Introduction
Bronchopulmonary dysplasia (BPD) is a neonatal
chronic lung disease characterized by impaired lung development
(1,2). The pathogenesis of BPD is complex,
and is thought to be associated with a number of features,
including genetic factors, premature birth, prenatal and postnatal
infections, mechanical ventilation, and oxygen toxicity (1,2).
Supplemental oxygen is an important aspect of treatment of
respiratory failure in premature neonates. However, oxidative
stress induced by hyperoxic exposure results in overproduction of
reactive oxygen metabolites (including hydrogen peroxide, singlet
oxygen, superoxide free radicals, and hydroxyl free radicals),
which overwhelms the immature antioxidant enzyme systems of
premature neonates, causes lung injury, impairs alveolarization and
leads to BPD (3,4).
The mammalian sirtuin (SIRT) family is comprised of
seven nicotinamide adenine dinucleotide (NAD)-dependent histone
deacetylase (HDAC) members (5). Of
these, SIRT1 is the most-studied protein and has a role in the
regulation of several physiopathological processes, including gene
expression, mitochondrial function, cellular metabolism and
response to oxidative stress (6–10).
Furthermore, SIRT1 is known to protect against oxidative
stress-induced cell proliferation and inhibit cell apoptosis
(7,8,11–13).
Conjugation of small ubiquitin-related modifier
(SUMO) to target proteins, which is also referred to as
SUMOylation, is an important post-translational modification that
regulates a variety of cellular processes (14). Similar to ubiquitylation,
SUMOylation requires E1 (activating), E2 (conjugating) and E3
(ligating) enzymes to conjugate SUMO to its substrates (15). The mammalian genome encodes four
SUMO isoforms (SUMO1, SUMO2, SUMO3, and SUMO4) (16,17).
Of these isoforms, SUMO2 and SUMO3 are similar in structure and are
referred to as SUMO2/3; SUMO1 is ~50% similar to SUMO2/3, whereas
SUMO4 shares 87% sequence identity with SUMO2/3 (16,17).
SUMO is crucial for oxidative stress responses and SUMOylation is
one of the biological processes triggered by oxidative stress
(15). An increasing number of
SUMOylated proteins have been identified in cells exposed to
stress, including oxidative stress, heat shock, DNA damage and
ethanol stress (14,18,19).
In a previous study, lower expression of SIRT1 in
leukocytes isolated from tracheal aspirate was indicated to be
associated with the development of BPD in premature infants
(20). In addition, SUMOylation of
SIRT1 increases its HDAC activity and serves as a molecular
regulator that determines the fate of cells exposed to
stress-induced DNA damage (5).
Subsequently, it was hypothesized that SUMOylation of SIRT1 in
premature neonates may be implicated in the development of BPD.
In the present study, the effects of hyperoxia on
the expression of SUMO and SIRT1 proteins were examined, and the
interactions of these proteins in premature neonates with BPD were
investigated.
Materials and methods
Subjects and study design
In the present prospective study, 40 premature
neonates (20 neonates with BPD and 20 gender-matched premature
neonates without BPD; 22 male and 18 female; mean gestational age,
29.1±0.6 weeks; Table I) were
enrolled at the Department of Neonatology in the Affiliated
Hospital of Southwest Medical University (Luzhou, China) between
July 1 2015 and August 31 2016. The following inclusion criteria
were used: Gestational age between 28 and 30 weeks; treatment with
non-invasive positive pressure ventilation or normal oxygen
inhalation; and availability of blood samples for western blot
analysis and immunoprecipitation assay. The following exclusion
criteria applied to the present study: Presence of congenital heart
diseases; multiple anomaly syndromes; chromosomal abnormalities; or
hospitalization time <28 days. No other pre-existing conditions
existed in the neonates included in the present study.
 | Table I.Baseline characteristics of
subjects. |
Table I.
Baseline characteristics of
subjects.
| Variable | BPD group | Non-BPD group |
|---|
| Number of neonates,
n | 20 | 20 |
| Male/female, n | 11/9 | 11/9 |
| Gestational age,
week | 28.9±0.5 | 29.2±0.5 |
| Body weight, g | 1,179.9±80.8 | 1,213.8±76.8 |
| Oxygen therapy |
|
Normoxia group, n | 0 | 6 |
|
Low-oxygen group, n | 0 | 12 |
|
Medium-oxygen group, n | 16 | 2 |
|
High-oxygen group, n | 4 | 0 |
BPD was defined as the requirement of supplemental
oxygen at the 28th postnatal day (4), and subjects were assigned to the BPD
or non-BPD group (20 per group) based on this diagnostic criterion.
According to fraction of inspired oxygen (FiO2),
obtained from ventilators, premature neonates were divided into low
(21%<FiO2<30% lasting more than 24 h), medium
(30%≤FiO2<40% lasting more than 24 h), high-oxygen
(FiO2≥40% lasting more than 24 h), and normoxia
(FiO2>21% lasting less than 24 h or
FiO2=21%) groups (12).
Residual venous blood samples at 28 days post hospitalization were
obtained for isolation of peripheral blood mononuclear cells
(PBMCs). The Ethics Committee at the Affiliated Hospital of
Southwest Medical University approved the present study. Written
informed consent was obtained from immediate family members of all
premature neonates.
Reagents and antibodies
Human peripheral blood lymphocyte separation
solution was purchased from Tianjin Haoyang Biological Manufacture
Co., Ltd. (Tianjin, China). Radioimmunoprecipitation assay (RIPA)
protein lysis buffer was obtained from Amyjet Scientific, Inc.
(Wuhan, China). A BCA protein assay kit was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Antibodies used
included: Rabbit anti-human SIRT1 antibody (Santa Cruz
Biotechnology Inc., Dallas, TX, USA), rabbit anti-human GAPDH
antibody (Beyotime Institute of Biotechnology, Inc., Jiangsu,
China), horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG) antibody (Shanghai YuanMu Biological
Technology Co., Ltd., Shanghai, China), anti-SUMO1 monoclonal
antibody (ab133352; Abcam, Cambridge, UK), anti-SUMO2/3 polyclonal
antibody (ab3742; EMD Millipore, Billerica, MA, USA), and
anti-SUMO4 monoclonal antibody (ab126606; Abcam).
Preparation of venous blood
samples
Residual venous blood samples of premature neonates
in the BPD and non-BDP groups were collected to isolate PBMCs by
Ficoll density gradient centrifugation. Blood samples (1.0–1.5 ml)
were placed in an anticoagulant tube, diluted and mixed with an
identical volume of normal saline, and then transferred to a
centrifuge tube with lymphocyte separation solution at the bottom.
Following centrifugation at 900 × g for 20 min at room temperature,
the mononuclear cell layer was transferred to another centrifuge
tube with a pipette and washed twice with normal saline. The
supernatant was discarded and the remaining PBMC pellets were
collected and stored at −80°C.
Protein extraction and western blot
analysis
PBMCs were solubilized in RIPA protein lysis buffer
for 30 min on ice followed by centrifugation at 900 × g for 15 min
at 4°C. The supernatant was collected and protein concentrations
were quantitated using the protein assay kit according to the
manufacturer's protocols. Equivalent protein extracts (1.8 µmol/l)
were separated by 10% SDS-PAGE and transferred electrophoretically
onto polyvinylidene difluoride (pore size, 0.2 µm) membranes.
Following incubation with blocking buffer containing 5% non-fat dry
milk for 1 h at room temperature, membranes were incubated
overnight with rabbit anti-SIRT1 (ab110304; 1:1,000), anti-SUMO1
monoclonal (1:800), anti-SUMO2/3 polyclonal (1:800), anti-SUMO4
monoclonal (1:800) or rabbit anti-GAPDH antibodies (1:3,000) at
4°C. The blots were washed three times for 5 min each with
TBS/Tween-20 (TBST), and subsequently incubated with HRP-labeled
goat anti-rabbit IgG antibody (1:3,000) for 1 h at room
temperature. Following three washes (5 min each) with TBST,
chemiluminescent signals were visualized using an enhanced
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and band intensity was analyzed using Quantity
One software (version 4.6.6; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The expression levels of target proteins were normalized
relative to that of GAPDH.
Immunoprecipitation assay
Protein extracts were prepared by adding the
radioimmunoprecipitation lysis buffer (Pierce; Thermo Fisher
Scientific, Inc.) containing protease inhibitors to PBMCs, and then
incubated with rabbit anti-SIRT1 antibody (SC-135792) or control
IgG antibody (Pierce, Rockford, IL, USA) overnight at 4°C. Once
antigen-antibody complexes were precipitated with protein
A-sepharose beads (Pierce; Thermo Fisher Scientific, Inc.) for
30–60 min at room temperature, immunoprecipitated proteins (1.8
µmol/l) were separated by 10% SDS-PAGE and incubated overnight at
4°C with anti-SUMO1 monoclonal (1:800), anti-SUMO2/3 polyclonal
(1:600) or anti-SUMO4 monoclonal antibody (1:600). SIRT1 was used
as the reference protein. Chemiluminescent signals were visualized
using the enhanced chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.) and band intensity was analyzed using Quantity
One software (version 4.6.6; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analyses
All statistical analyses were performed using SPSS
for Windows (version 17.0; SPSS, Inc., Chicago, IL, USA). Data
pertaining to normally distributed variables were expressed as the
mean ± or + standard deviation as indicated, and between-group
differences were assessed using Student's t-test. Multi-group
comparisons were performed using one-way analysis of variance with
Dunnett's post hoc test. Categorical data were compared using
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
The present study included 40 premature neonates (22
male and 18 female; mean gestational age, 29.1±0.6 weeks; Table 1). Of the 20 neonates in the BPD
group, 16 neonates were indicated in the medium-oxygen group and 4
neonates were identified in the high-oxygen group. However, in the
non-BPD group, 6 neonates were in the normoxia group, 12 in the
low-oxygen group and 2 in the medium-oxygen group. No significant
differences were observed between the BPD and non-BPD groups with
regards to gestational age and body weight.
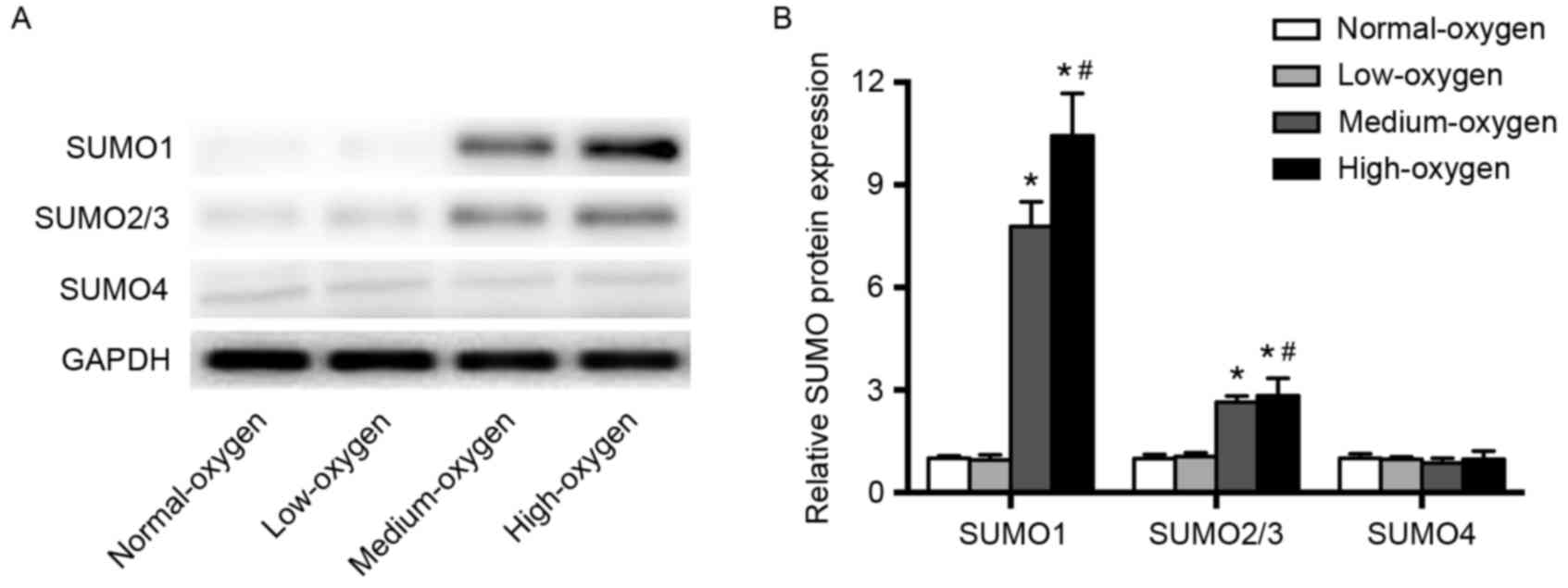
SUMO expression after inhalation of
different concentrations of oxygen
Western blot analysis was performed to examine the
expression levels of SUMO proteins in PBMCs of premature infants.
The expression levels of SUMO1 and SUMO2/3 proteins in the normoxia
group were significantly lower than those in the medium- and
high-oxygen groups (P<0.01), but were comparable to those in the
low-oxygen group (Fig. 1). SUMO4
expression levels were comparable among the normoxia, low-, middle-
and high-oxygen groups (Fig. 1).
These results indicate that oxygen inhalation with
FiO2≥30% significantly upregulated SUMO1 and SUMO2/3
expression levels in premature infants.
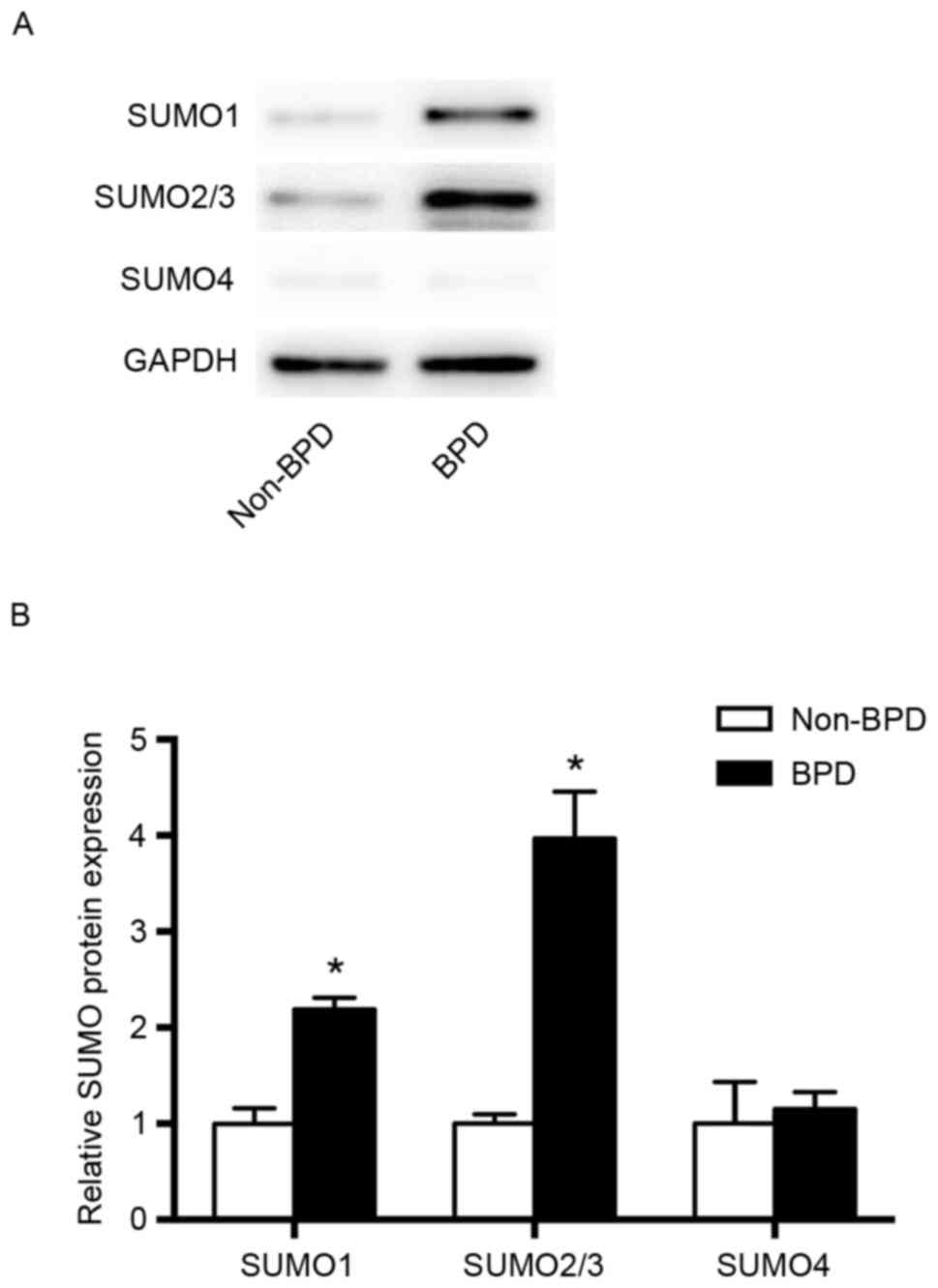
Comparison of SUMO expression in the
BPD and non-BPD groups
The expression levels of SUMO1 and SUMO2/3 proteins
in the BPD group were significantly higher compared with those in
the non-BPD group (P<0.01), whereas no significant difference in
SUMO4 expression was observed between groups (Fig. 2).
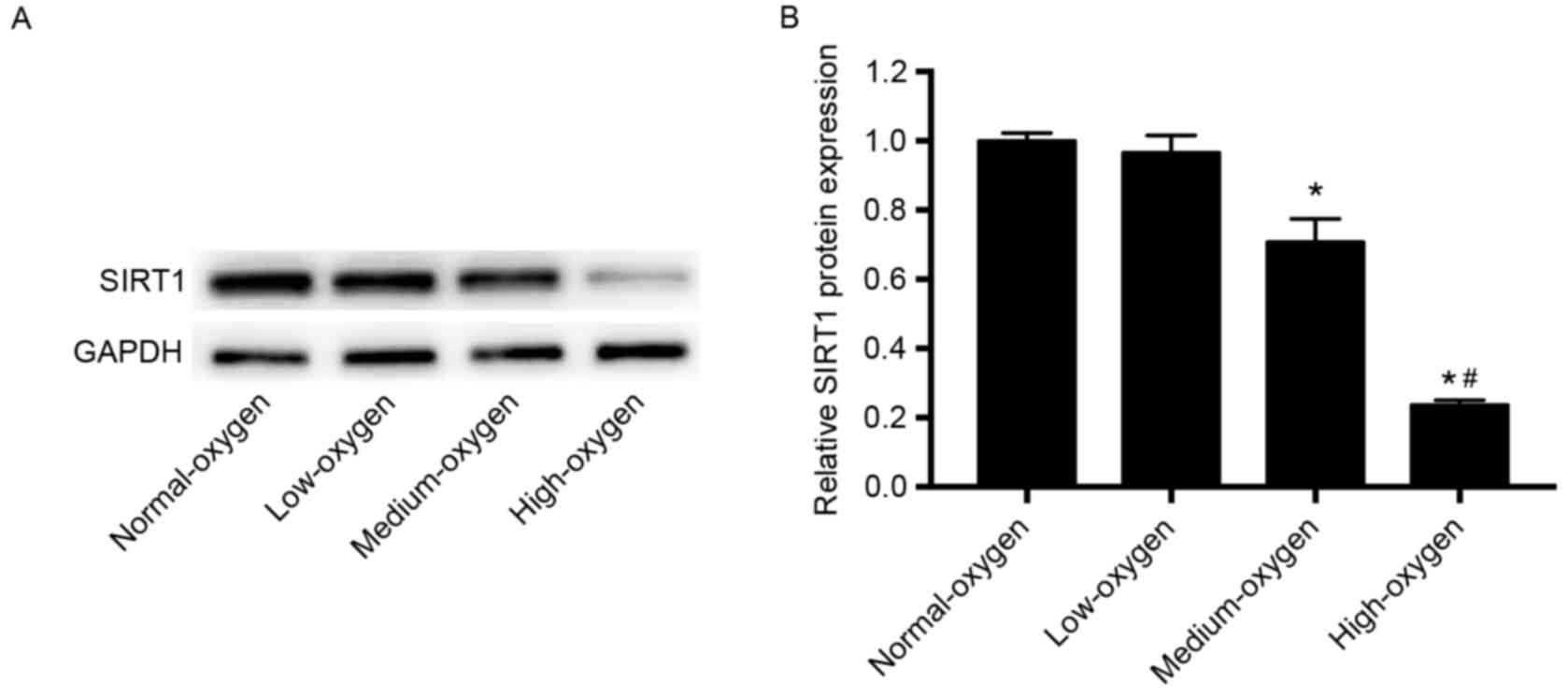
SIRT1 expression following inhalation
of different concentrations of oxygen
The expression levels of SIRT1 protein in the
medium- and high-oxygen groups were significantly lower compared
with those in the normoxia group (P<0.01; Fig. 3). SIRT1 expression levels in the
low-oxygen group were lower than that in the normoxia group;
however, the difference was not statistically significant (Fig. 3). These results suggest that oxygen
inhalation with FiO2≥30% may significantly downregulate
SIRT1 expression in preterm infants.
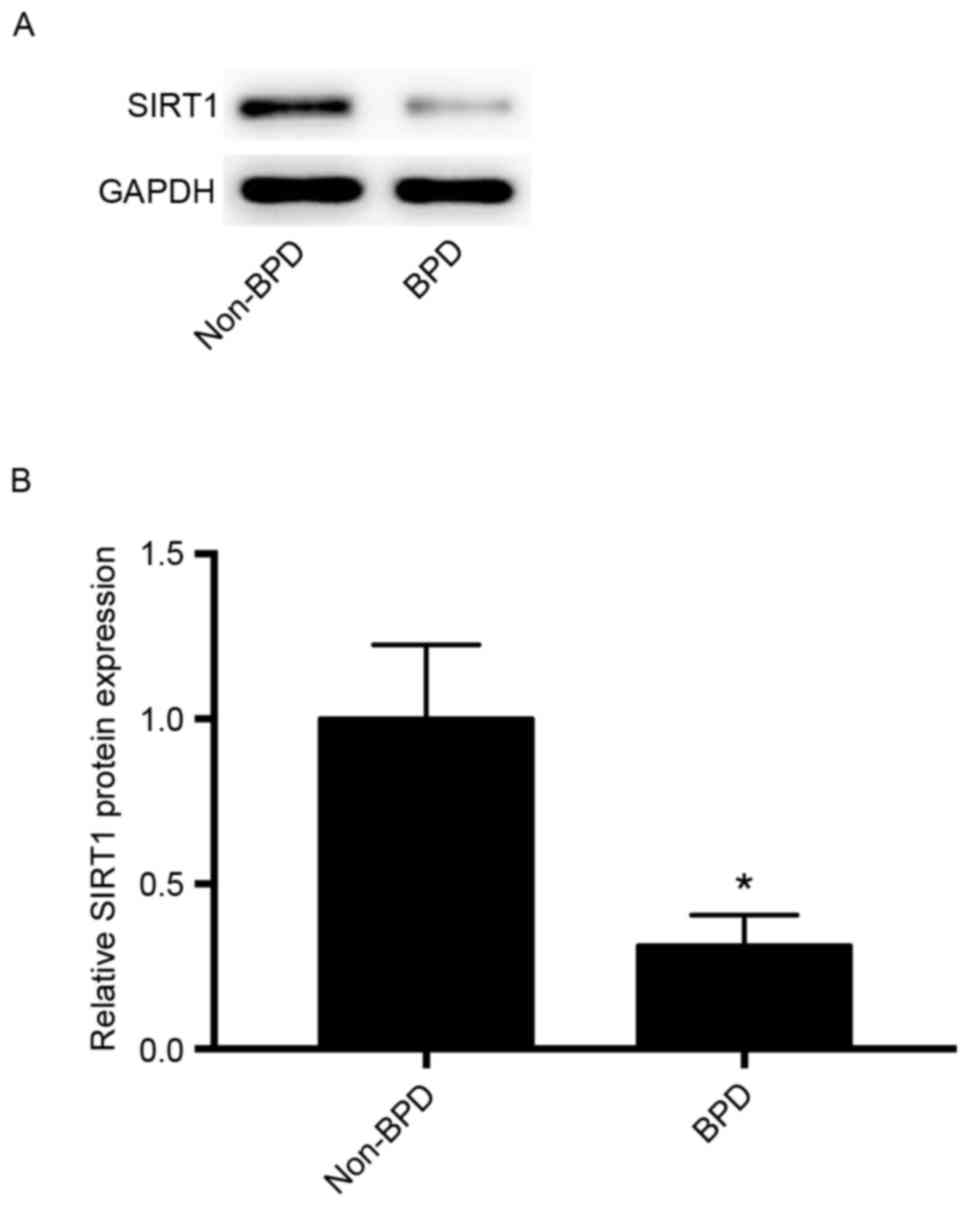
Comparison of SIRT1 expression in the
BPD and non-BPD groups
SIRT1 expression was compared in the BPD and non-BPD
groups to assess its potential role in BPD. As indicated in
Fig. 4, the expression levels of
SIRT1 protein in the BPD group was significantly lower than that in
the non-BPD group (P<0.01).
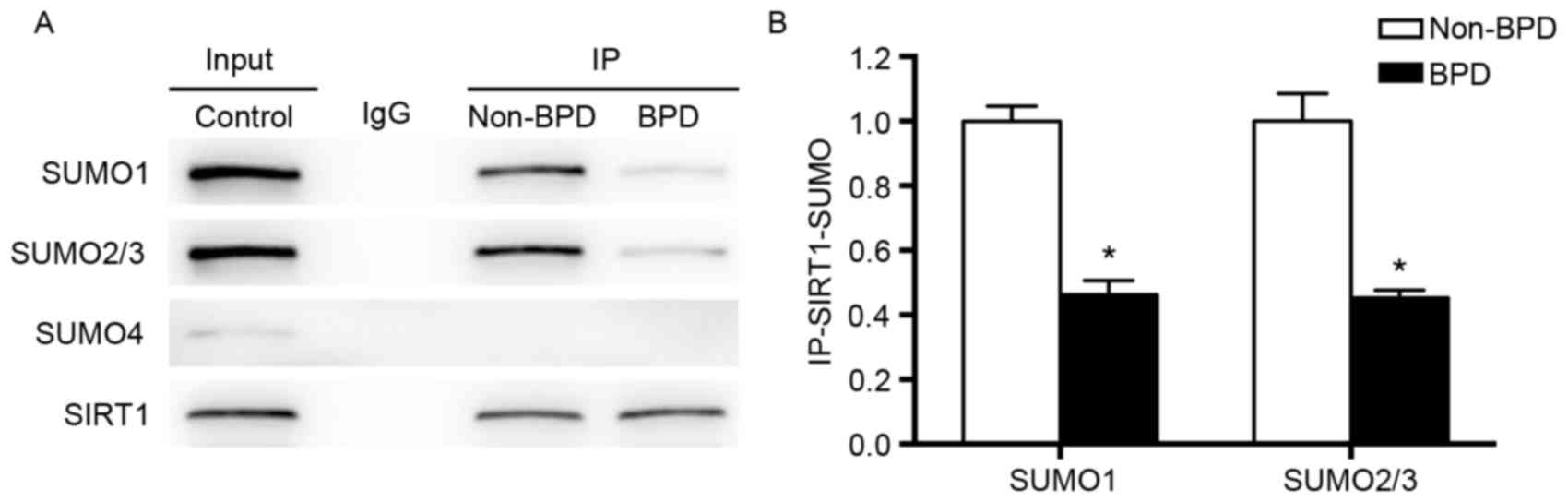
Immunoprecipitation assay of
interactions between SUMO and SIRT1
Interactions between SUMO and SIRT1 were detected by
immunoprecipitation with anti-SIRT1 antibody and followed by
western blot analysis using anti-SUMO1, anti-SUMO2/3 or anti-SUMO4
antibodies. As indicated in Fig.
5, SIRT1 interacted with SUMO1 and SUMO2/3 in both the non-BPD
and BPD groups. However, SIRT1-SUMO4 interaction was not observed
in either the non-BPD or BPD group. Both SIRT1-SUMO1 and
SIRT1-SUMO2/3 interactions in the BPD group were significantly
weaker than those in the non-BPD group (P<0.01; Fig. 5).
Discussion
In the present study, the expression levels of SUMO
and SIRT1 in PBMCs of premature neonates who were administered
different concentrations of oxygen were investigated. Furthermore,
the expression levels of SIRT1 and SUMO proteins and their
interactions in BPD and non-BPD groups were determined. The present
findings demonstrated that oxygen inhalation with
FiO2≥30% significantly upregulated SUMO1 and SUMO2/3
expression, but downregulated SIRT1 expression. Compared with the
non-BPD group, the expression of SIRT1 protein and SUMOylation of
SIRT1 by SUMO1 and SUMO2/3 was significantly attenuated in the BPD
group. To the best of our knowledge, SUMOylation of SIRT1 in
premature neonates with BPD has not been previously
investigated.
Supplemental oxygen is an important aspect of
treatment of premature neonates with respiratory failure. However,
hyperoxic exposure triggers oxidative stress, results in lung
injury and contributes to the development of BPD (3,4).
Infants with BPD who were administered higher concentrations of
supplemental oxygen were previously demonstrated to have more
persistent lung disease (4).
Increased oxidative stress has also been detected in adolescents
with BPD (2). SUMO is crucial for
the cellular response to oxidative stress (19). Huang et al (21) previously reported that exposure to
high glucose upregulated SUMO1 and SUMO2/3 expression levels in a
time- and dose-dependent. manner. In the present study, oxygen
inhalation with FiO2≥30% significantly increased SUMO1
and SUMO2/3 expression levels in PBMCs of preterm infants. However,
no significant difference was observed between the normoxia, low-,
middle- and high-oxygen groups with respect to SUMO4 expression.
Although previous studies have indicated a role of SUMO4 in the
modulation of cellular response to stress, its function is not
well-characterized (16). In the
present study, expression levels of SUMO1 and SUMO2/3 in the BPD
group were significantly higher compared with those in the non-BPD
group. These findings indicate that SUMO1 and SUMO2/3 may serve as
oxidative stress-related proteins and may have a role in the
pathogenesis of BPD in premature neonates. In a previous study by
Yuan et al (22),
individual deletion of either SUMO1 or SUMO2/3 did not affect the
development of zebra fish, whereas inactivation of all three SUMOs
triggered p53-dependent apoptosis and resulted in severe
developmental defects.
HDAC is associated with the regulation of cell
differentiation and proliferation. Decreased HDAC activity has been
indicated to cause cell cycle arrest and suppress alveolar cell
proliferation (23). SIRT1 is a
NAD-dependent HDAC, which has a crucial role in the cellular
response to nutritional and metabolic disorders, inflammation,
hypoxia and oxidative stress (24–26).
In a neonatal rat model of lung injury, exposure to hyperoxia was
revealed to induce the downregulation of HDAC expression (27,28).
Similar results were obtained in the present study, in that the
expression levels of SIRT1 in the medium- and high-oxygen groups
were significantly lower compared with those in the normoxia group.
Furthermore, SIRT1 has been indicated to protect against oxidative
stress and inhibit apoptosis (7,8).
Abnormal expression of SIRT1 was recently observed
in patients with pulmonary fibrosis, pulmonary inflammatory
diseases or lung tumors (29–31).
Mody et al (20) previously
performed immunocytochemistry analysis to detect SIRT1 expression
in tracheal aspirate leukocytes of 51 premature infants, and
demonstrated SIRT1 to be less localized in the nuclei of tracheal
aspirate mononuclear cells in infants with BPD compared with those
who did not develop BPD. In the present study, SIRT1 expression in
PBMCs of preterm infants in the BPD group was significantly lower
than that in the non-BPD group. Additionally, SIRT1 has been
suggested to promote the survival of both aging and cancerous cells
(32). Lower SIRT1 in tracheal
aspirate leukocytes has previously been indicated to be associated
with the development of BPD in premature infants (20). In addition, SIRT1 is considered as
a potential therapeutic target in the context of diseases including
cancer, Alzheimer's diseases and diabetes (7).
SUMOylation is a reversible posttranslational
modification that contributes to various cellular processes,
including genome stability, gene expression, RNA processing,
protein synthesis and repair of DNA damage, and has a crucial role
in a variety of human diseases, including brain ischemia, heart
diseases, cancer and degenerative diseases (14,15).
SUMOylation of target proteins leads to alterations of their
subcellular localization, activity and stability (17). Furthermore, SUMOylation of SIRT1 by
SUMO1 was identified in DU145 prostate cancer cells, and HDAC
activity of SIRT1 was enhanced after SUMOylation (5). A recent study by Han et al
(32) indicated that SUMOylation
of SIRT1 improved the stability of SIRT1 protein. In the present
study, SUMOylation of SIRT1 by SUMO1 and SUMO2/3 was demonstrated
in PBMCs of premature neonates; however, no notable interaction was
observed between SUMO4 and SIRT1. A previous study has also
indicated that the possible role of SUMO4 in SUMOylation is yet to
be understood (17). In a previous
study by Yang et al (5),
decreased SUMOylation of SIRT1 in response to acute DNA damage
attenuated its HDAC activity, enhanced the activity of its
pro-apoptotic substrates and ultimately resulted in cell death. In
the present study, SUMOylation of SIRT1 by SUMO1 and SUMO2/3 in the
BPD group was significantly attenuated when compared with that in
the non-BPD group. These findings suggest that decreased
SUMOylation of SIRT1 may be associated with the pathogenesis of BPD
in premature neonates.
This prospective study has limitations. Firstly, the
expression of SIRT1 and SUMO proteins and their interactions were
examined in PBMCs rather than in lung biopsy tissues. However,
PBMCs are widely distributed in the lung tissue, and likely have a
role in the development of BPD. Secondly, only a small sample of
premature neonates was included in the present study.
In conclusion, oxygen inhalation with
FiO2≥30% was significantly associated with the
downregulation of SIRT1 expression in PBMCs of premature neonates.
Decreased expression of SIRT1, and its interactions with SUMO1 and
SUMO2/3 may be associated with the development of BPD. As the
present study is only a preliminary work, further investigation
with a larger sample size is required to elucidate the mechanism of
SIRT1-associated SUMOylation in the development of BPD.
Acknowledgements
The present study was supported by grants from
projects in the National Natural Science Foundation of China (grant
no. 81571480) and the Joint Special Fund of Luzhou Municipal
Government and Luzhou Medical College (grant no. 2013LZLY-J08).
References
|
1
|
Bhandari A and Bhandari V: Pitfalls,
problems, and progress in bronchopulmonary dysplasia. Pediatrics.
123:1562–1573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niedermaier S and Hilgendorff A:
Bronchopulmonary dysplasia-an overview about pathophysiologic
concepts. Mol Cell Pediatr. 2:22015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Wall SB, Ren C, Velten M, Hill CL,
Locy ML, Rogers LK and Tipple TE: Thioredoxin reductase inhibition
attenuates neonatal hyperoxic lung injury and enhances nuclear
factor E2-Related Factor 2 activation. Am J Respir Cell Mol Biol.
55:419–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali Z, Schmidt P, Dodd J and Jeppesen DL:
Bronchopulmonary dysplasia: A review. Arch Gynecol Obstet.
288:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Fu W, Chen J, Olashaw N, Zhang X,
Nicosia SV, Bhalla K and Bai W: SIRT1 sumoylation regulates its
deacetylase activity and cellular response to genotoxic stress. Nat
Cell Biol. 9:1253–1262. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang HC and Guarente L: SIRT1 mediates
central circadian control in the SCN by a mechanism that decays
with aging. Cell. 153:1448–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ou X, Lee MR, Huang X, Messina-Graham S
and Broxmeyer HE: SIRT1 positively regulates autophagy and
mitochondria function in embryonic stem cells under oxidative
stress. Stem Cells. 32:1183–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruan Y, Dong C, Patel J, Duan C, Wang X,
Wu X, Cao Y, Pu L, Lu D, Shen T and Li J: SIRT1 suppresses
doxorubicin-induced cardiotoxicity by regulating the oxidative
stress and p38MAPK pathways. Cell Physiol Biochem. 35:1116–1124.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hori YS, Kuno A, Hosoda R and Horio Y:
Regulation of FOXOs and p53 by SIRT1 modulators under oxidative
stress. PLoS One. 8:e738752013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang P, Yang X, Wang W, Zhang J, He
Y, Zhang W, Jing T, Wang B and Lin R: SIRT1 inhibits inflammatory
response partly through regulation of NLRP3 inflammasome in
vascular endothelial cells. Mol Immunol. 77:148–156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong X, Guan J, Li J, Wei J and Wang R:
P66Shc-SIRT1 regulation of oxidative stress protects against
cardio-cerebral vascular disease. Mol Neurobiol. 54:5277–5285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Li Q, Kang L, Lei X, Zhai X, Zhao
S, Zhang C and Dong W: Resveratrol inhibits hyperxia-induced cell
apoptosis through up-regulating SIRT1 expression in HPAECs. Xi Bao
Yu Fen Zi Mian Yi Xue Za Zhi. 31:590–595. 2015.(In Chinese).
PubMed/NCBI
|
|
13
|
Yang X, Dong W, Li Q, Kang L, Lei X, Zhang
L, Lu Y and Zhai X: Hyperoxia induces reactive oxygen species
production and promotes SIRT1 nucleocytoplasmic shuttling of
peripheral blood mononuclear cells in premature infants in vitro.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 31:1669–1676. 2015.(In
Chinese). PubMed/NCBI
|
|
14
|
Feligioni M and Nistico R: SUMO: A
(oxidative) stressed protein. Neuromolecular Med. 15:707–719. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W and Paschen W: SUMO proteomics to
decipher the SUMO-modified proteome regulated by various diseases.
Proteomics. 15:1181–1191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chymkowitch P, Nguéa PA and Enserink JM:
SUMO-regulated transcription: Challenging the dogma. Bioessays.
37:1095–1105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sriramachandran AM and Dohmen RJ:
SUMO-targeted ubiquitin ligases. Biochim Biophys Acta. 1843:75–85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agbor TA and Taylor CT: SUMO, hypoxia and
the regulation of metabolism. Biochem Soc Trans. 36:445–448. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enserink JM: Sumo and the cellular stress
response. Cell Div. 10:42015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mody K, Saslow JG, Kathiravan S, Eydelman
R, Bhat V, Stahl GE, Pyon K, Bhandari V and Aghai ZH: Sirtuin1 in
tracheal aspirate leukocytes: Possible role in the development of
bronchopulmonary dysplasia in premature infants. J Matern Fetal
Neonatal Med. 25:1483–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang W, Xu L, Zhou X, Gao C, Yang M, Chen
G, Zhu J, Jiang L, Gan H, Gou F, et al: High glucose induces
activation of NF-κB inflammatory signaling through IκBα sumoylation
in rat mesangial cells. Biochem Biophys Res Commun. 438:568–574.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan H, Zhou J, Deng M, Liu X, Le Bras M,
de The H, Chen SJ, Chen Z, Liu TX and Zhu J: Small
ubiquitin-related modifier paralogs are indispensable but
functionally redundant during early development of zebrafish. Cell
Res. 20:185–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang YX, Zhao J, Song QH, Zheng LH, Fan
C, Liu TT, Bao YL, Sun LG, Zhang LB and Li YX: Virtual screening
and experimental validation of novel histone deacetylase
inhibitors. BMC Pharmacol Toxicol. 17:322016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Ni J, Guo R and Li W: In patients
with coronary artery disease and type 2 diabetes, SIRT1 expression
in circulating mononuclear cells is associated with levels of
inflammatory cytokines but not with coronary lesions. Biomed Res
Int. 2016:87348272016.PubMed/NCBI
|
|
25
|
Zhu Y, Sun Y, Guan W, Yan Y, Zhang W, Bai
L, Kong H and Li F: Lycium barbarum polysaccharides enhances SIRT1
expression and decreases MMP-9 and HIF-1α expressions in hypoxic
pulmonary vascular smooth muscle cells. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 32:906–910. 2016.(In Chinese). PubMed/NCBI
|
|
26
|
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ,
Li T, Fan J, Peng ZW and Yan WJ: Nrf2/antioxidant defense pathway
is involved in the neuroprotective effects of Sirt1 against focal
cerebral ischemia in rats after hyperbaric oxygen preconditioning.
Behav Brain Res. 309:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cetinkaya M, Cansev M, Cekmez F, Tayman C,
Canpolat FE, Kafa IM, Yaylagul EO, Kramer BW and Sarici SU:
Protective effects of valproic acid, a histone deacetylase
inhibitor, against hyperoxic lung injury in a neonatal rat model.
PLoS One. 10:e01260282015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korfei M, Skwarna S, Henneke I, MacKenzie
B, Klymenko O, Saito S, Ruppert C, von der Beck D, Mahavadi P,
Klepetko W, et al: Aberrant expression and activity of histone
deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax.
70:1022–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conti V, Corbi G, Manzo V, Pelaia G,
Filippelli A and Vatrella A: Sirtuin 1 and aging theory for chronic
obstructive pulmonary disease. Anal Cell Pathol (Amst).
2015:8973272015.PubMed/NCBI
|
|
30
|
Ahmad T, Sundar IK, Tormos AM, Lerner CA,
Gerloff J, Yao H and Rahman I: Shelterin telomere protection
protein 1 reduction causes telomere attrition and cellular
senescence via sirtuin 1 deacetylase in chronic obstructive
pulmonary disease. Am J Respir Cell Mol Biol. 56:38–49. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu HY, Li QR, Cheng XF, Wang GJ and Hao
HP: NAMPT inhibition synergizes with NQO1-targeting agents in
inducing apoptotic cell death in non-small cell lung cancer cells.
Chin J Nat Med. 14:582–589. 2016.PubMed/NCBI
|
|
32
|
Han X, Niu J, Zhao Y, Kong Q, Tong T and
Han L: HDAC4 stabilizes SIRT1 via sumoylation SIRT1 to delay
cellular senescence. Clin Exp Pharmacol Physiol. 43:41–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|