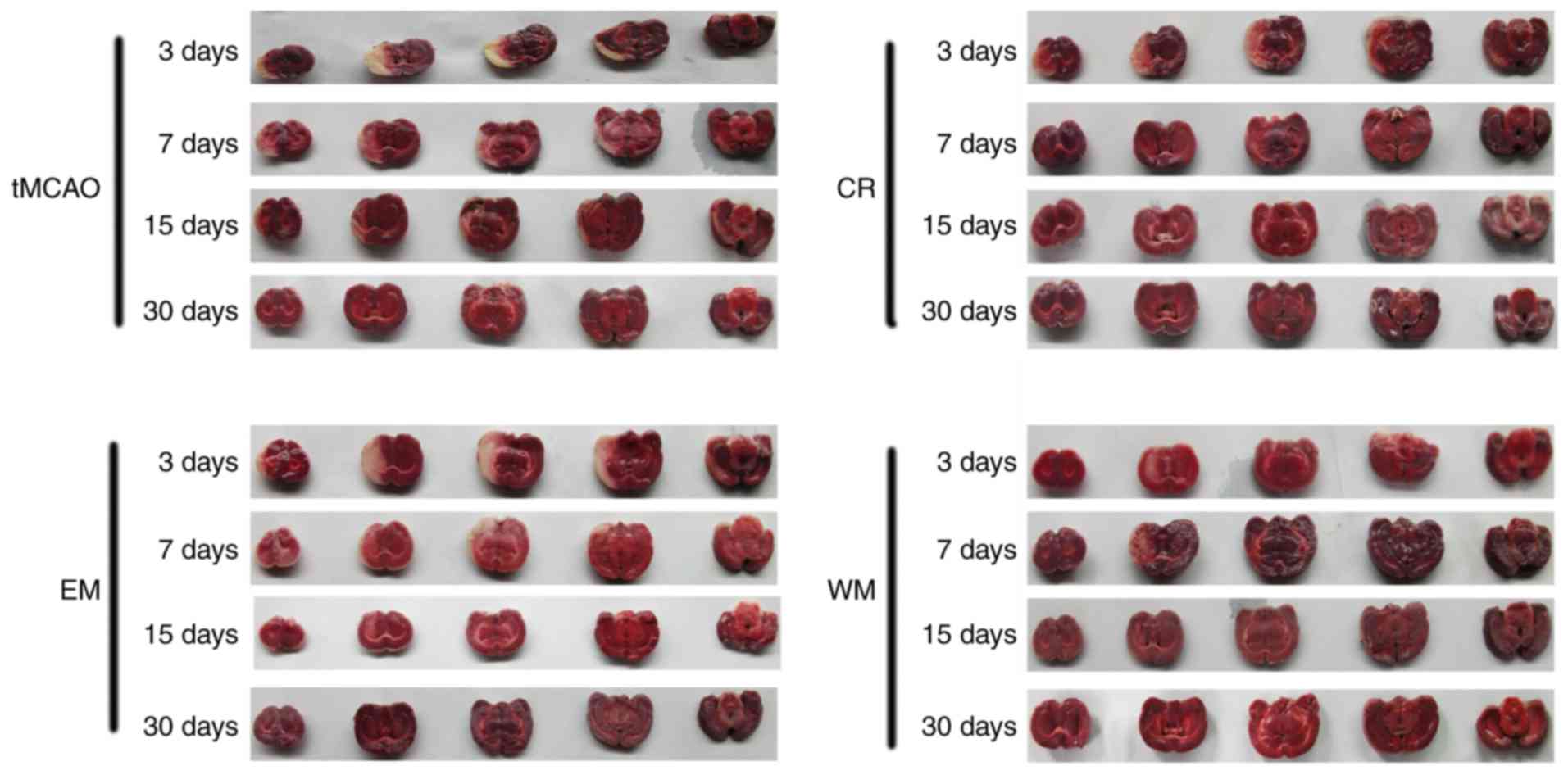 | Figure 2.TTC staining in each group. After the
tMCAO, the rats were randomly divided into 4 groups (n=18 rats in
each group): The tMCAO group (no specific training), and the CR, EM
and WM groups with different training methods. The rats in each
groups were sacrificed and the brains were stained with TTC. TTC,
2,3,5-triphenyltetrazolium chloride; tMCAO, temporary middle
cerebral artery occlusion; CR, control rehabilitation; EM,
environment modification; WM, willed movement. |
 | Table V.Infarct volume indicated by
2,3,5-triphenyltetrazolium chloride staining. |
Table V.
Infarct volume indicated by
2,3,5-triphenyltetrazolium chloride staining.
|
| Time after tMCAO
(days) |
|---|
|
|
|
|---|
| Groups | 3 | 7 | 15 | 30 |
|---|
| MCAO |
0.20±0.10 |
0.18±0.19 |
0.15±0.18 |
0.08±0.14 |
| EM |
0.18±0.25 |
0.17±0.13 |
0.14±0.18 |
0.08±0.25 |
| CR |
0.19±0.15 |
0.15±0.14a |
0.11±0.14a |
0.07±0.21 |
| WM |
0.17±0.19 |
0.16±0.08 |
0.12±0.16a |
0.03±0.78a–c |
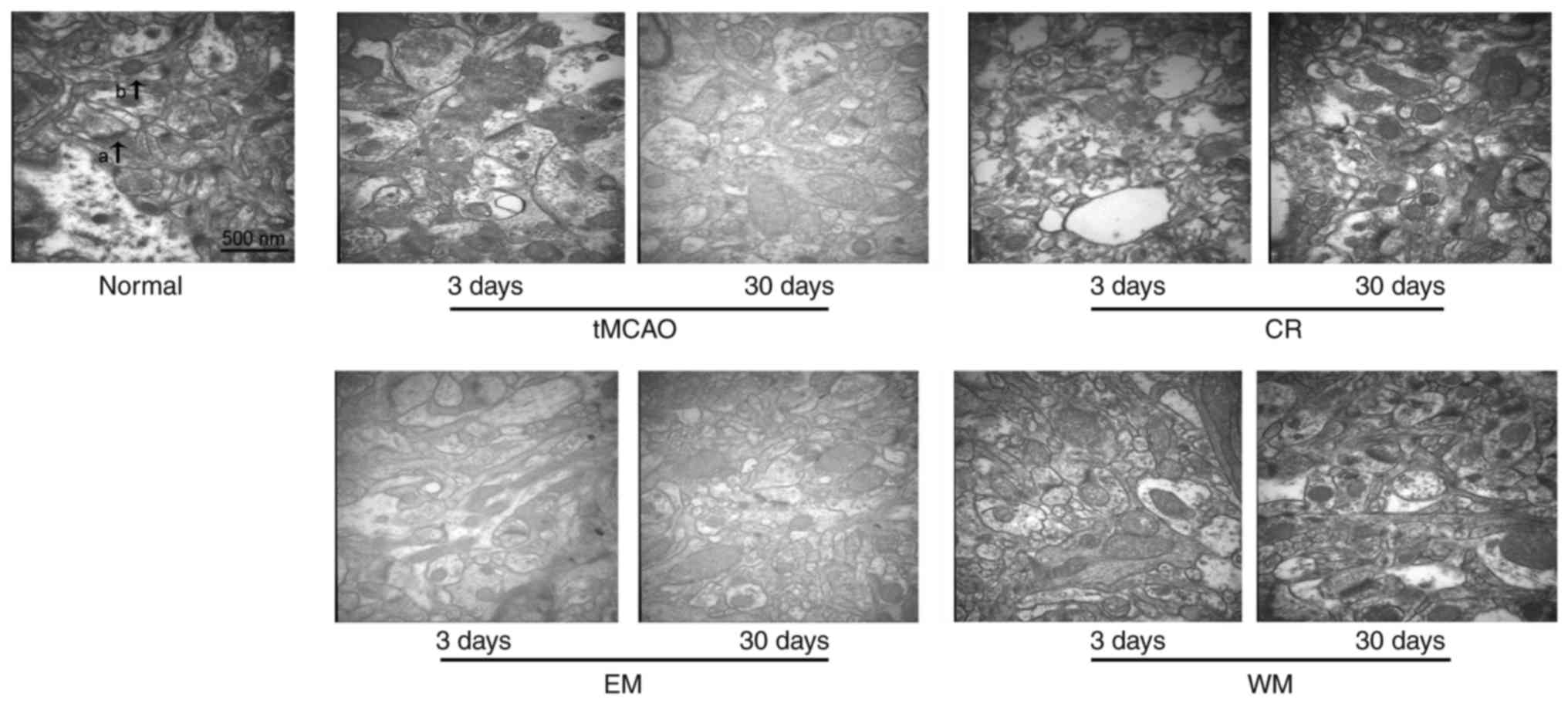
TEM observation
TEM observation indicated the ultrastructural
changes of nerve cells after stroke (Fig. 3). A total of 3 days after tMCAO,
there were few synaptic junctions present in each group. WM and CR
groups exhibited notable increases in the number of synaptic
junctions 30 days after stroke, however the increase was moderate
in the EM and tMCAO groups.
Location and expression of indicated
protein in ischemic penumbra of brain
Immunohistochemical staining demonstrated the
location and expression levels of GluR2 and GRASP-1 in the neurons.
GluR2 and GRASP-1 were expressed in the cytoplasm and the cell
nucleus. The proportion of GluR2-stained neurons was higher in CR
(47%), EM (53%) and WM (82%) groups compared with that of the tMCAO
(38%) group. There were no significant differences in
GRASP-1-stained cells among tMCAO, CR and EM groups. The WM group
exhibited increased GRASP-1 staining compared with the other groups
(Fig. 4A).
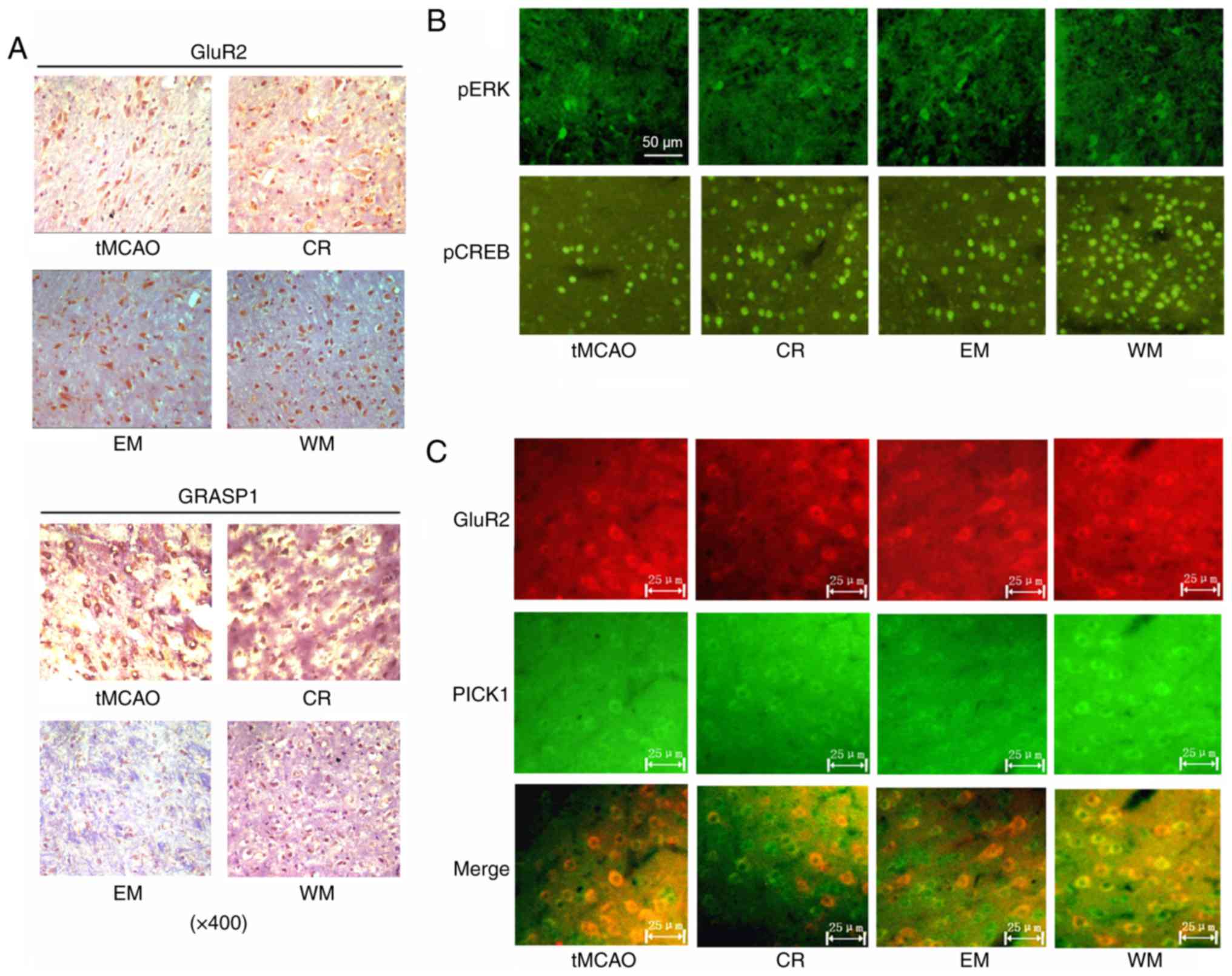 | Figure 4.Immunohistochemistry and
immunofluorescence. After the tMCAO, the rats were randomly divided
into 4 groups (n=18 rats in each group): The tMCAO group (no
specific training), and the CR, EM and WM groups with different
training methods. The cortical area and its adjacent corpus
callosum in the ischemic hemisphere were evaluated with (A)
immunohistochemistry and (B and C) immunofluorescence methods to
detect the expression of pERK, pCREB, GluR2, GRASP-1 and PICK1.
tMCAO, temporary middle cerebral artery occlusion; CR, control
rehabilitation; EM, environment modification; WM, willed movement;
p-, phosphorylated; ERK, extracellular signal-related kinase; CREB,
cyclic adenosine monophosphate response element-binding protein 1;
GluR2, glutamate receptor 2; GRASP-1, glutamate receptor
interacting protein 1-associated protein 1; PICK1, protein
interacting with C-kinase 1. |
Neurons with pERK and pCREB staining presented with
green fluorescence. pERK staining was present in the cytoplasm and
cell nucleus of neurons, while pCREB staining was present in the
cell nucleus. The WM group exhibited an increase in pERK-stained
neurons compared with other groups. The proportion of pCREB-stained
neurons was ~28, 75, 67 and 83% in the tMCAO, CR, EM and WM groups,
respectively (Fig. 4B).
GluR2 and PICK1 expression was detected with
immunofluorescence. Neurons with GluR2 and PICK1 staining were
observed to be increased in the WM group compared with the tMCAO
group (Fig. 4C).
Western blotting was conducted in order to quantify
expression of these proteins. pERK and pCREB protein levels were
increased in CR (P<0.01), EM (P<0.05), and WM (P<0.001)
groups compared with the tMCAO group. In addition, expression
levels of GluR2, GRASP-1 and PICK1 were increased in CR
(P<0.05), EM (P<0.05) and WM (P<0.01) groups (Fig. 5).
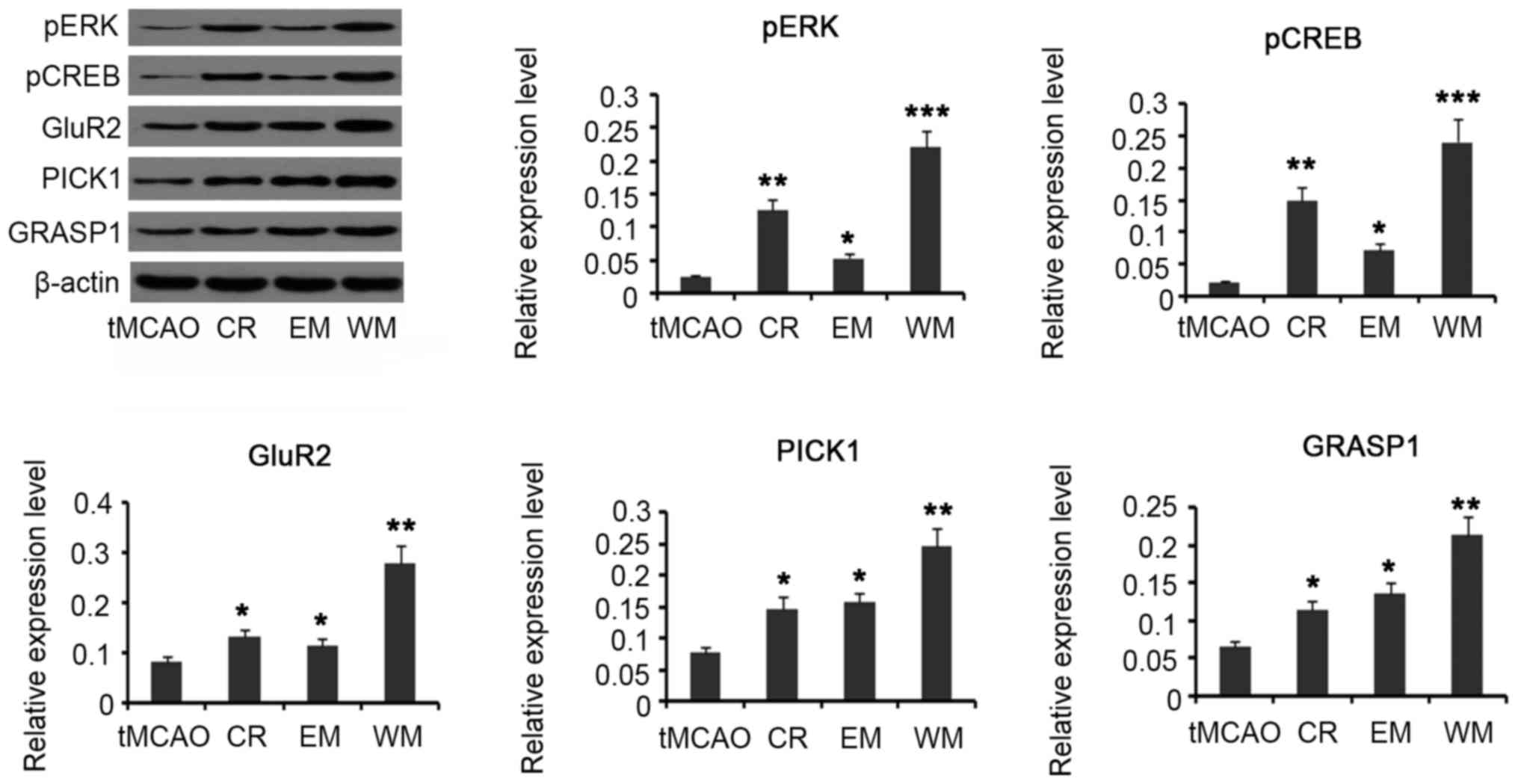 | Figure 5.Quantification of protein level with
western blotting. Subsequent to tMCAO, the rats were randomly
divided into 4 groups (n=18 rats in each group): The tMCAO group
(no specific training), and the CR, EM and WM groups with different
training methods. Expression of pERK, pCREB, GluR2, GRASP-1 and
PICK1 levels in the cortex adjacent to the injury brain were
evaluated with western blotting. *P<0.05 and **P<0.01 vs.
tMCAO group. tMCAO, temporary middle cerebral artery occlusion; CR,
control rehabilitation; EM, environment modification; WM, willed
movement; p-, phosphorylated; ERK, extracellular signal-related
kinase; CREB, cyclic adenosine monophosphate response
element-binding protein 1; GluR2, glutamate receptor 2; GRASP-1,
glutamate receptor interacting protein 1-associated protein 1;
PICK1, protein interacting with C-kinase 1. |
Discussion
Post-stroke neurological injury is an important
health problem associated with a high disability and mortality rate
worldwide (1). Stroke is
associated with cerebrovascular bleeding, inflammatory reaction and
stress response, which causes neuronal apoptosis or death and
functional loss of motion, communication and cognition (2). Neurorehabilitation training
represents a therapeutic option to help stroke patients improve the
impaired nervous system and motor control, according to the
activity-dependent neural plasticity. Exercise training has been
considered to improve neuroplasticity, which leads to enhancement,
compensation and replacement of the remaining function of nerve
(24). Synaptic activity is
decreased following brain injury, however exercise training
increases presynaptic release of neurotransmitters, in addition to
upregulating postsynaptic response to those neurotransmitters,
which can, at least in part, restore neural function following an
injury (26). In addition to
synaptic activity regulation, new synapse formation, which may
compensate for the lost structural circuits, can be triggered by
stroke. This self-regulation of synaptic plasticity is activity-
and experience-dependent (27).
Motion-rehabilitation training can increase the connectivity
between multiple brain regions that are disconnected after stroke,
leading to improved functional outcomes.
In the present study, rats in the WM group exhibited
greater improvements in neurobehavioral performance compared with
the other groups. The WM group exhibited an evident reduction in
brain damage as indicated by TTC staining. TEM observation
indicated that there were increased synaptic junctions following WM
training for a period of time (one month). A previous study
demonstrated that WM training was more effective in enhancing the
PICK1-mediated synaptic plasticity in the area adjacent to the
damage region of ischemic rats when compared with swimming training
(28). Traditional
neurorehabilitation training highlights the intensity, however
fails to take into account the voluntary participation of patients.
Ploughman et al (29)
observed that although the more intense, motorized running exercise
induced a rapid increase in BDNF, the elevation was more
short-lived compared to that of voluntary running. In addition,
intense motorized running triggered a more pronounced increase in
the stress hormone, corticosterone, however reduction in pCREB and
synapsin-I in hippocampus were observed beginning 30 to 60 min
subsequent to the exercise (29).
It is, thus, suggested that frequent voluntary running with a lower
intensity should be used in the clinic, which may have a delayed
but sustained effect supporting brain remodeling after stroke.
In a previous study, it was identified that the
signal transducer and activator of transcription 3 (STAT3) pathway
was involved in the regulation of BDNF, PICK1 and SYP and mediates
the improvements in neuroplasticity that result from WM training
(30). The present study
additionally identified that the ERK/CREB pathway was notably
activated following WM training. Although CR and EM groups also
exhibited ERK/CREB pathway activation, this was not as effective as
that of the WM group. Notably, BDNF and SYP have been observed to
be positively regulated by the ERK pathway (8,9), it
is suggested that STAT3 and ERK signaling may exert synergistic or
additional effects on the promotion of BDNF and SYP. The ERK/CREB
pathway serves as a key signaling pathway for neuroprotection and
endogenous neurogenesis. It was reported that CREB activation
serves a critical part in DNA repair and anti-oxidation, thereby
protecting neurons from damage induced by cerebral ischemia and
reperfusion (9). Neurogenesis
mediated by the ERK/CREB pathway is involved in structural and
functional neuroplasticity, therefore, it is suggested that
ERK/CREB activation triggered by WM training is important to reduce
brain damage after stroke, and improves neurobehavioral
performance.
It has been previously observed that AMPAR is
involved in neuroplasticity improved by WM training (21). In the mammalian central nervous
system, AMPA-type glutamate receptors mediate the majority of fast
excitatory synaptic transmission. AMPARs are tetramers made up of
combinations of four subunits: GluR1, GluR2, GluR3 and GluR4,
andGluR1 and GluR4 mRNA of rats in the WM group have been observed
to be significantly upregulated in the ischemia penumbra region at
the subacute stage (21). The
present study used western blotting, immunofluorescence and
immunohistochemistry to indicate that GluR2 was also upregulated
following WM training. GluR2 is a critical subunit in determining
mammalian AMPAR function; GluR2 is involved in the regulation of
the Ca2+ permeability, single-channel conductance and
AMPAR trafficking and assembling. AMPAR trafficking, namely
redistribution in and out of the synapse, has emerged as an
important mechanism for synaptic plasticity, such as LTP and
long-term depression (LTD) (16).
Increased delivery of AMPARs to the postsynaptic membrane leads to
LTP, while net removal of AMPARs by internalization from the
surface appears to underlie LTD (17). GRASP-1 affects
GluR2/3-GRIPinteraction and regulates AMPAR trafficking to the
synaptic membrane, thus it is associated to AMPAR-dependent LTP
(14). PICK1is an AMPAR-binding
protein, serving an essential role in regulating LTD in cerebral
and hippocampal synapses. A previous study identified that WM
training induces a PICK1-dependent LTD in rats subjected to focal
cerebral ischemia (30). In the
present study, it was observed that GRASP-1 and PICK1 expression
were induced by WM training, suggesting that WM training is
involved in LTP and LTD in regulating synaptic plasticity.
Collectively, the present study demonstrated that WM
training confers greater effects on improving neurobehavioral
performance compared with traditional training in a rotating wheel.
The WM group exhibited evident reduction in brain damage with
increased synaptic junctions. Study of the molecular mechanism
indicated that WM training promotes the ERK/CREB pathway and
GluR2/GRASP-1/PICK1 cascades. These signaling pathways were
involved in neuroprotection, endogenous neurogenesis and synaptic
plasticity of LTP and LTD. The current study lays a preliminary
foundation for future research on therapeutic intervention of WM
training against stroke-induced neuronal damage.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 30973167),
China Postdoctoral Science Foundation (grant no. 2011m501301),
Postdoctoral Science Foundation of Hunan Province and the
Postdoctoral Science Foundation of Central South University.
References
|
1
|
Pan L, Song A, Duan S and Yu Z:
Patient-centered robot-aided passive neurorehabilitation exercise
based on safety-motion decision-making mechanism. Biomed Res Int.
2017:41859392017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Zhang X, Xue L, Hao C, Liao W and
Wan Q: Treatment with enriched environment reduces neuronal
apoptosis in the periinfarct cortex after cerebral
ischemia/reperfusion injury. Cell Physiol Biochem. 41:1445–1456.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker K, Berretta A, Saenger S,
Sivaramakrishnan M, Shirley SA, Metzger F and Clarkson AN:
PEGylated insulin-like growth factor-I affords protection and
facilitates recovery of lost functions post-focal ischemia. Sci
Rep. 7:2412017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi DH, Ahn JH, Choi IA, Kim JH, Kim BR
and Lee J: Effect of task-specific training on Eph/ephrin
expression after stroke. BMB Rep. 49:635–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanchez-Mendoza EH and Hermann DM:
Correlates of post-stroke brain plasticity, relationship to
pathophysiological settings and implications for human
proof-of-concept studies. Front Cell Neurosci. 10:1962016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang QP, Shen Q, Wu LX, Feng XL, Liu H, Wu
B, Huang XS, Wang GQ, Li ZH and Liu ZJ: STAT3 signal that mediates
the neural plasticity is involved in willed-movement training in
focal ischemic rats. J Zhejiang Univ Sci B. 17:493–502. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye Y, Zhao Z, Xu H, Zhang X, Su X, Yang Y,
Yu X and He X: Activation of sphingosine 1-phosphate receptor 1
enhances hippocampus neurogenesis in a rat model of traumatic brain
injury: An involvement of MEK/Erk signaling pathway. Neural Plast.
2016:80721562016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Zhu J, Zhou L and Wan L: RACK1
promotes maintenance of morphine-associated memory via activation
of an ERK-CREB dependent pathway in hippocampus. Sci Rep.
6:201832016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Li X, Guo C, Li L, Wang Y, Zhang Y,
Chen Y, Liu W and Gao L: Long-term neurocognitive dysfunction in
offspring via NGF/ERK/CREB signaling pathway caused by ketamine
exposure during the second trimester of pregnancy in rats.
Oncotarget. 8:30956–30970. 2017.PubMed/NCBI
|
|
10
|
Qu H, Zhao M, Zhao S, Xiao T, Tang X, Zhao
D, Jolkkonen J and Zhao C: Forced limb-use enhances brain
plasticity through the cAMP/PKA/CREB signal transduction pathway
after stroke in adult rats. Restor Neurol Neurosci. 32:597–609.
2014.PubMed/NCBI
|
|
11
|
Li H, Wang J, Wang P, Rao Y and Chen L:
Resveratrol reverses the synaptic plasticity deficits in a chronic
cerebral hypoperfusion rat model. J Stroke Cerebrovasc Dis.
25:122–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pregi N, Belluscio LM, Berardino BG,
Castillo DS and Cánepa ET: Oxidative stress-induced CREB
upregulation promotes DNA damage repair prior to neuronal cell
death protection. Mol Cell Biochem. 425:9–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Isaac JT, Ashby MC and McBain CJ: The role
of the GluR2 subunit in AMPA receptor function and synaptic
plasticity. Neuron. 54:859–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dixon RM, Mellor JR and Hanley JG:
PICK1-mediated glutamate receptor subunit 2 (GluR2) trafficking
contributes to cell death in oxygen/glucose-deprived hippocampal
neurons. J Biol Chem. 284:14230–14235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanley JG: Actin-dependent mechanisms in
AMPA receptor trafficking. Front Cell Neurosci. 8:3812014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bakshi K, Kosciuk M, Nagele RG, Friedman E
and Wang HY: Prenatal cocaine exposure increases synaptic
localization of a neuronal RasGEF, GRASP-1 via hyperphosphorylation
of AMPAR anchoring protein, GRIP. PLoS One. 6:e250192011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoogenraad CC and van der Sluijs P:
GRASP-1 regulates endocytic receptor recycling and synaptic
plasticity. Commun Integr Biol. 3:433–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye B, Yu WP, Thomas GM and Huganir RL:
GRASP-1 is a neuronal scaffold protein for the JNK signaling
pathway. FEBS Lett. 581:4403–4410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiu SL, Diering GH, Ye B, Takamiya K,
Chen CM, Jiang Y, Niranjan T, Schwartz CE, Wang T and Huganir RL:
GRASP1 regulates synaptic plasticity and learning through endosomal
recycling of AMPA receptors. Neuron. 93:1405–1419.e8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Institute for Laboratory Animal
ResearchGuide for the care and use of laboratory animals. 8th
edition. Washington (DC): National Academies Press; 2011,
PubMed/NCBI
|
|
21
|
Tang Q, Yang Q, Hu Z, Liu B, Shuai J, Wang
G, Liu Z, Xia J and Shen X: The effects of willed movement therapy
on AMPA receptor properties for adult rat following focal cerebral
ischemia. Behav Brain Res. 181:254–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andrabi SS, Parvez S and Tabassum H:
Progesterone induces neuroprotection following reperfusion-promoted
mitochondrial dysfunction after focal cerebral ischemia in rats.
Dis Model Mech. 10:787–796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shu S, Li CM, You YL, Qian XL, Zhou S and
Ling CQ: Electroacupuncture ameliorates cerebral
ischemia-reperfusion injury by regulation of autophagy and
apoptosis. Evid Based Complement Alternat Med. 2016:72974252016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Wang X, Zhang J, Dang C, Liu G,
Liang Z, Huang G, Zhao W and Zeng J: Tongxinluo enhances
neurogenesis and angiogenesis in peri-infarct area and
subventricular zone and promotes functional recovery after focal
cerebral ischemic infarction in hypertensive rats. Evid Based
Complement Alternat Med. 2016:85495902016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng Y, Wei H, Sun R, Tian Z and Zheng X:
Rapid method for protein quantitation by Bradford assay after
elimination of the interference of polysorbate 80. Anal Biochem.
494:37–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu J, Wang H, Deng L and Li J: Exercise
training promotes functional recovery after spinal cord injury.
Neural Plast. 2016:40395802016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng A, Hou Y and Mattson MP:
Mitochondria and neuroplasticity. ASN Neuro. 2:e000452010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nie J, Yang X, Tang Q, Shen Q and Li S:
Willed-movement training reduces brain damage and enhances synaptic
plasticity related proteins synthesis after focal ischemia. Brain
Res Bull. 120:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ploughman M, Granter-Button S, Chernenko
G, Attwood Z, Tucker BA, Mearow KM and Corbett D: Exercise
intensity influences the temporal profile of growth factors
involved in neuronal plasticity following focal ischemia. Brain
Res. 1150:207–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Q, Tan L, Yang X, Shen Q, Huang X,
Wang G, Chen H, Nie J, Li S and Wu L: Willed-movement training
reduces motor deficits and induces a PICK1-dependent LTD in rats
subjected to focal cerebral ischemia. Behav Brain Res. 256:481–487.
2013. View Article : Google Scholar : PubMed/NCBI
|