Introduction
Acute myocardial infarction (AMI) is a disease,
which seriously endangers human health. A large number of
myocardial cells in the infarcted region are lost due to ischemia
and hypoxia (1). As the numbers of
myocardial cells decrease, cardiac function is subjected to a
progressive decrease in the ventricular remodeling process. This is
due to the necrotic cardiac muscle tissue being unable to recover,
and being substituted only by collapsed scar tissue without
contraction function (2). At
present, the therapeutic methods for AMI mainly include drug
therapy, interventional therapy and surgery, which can only improve
the damaged myocardium and reduced myocardial function following
ischemia, even with recovery in blood flow (3). However, these types of therapy do not
recover the functions of necrotic myocardium. With the process of
ventricular remodeling, this eventually leads to heart failure and
a decrease in patient quality of life (4). The clinical symptoms and prognosis of
patients can be improved by the use of drugs, a ventricular assist
device and heart transplantation. However, studies have shown that,
following a diagnosis of congestive heart failure, 20% of patients
succumb to mortality within 1 year (1).
AMI is a pathological process accompanied by an
inflammatory response. Inflammatory cytokines are important in
ventricular remodeling following myocardial infarction (5). At the early stage of infarction, the
local inflammatory reaction is prominent and, with high levels of
inflammatory cell infiltration, inflammatory cells and inflammatory
cytokine can adversely affect cell-transplant therapy (6).
Oxidative stress refers to the pathological process
of excessive oxygen and/or a reduction in antioxidation ability,
and disturbance in the balance between the oxidation system and
antioxidant system generated by reactive oxygen leads to latent
injury (7). In previous years, a
number of studies have shown that oxidative stress is an essential
mechanism for the genesis and development of cardiovascular disease
(8).
Peroxisome proliferator-activated receptor (PPAR) is
a ligand-activated transcription factor. It belongs to the nuclear
receptor super-family (9). PPARs
are divided into three sub-types, namely PPARα, PPARβ and PPAR-γ
(10). Studies have shown that
PPARs can inhibit the inflammatory response in vitro and
in vivo (10,11). PPAR-γ is expressed in endothelial
cells of the aorta and carotid artery, and in umbilical vein
endothelial cells. Its ligand can inhibit the expression of
intercellular adhesion molecule 1 (ICAM-1) induced by cytokines
(11). PPAR-γ is also expressed in
human vascular endothelial cells, vascular smooth muscle cells and
mononuclear macrophages. PPAR-γ ligands can prevent the
accumulation of inflammatory cells through inhibiting the
expression of interleukin (IL)-8, monocyte chemoattractant protein
1 (MCP-1) and ICAM-1 (9,12).
Studies have shown that the content of apoptotic
factors in infarcted mice myocardial cells can be inhibited through
drug pretreatment, including the expression of P53, B-cell lymphoma
2 (Bcl-2)-associated X protein (Bax) and Fas (13,14).
Results have shown that, in cases showing an effective increase in
the expression of Bcl-2, cell apoptosis was markedly decreased.
Cytochrome c forms apoptotic bodies following release, and
has effects on caspase-9 protein composition (15). Cell apoptosis is eventually induced
through activating downstream caspase-3 and other cells (15). This indicates that decreasing the
activity or reducing the expression levels of caspase-3 and
caspase-9 protease can effectively inhibit myocardial cell
apoptosis (16). The Bcl-2 family
can regulate the release of cytochrome c in the
mitochondria. Therefore, the activation of apoptotic cytochrome
c can be inhibited to inhibit the genesis of cell apoptosis
(15).
Hesperetin is the main pharmacological ingredient of
the fruit of the citrus family, rutaceae. It is flavanone, which is
originates mainly from the hydrolysate of hesperidin. The glucoside
can generate hesperetin from hydrolyzation, and function in the
human intestinal flora. It is enriched in pericarpium citri
reticulatae viride, pericarpium citri reticulatae and immature
bitter orange. Its pharmacologic actions include stomach, phlegm
removal, cough and cold relief, diuretic, antiviral and antibiotic
effects and stomach pain relief. Studies have shown hesperetin has
potent effects against oxidative stress, inflammation and allergy.
The present study investigated the preventive effect of hesperidin
in the modulation of AMI-induced inflammatory responses and
antioxidant status.
Materials and methods
Animals and treatment
Experiments were performed in accordance with the
animal ethics guidelines of the Institutional Animal Ethics
Committee of Jinan University 2nd Clinical Medicine College
(Shenzhen, China). C57BL/6 male mice (18–20 g; 5–6 weeks old) were
purchased from Shenzhen Advanced Animal Study Service Center
(Shenzhen, China) and housed in sterilized polypropylene rat cages,
under a 12/12-h light/dark cycle, at an ambient temperature of
22–23°C and ambient humidity of 55–60%, under
specific-pathogen-free conditions.
The mice were randomly divided into four equal
groups, each containing eight mice: Control group, AMI group, 50
mg/kg/d histamine group and 100 mg/kg/d histamine group. Anesthesia
was performed by inhalation of 1.0–2.0% isoflurane gas. A left
thoracotomy was performed to expose the heart, and myocardial
infarction was induced and ligated using an 8–0 silk suture at the
left anterior descending coronary artery. Following closure of the
chest wall, the mice were extubated. The mice were administered
orally with PBS, 50 or 100 mg/kg of histamine for 2 weeks in the
AMI group, 50 mg/kg/d histamine group and 100 mg/kg/d histamine
group, respectively. Following treatment with histamine, the
animals were sacrificed under 1.0–2.0% isoflurane gas. The body
weights and heart weights were recorded, and were stored at −80°C
until analysis.
Measurement of infarct size
Following histamine administration and sacrifice of
the animals under 1.0–2.0% isoflurane gas, the hearts were removed
and weighed, and sliced into 4.0-mm thick sections perpendicularly.
The sections were then incubated with 1% triphenyltetrazolium
chloride (Sigma; Merck Millipore; Darmstadt, Germany) in phosphate
solution for 10 min at 37°C. Infarct size was determined by
computer morphometry using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Biochemical assay
Following histamine treatment, serum samples from
the mice were collected after centrifugation (2,000 × g for 10 min
at 4°C) to determine the levels of creatine kinase (CK)-MB, tumor
necrosis factor (TNF-α), IL-1β, IL-6, MCP-1, ICAM-1,
malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD)
and caspase-3/9 using ELISA kits.
Western blot analysis
Following histamine treatment, the hearts were
removed and homogenized in cytoplasmic and protease inhibitors
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The lysates
were centrifuged at 12,000 × g for 10 min at 4°C to analyze protein
concentrations using a BCA assay (Beyotime Institute of
Biotechnology, Haimen, China). Total protein (50 µg) was resolved
by SDS-PAGE (6–10%) and the samples were blocked with 5% non-milk
fat in TBST for 1 h at 37°C and immunoblotted with p53 (catalog no.
2524; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
Bax (catalog no. 14796; 1:2,000, Cell Signaling Technology, Inc.),
Bcl-2 (catalog no. 3498; 1:2,000, Cell Signaling Technology, Inc.),
PPAR-γ (catalog no. 2435; 1:2,000; Cell Signaling Technology, Inc.)
and GAPDH (cat. no. AF0006; 1:2,000; Beyotime Institute of
Biotechnology) following transfer onto polyvinylidene difluoride
membranes at 4°C overnight. Following washing, the membranes were
incubated with secondary antibodies conjugated to horseradish
peroxidase (cat. no. A0208; 1:5,000; Beyotime Institute of
Biotechnology) for 1 h at 37°C, and the bands were developed with
ECL Plus Western blot detection reagents (GE Healthcare Life
Sciences, Chalfont, UK).
Statistical analysis
All values are presented as the mean ± standard
deviation using SPSS software version 17.0 (SPSS, Inc., Chicago,
IL, USA). Comparisons between two groups were assessed using
Student's t test or two-way analysis of variance followed by
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preventive effect of hesperidin on
infarct size of AMI mice
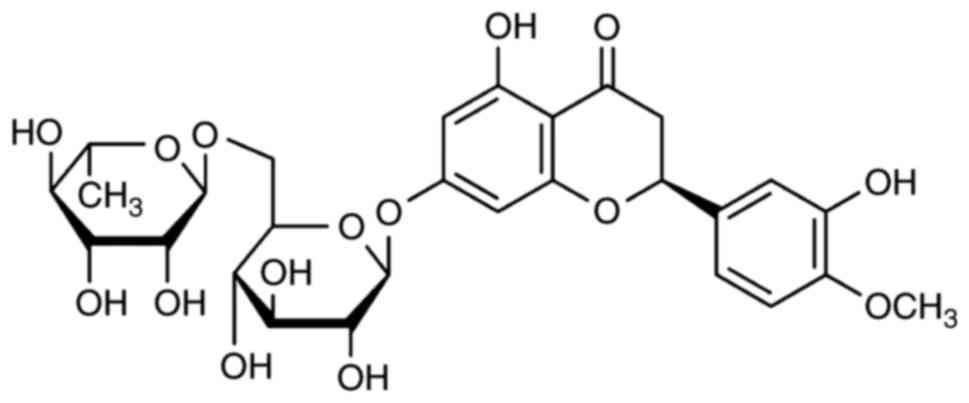
The constitutional formula of hesperidin is shown in
Fig. 1. The present study
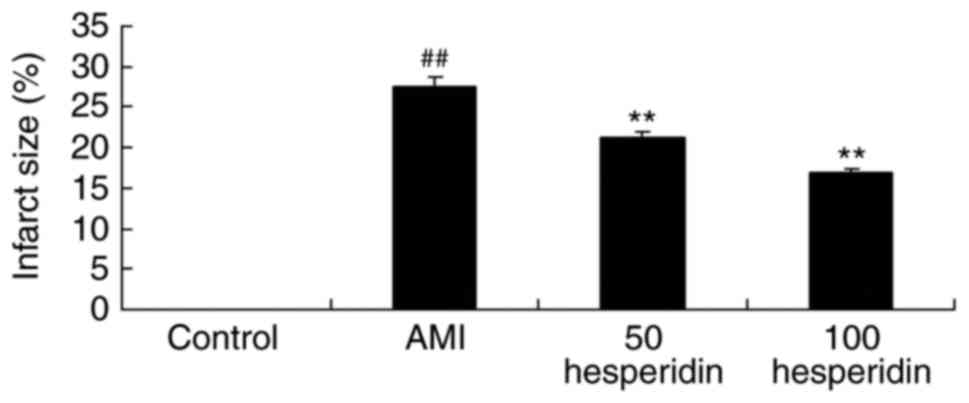
investigated the preventive effect of hesperidin on infarct size in
AMI mice. A significant increase in infarct size was found in the
AMI mice, compared with that in the control group (Fig. 2). Treatment with 50 and 100 mg/kg
hesperidin significantly inhibited infarct size in the AMI mice,
compared with AMI model group (Fig.
2).
Preventive effect of hesperidin on
heart weight/body weight ratio and activity of creatine kinase
(CK-MB) in AMI mice
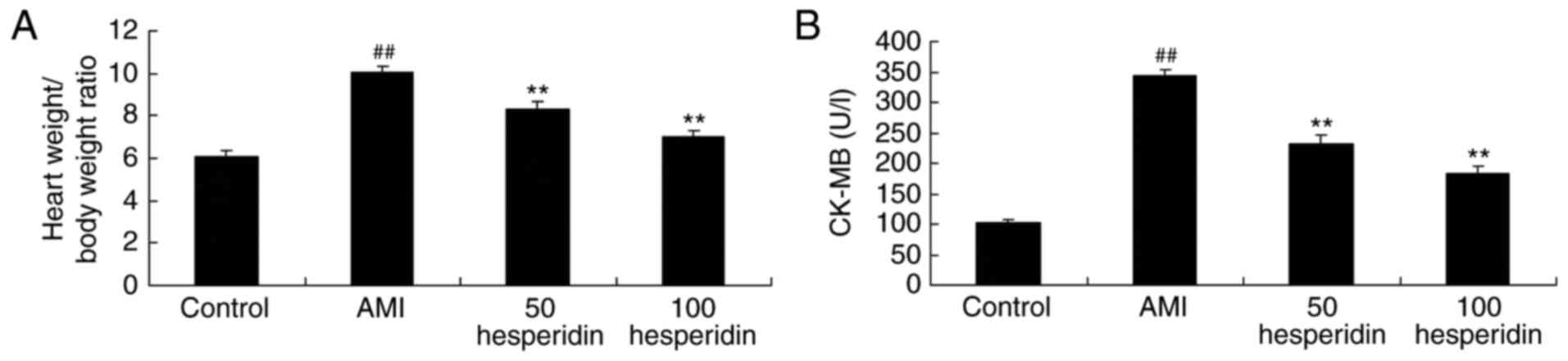
The present study analyzed whether the preventive
effect of hesperidin affected the heart weight/body weight ratio
and activity of CK-MB in AMI mice. Compared with the control group,
there were significant increases in the heart weight/body weight
ratio and activity of CK-MB in the AMI mice (Fig. 3A and B). However, treatment with 50
and 100 mg/kg hesperidin significantly inhibited the heart
weight/body weight ratio and the activity of CK-MB in the AMI mice,
compared with the AMI model group (Fig. 3A and B).
Preventive effect of hesperidin on
inflammatory responses in AMI mice
The present study examined the anti-inflammatory
effect of hesperidin in AMI mice. The activities of TNF-α, IL-1β
and IL-6 were analyzed using ELISA kits. In the AMI mice,
significant increases in the activities of TNF-α, IL-1β and IL-6
were found, compared with those in the control group (Fig. 4A-C). The increases in the
activities of TNF-α, IL-1β and IL-6 were significantly prevented by
treatment with 50 and 100 mg/kg hesperidin in the AMI mice,
compared with activities in the AMI model group (Fig. 4A-C).
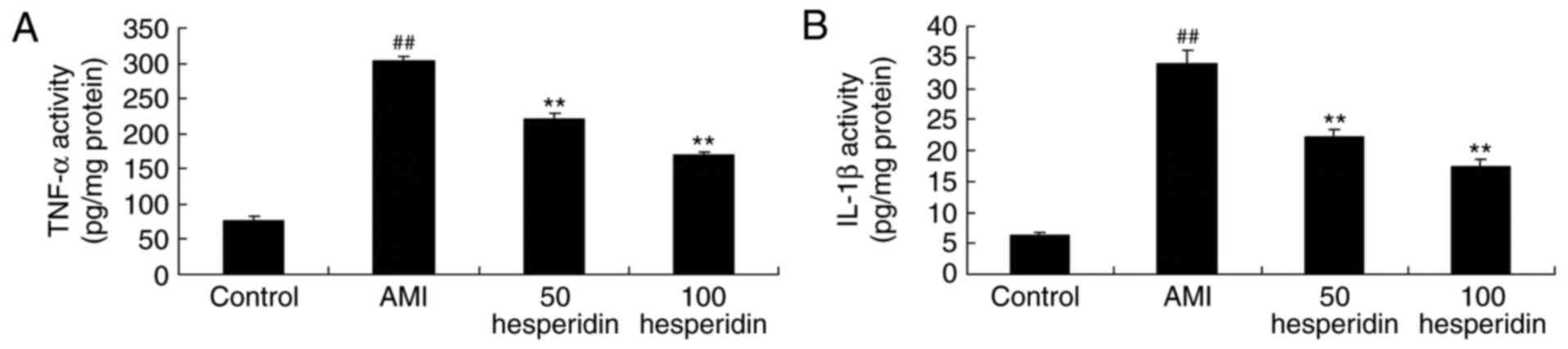 | Figure 4.Preventive effect of hesperidin on
inflammatory responses of AMI mice. Preventive effect of hesperidin
on the activities of (A) TNF-α, (B) IL-1β and (C) IL-6 in AMI mice.
##P<0.01, compared with control group; **P<0.01,
compared with AMI model mice group. AMI, acute myocardial
infarction; Control, control group; 50 hesperidin, 50 mg/kg
hesperidin-treated group; 100 hesperidin, 100 mg/kg
hesperidin-treated group; TNF-α, tumor necrosis factor-α, IL,
interleukin. |
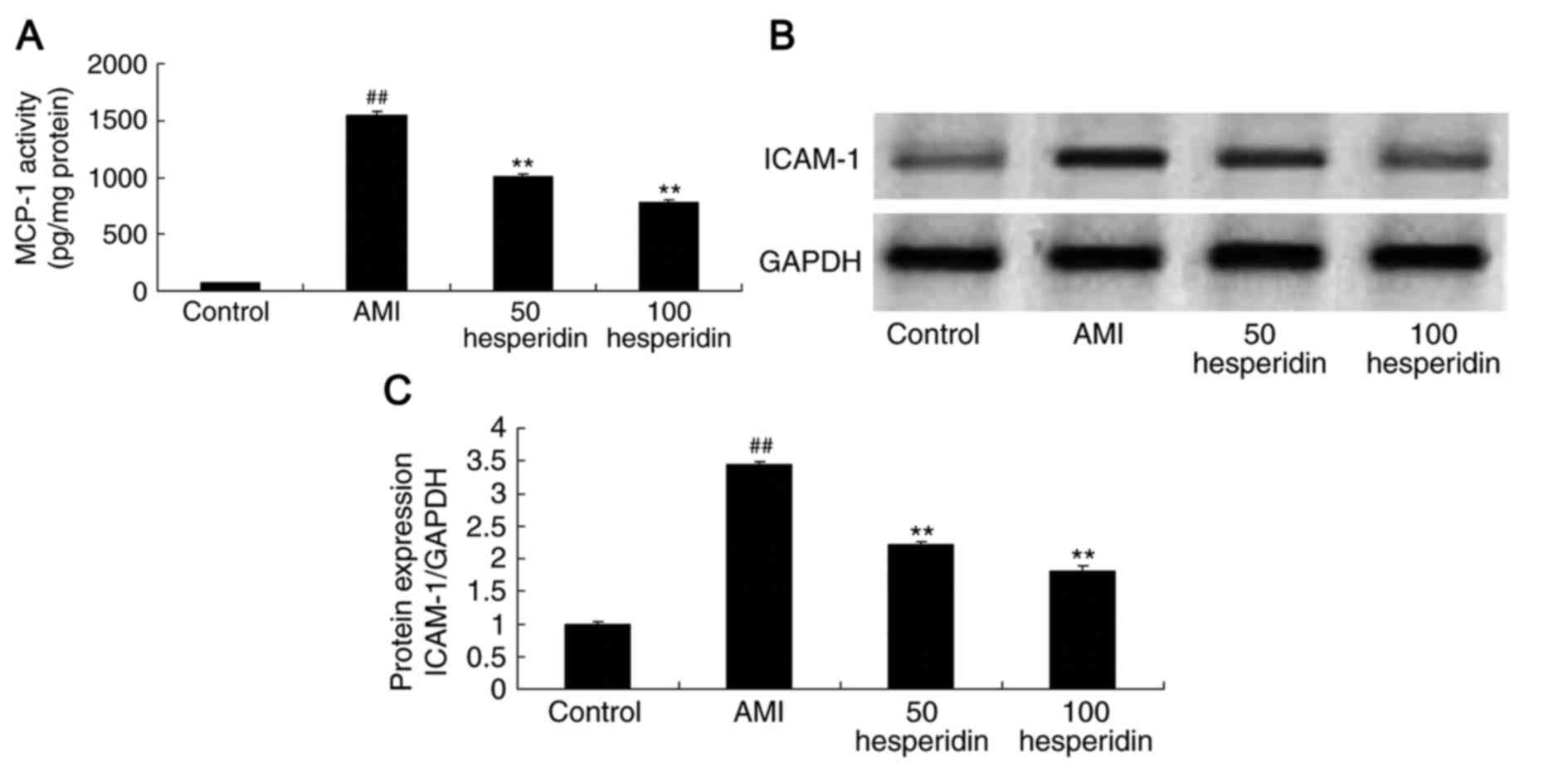
Preventive effect of hesperidin on the
expression of MCP-1 and ICAM-1 in AMI mice
In order to identify the anti-inflammatory effect of
hesperidin in AMI mice, the activity of MCP-1 and protein
expression of ICAM-1 were analyzed. Similar to the other findings
in the mice, the activity of MCP-1 and the protein expression of
ICAM-1 were higher in the AMI mice, compared with those in the
control group (Fig. 5A-C).
Treatments with 50 and 100 mg/kg hesperidin significantly
suppressed the MCP-1 level and protein expression of ICAM-1 in the
AMI mice, compared with those in the AMI model group (Fig. 5A-C).
Preventive effect of hesperidin on the
antioxidant status of AMI mice
To investigate the antioxidant effect of hesperidin
on AMI mice, the activities of MDA, SOD and CAT were analyzed using
ELISA assays. The activity of MDA was increased, and the activities
of SOD and CAT were reduced in the AMI mice, compared with those in
the control group (Fig. 6A-C).
Treatment with 50 and 100 mg/kg hesperidin significantly inhibited
the activity of MDA, and promoted the activities of SOD and CAT in
AMI mice, compared with the AMI model group (Fig. 6A-C).
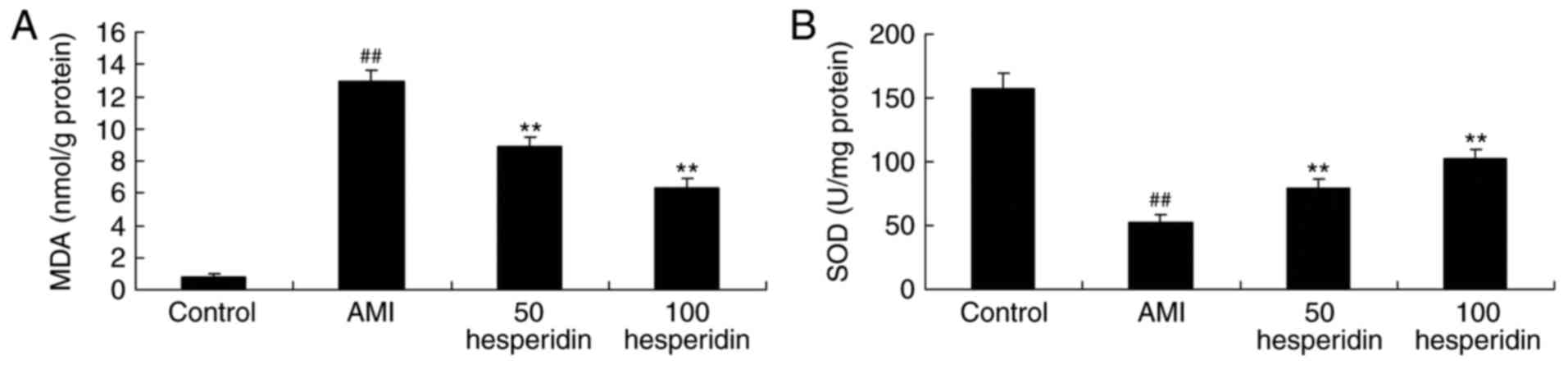 | Figure 6.Preventive effect of hesperidin on
the antioxidant status of AMI mice. Preventive effect of hesperidin
on activities of (A) MDA, (B) SOD and (C) CAT in AMI mice.
##P<0.01, compared with control group; **P<0.01,
compared with AMI model mice group. AMI, acute myocardial
infarction; Control, control group; 50 hesperidin, 50 mg/kg
hesperidin-treated group; 100 hesperidin, 100 mg/kg
hesperidin-treated group; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, catalase. |
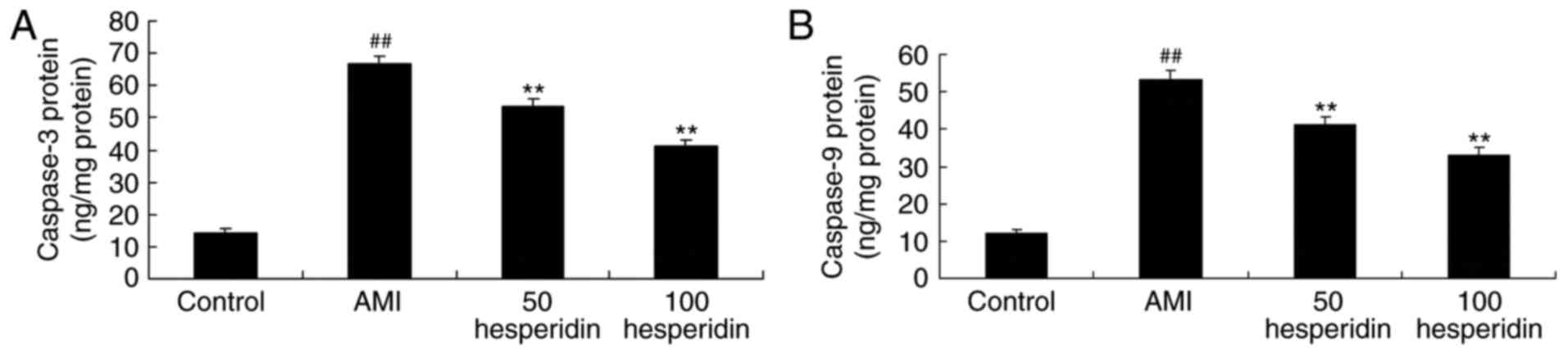
Preventive effect of hesperidin on
caspase-3 and caspase-9 of AMI mice
To evaluate the preventive effect of hesperidin on
cardiomyocyte apoptosis in AMI mice, the activities of caspase-3
and caspase-9 were measured using an ELISA assay. The results of
the ELISA assay revealed that the activities of caspase-3 and
caspase-9 in the AMI mice were higher, compared with those in the
control group mice (Fig. 7).
Treatment with 50 and 100 mg/kg hesperidin significantly reduced
the activities of caspase-3 and caspase-9 in the AMI mice, compared
with those in the AMI model group mice (Fig. 7).
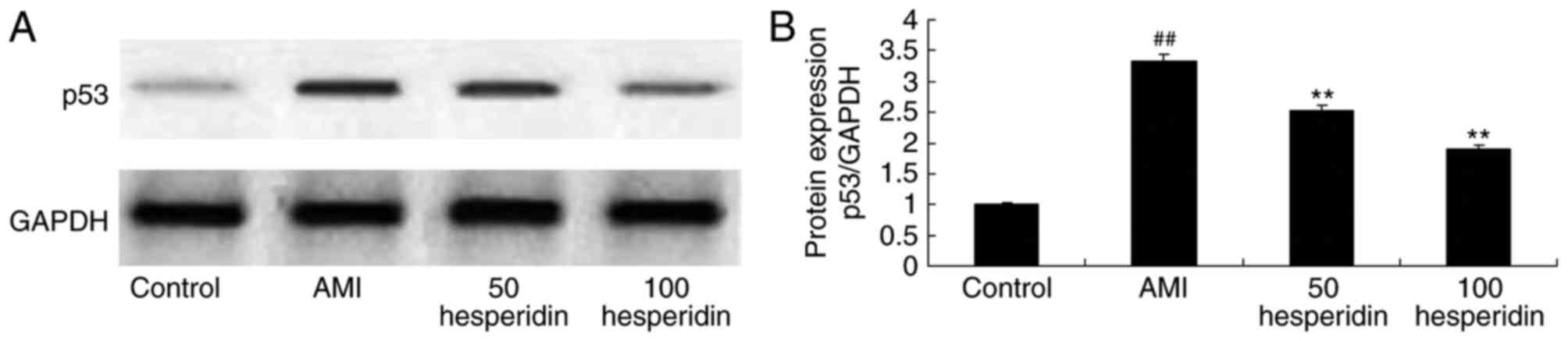
Preventive effect of hesperidin on the
protein expression of p53 in AMI mice
To clarify the preventive effect of hesperidin on
the mechanism of cardiomyocyte apoptosis in AMI mice, the protein
expression of p53 was measured using western blot analysis. A
significant increase in the protein expression of p53 was observed
in the AMI mice, compared with that in the control group mice
(Fig. 8A and B). Treatment with
hesperidin (50 and 100 mg/kg) significantly suppressed the protein
expression of p53 in the AMI mice, compared with that in the AMI
model group mice (Fig. 8A and
B).
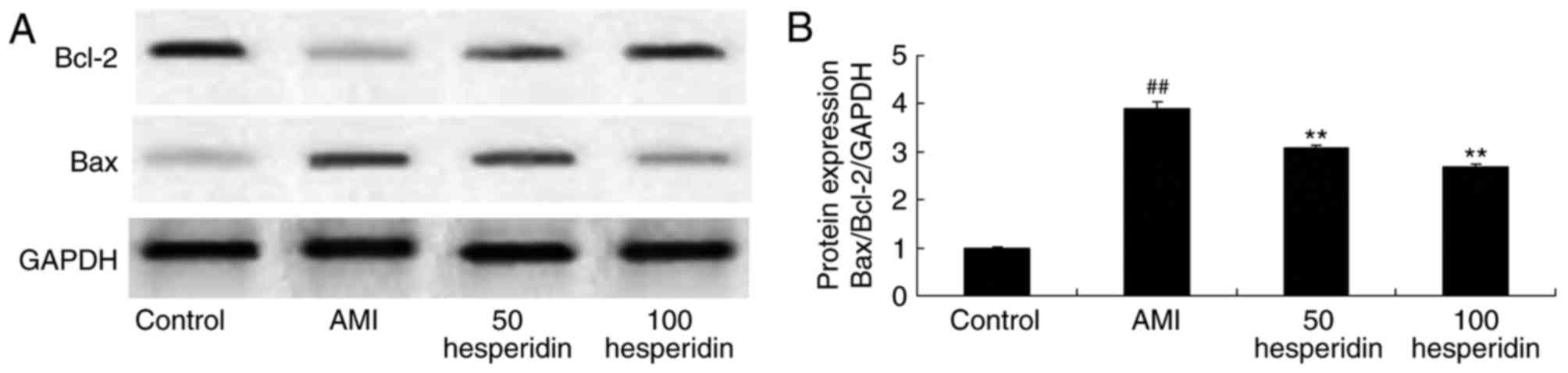
Preventive effect of hesperidin on the
protein expression of Bax/Bcl-2 in AMI mice
To further clarify the preventive effect of
hesperidin on the mechanism of cardiomyocyte apoptosis of AMI mice,
the protein expression of Bax/Bcl-2 in AMI mice was measured using
a western blot analysis. The protein expression of Bax/Bcl-2 in the
AMI mice was significantly increased, compared with that in the
control group. Administration with 50 and 100 mg/kg of hesperidin
significantly inhibited the protein expression of Bax/Bcl-2 in the
AMI mice, compared with that in the AMI model group mice (Fig. 9A and B).
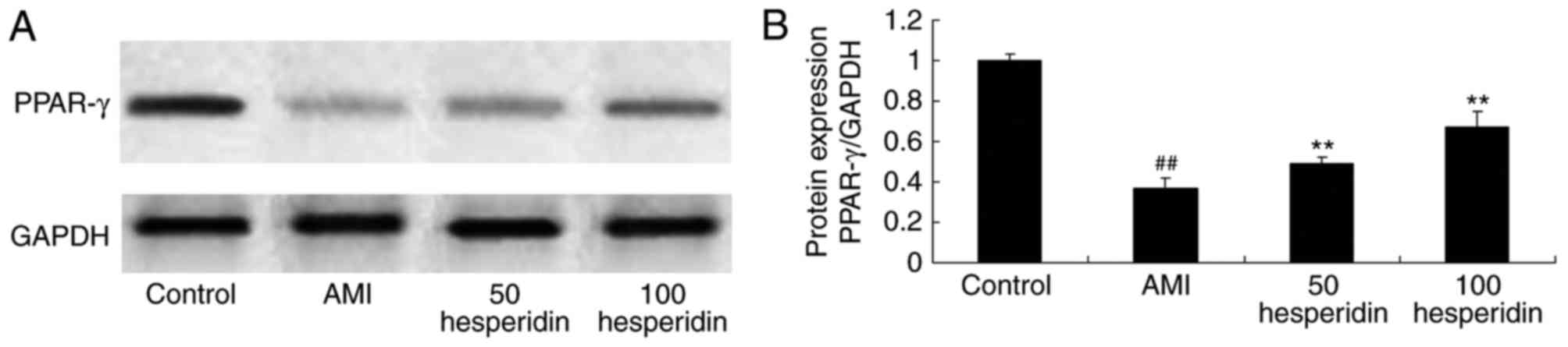
Preventive effect of hesperidin on the
protein expression of PPAR-γ in AMI mice
To examine the preventive effect of hesperidin in
AMI, the protein expression of PPAR-γ was analyzed in AMI mice
treated with hesperidin. The protein expression of PPAR-γ was
significantly suppressed in the AMI mice, compared with the control
group mice (Fig. 10A and B).
Treatment with 50 and 100 mg/kg of hesperidin significantly
increased the protein expression of PPAR-γ in the AMI mice,
compared with that in the AMI model group mice (Fig. 10A and B).
Discussion
In the majority of developed countries, AMI is the
primary contributor to human mortality rates. AMI therapy,
including early reperfusion, reduces the mortality rates of
patients with AMI substantially (17). However, left ventricular remodeling
and heart failure have become important health concerns. At
present, the therapeutic methods for cardiac remodeling following
myocardial infarction are limited (18). Consequently, the identification of
key targets and therapeutic methods for poor left ventricular
remodeling has become a priority. The results from the present
study showed that hesperidin significantly inhibited the infarct
size, heart weight/body weight ratio and activity of CK-MB in AMI
mice.
The inflammatory reaction is a complex dynamic
pathological process caused by tissue damage and infection
(19). Typical inflammatory
responses can lead to vasodilation, increased blood flow, increased
vascular permeability through the release of a series of
inflammatory factors (20). AMI is
the process of myocardial ischemic necrosis caused by acute
coronary artery occlusion, and is accompanied by tissue necrosis
and inflammatory reaction (21).
Studies have shown that several inflammatory mediums are involved
in the pathological process of AMI, including increases in TNF-α,
IL-1β, IL6, prostaglandin and leukotrienes (20,22).
In acute inflammatory reactions, multinuclear leukocyte
accumulation and infiltration, and increased reactive protein
generation are observed (21).
However, severe inflammation caused by AMI is detrimental to the
survival of transplanted cells. The release of a large quantity of
acute inflammatory medium aggravates ischemia reperfusion injury of
the myocardium, and affects the survival and function of the
transplanted cells (23). The
results of the present study indicated that 50 and 100 mg/kg
hesperidin significantly prevented the increases in the activities
of TNF-α, IL-1β and IL-6, activity of MCP-1 and protein expression
of ICAM-1 in the AMI mice. Rotimi et al reported that
hesperidin also prevents lipopolysaccharide-induced oxidative
stress and inflammation in rats (24).
Oxidative stress refers to damage of the
oxidant/antioxidant equilibrium. Oxidative stress is a common
characteristic of diabetes and hypertension. During the process of
ischemia reperfusion injury, H2S can inhibit the
generation of myocardial cell mitochondrial cytochrome c
oxidase, reduce the generation of O2−, and
protect the structure and functions of mitochondria (25). Oxidative stress, intracellular
ionized calcium overload and mitochondrial dysfunction are
essential factors leading to myocardial cell apoptosis (12). Oxidative stress is a factor, which
has been of particular concern. In myocardial infarction and other
pathological states, the generation of oxygen ions under a
reduction state, namely reactive oxygen species (ROS), exceeds the
unsteady state of cell endogenous detoxification and/or utilization
capability (7). The excess ROS in
the cells lead to the injury-induced modification of important
macromolecules in the cell, lipid peroxidation leads to changes in
membrane structure and function, oxidation of proteins mercapto and
amidogen lead to a loss of activity of important enzymes, and DNA
injury leads to cell mutation (7,8). The
present study demonstrated that treatment with 50 and 100 mg/kg
hesperidin significantly inhibited the activity of MDA, and
increased the activities of SOD and CAT in AMI mice. Zhang et
al reported that hesperidin also inhibited oxidative stress in
isoniazid- and rifampicin-induced liver injury in rats (26).
Cell apoptosis is an essential biological activity
(27). It is an organic component
essential for human and animal survival (27), and is a normal physiological
process. If the transmission signal pathway regulating cell
apoptosis is seriously damaged, it can lead to a series of human
diseases, including cancer, and various types of infectious
diseases (28). Bcl-2 family
members may directly induce cell apoptosis, thus being essential
membrane protein molecules for cell apoptosis (28). In addition, cell apoptosis can be
controlled through different intracellular and extracellular
signals. It has been detected that certain genes can promote
apoptotic cytokines, including Bax, P53 and Fas, and certain genes
can inhibit apoptotic cytokines (14). The initiation and inhibition of
apoptotic proteins is decisive of the cell apoptotic ratio in the
body (13). If the protein
expression of Bcl-2 is higher than that of Bax, it is conducive to
the promoting cell survival. By contrast, it can accelerate cell
apoptosis (13). Siddiqi et
al suggested that hesperidin ameliorates
trichloroethylene-induced nephrotoxicity through altering the
expression of caspase-3, bax and bcl-2 in Wistar rats (29). The present study indicated that
hesperidin significantly prevented the activities of caspase-3 and
caspase-9, and suppressed the protein expression of p53 and
Bax/Bcl-2 in AMI mice. Justin Thenmozhi et al demonstrated
that hesperidin also ameliorates oxidative stress and apoptosis in
Alzheimer's disease in rats (30).
PPAR-γ is a ligand-activated transcription factor,
which can regulate gene expression through the function of specific
DNA reaction components (31). Its
functions include transforming various homeostatic changes,
medicinal or nutritive components, and various inflammatory stimuli
into intracellular signals (31).
It is an important messenger in regulating energy metabolism, cell
differentiation, proliferation, apoptotic and inflammatory
reactions, endogenous synthesis and the secretion of active
materials (32). It was previously
suggested that PPAR-γ only existed in the differentiation of
adipose cells, however, it has since been reported that PPAR-γ
exists extensively on a variety of cells, including myocardial
cells, myocardial fibroblasts, mononuclear/macrophages and vascular
smooth muscle cells (9). In
addition to its involvement in adipose cell differentiation, PPAR-γ
also regulates gene transcription in the cardiovascular system, and
the gene regulates lipogenesis and expression of the diabetes
obesity gene (33). It is also
involved in the transcriptional regulation of protein-coding genes,
which are associated with a variety of physiological activities,
including inflammatory reactions, immunoregulation, cell cycle
regulation and tumor cell differentiation (33). PPAR-γ exists on a variety of
ligands and activators. Studies have shown that PPAR-γ is important
in resistance to ischemia-reperfusion injury, and is an important
regulatory factor for cell inflammation and ischemia (11). In the present study, hesperidin
significantly induced the protein expression of PPAR-γ in AMI mice.
Agrawal et al showed that hesperidin also exhibited
cardioprotective effects in an ischemic heart disease model in
diabetic rats through the PPAR-γ pathway (34).
In conclusion, the present study confirmed that the
preventive effect of hesperidin modulated AMI, inflammatory
responses and antioxidant status in AMI mice through the expression
of PPAR-γ and Bcl-2. In this context, hesperidin may be clinically
useful for the treatment of AMI.
Acknowledgements
This study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 9151802001000011).
References
|
1
|
Spatz ES, Beckman AL, Wang Y, Desai NR and
Krumholz HM: Geographic variation in trends and disparities in
acute myocardial infarction hospitalization and mortality by income
levels, 1999–2013. JAMA Cardiol. 1:255–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reinstadler SJ, Baum A, Rommel KP, Eitel
C, Desch S, Mende M, Metzler B, Poess J, Thiele H and Eitel I:
ST-segment depression resolution predicts infarct size and
reperfusion injury in ST-elevation myocardial infarction. Heart.
101:1819–1825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellaton C, Cayla G, Silvain J, Zeymer U,
Cohen M, Goldstein P, Huber K, Pollack C Jr, Kerneis M, Collet JP,
et al: Incidence and consequence of major bleeding in primary
percutaneous intervention for ST-elevation myocardial infarction in
the era of radial access: An analysis of the international
randomized acute myocardial infarction treated with primary
angioplasty and intravenous enoxaparin or unfractionated heparin to
lower ischemic and bleeding events at short- and long-term
follow-up trial. Am Heart J. 170:778–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemkes J, Nijveldt R, Beek AM, Knaapen P,
Hirsch A, Meijers J, Allaart CP, van Rossum A and van Royen N:
Evaluating the optimal timing of revascularisation in patients with
transient ST-segment elevation myocardial infarction: Rationale and
design of the TRANSIENT trial. J Cardiovasc Transl Res. 7:590–596.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alestalo K, Miettinen JA, Vuolteenaho O,
Huikuri H and Lehenkari P: Bone marrow mononuclear cell
transplantation restores inflammatory balance of cytokines after ST
segment elevation myocardial infarction. PLoS One. 10:e01450942015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aydin MU, Aygul N, Altunkeser BB, Unlu A
and Taner A: Comparative effects of high-dose atorvastatin versus
moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and
inflammatory markers in ST elevation myocardial infarction.
Atherosclerosis. 239:439–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bashar T and Akhter N: Study on oxidative
stress and antioxidant level in patients of acute myocardial
infarction before and after regular treatment. Bangladesh Med Res
Counc Bull. 40:79–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitano D, Takayama T, Nagashima K, Akabane
M, Okubo K, Hiro T and Hirayama A: A comparative study of
time-specific oxidative stress after acute myocardial infarction in
patients with and without diabetes mellitus. BMC Cardiovasc Disord.
16:1022016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ibarra-Lara Mde L, Sánchez-Aguilar M,
Soria E, Torres-Narváez JC, Del Valle-Mondragón L, Cervantes-Pérez
LG, Pérez-Severiano F, Ramírez-Ortega Mdel C, Pastelín-Hernández G,
Oidor-Chan VH and Sánchez-Mendoza A: Peroxisome
proliferator-activated receptors (PPAR) downregulate the expression
of pro-inflammatory molecules in an experimental model of
myocardial infarction. Can J Physiol Pharmacol. 94:634–642. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lima Ede A, Lima MM, Marques CD, Duarte
AL, Pita Ida R and Pita MG: Peroxisome proliferator-activated
receptor agonists (PPARs): A promising prospect in the treatment of
psoriasis and psoriatic arthritis. An Bras Dermatol. 88:1029–1035.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lincoff AM, Tardif JC, Neal B, Nicholls
SJ, Rydén L, Schwartz GG, Malmberg K, Buse JB, Henry RR, Wedel H,
et al: Evaluation of the dual peroxisome proliferator-activated
receptor α/γ agonist aleglitazar to reduce cardiovascular events in
patients with acute coronary syndrome and type 2 diabetes mellitus:
Rationale and design of the AleCardio trial. Am Heart J.
166:429–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ata MA, Khand F, Shaikh SS and Ata MA:
Effect of smoking on serum xanthine oxidase and malondialdehyde
levels in patients with acute myocardial infarction. J Pak Med
Assoc. 65:39–42. 2015.PubMed/NCBI
|
|
13
|
Radhiga T, Rajamanickam C, Sundaresan A,
Ezhumalai M and Pugalendi KV: Effect of ursolic acid treatment on
apoptosis and DNA damage in isoproterenol-induced myocardial
infarction. Biochimie. 94:1135–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarr KU, Eschricht S, Burkly LC, Preusch
M, Katus HA, Frey N and Chorianopoulos E: TNF-like weak inducer of
apoptosis aggravates left ventricular dysfunction after myocardial
infarction in mice. Mediators Inflamm. 2014:1319502014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Wang Z, Feng SJ, Xu L, Shi HX,
Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, et al: PEDF improves
cardiac function in rats with acute myocardial infarction via
inhibiting vascular permeability and cardiomyocyte apoptosis. Int J
Mol Sci. 16:5618–5634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ning Y, Li Z and Qiu Z: FOXO1 silence
aggravates oxidative stress-promoted apoptosis in cardiomyocytes by
reducing autophagy. J Toxicol Sci. 40:637–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hofma SH, Smits PC, Brouwer J, Velders MA,
van't Hof AW, Queré M, de Vries CJ and van Boven AJ: Long-term
follow-up of second-generation everolimus-eluting stents versus
first-generation sirolimus-eluting stents in acute myocardial
infarction: Three-year results of the XAMI trial. EuroIntervention.
10:1280–1283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magro M, Raber L, Heg D, Taniwaki M,
Kelbaek H, Ostojić M, Baumbach A, Tüller D, von Birgelen C, Roffi
M, et al: The MI SYNTAX score for risk stratification in patients
undergoing primary percutaneous coronary intervention for treatment
of acute myocardial infarction: A substudy of the COMFORTABLE AMI
trial. Int J Cardiol. 175:314–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padilla S, Masiá M, García N, Jarrin I,
Tormo C and Gutiérrez F: Early changes in inflammatory and
pro-thrombotic biomarkers in patients initiating antiretroviral
therapy with abacavir or tenofovir. BMC Infect Dis. 11:402011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ehrenpreis ED, Zhou Y, Alexoff A and
Melitas C: Effect of the diagnosis of inflammatory bowel disease on
risk-adjusted mortality in hospitalized patients with acute
myocardial infarction, congestive heart failure and pneumonia. PLoS
One. 11:e01589262016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toldo S, Marchetti C, Mauro AG, Chojnacki
J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN and
Abbate A: Inhibition of the NLRP3 inflammasome limits the
inflammatory injury following myocardial ischemia-reperfusion in
the mouse. Int J Cardiol. 209:215–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prondzinsky R, Unverzagt S, Lemm H,
Wegener N, Heinroth K, Buerke U, Fiedler M, Thiery J, Haerting J,
Werdan K and Buerke M: Acute myocardial infarction and cardiogenic
shock: Prognostic impact of cytokines: INF-γ, TNF-α, MIP-1β, G-CSF,
and MCP-1β. Med Klin Intensivmed Notfmed. 107:476–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davies NM, Smith GD, Windmeijer F and
Martin RM: COX-2 selective nonsteroidal anti-inflammatory drugs and
risk of gastrointestinal tract complications and myocardial
infarction: An instrumental variable analysis. Epidemiology.
24:352–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rotimi SO, Bankole GE, Adelani IB and
Rotimi OA: Hesperidin prevents lipopolysaccharide-induced
endotoxicity in rats. Immunopharmacol Immunotoxicol. 38:364–371.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stamboul K, Lorin J, Lorgis L, Guenancia
C, Beer JC, Touzery C, Rochette L, Vergely C, Cottin Y and Zeller
M: Atrial fibrillation is associated with a marker of endothelial
function and oxidative stress in patients with acute myocardial
infarction. PLoS One. 10:e01314392015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Zhu J, Zhou Y, Wei Y, Xi L, Qin
H, Rao Z, Han M, Ma Y and Wu X: Hesperidin alleviates oxidative
stress and upregulates the multidrug resistance protein 2 in
isoniazid and rifampicin-induced liver injury in rats. J Biochem
Mol Toxicol. 30:342–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aonuma T, Takehara N, Maruyama K, Kabara
M, Matsuki M, Yamauchi A, Kawabe J and Hasebe N:
Apoptosis-resistant cardiac progenitor cells modified with
apurinic/apyrimidinic endonuclease/redox factor 1 gene
overexpression regulate cardiac repair after myocardial infarction.
Stem Cells Transl Med. 5:1067–1078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tseng CY, Wang JS, Chang YJ, Chang JF and
Chao MW: Exposure to high-dose diesel exhaust particles induces
intracellular oxidative stress and causes endothelial apoptosis in
cultured in vitro capillary tube cells. Cardiovasc Toxicol.
15:345–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddiqi A, Nafees S, Rashid S, Sultana S
and Saidullah B: Hesperidin ameliorates trichloroethylene-induced
nephrotoxicity by abrogation of oxidative stress and apoptosis in
wistar rats. Mol Cell Biochem. 406:9–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thenmozhi Justin A, William Raja TR,
Manivasagam T, Janakiraman U and Mohamed Essa M: Hesperidin
ameliorates cognitive dysfunction, oxidative stress and apoptosis
against aluminium chloride induced rat model of Alzheimer's
disease. Nutr Neurosci. 20:360–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Y, Zhang ZH, Wei SG, Weiss RM and
Felder RB: Peroxisome proliferator-activated receptor-γ regulates
inflammation and renin-angiotensin system activity in the
hypothalamic paraventricular nucleus and ameliorates peripheral
manifestations of heart failure. Hypertension. 59:477–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuda S, Kobayashi H, Iwasa M, Kawamura
I, Sumi S, Narentuoya B, Yamaki T, Ushikoshi H, Nishigaki K,
Nagashima K, et al: Antidiabetic drug pioglitazone protects the
heart via activation of PPAR-gamma receptors, PI3-kinase, Akt, and
eNOS pathway in a rabbit model of myocardial infarction. Am J
Physiol Heart Circ Physiol. 296:H1558–H1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo MY, Ou HC, Lee WJ, Kuo WW, Hwang LL,
Song TY, Huang CY, Chiu TH, Tsai KL, Tsai CS and Sheu WH: Ellagic
acid inhibits oxidized low-density lipoprotein (OxLDL)-induced
metalloproteinase (MMP) expression by modulating the protein kinase
C-α/extracellular signal-regulated kinase/peroxisome
proliferator-activated receptor γ/nuclear factor-κB
(PKC-α/ERK/PPAR-γ/NF-κB) signaling pathway in endothelial cells. J
Agric Food Chem. 59:5100–5108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agrawal YO, Sharma PK, Shrivastava B, Ojha
S, Upadhya HM, Arya DS and Goyal SN: Hesperidin produces
cardioprotective activity via PPAR-γ pathway in ischemic heart
disease model in diabetic rats. PLoS One. 9:e1112122014. View Article : Google Scholar : PubMed/NCBI
|