Introduction
Renal cell carcinoma (RCC) has obvious resistance to
apoptosis induced by chemical and immunological preparations and
radiation, and this is why the non-surgical treatment for RCC is
unlikely to be effective (1,2).
However, the mechanism of RCC in apoptosis resistance is still
unclear. Recently, molecular targeting therapy employing
multi-kinase inhibitors (MKIs) such as tyrosine kinase inhibitors
has raised hope for patients with advanced RCC; sorafenib and
sunitinib (3,4) are becoming first-line treatments for
metastatic RCC. However, if the patients develop resistance to
MKIs, they rapidly succumb to the disease. It is now of utmost
importance to find another way to control cancer progression.
Manipulation of the apoptotic mechanisms is one promising approach
(5).
Different apoptotic signals such as chemicals can
induce cell apoptosis in two ways, one of which is death receptor
pathway (extrinsic), the other one is the mitochondrial pathway
(intrinsic). Cluster of differentiation (CD)95 antibodies (CH11)
are Fas ligands which can induce apoptosis through death receptor
pathway (6,7). Topotecan and Etoposide (8) are anti-cancer drugs which can inhibit
DNA topoisomerase I and II and induce apoptosis through the
mitochondrial pathway (9,10). As an anti-cancer and anti-HIV drug,
caffeic acid phenethyl ester (CAPE) is a nuclear factor (NF)-κB
inhibitor. As NF-κB targets, the expression of inhibitor of
apoptosis proteins (IAPs) can be promoted by NF-κB, so CAPE induces
apoptosis through suppression of IAPs expression (11). However, regardless of which
apoptosis pathway is active, the glutathione protease family
(caspase) such as caspase-3, −7 and-9 must be activated, causing a
chain reaction leading to apoptosis (12). More importantly, caspase-3 and −7
activation is the key to apoptosis; therefore, once they are
activated, apoptosis can be carried out normally. IAPs directly
bind to caspases and inhibit their activation, serving a vital role
in the regulation of cell apoptosis. Caspase inhibitor XIAP is the
most effective among the IAPs family. It can restrain apoptosis by
suppressing the apoptosis initiation factor caspase-9 and effector
caspase-3 and −7 (13). Therefore,
XIAP expression levels may directly determine the sensitivity of
tumor cells to apoptosis.
RNA interference (RNAi) can efficiently and
specifically inhibit homologous gene expression (14–17).
It interferes with the homologous sequences and gene expression at
the transcriptional level, and causes the specific degradation of
homologous mRNA, corresponding to silencing gene expression. RNAi
technologies can effectively inhibit XIAP expression (18,19).
It may reduce the experimental error, and improve the experimental
accuracy and credibility.
The present study induced apoptosis in different RCC
cell lines, which exhibit varying expression levels of XIAP,
through death receptor, mitochondrial and NF-κB signaling pathways.
Furthermore, RNAi was used to reduce the expression of XIAP in XIAP
over-expressing RCC cells in order to study its role in apoptosis,
and to investigate the mechanism of RCC cells in apoptosis
resistance. This way, its potential application value in tumor gene
therapy was investigated.
Materials and methods
Cell culture
The established RCC cell lines ClearCa-2 and
ClearCa-6 were obtained from Heinrich-Heine University (Dusseldorf,
Germany). The Caki1 cell line was purchased from China
Infrastructure of Cell Line Resources (Beijing, China), and
cultured as described previously (20). CH11 (CD95-specific CH11 antibody)
was purchased from Immunotech; Beckman Coulter, Inc. (Brea, CA,
USA); Etoposide was from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany), CAPE and Topotecan hydrochloride was from Merck KGaA.
Western blotting
Western blotting was performed on the cell lines, as
described previously (20). A
horseradish peroxidase-labelled secondary antibody (cat. no. 0101;
1:10,000; 37°C for 40 min) was used and blots were visualized using
a Super Signal West Pico Substrate (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. β-actin
was used as a loading control. The images were analyzed using
UN-SCAN-Itgel Automated Digitizing System software (version 5.1 for
Windows; Silk Scientific Inc., Orem, UT, USA). The following
antibodies were used: Anti-XIAP (cat. no. 2042; 1:10,000; 37°C for
40 min) from Sigma-Aldrich; Merck KGaA; polyclonal anti-inhibitor
of apoptosis 1 (c-IAP1; cat. no. 4952; 1:10,000; 37°C for 40 min),
anti-survivin (cat. no. 2802; 1:10,000; 37°C for 40 min) from Cell
Signaling Technology Inc. (Danvers, MA, USA); anti-c-IAP2 (clone
F30-2285; 1:10,000; 37°C for 40 min) from BD Biosciences (Franklin
Lakes, NJ, USA) and anti-β-actin (cat. no. 8227; 1:20,000; 37°C for
40 min) from Abcam (Cambridge, MA, USA).
RNAi
The XIAP-targeting short hairpin RNA vector was
generated through literature review (21). The target sequence is 50-AGG TGA
AGG TGA TAA AGT A-30 (22)
Transfection was carried out using Lipofectamine 2000 transfection
reagent (Gibco; Thermo Fisher Scientific, Inc.) and a BLOCK-iT™ U6
RNAi Entry Vector kit (Kang Wei Technology, China; http://www.cwbiotech.com). For generation of stable
transfectant clones, the transfected cells were selected with G418
for 3–4 weeks. A total of three selected clones were screened for
XIAP expression (clone nos 1–3), and clone no. 2 was randomly
selected for further experiments. G418-resistant mock transfectants
were also isolated, produced by transfection of the plasmid without
XIAP-targeting sequence.
Measurement of cell viability and cell
apoptosis
CH11 (cat. no. 49516; 37°C for 24 h) from Abcam
(Cambridge, MA, USA), Topotecan (cat. no. d1916; 37°C for 24 h)
from Baomanbio (Shanghai, China), CAPE (cat. no. 211200; 37°C for
24 h) from Calbiochem; Merck KGaA (Darmstadt, Germany), and
Etoposide (cat. no. 341205; 37°C for 24 h) from Calbiochem were
used to induce apoptosis, and cell viability was detected by
counting cells under the optical microscope. Trypan blue (cat. no.
GD-jk1413; 37°C for 1 min) from Guduo (Shanghai, China) was used to
detect viable cells or cell death according to the manufacturer's
protocol. In additional experiments, in order to improve the
experiment efficiency, an MTT kit was used (Gibco; Thermo Fisher
Scientific, Inc.) and flow cytometry (BD Biosciences) were used to
detect early apoptosis according the manufacturer's protocol.
Statistical analysis
SPSS software version 13.0 was used (SPSS, Inc.,
Chicago, IL, USA). All data are expressed as the mean ± standard
deviation of three independent experimental replicates. Statistical
analysis was performed using a one-way analysis of variance
followed by the Least Significant difference post hoc test or
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Basal expression of XIAP is
significantly different between ClearCa-2 and ClearCa-6 cell
lines
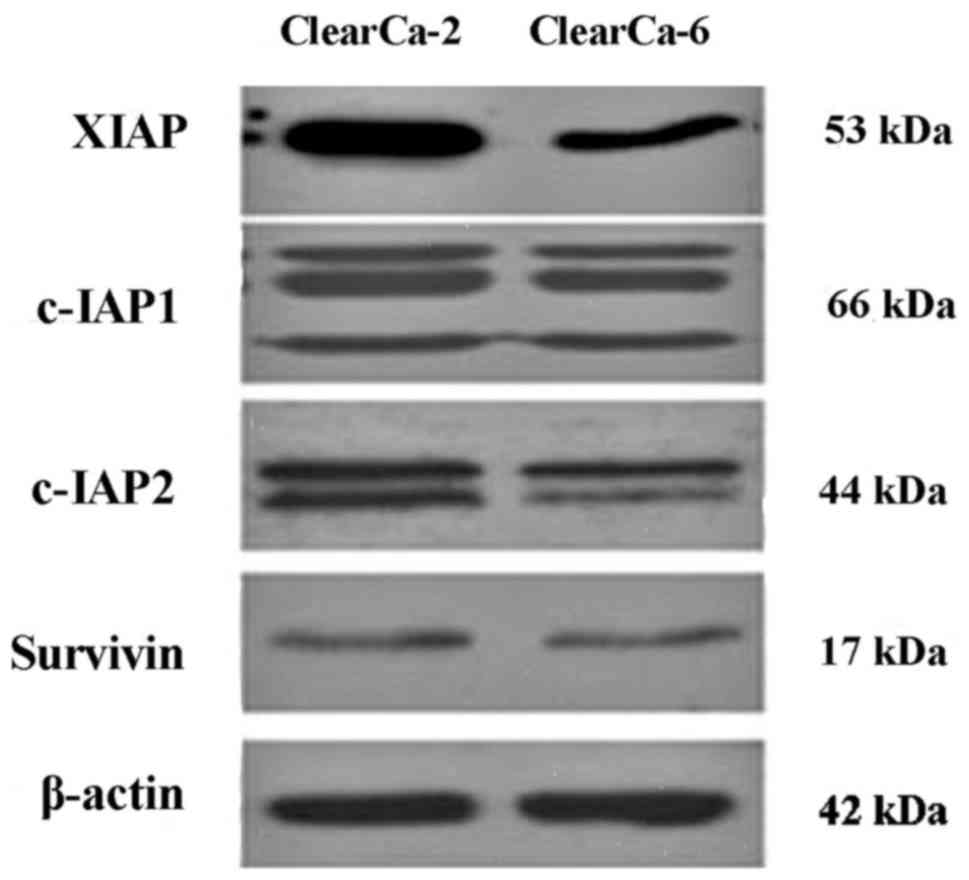
The basal protein expression of XIAP, cIAP-1, cIAP-2
and survivin, which are the main members of the IAPs family, were
analyzed by western blot analysis. It was demonstrated that there
were no differences between cIAP-1 and survivin expression levels
in both cell lines, and cIAP-2 had a slight difference, but the
expression XIAP between the two cell lines was very different. The
expression of XIAP in ClearCa-2 was much higher than ClearCa-6
(Fig. 1).
ClearCa-2 cells with high expression
XIAP are resistant to apoptosis; however, ClearCa-6 cells with
low-expression XIAP are sensitive to apoptosis
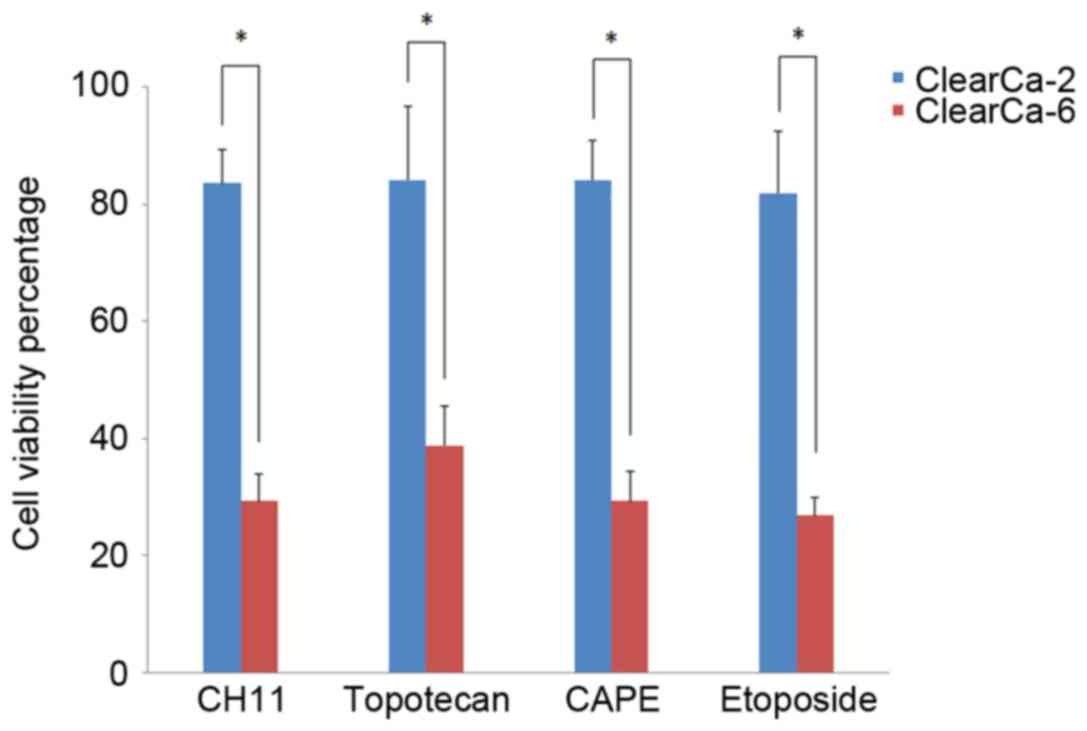
Because of the complicated mechanism of apoptosis,
four different drugs which could induce cell apoptosis through
different pathways were used. The results demonstrated that
ClearCa-2 cells with higher expression of XIAP were resistant to
apoptosis induced by all four drugs (Fig. 2). Following drug treatment for 24
h, the cell viabilities (compared with the control group) were
83.54% for CH11, 84.07% for Topotecan, 84.04% for CAPE, and 81.85%
for Etoposide. However, ClearCa-6 cells with lower expression of
XIAP were sensitive to apoptosis induced by all four drugs
(Fig. 2). This difference in the
expression levels between the two cell lines was statistically
significant (P<0.05).
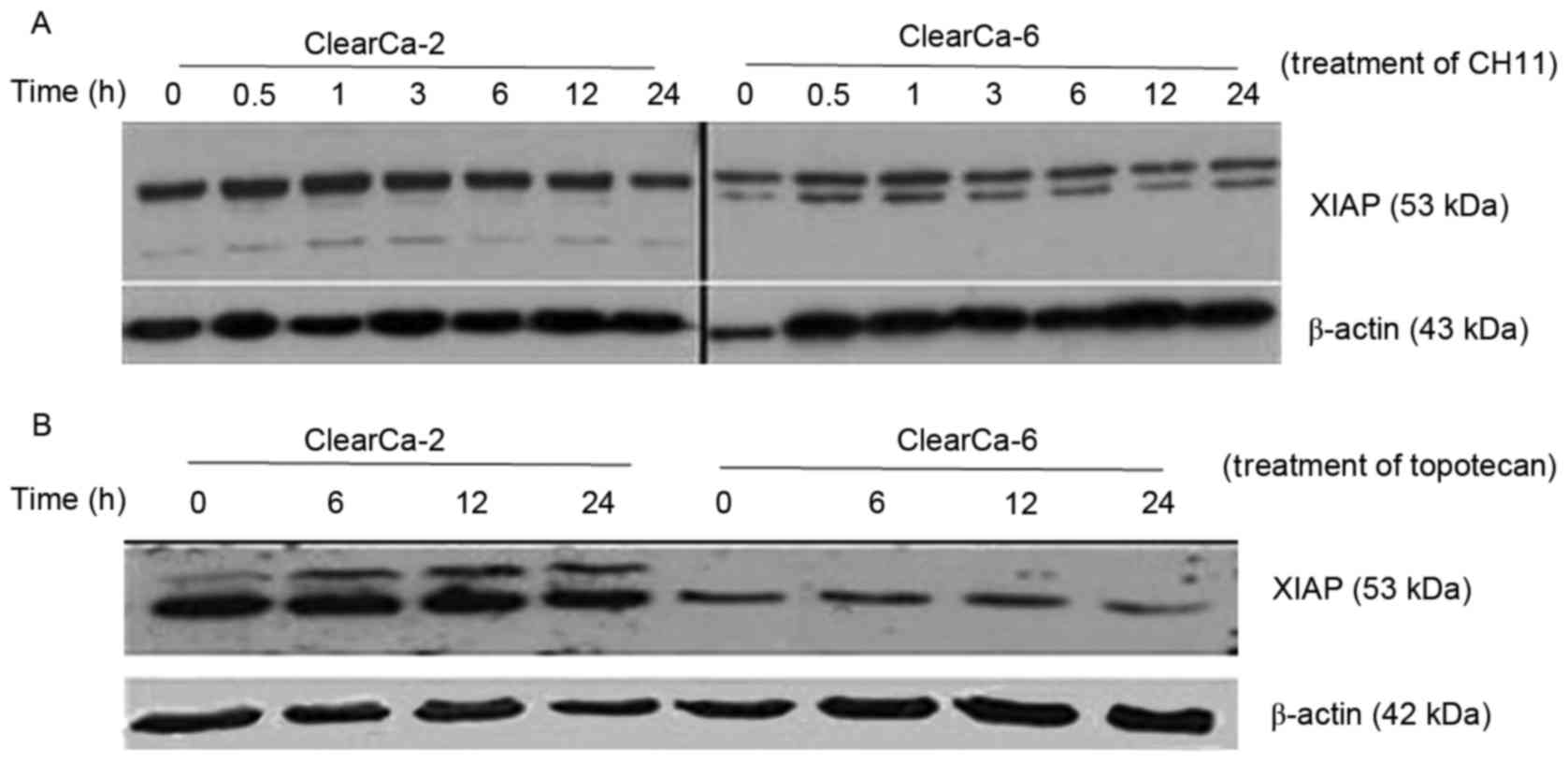
Different apoptotic signals did not
impact on the expression of XIAP during apoptosis
ClearCa-2 and −6 cell lines demonstrated a different
sensitivity to apoptosis, because of different basal protein
expression levels of XIAP. However, whether these levels would
change during apoptosis induced by different drug sat different
time points or not was investigated. Following 24 h treatment with
CH11 (Fig. 3A) and Topotecan
(Fig. 3B), both cell lines had no
change in XIAP expression but XIAP expression levels were higher in
ClearCa-2 cells compared with the ClearCa-6 cells.
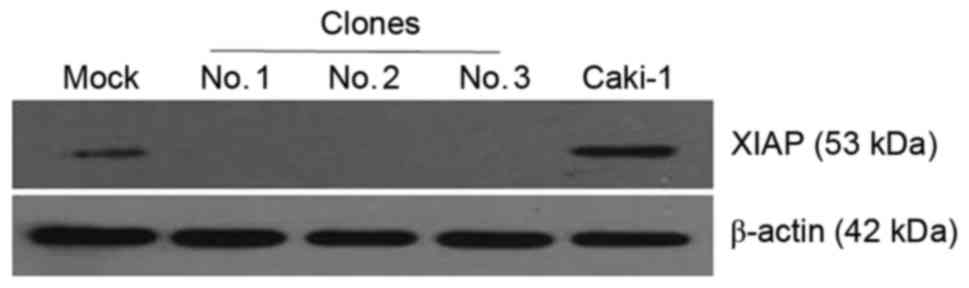
RNAi technology inhibits the
expression of XIAP in the Caki-1 cell line
To further determine the role XIAP serves in RCC
cell apoptosis, another XIAP-high-expression RCC cell line was
studied (Caki-1), by inhibiting XIAP expression through RNAi. A
total of three stable transfection cell lines were used (clone nos.
1–3), and western blot analysis was performed to detect XIAP
expression in them. Expression levels of XIAP were effectively
silenced in all three clone cell lines, but in the parental and the
mock group, the expression of XIAP was normal (Fig. 4). Therefore, clone no. 2 was
selected in further experiments, and was termed as XIAP
no-expression Caki-1 cells.
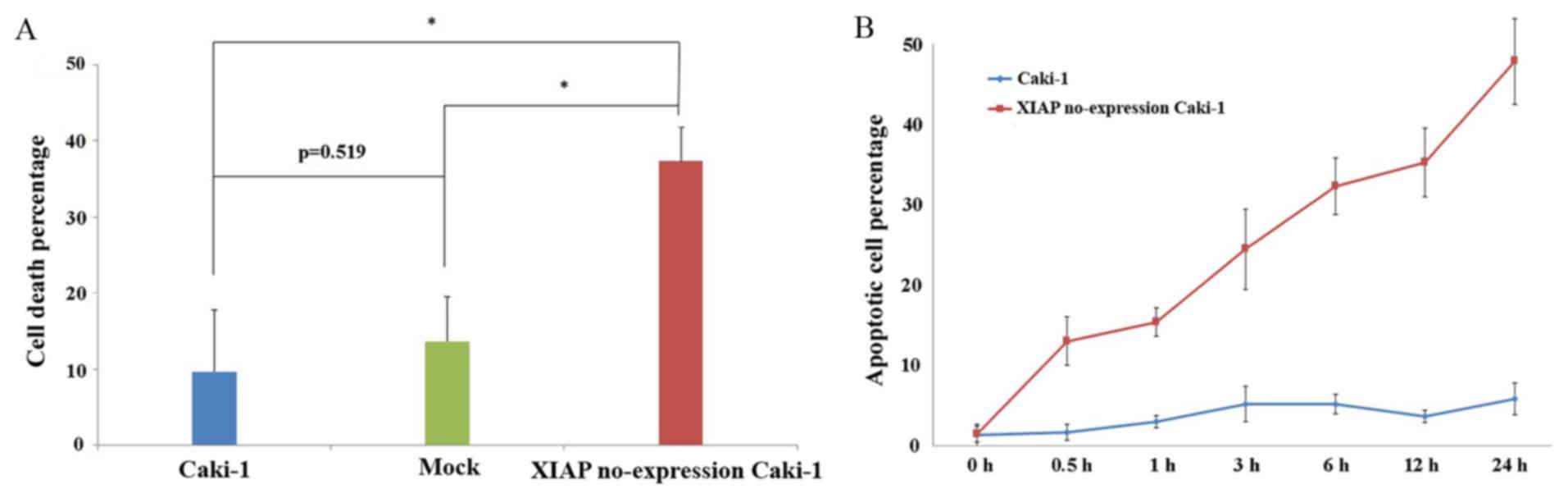
Sensitivity of Caki-1 cells not
expressing XIAP to Etoposide-induced apoptosis is greatly
enhanced
It was demonstrated that RCC cells were more
sensitive to Etoposide-induced apoptosis than with the other drugs;
therefore, XIAP no-expression Caki-1 cells, Caki-1 cells and Mock
cells (transfected by plasmid without XIAP interference gene) were
treated with Etoposide for 24 h, and the cell death percentage was
measured by MTT. It was demonstrated that the cell death percentage
of XIAP no-expression Caki-1 cells was much higher than the Caki-1
and Mock cells, (P<0.05; Fig.
5). Between Caki-1 and mock cells, there was no statistical
significance in the cell death percentage (P=0.519; Fig. 5A). It was also observed that
following the treatment of Etoposide, the early apoptosis rate in
XIAP no-expression Caki-1 cells was much higher than in the Caki-1
cells. At 0, 0.5, 1, 3, 6, 12 and 24 h, the early apoptosis rates
of XIAP no-expression Caki-1 cells were 1.23, 11.7, 13.87, 22.07,
29.14, 31.81 and 43.21%, respectively. However, the early apoptosis
rates of Caki-1 cells were 1.16, 1.48, 2.62, 4.61, 4.61, 3.26, and
5.20%, respectively (Fig. 5B).
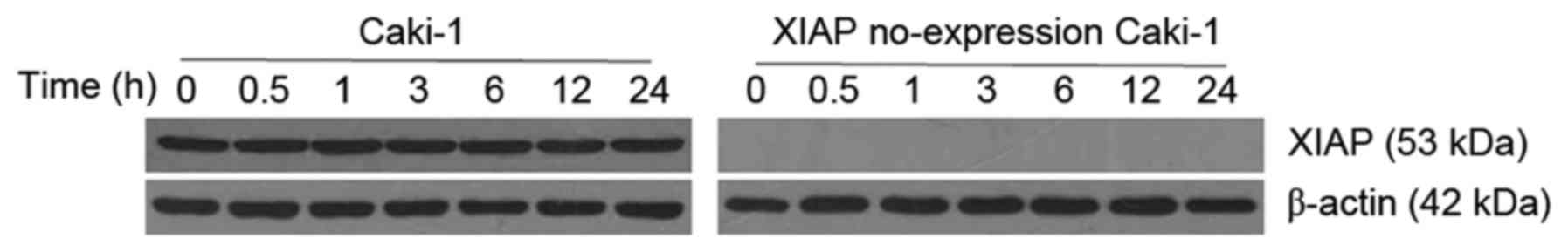
Expression of XIAP is stable during
apoptosis induced by Etoposide
In order to observe the level of expression of XIAP
in the progress of apoptosis, the expression of XIAP in Caki-1 and
XIAP no-expression Caki-1 cells was detected by western blot
analysis, at different time points during apoptosis induced by
Etoposide. It was observed that there was no change in the XIAP
protein expression levels, as was also observed in the ClearCa-2 an
ClearCa-6 cell lines (Fig. 6).
Discussion
In recent years, many apoptosis controlling genes
have been reported (23–25). XIAP, an important member of the
anti-apoptotic IAPs family, has a strong inhibitory effect on
caspase-3, −7, and −9 and it can also inhibit pro-caspase-3. It can
also affect signaling pathways, such as c-Jun N-terminal kinase
where, it is thought to serve a key role in the regulation of
apoptosis (26–28). Many malignant tumors, such as
breast, ovarian, lung, pancreatic and prostate cancer, express high
levels of XIAP (29–34). Some reports have mentioned that
increasing expression levels of XIAP through transfection can
enhance their resistance to apoptosis induced by the death
receptors or gamma-rays. Conversely, reducing the expression of
XIAP increases the sensitivity of cancer cells to apoptosis
(35,36). A previous study illustrated that
XIAP is expressed in different histologic types of RCC, and further
confirmed the universality of XIAP expression in human cancer
(37). Notably, the expression of
XIAP in RCC is increased, from early to late cancer stages, at both
mRNA and protein levels (37).
This indicated that tumor progression coincided with higher
expression of XIAP. Similar reports have been presented regarding
non-small cell lung cancer and acute myeloid leukemia (38). High expression levels of XIAP may
reduce the sensitivity of RCC cells to apoptosis, and provide
favorable conditions for tumor cell survival and development.
The present study demonstrated that the expression
levels of XIAP have important effects in RCC cells apoptosis. The
ClearCa-2 and ClearCa-6 RCC cell lines have different basal protein
expression levels of XIAP. Apoptosis may be induced through
extrinsic or intrinsic signaling pathways, and ultimately activate
caspases leading to apoptosis. A total of four drugs were chosen
that function through the extrinsic death receptor pathway (CH11),
the intrinsic mitochondrial pathway (Etoposide and Topotecan) and
the nuclear factor (NF)-κB inhibiting IAPs (CAPE) induced
apoptosis. It was demonstrated that the ClearCa-6 cell line was
sensitive to apoptosis, whereas ClearCa-2 cells were not, and the
intrinsic mitochondrial pathway was the strongest in inducing
apoptosis. Furthermore, another RCC cell line was employed, termed
Caki-1, which has also high expression of XIAP, to verify our
conclusion that XIAP may have important effects in RCC cell
apoptosis (22). The protein
expression of XIAP in the Caki-1 cells was eliminated through RNAi,
and it was confirmed that the sensitivity of RCC cells to apoptosis
was increased with decreased expression of XIAP.
Previous studies have reported that the expression
of XIAP increases following exposure of tumor cells to tumor
necrosis factor-related apoptosis-inducing ligand or gamma-rays
(39); however, other reports
demonstrated the opposite result (40,41).
In the present study, CH11 and Topotecan induced apoptosis, but had
no effect in the expression of XIAP in either ClearCa-2 or
ClearCa-6 cell lines. The expression of XIAP in both cell lines was
stable during apoptosis, although ClearCa-2 cells expressed much
higher XIAP than the ClearCa-6 cells. To verify this result, the
expression of XIAP was investigated in Caki-1 cells and
no-expression XIAP Caki-1 cells during apoptosis induced by
Etoposide, and the same phenomenon was observed. Therefore, it is
evident that that the role of XIAP in apoptosis is very
important.
RNAi can effectively suppress protein expression,
with a range in the inhibition rate (14–17).
Regarding XIAP, Wang et al (18) has reported that RNAi could reduce
the expression of XIAP by 56.2% in laryngeal carcinoma cells. Cao
et al (19) has reported
that RNAi could reduce its expression by 79.86% in pancreatic
carcinoma cells. Regarding the RCC cells, Bilim et al
(22) used RNAi to decrease XIAP
by 85.3%, in Caki-1 cells. In the present study, the interference
plasmid transfected Caki-1 cells and it completely inhibited XIAP
expression by 100%. To the best of our knowledge, no previous study
has ever reported such an effect. This study provides novel
insights on XIAP and gene therapy in clinical practice.
In conclusion, there are so many factors inducing
apoptosis, and the present study only studied the effect of XIAP on
apoptosis. There was a difference in the basal expression of XIAP
in two RCC cell lines, and those with higher expression of XIAP
resisted apoptosis. At the same time, reducing the expression of
XIAP enhanced the sensitivity to apoptosis. However, the underlying
mechanism(s) of this phenomenon need to be elucidated further.
Acknowledgements
The present study was a part of ‘Implication of
Smac/DIABLO to Resistance to Apoptosis in Renal Cell Carcinoma Cell
Lines’ (grant no. KM201310025017), the Beijing Municipal Commission
of Education, Science and Technology Plan, the ‘Comparison of
apoptosis – sensitizing mechanisms of XIAP differentially expressed
renal cell carcinoma cells’ (grant no. 81441073) and the National
Natural Science Foundation of China.
References
|
1
|
Yagoda A, Abi-Rached B and Petrylak D:
Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin
Oncol. 22:42–60. 1995.PubMed/NCBI
|
|
2
|
Spencer WF, Linehan WM, Walther MM, Haas
GP, Lotze MT, Topalian SL, Yang JC, Merino MJ, Lange JR, Pockaj BA,
et al: Immunotherapy with interleukin-2 and alpha-interferon in
patients with metastatic renal cell cancer with in situ primary
cancers: A pilot study. J Urol. 147:24–30. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Hanbury DC, Kuczyk MA,
Merseburger AS, Mulders PF, Patard JJ and Sinescu IC: European
Association of Urology Guideline Group for renal cell carcinoma.
Renal cell carcinoma guideline. Eur Urol. 51:1502–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ and Bukowski RM: Targeted
therapy for metastatic renal cell carcinoma. J Clin Oncol.
24:5601–5608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
6
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watabe M, Hishikawa K, Takayanagi A,
Shimizu N and Nakaki T: Caffeic acid phenethyl ester induces
apoptosis by inhibition of NFkappaB and activation of Fas in human
breast cancer MCF-7 cells. J Biol Chem. 279:6017–6026. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang Lu, Dong Li and Fuchu He: Advances
in bioinformatics of ubiquitination of protein. Hereditas.
35:17–26. 2013.(In Chinese). PubMed/NCBI
|
|
9
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kothakota S, Azuma T, Reinhard C, Klippel
A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ
and Williams LT: Caspase-3-generated fragment of gelsolin: Effector
of morphological change in apoptosis. Science. 278:294–298. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watabe M, Hishikawa K, Takayanagi A,
Shimizu N and Nakaki T: Caffeic acid phenethyl ester induces
apoptosis by inhibition of NFkappaB and activation of Fas in human
breast cancer MCF-7 cells. J Biol Chem. 279:6017–6026. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holcik M and Korneluk RG: XIAP, the
guardian angel. Nat Rev Mol Cell Biol. 2:550–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada T, Horinaka M, Shinnoh M, Yoshioka
T, Miki T and Sakai T: A novel HDAC inhibitor OBP-801 and a PI3K
inhibitor LY294002 synergistically induce apoptosis via the
suppression of survivin and XIAP in renal cell carcinoma. Int J
Oncol. 43:1080–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao SL, Cui TT, Zhao W, Zhang WW, Xie ZL,
Wang CH, Jia HS and Liu Q: RNAi-based knockdown of multidrug
resistance-associated protein 1 is sufficient to reverse multidrug
resistance of human lung cells. Asian Pac J Cancer Prev.
15:10597–105601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo SY, Zhu XD, Ge LY, Qu S, Li L, Su F
and Guo Y: RNAi-mediated knockdown of the c-jun gene sensitizes
radioresistant human nasopharyngeal carcinoma cell line CNE-2R to
radiation. Oncol Rep. 33:1155–1160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patrick J: Wightman, George R. Jackson and
Katrina M. Dipple: Disruption of glycerol metabolism by RNAi
targeting of genes encoding glycerol kinase results in a range of
phenotype severity in Drosophila. PLoS One. 8:e716642013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Li B, Wang X, Lin F, Gao P, Cheng
SY and Zhang HZ: Inhibiting XIAP expression by RNAi to inhibit
proliferation and enhance radiosensitivity in laryngeal cancer cell
line. Auris Nasus Larynx. 36:332–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao LP, Song JL, Yi XP and Li YX: Double
inhibition of NF-κB and XIAP via RNAi enhances the sensitivity of
pancreatic cancer cells to gemcitabine. Oncol Rep. 29:1659–1665.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomita Y, Bilim V, Kawasaki T, Takahashi
K, Okan I, Magnusson KP and Wiman KG: Frequent expression of Bcl-2
in renal-cell carcinomas carrying wild-type p53. Int J Cancer.
66:322–325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bilim V, Yuuki K, Itoi T, Muto A, Kato T,
Nagaoka A, Motoyama T and Tomita Y: Double inhibition of XIAP and
Bcl-2 axis is beneficial for retrieving sensitivity of renal cell
cancer to apoptosis. Br J Cancer. 98:941–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilim V, Yuuki K, Itoi T, Muto A, Kato T,
Nagaoka A, Motoyama T and Tomita Y: Double inhibition of XIAP and
Bcl-2 axis is beneficial for retrieving sensitivity of renal cell
cancer to apoptosis. Br J Cancer. 98:941–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sioud M: siRNA and miRNA Gene Silencing:
From Bench to Bedside. Humana Press; New York, NY: 2009, View Article : Google Scholar
|
|
24
|
Vorburger SA, Pataer A, Swisher SG and
Hunt KK: Gene therapy for cancer. Humana Press; Totowa, NJ:
2007
|
|
25
|
Potten CS, Wilson JW and Booth C:
Apoptosis genes. Kluwer Academic; Boston: 1998
|
|
26
|
Arroyo JA, Li C, Schlabritz-Loutsevitch N,
McDonald T, Nathanielsz P and Galan HL: Increased placental XIAP
and caspase 3 is associated with increased placental apoptosis in a
baboon model of maternal nutrient reduction. Am J Obstet Gynecol.
203(364): e13–8. 2010.
|
|
27
|
Hörnle M, Peters N, Thayaparasingham B,
Vörsmann H, Kashkar H and Kulms D: Caspase-3 cleaves XIAP in a
positive feedback loop to sensitize melanoma cells to TRAIL-induced
apoptosis. Oncogene. 30:575–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Zhou ZG, Zhou B, Wang R, Yan H and
Li Y: Downregulation of GRP78 and XIAP is correlated with apoptosis
during cerulein-induced acute pancreatitis in rats via regulation
of caspase activation. Mol Med Rep. 7:725–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng YJ, Jiang HS, Hsu SL, Lin LC, Wu CL,
Ghanta VK and Hsueh CM: XIAP-mediated protection of H460 lung
cancer cells against cisplatin. Eur J Pharmacol. 627:75–84. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danquah M, Duke CB III, Patil R, Miller DD
and Mahato RI: Combination therapy of antiandrogen and XIAP
inhibitor for treating advanced prostate cancer. Pharm Res.
29:2079–2091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Moraes Nestal G, Vasconcelos FC, Delbue
D, Mognol GP, Sternberg C, Viola JP and Maia RC: Doxorubicin
induces cell death in breast cancer cells regardless of Survivin
and XIAP expression levels. Eur J Cell Biol. 92:247–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castells M, Milhas D, Gandy C, Thibault B,
Rafii A, Delord JP and Couderc B: Microenvironment mesenchymal
cells protect ovarian cancer cell lines from apoptosis by
inhibiting XIAP inactivation. Cell Death Dis. 4:e8872013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zai HY, Yi XP, Li YX, You XY, Cao LP and
Liu H: X-linked inhibitor of apoptosis protein (XIAP) and Survivin
suppression on human pancreatic cancer cells Panc-1 proliferation
and chemosensitivety. Beijing da xue xue Bao. 45:242–249.
2013.PubMed/NCBI
|
|
34
|
Ning ZR, Li S, Guo YW and Fang DJ:
Expression and clinical significance of Cox-2 and XIAP in malignant
tumors of the salivary gland. Shanghai Kou Qiang Yi Xue.
23:317–321. 2014.PubMed/NCBI
|
|
35
|
Spahn A, Blondeau N, Heurteaux C, Dehghani
F and Rami A: Concomitant transitory up-regulation of X-linked
inhibitor of apoptosis protein (XIAP) and the heterogeneous nuclear
ribonucleoprotein C1-C2 in surviving cells during neuronal
apoptosis. Neurochem Res. 33:1859–1868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holt SV, Brookes KE, Dive C and Makin GW:
Down-regulation of XIAP by AEG35156 in paediatric tumour cells
induces apoptosis and sensitises cells to cytotoxic agents. Oncol
Rep. 25:1177–1181. 2011.PubMed/NCBI
|
|
37
|
Yan Y, Mahotka C, Heikaus S, Shibata T,
Wethkamp N, Liebmann J, Suschek CV, Guo Y, Gabbert HE, Gerharz CD
and Ramp U: Disturbed balance of expression between XIAP and
Smac/DIABLO during tumour progression in renal cell carcinomas. Br
J Cancer. 91:1349–1357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
39
|
Ramp U, Caliskan E, Mahotka C, Krieg A,
Heikaus S, Gabbert HE and Gerharz CD: Apoptosis induction in renal
cell carcinoma by TRAIL and gamma-radiation is impaired by
deficient caspase-9 cleavage. Br J Cancer. 88:1800–1807. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ng CP, Zisman A and Bonavida B: Synergy is
achieved by complementation with Apo2L/TRAIL and actinomycin D in
Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: Role of
XIAP in resistance. Prostate. 53:286–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Flanagan L, Sebastia J, Delgado ME, Lennon
JC and Rehm M: Dimerization of Smac is crucial for its
mitochondrial retention by XIAP subsequent to mitochondrial outer
membrane permeabilization. Biochim Biophys Acta. 1813:819–826.
2011. View Article : Google Scholar : PubMed/NCBI
|