Introduction
Breast cancer is one of the most frequently
occurring malignant tumors affecting women worldwide (1). The incidence of breast cancer is
increasing, with a demonstrated younger age of onset in recent
years (2). Each year >1 million
cases are newly diagnosed, and there are >400,000 cases of
mortality (3). The incidence rate
of breast cancer in China is increasing, and it is currently the
most frequently occurring malignant tumor in females. Metastasis is
the final stage of development in breast cancer and is the leading
cause of mortality in patients. Therefore, identifying strategies
that inhibit breast cancer cell growth, invasion and metastasis are
critical for the successful treatment of breast cancer, and are the
focus of primary research. Recent studies have facilitated the
development of more effective therapeutic strategies for breast
cancer treatment in areas including surgery, chemotherapy,
radiotherapy, endocrine therapy and targeted molecular treatment
(4–8). These have resulted in an increase in
the curative effects and survival rates of breast cancer patients
to varying degrees (4–8). At present, the primary treatment for
the inhibition of breast cancer metastasis is chemotherapy;
however, the results are generally unsatisfactory. In addition,
currently available chemotherapeutic agents are unable to
effectively distinguish tumor cells from healthy human cells, which
leads to adverse and toxic side effects. Therefore, the
identification of a highly potent antitumor treatment with low
toxicity is a primary focus of research.
The use of traditional Chinese medicine (TCM) as a
therapeutic strategy for various diseases is of great value in
China. It has been demonstrated previously that numerous monomer
compositions extracted from TCMs exhibit antitumor effects, such as
curcumin (9) and puerarin
(10). Paeoniflorin (PF) was first
isolated from the peony plant (Ranunculaceae family) in
1963, and is the primary active monomer component in peonies. PF is
a monoterpene glycoside compound that exhibits anti-inflammatory,
immune regulatory and neuroprotective properties (11–13).
In addition, previous studies have demonstrated that that PF may
exhibit antitumor effects, including inhibitory effects on the
proliferation, invasion and metastasis of lung (14), gastric (15), liver (16) and cervical cancer cells (17). Zhang et al (18) revealed that PF inhibited the
proliferation and invasion of breast cancer cells by inhibiting the
Notch-1 signaling pathway.
The aim of the present study was to investigate the
effects of PF on the MCF-7 breast cancer cell line. Cells were
treated with increasing concentrations of PF for different
durations in order to examine the effects of PF on their
proliferation and invasion rates. It has been previously
demonstrated that silencing of notch homolog-1 (NOTCH-1) decreases
the invasive ability of breast cancer cells (19), and inhibition of the expression of
NOTCH-1 inhibits breast cancer cell proliferation (20). These results suggest that the
NOTCH-1 signaling pathway may be important in the regulation of
breast cancer cell proliferation and invasion. Therefore, the
present study examined the expression levels of key genes of the
NOTCH-1 signaling pathway, in order to identify the potential
mechanisms underlying the effect of PF on breast cancer cells.
Materials and methods
Instruments and reagents
The MCF-7 breast cancer cell line was obtained from
American Type Culture Collection (Manassas, VA, USA). RPMI-1640
culture medium, bovine serum albumin (BSA) and fetal bovine serum
(FBS) was obtained from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). PF was purchased from the National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China). The Cell Counting kit (CCK)-8 assay was purchased from
Peptide Institute, Inc. (Osaka, Japan), the transwell chamber
system was obtained from Corning Incorporated (Corning, NY, USA),
the Nanodrop2000 nucleic acid protein detector was purchased from
Thermo Fisher Scientific, Inc., the RNA extraction reagent,
TRIzol®, was purchased from Invitrogen; Thermo Fisher
Scientific, Inc., the SYBR Green Mixture was purchased from Toyobo
Life Science (Osaka, Japan), and the real-time fluorescence
quantitative reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) kit was obtained from Takara Bio, Inc., (Otsu,
Japan). The gel imaging system and the ViiA7 Real-Time PCR System
were obtained from Applied Biosystems; Thermo Fisher Scientific
Inc., the total protein extraction kit was purchased from BestBio
(Shanghai, China), and the Coomassie Brilliant Blue protein assay
kit was obtained from Shanghai Meiji Biological Technology Co. Ltd.
(Shanghai, China). In addition, the SDS-PAGE gel,
phosphate-buffered saline plus 0.1% Tween-20 (PBST) solution
(Nanjing Senbeijia Biological Technology Co., Ltd., Nanjing,
China), vertical polyacrylamide electrophoresis apparatus and the
GIS-2020D gel imaging analysis system were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Antibodies for
NOTCH-1 (cat. no. ab52627), Hes family basic helix-loop helix
transcription Factor (HES)-1 (cat. no. ab108937), β-actin (cat. no.
ab8226) and horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (cat. no. ab6721) were purchased
from Abcam (Cambridge, MA, USA).
MCF-7 cell culture
The MCF-7 cells were cultured in RPMI-1640 medium
supplemented with streptomycin and penicillin (1,000 IU/l) and 10%
FBS. The cells were then placed in an incubator at 37°C with 5%
CO2, and the cells were passaged once every 2–3 days.
During passaging, the culture medium in the culture dish was
discarded and the cells were washed with PBS twice. Following
incubation of cells with trypsin for 1 min, the adherent cells were
gently pipetted into single cell suspensions, which were then
centrifuged at 500 × g at room temperature for 5 min. The
supernatants were subsequently discarded and the cell pellet was
re-suspended in 1 ml medium; 200 µl of cell suspension was used for
subculture.
CCK-8 cell proliferation assay
Cells growing in the logarithmic phase were digested
and counted, then 1×104 cells/well were transferred to a
96-well plate. The cells were allowed to adapt to the culture
environment for 1 h, before they were treated with 0, 7.5, 15 and
30 µM PF for 24, 48 and 72 h. For each dose, there were five
replicate wells, and a blank control group was included in each
assay. Following the treatment intervention, the cells were washed
with PBS three times before 100 µl CCK-8 mixture (1:10, CCK-8
reagent: medium) was added to the wells, which were then incubated
in a 5% CO2 incubator at 37°C for 2 h. The absorbance
(A) values were measured at a wavelength of 450 nm using a
microplate reader, and the rate of cell survival was calculated
using the following formula: Cell survival rate=(Atreated
group-Ablank control group)/(Acontrol
group-Ablank control group).
Transwell invasion assay
A total of 50 mg/l Matrigel was diluted in
serum-free medium at a ratio of 1:8. The inner surface of the
membrane at the bottom of the transwell chamber was coated with the
Matrigel matrix mixture, and the chamber was placed in an incubator
for 30 min at 37°C before it was transferred to a safety cabinet
for air-drying overnight. The chamber was blocked with serum-free
RPMI-1640 medium containing 10 g/l BSA for 30 min. MCF-7 cells were
serum-starved for 24 h before they were digested, collected and
centrifuged at 500 × g for 5 min at room temperature. The
supernatant was discarded and the cell pellet was washed with PBS
twice before the cells were re-suspended in serum-free RPMI-1640
medium containing 10 g/l BSA. The cell concentration was adjusted
to 5×107 cells/l. A total of 200 µl cell suspension was
added into the lower transwell chamber, and 600 µl RPMI-1640
culture medium supplemented with 10% FBS was added into the lower
chamber. Cells were incubated for 6 h to allow them to adapt to the
culture conditions before PF was added to the lower chamber at
concentrations of 0, 7.5, 15 and 30 µM. A total of 5 replicate
wells for each group were analyzed, and each assay included a blank
control. Following the addition of PF, the plates were incubated
for 48 h at 37°C. Cells that remained in the upper chamber of the
transwell invasion system were removed using a cotton swab, and the
wells were washed with PBS twice. The cells on the underside of the
upper chamber were fixed with methanol (100%) for 15 min at room
temperature, stained with 1% crystal violet for 10 min at room
temperature, and then observed under an inverted microscope for
analysis and counting; for each group a total of five fields were
analyzed under the 400-fold microscope. A total of three
independent repeats of this assay were performed.
Detection of NOTCH-1 and HES-1 mRNA
expression levels
Following treatment of MCF-7 (5×105)
cells with 0, 7.5, 15 and 30 µM PF for 48 h, the cells were
collected and RNA was extracted using TRIzol® reagent
according to the manufacturer's instructions. The purity and
content of RNA was determined using the Nanodrop 2000 protein and
nucleic acid analyzer. The integrity of RNA was detected via 1%
agarose gel electrophoresis. A total of 1 µg RNA was reverse
transcribed to cDNA using an RNA reverse transcription kit (Takara
Bio, Inc.), according to the manufacturer's protocol. The reaction
mixture for the quantitative (q)PCR reaction consisted of the
following: 5 µl SYBR Green Mixture (2X), 0.5 µl cDNA, 0.5 µl
forward primer (10 µM), 0.5 µl reverse primer (10 µM) and 3.5 µl
ddH2O. The thermocycling conditions for the qPCR
reaction were as follows: Pre-denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing and extension at 60°C for 60 sec. The following primers
were used: Notch-1, forward, 5′-CACTGTGGGCGGGTCC-3′ and reverse,
5′-GTTGTATTGGTTCGGCACCAT-3; HES-1, forward,
5′-TAGCTCGCGGCATTCCAAGC-3′ and reverse,
5′-GTGCTCAGCGCAGCCGTCATCT-3′; β-actin, forward,
5′-CCACACTGTGCCCATCTACG-3′ and reverse,
5′-AGGATCTTCATGAGGTAGTCAGTCAG-3′. This was performed using the
ViiA7 Real-Time PCR System. Experiments were performed in
triplicate for each group and β-actin was used as the internal
reference gene. The relative expression level of mRNA was determine
using the by 2−∆∆Cq method (21).
Detection of NOTCH-1 and HES-1 protein
expression levels
Following treatment of MCF-7 (5×105)
cells with 0, 7.5, 15, and 30 µM PF for 48 h, the cells were lysed
in lysis buffer (20 mM Tris-HCl, pH 8.0, 100 mM KCl and 0.2 mM
EDTA) and centrifuged at 2,600 × g for 30 min at room temperature.
Total proteins were then extracted from the supernatant using the
BestBio kit. The protein concentration was determined using the
Coomassie Brilliant Blue protein assay kit, and the buffers were
added into protein solutions and boiled. Protein samples (40 µg)
were loaded onto a 6–12% SDS-PAGE gel and electrophoresed.
Following the addition of transfer buffers, the gel was transferred
to a nitrocellulose membrane. The membrane was then blocked with
PBST containing 5% skim milk powder and placed on a shaker at room
temperature for 2 h. PBST was used to wash the membrane three times
before the primary antibodies were added and incubated on a shaker
in the refrigerator at 4°C overnight. The following primary
antibodies were used: NOTCH-1 (1:1,000), HES-1 (1:2,000) and
β-actin (1:2,000). The membrane was then washed with PBST for 30
min and incubated for 1 h at the room temperature with the
HRP-conjugated goat anti-rabbit secondary antibody (1:10,000).
Following washing with PBST three times, the electroluminescence
detection reagent (Thermo Fisher Scientific, Inc.) was evenly
spread on the nitrocellulose membrane. The optical densities of
NOTCH-1, HES-1 and β-actin protein bands were analyzed using the
GIS-2020D gel imaging analysis system, and the ratio of NOTCH-1 and
HES-1 protein band optical densities were compared with the β-actin
protein band optical density to determine the expression
intensities of the NOTCH-1 and HES-1 proteins.
Statistical analysis
SPSS software (version, 13.0; SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis, and normally
distributed data are presented as the mean ± standard deviation.
One-way analysis of variance (ANOVA) was used to measure the effect
of PF on cell invasion capacity and the mRNA expression levels of
NOTCH1 and HES-1. Repeated measures ANOVA was used to analyze the
effect of PF on the proliferation of MCF-7 cells at different time
points, and Mauchly's sphericity test was used to validate the
results. The post hoc Dunnett's test was used for multiple
comparisons. GraphPad Prism 5.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to generate the graphs. P<0.05 was
considered to indicate a statistically significant difference.
Results
PF inhibits cell proliferation
MCF-7 cells were treated with increasing
concentrations of PF at various time points, and the effect of PF
on the proliferation of MCF-7 cells was determined using the CCK-8
assay. The results of the repeated measures ANOVA test indicated
that statistically significant differences among all time points
were observed (F=600.02; P<0.05). In addition, it was revealed
that PF significantly inhibited the proliferation of MCF-7 cells in
a time- and concentration-dependent manner (F=100.93; P<0.05;
Table I). PF was observed to
inhibit the proliferation of MCF-7 cells in a dose- and
time-dependent manner (Table I.
The inhibitory effect of 30 µM PF on the proliferation of cells was
most pronounced when the cells were treated for 72 h.
 | Table I.Effect of paeonifiorin on MCF-7 cell
proliferation. |
Table I.
Effect of paeonifiorin on MCF-7 cell
proliferation.
|
| Time (h) |
|---|
|
|
|
|---|
| Concentration
(µM) | 24 | 48 | 72 |
|---|
| 0 |
1.00±0.042 |
1.00±0.043 |
1.00±0.041 |
| 7.5 |
0.89±0.038a |
0.81±0.038a,b |
0.74±0.040a–c |
| 15 |
0.80±0.041a |
0.73±0.039a,b |
0.61±0.044a–c |
| 30 |
0.71±0.043a |
0.65±0.041a,b |
0.52±0.037a–c |
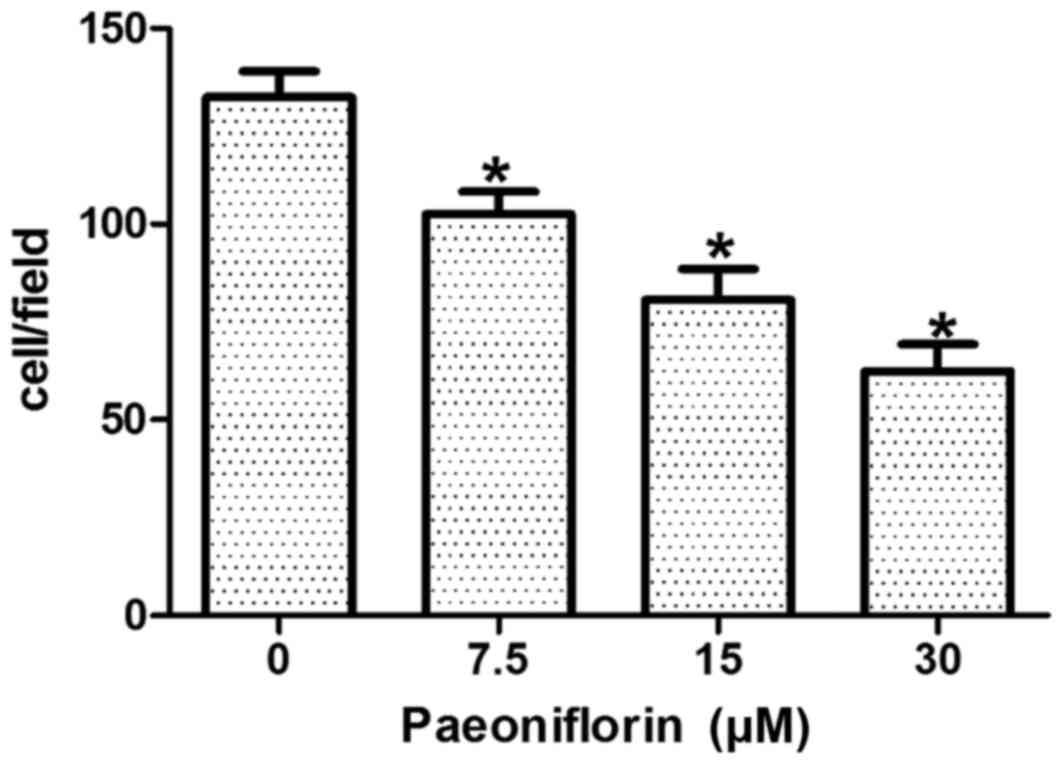
PF inhibits cell invasion
capacity
MCF-7 cells were treated for 48 h with 0, 7.5, 15
and 30 µM PF, and the transwell assay system was used to detect the
invasion ability of the cells. The number of cells that traversed
the membrane was 132.44±6.58 in the control group, whereas
102.56±5.74, 80.72±7.82 and 62.46±6.88 cells were observed to
traverse the membrane following treatment with 7.5, 15 and 30 µM
PF, respectively (Fig. 1). The
results demonstrated that a statistically significant and
dose-dependent reduction in cell invasion capabilities was observed
in PF-treated cells when compared with control cells (F=99.51;
P<0.05; Fig. 1).
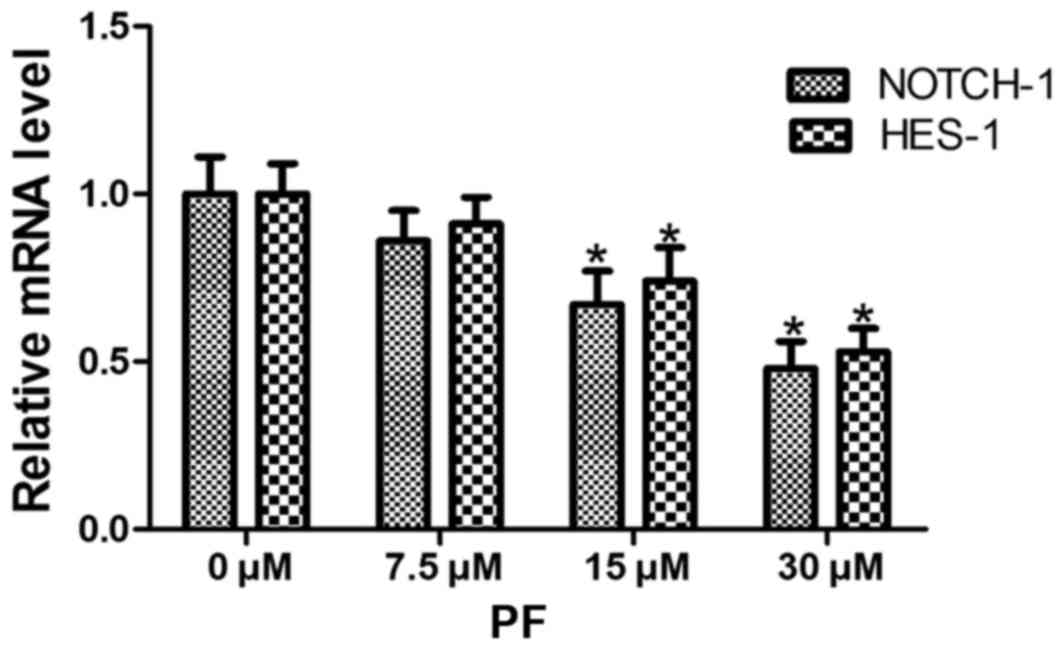
PF decreases NOTCH-1 and HES-1 mRNA
expression levels
The 0 µM PF-treated group served as a control, and
the β-actin gene served as an internal reference gene for the
analysis of NOTCH-1 and HES-1 mRNA levels. Following
treatment with PF for 48 h, the mRNA levels of NOTCH-1 and
HES-1 were determined using RT-qPCR. Different
concentrations of PF significantly decreased NOTCH-1 and HES-1 mRNA
expression (NOTCH-1, F=29.13, P<0.05; HES-1, F=29.97,
P<0.05). When compared with the results of the control group,
the mRNA expression levels NOTCH-1 and HES-1 decreased in a
dose-dependent manner, and were significantly different in the 15
and 30 µM PF groups (P<0.05). No significant differences were
observed between treatment with 7.5 µM PF and the control (0 µM PF)
group (Fig. 2).
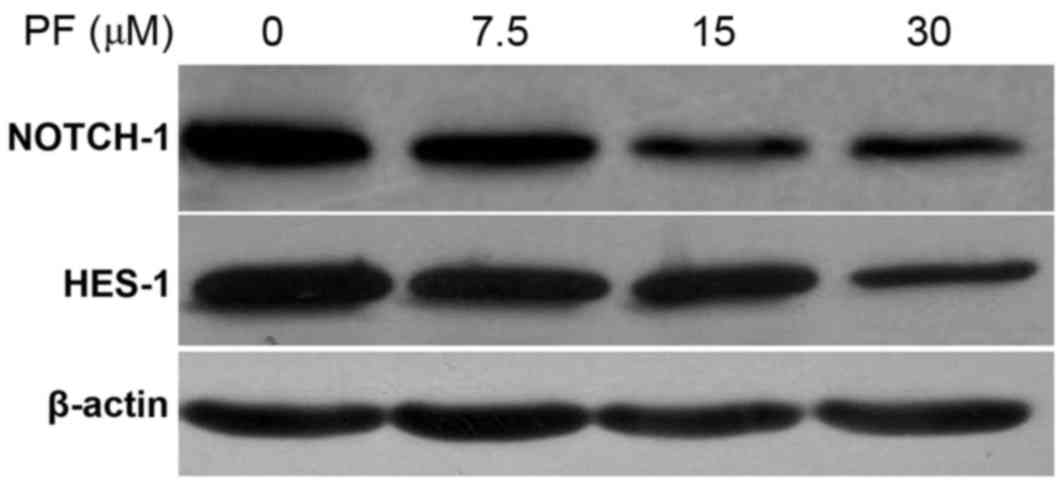
PF decreases NOTCH-1 and HES-1 protein
expression levels
Following treatment of MCF-7 cells with 0, 7.5, 15
and 30 µM PF for 48 h, the protein expression levels of NOTCH-1 and
HES-1 were detected via western blotting. The results are presented
in Table II and Fig. 3. The pattern of NOTCH-1 and HES-1
protein expression following PF treatment was similar to the
pattern of mRNA expression. The protein expression levels of
NOTCH-1 and HES-1 decreased with the increasing concentrations of
PF (Table II; Fig. 3). A statistically significant
difference in NOTCH-1 (t=22.15; P<0.05) and HES-1 (t=19.81;
P<0.05) protein expression was observed at all concentrations
(7.5, 15 and 30 µM) of PF tested when compared with the untreated
controls (0 µM PF; Table II).
 | Table II.Effect of paeoniflorin treatment on
HES-1 and NOTCH-1 protein expression levels. |
Table II.
Effect of paeoniflorin treatment on
HES-1 and NOTCH-1 protein expression levels.
|
| Paeoniflorin
concentration (µM) |
|---|
|
|
|
|---|
| Protein | 0 | 7.5 | 15 | 30 |
|---|
| NOTCH-1 |
1.12±0.06 |
0.98±0.05a |
0.61±0.07a |
0.55±0.04a |
| HES-1 |
1.08±0.08 |
0.94±0.07a |
0.79±0.06a |
0.56±0.05a |
Discussion
At present, the primary cause of mortality in breast
cancer is the recurrence and metastasis of tumors. Therefore,
inhibiting the invasion and metastasis of cancer cells is of great
importance. Current strategies that inhibit breast cancer
metastasis predominantly involve chemotherapeutic agents; however,
the low specificity of this procedure results in adverse and toxic
side effects. Medicinal plants used for the treatment of tumors
exhibit few toxic side effects, and may therefore be a more
effective treatment when compared with typical chemotherapy drugs
(22,23). Numerous studies have demonstrated
that PF exhibits antitumor effects (24,25),
and may be used for the treatment of cervical, lung and gastric
cancers. However, the role of PF in breast cancer remains to be
elucidated. Therefore, the present study investigated the role of
PF in the inhibition of MCF-7 breast cancer cell growth and
invasion in vitro.
The results of the present study demonstrated that
increasing concentrations and durations of treatment with PF were
associated with a decrease in the survival rate of MCF-7 cells. The
inhibitory effect of PF on MCF-7 cells was most significant
following treatment with 30 µM PF for 72 h, and the rate of growth
inhibition was ~52%. Therefore, PF demonstrated a significant
inhibitory effect on the proliferation of MCF-7 cells, in a dose-
and time-dependent manner. In addition, following treatment of
MCF-7 cells with PF for 48 h, cell invasion decreased with an
increasing dose, which suggests that PF successfully inhibited the
invasive ability of MCF-7 cells. Zhang et al (18) suggested that the proliferation and
invasion of MCF-7 and MDA-MB-231 breast cancer cells are inhibited
by PF at concentrations of 10, 20 and 40 µM, which is consistent
with the results of the present study. Therefore, the results of
the current study together with those of Zhang et al
(18), provide a theoretical
foundation for the future application of PF in the treatment of
breast cancer.
The NOTCH signaling pathway is a conserved signal
transduction pathway, which is important for cell development
(26,27) and apoptosis (28,29).
Out of the four mammalian NOTCH receptors, NOTCH-1 is the primary
receptor (30). It has previously
been demonstrated that the NOTCH-1 signaling pathway is associated
with the proliferation, invasion and metastasis of breast cancer
cells (20,31,32);
therefore, inhibition of this pathway may subsequently inhibit
these characteristics of breast cancer cells. The authors of the
present study investigated the potential role of the NOTCH
signaling pathway in the inhibition of breast cancer cell
proliferation and invasion following treatment with PF. Following
exposure of MCF-7 cells to PF for 48 h, the mRNA and protein
expression levels of NOTCH-1 and HES-1, which are involved in the
NOTCH-1 signaling pathway, were significantly decreased in a
dose-dependent manner. The results indicated that PF inhibited the
proliferation and invasion of breast cancer cells potentially via
inhibition of NOTCH-1 signal transduction. Zhang et al
(18) revealed that when
MDA-MB-231 breast cancer cells were treated with PF for 48 h at
concentrations of 10, 20 and 40 µM, the mRNA and protein expression
levels of NOTCH-1 were significantly reduced. In addition, when the
NOTCH-1 gene was silenced, the proliferation of breast cancer cells
was suppressed (18). The results
of the study by Zhang et al (18) together with the results of the
present study, confirmed that the proliferation and invasion
abilities of breast cancer cells are inhibited by PF, and that
inhibition of the NOTCH-1 signaling pathway may be involved.
In conclusion, the present study provided further
evidence to demonstrate that PF inhibits the proliferation and
invasion of breast cancer cells via inhibition of NOTCH-1 signal
transduction. However, the mechanisms underlying these effects may
be more complex. Further investigation is required to elucidate the
precise mechanism by which PF mediates inhibition of breast cancer
cell growth and invasion, in order to provide a theoretical basis
for the application of PF in the clinical treatment of breast
cancer.
References
|
1
|
Hortobagyi GN, de la Garza Salazar J,
Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC,
Martin M, Namer M, et al: The global breast cancer burden:
Variations in epidemiology and survival. Clin Breast Cancer.
6:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coughlin SS and Ekwueme DU: Breast cancer
as a global health concern. Cancer Epidemiol. 33:315–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zafrakas M, Papasozomenou P and
Emmanouilides C: Sorafenib in breast cancer treatment: A systematic
review and overview of clinical trials. World J Clin Oncol.
7:331–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeichner SB, Terawaki H and Gogineni K: A
review of systemic treatment in metastatic triple-negative breast
cancer. Breast Cancer (Auckl). 10:25–36. 2016.PubMed/NCBI
|
|
6
|
Gao J and Swain SM: Pertuzumab for the
treatment of breast cancer: A safety review. Expert Opin Drug Saf.
15:853–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castaneda SA and Strasser J: Updates in
the treatment of breast cancer with radiotherapy. Surg Oncol Clin N
Am. 26:371–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan HG, Malmgren JA and Atwood MK:
Triple-negative breast cancer in the elderly: Prognosis and
treatment. Breast J. 2017.Doi: 10.1111/tbj.12813. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YT, Lin YW, Chiu HM and Chiang BH:
Curcumin induces apoptosis of colorectal cancer stem cells by
coupling with CD44 marker. J Agric Food Chem. 64:2247–2253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim ID and Ha BJ: Paeoniflorin protects
RAW 264.7 macrophages from LPS-induced cytotoxicity and
genotoxicity. Toxicol In Vitro. 23:1014–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q,
Tan J and Ma Q: Protective effect of paeoniflorin on inflammation
and apoptosis in the cerebral cortex of a transgenic mouse model of
Alzheimer's disease. Mol Med Rep. 13:2247–2252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong SZ, Ge QH, Li Q, Qu R and Ma SP:
Peoniflorin attentuates Abeta((1–42))-mediated neurotoxicity by
regulating calcium homeostasis and ameliorating oxidative stress in
hippocampus of rats. J Neurol Sci. 280:71–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Chen GL, Li YJ, Chen Y and Lin FZ:
Paeoniflorin inhibits macrophage-mediated lung cancer metastasis.
Chin J Nat Med. 13:925–932. 2015.PubMed/NCBI
|
|
15
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
17
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Yuan Y, Cui J, Xiao T and Jiang
D: Paeoniflorin inhibits proliferation and invasion of breast
cancer cells through suppressing Notch-1 signaling pathway. Biomed
Pharmacother. 78:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Zhang J, Xiong N, Li S, Chen Y, Yang
H, Wu C, Zeng H and Liu Y: Notch-1 signaling activates NF-kappaB in
human breast carcinoma MDA-MB-231 cells via PP2A-dependent AKT
pathway. Med Oncol. 33:332016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pei J and Wang B: Notch-1 promotes breast
cancer cells proliferation by regulating LncRNA GAS5. Int J Clin
Exp Med. 8:14464–14471. 2015.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia X, Cole SPC, Cai T and Cai Y: Effect
of traditional Chinese medicine components on multidrug resistance
in tumors mediated by P-glycoprotein. Oncol Lett. 13:3989–3996.
2017.PubMed/NCBI
|
|
23
|
Jiao L, Dong C, Liu J, Chen Z, Zhang L, Xu
J, Shen X, Che J, Yang Y, Huang H, et al: Effects of Chinese
medicine as adjunct medication for adjuvant chemotherapy treatments
of non-small cell lung cancer patients. Sci Rep. 7:465242017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang LL, Zhang SL and Wang SZ: Relevant
study on apoptosis of cervical cancer HeLa cells induced by
paeoniflorin. Zhonghua Yi Xue Za Zhi. 90:3371–3375. 2010.(In
Chinese). PubMed/NCBI
|
|
25
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XY, Zhai WJ and Teng CB: Notch
Signaling in pancreatic development. Int J Mol Sci. 17:E482015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duval F, Mathieu M and Labrecque N: Notch
controls effector CD8+ T cell differentiation. Oncotarget.
6:21787–21788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang TP: Notch, apoptosis and cancer. Adv
Exp Med Biol. 727:199–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding Y and Shen Y: Notch increased
vitronection adhesion protects myeloma cells from drug induced
apoptosis. Biochem Biophys Res Commun. 467:717–722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma D, Dong X, Zang S, Ma R, Zhao P, Guo D,
Dai J, Chen F, Ye J and Ji C: Aberrant expression and clinical
correlation of Notch signaling molecules in breast cancer of
Chinese population. Asia Pac J Clin Oncol. 7:385–391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun DW, Zhang HD, Mao L, Mao CF, Chen W,
Cui M, Ma R, Cao HX, Jing CW, Wang Z, et al: Luteolin inhibits
breast cancer development and progression in vitro and in vivo by
suppressing Notch signaling and regulating MiRNAs. Cell Physiol
Biochem. 37:1693–1711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-kappaB activation. PLoS One. 9:e959122014.
View Article : Google Scholar : PubMed/NCBI
|