Introduction
Although fractures heal, bone nonunion remains one
of the major difficulties for bone fracture treatment (1). There were ~10,000 patients suffering
from bone fractures in the USA in 2012 (2). Bone nonunion causes significant
discomfort for patients, with body pain and mental distress, and
increases the burden for the patient, society and the economy
(3).
The typical therapeutic methods for bone nonunion
include sclerosis bone excision, bone nonunion tissue removal,
medullary cavity excavation, bone grafting and fixation (4). During the treatment process, many
studies have detected that the majority of hypertrophic nonunion
cases achieve bony union through compressive fixation; and atrophic
nonunion is generally accompanied by bone defects (5). Bone marrow mesenchymal stem cell
(BMMSC) implantation is not exposed and some bone nonunion can be
healed (6). However, the mechanism
underlying the reason that bone nonunion may be healed without
direct treatment of the fracture site remains unclear.
Studies have revealed that fracture healing is a
complicated and consecutive process (7,8). At
bone nonunion, hematoma and tissues between the fracture ends
gradually develop into tissues between the fracture ends of bone
nonunion (9). Tissues and hematoma
join the fracture ends and in vitro its reaction is similar
to that of bone mesenchymal stem cells. Such cells are separated
from tissues between fracture ends of bone nonunion (10). BMMSCs have multiple potentialities
and present positive expression to surface antigen of bone
mesenchymal stem cells (8).
Extracorporeal shock wave therapy (ESWT) is an
emerging treatment strategy, which is effective for muscle and
joint diseases (11). It is a
mechanical pulse pressure wave that is mediated by a physical
mechanism. It predominantly includes four types of shockwave
generator: Liquid electric, magnetic, piezoelectric and air
pressure ballistic (12). The
former three are types of focused shockwave and the latter is
radial. The former three types to work on the area that requires
treatment through focused shockwaves via X-ray or ultrasonic
location (12,13). The radial shockwave is to treat
larger areas of damaged tissue (14).
Purinergic receptor P2X 7 (P2X7) receptor is widely
expressed in a variety of tissues and exerts diverse physiological
functions (15). It mediates
intracellular signal transduction and intercellular signal
communication, and performs significant physiological actions
(16). For example, the ion
channel formed by the P2X7 receptor mediates the quick response of
cells on extracellular signals (17). P2X7 receptor activation by ATP
mediates intercellular communication, and causes a series of
physiological functions, such as cell apoptosis and necrosis
induced by macrophage fusion, induction of lymphocyte membrane
bubble formation, promotion of neurotransmitter release at nerve
endings, and expression and release of cytokines and chemokines
(15,18). ATP activates multiple intracellular
signaling pathways mediated by P2X7 (15).
Numerous endogenous cytokines induce differentiation
of bone mesenchymal stem cells (BMSCs) into chondrocytes (19). A recent study demonstrated that
cytokines promoting the differentiation of BMSCs into chondrocytes
include cartilage-derived morphogenetic protein, insulin-like
growth factor (IGF), bone morphogenetic protein (BMP) and
transforming growth factor-β (TGF-β) (20). Another study adopted IGF-I to
successfully induce the differentiation of mesenchymal stem cells
(MSCs) into chondrocytes, the majority of which used BMP-2/4 as
factors to induce the differentiation of MSCs into chondrocytes
(21).
For example, P2X7 receptor mediates the
interleukin-1 (IL-1) maturity of monocytes and macrophages, IL-1
release and precursor protein processing (18). The detection of activated P2X7
receptor excites and conducts associated signaling pathways of
phospholipase D (22). In
addition, P2X7 mediates proliferation, differentiation and other
reactions of extracellular signals by activating mitogen-activated
protein kinases. The aim of the present study was to evaluate the
effect of ESWT combined with bone marrow mesenchymal stem cell
(BMMSC) transplantation to improve bone repair in a rabbit bone
nonunion model.
Materials and methods
Experimental animals
A total of 24 purebred New Zealand rabbits (age, 5–6
months; weight, 2.5–3.0 kg) were randomly divided into three
groups: Sham (n=8), model (n=8) and combination (n=8). Rabbits were
anesthetized via an intramuscular injection of ketamine (50 mg/kg)
and positioned on an operating table. The right forelimb was shaved
and disinfected using iodine. Skin was sliced (2.5–3.0 cm in
length) to expose the radius. In the bone nonunion model and
combination group, a 15-mm length of bone was removed from the
mid-radius and controlled to ≤0.1 mm using Vernier calipers. A
rongeur was used for gouging the bone stump and the injury was
sealed using bone wax. The wound was closed using no. 1 silk
sutures and disinfected with iodine, and the rabbits were
administered oral amoxicillin for 12 days. The sutures were then
removed. The present study was approved by the Institute of Animal
Care and Use Committee at Southern Medical University (Guangdong,
China).
Study design
Bone marrow was extracted from the tibia of the New
Zealand rabbits (n=2-3) and mechanically disintegrated and diluted
with Sigma-Aldrich Dulbecco's modified Eagle's medium-low glucose
(DMEM-LG; Merck KGaA, Darmstadt, Germany). DMEM-LG was centrifuged
at 500 × g for 5 min at 4°C. Cells were resuspended using standard
growth medium (GM) consisting of DMEM-LG supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin-streptomycin and 0.5%
Fungizone® solution (Thermo Fisher Scientific, Inc.) and
seeded on one 100-mm culture dish. Fresh GM was replaced every 2–3
days. BMMSCs was induced for 2 weeks and then was used to study.
XY-K-SHOCKMASTER-500 ESW Power (Xiangyu Medical Equipment Company,
Anyang, China) was used to perform the ESWT therapy (12 kW, 0.45
mJ/mm2, 1,000 shockwaves, 60 per min). BMMSCs
(2×106/day) were injected at the wound site. The ESWT
session (which was painless) lasted ~6 min and was performed once
per day every 5 days for 15 days.
Biomechanical testing
Mechanical strength [Newton (N)] and fracture
stiffness (N/mm) were measured using three-point bending with a
mechanical testing machine (Zwick/Roell, 1446; Zwick GmbH & Co.
KG, Ulm, Germany) and calculated with regard to the radius of the
bone. The bearing distance between the two points of support was 21
mm, and three-point cantilever bending was applied at 2 mm/min with
the fulcrum placed over the fracture callus.
Histological scoring
The right forelimb was fixed in 10% neutral buffered
formalin for 4–5 days, decalcified for 15–20 days in 10% formic
acid and embedded in paraffin. Bone was cut into 5 µm longitudinal
sections and stained with hematoxylin and eosin at 37°C for 20 min.
Histological scoring was performed under a light microscope
(magnification, ×100) as previously described (1).
Alkaline phosphatase (ALP) miRNA
expression analysis by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the bone tissue of three
groups using Invitrogen TRIzol (Thermo Fisher Scientific, Inc.).
cDNA was reverse-transcribed using a One Step SYBR RT-PCR kit
(Takara Bio, Inc., Otsu, Japan). qPCR was performed using SYBR
Premix Ex Taq (Takara Bio, Inc.) with a ViiA7 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequences
of primers for ALP were as follows: forward-GTT TTC TGT TCT GTA AGA
CGG G and reverse-GCC GTT AAT TGA CGT TCC GA. Conditions were as
follows: 5 min at 95°C (one cycle); 30 sec at 94°C; 30 sec at 60°C
and 30 sec at 72°C (40 cycles); then 72°C for 5 min.
ELISA
Peripheral blood was collected from the eye socket
of the rabbits and centrifuged at 4,000 × g for 10 min at 4°C to
separate the serum. A BCA assay (P0009; Beyotime Institute of
Biotechnology, Haimen, China) was used to measure the protein
concentration according to manufacturer's protocols. Protein (10
µg) was used to measure the ALP activity, IGF-1 and vascular
endothelial growth factor (VEGF) expression levels, and TGF-β
contents were measured using ELISA kits (A059-2, H041, H044 and
H034 respectively; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's protocols.
Western blotting
Bone tissue was lysed in RIPA buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) to extract proteins. Equal
quantities (50 µg) of protein extracts were loaded and separated by
6–10% SDS-PAGE using 12% acrylamide gradients (80 V for 30 min; 120
V for 60 min) and transferred electrophoretically onto a
polyvinylidene difluoride membrane (GE Healthcare Life Sciences,
Little Chalfont, UK). Membranes were blocked with 5% nonfat dry
milk in tris-buffered saline containing 0.05% Tween-20 and
incubated with anti-osteopontin (OPN; sc-20788; 1:500), anti-runt
related transcription factor 2 (RUNX-2; sc-10758; 1:500),
anti-collagen type I a1 chain (COL1-A1; sc-28657; 1:500),
anti-BMP-2 (sc-402; 1:500), anti-BMP-4 (sc-9003; 1:500), P2X7
(sc-25698; 1:500) and GAPDH (sc-25778; 1:500; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Horseradish
peroxidase-conjugated anti-mouse immunoglobulin G (7074; 1:2,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) was incubated
for 1 h at room temperature. Protein bands were visualized by
enhanced chemiluminescence (P0018; Beyotime Institute of
Biotechnology).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between groups were calculated by one-way analysis of
variance and Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
ESWT combined with BMMSC
transplantation increases mechanical strength, fracture stiffness
and histological scoring in a rabbit bone nonunion model
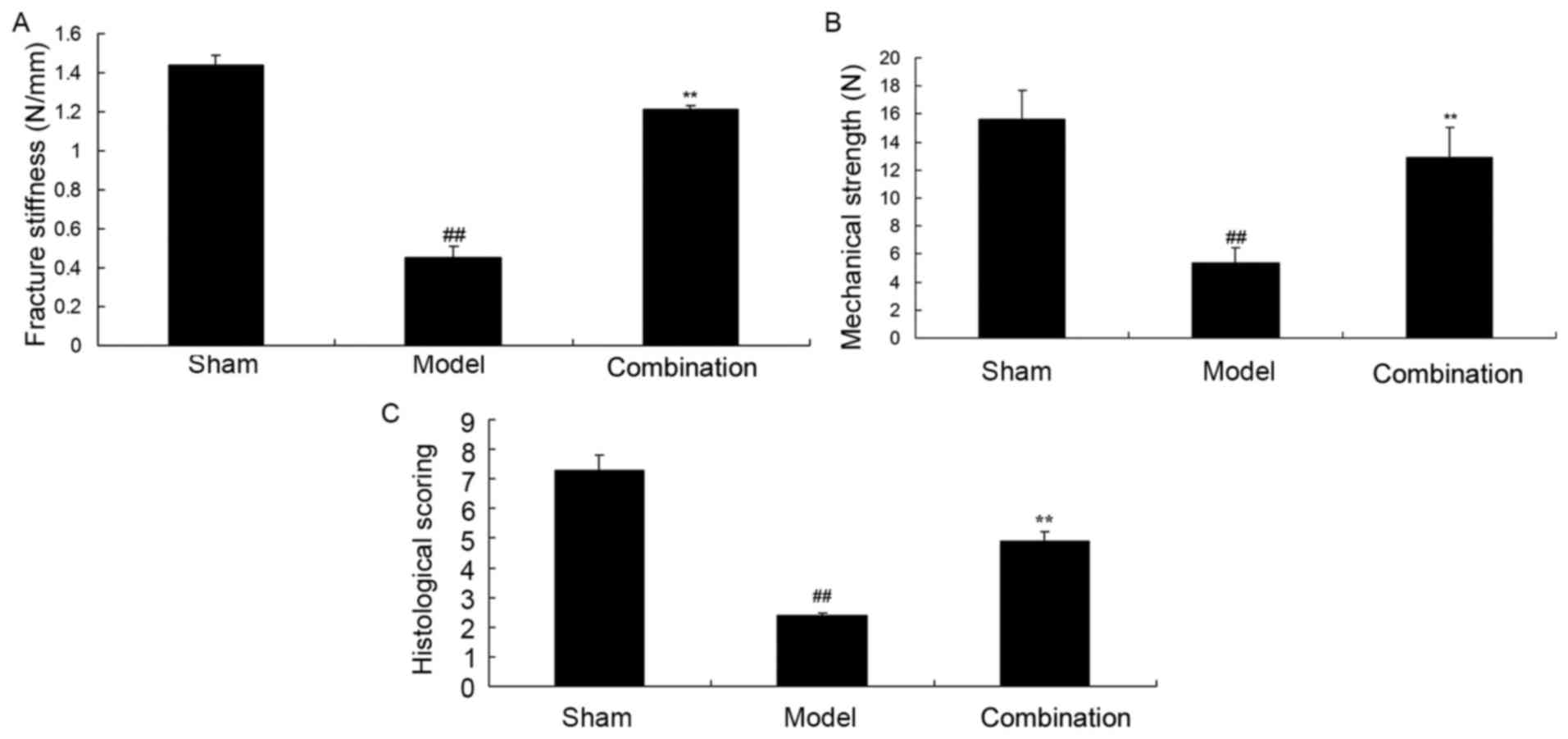
Fig. 1 demonstrated
that mechanical strength, fracture stiffness and histological
scoring were significantly inhibited in a rabbit bone nonunion
model, when compared with the sham group (P<0.01). Furthermore,
ESWT combined with BMMSC significantly decreased the inhibition of
mechanical strength, fracture stiffness and histological scoring in
the rabbit bone nonunion model (P<0.01; Fig. 1).
ESWT combined with BMMSC
transplantation increases ALP activity and miRNA expression levels
in a rabbit bone nonunion model
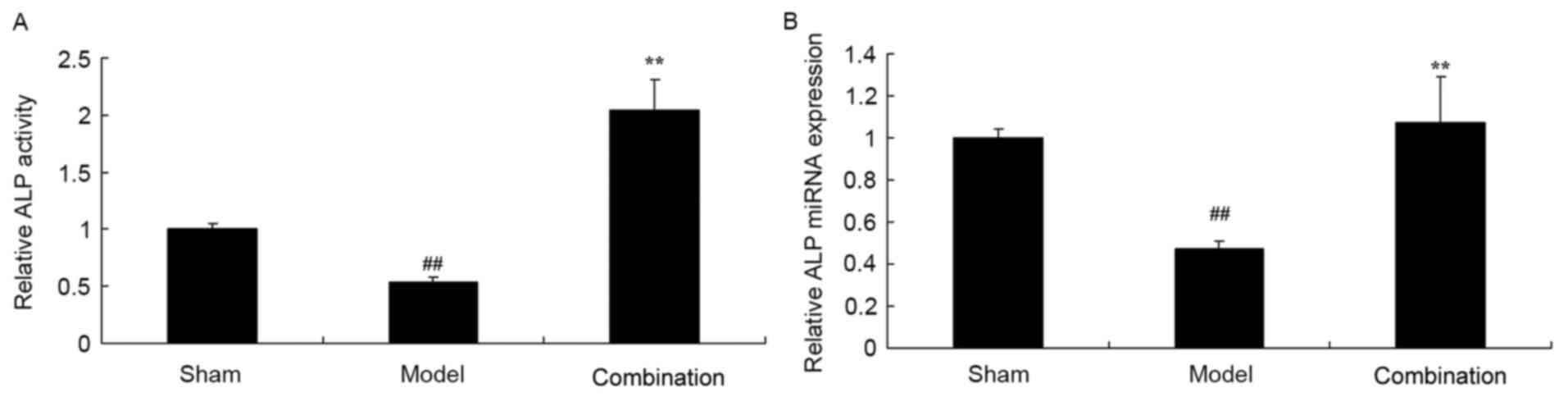
The results of ELISA and PCR demonstrated that ALP
activity and miRNA expression levels were significantly decreased
in a rabbit bone nonunion model group, as compared with the sham
control group (P<0.01; Fig. 2).
Furthermore, ESWT combined with BMMSC significantly enhanced ALP
activity and miRNA expression levels in the rabbit bone nonunion
model (P<0.01; Fig. 2).
ESWT combined with BMMSC
transplantation increases OPN, RUNX-2 and COL1-A1 protein
expression levels in a rabbit bone nonunion model
After 15 days of treatment, OPN, RUNX-2 and COL1-A1
protein expression levels in the rabbit bone nonunion model were
significantly suppressed, when compared with the sham group
(P<0.01; Fig. 3). ESWT combined
with BMMSC transplantation significantly induced OPN, RUNX-2 and
COL1-A1 protein expression levels in the rabbit bone nonunion model
(P<0.01; Fig. 3).
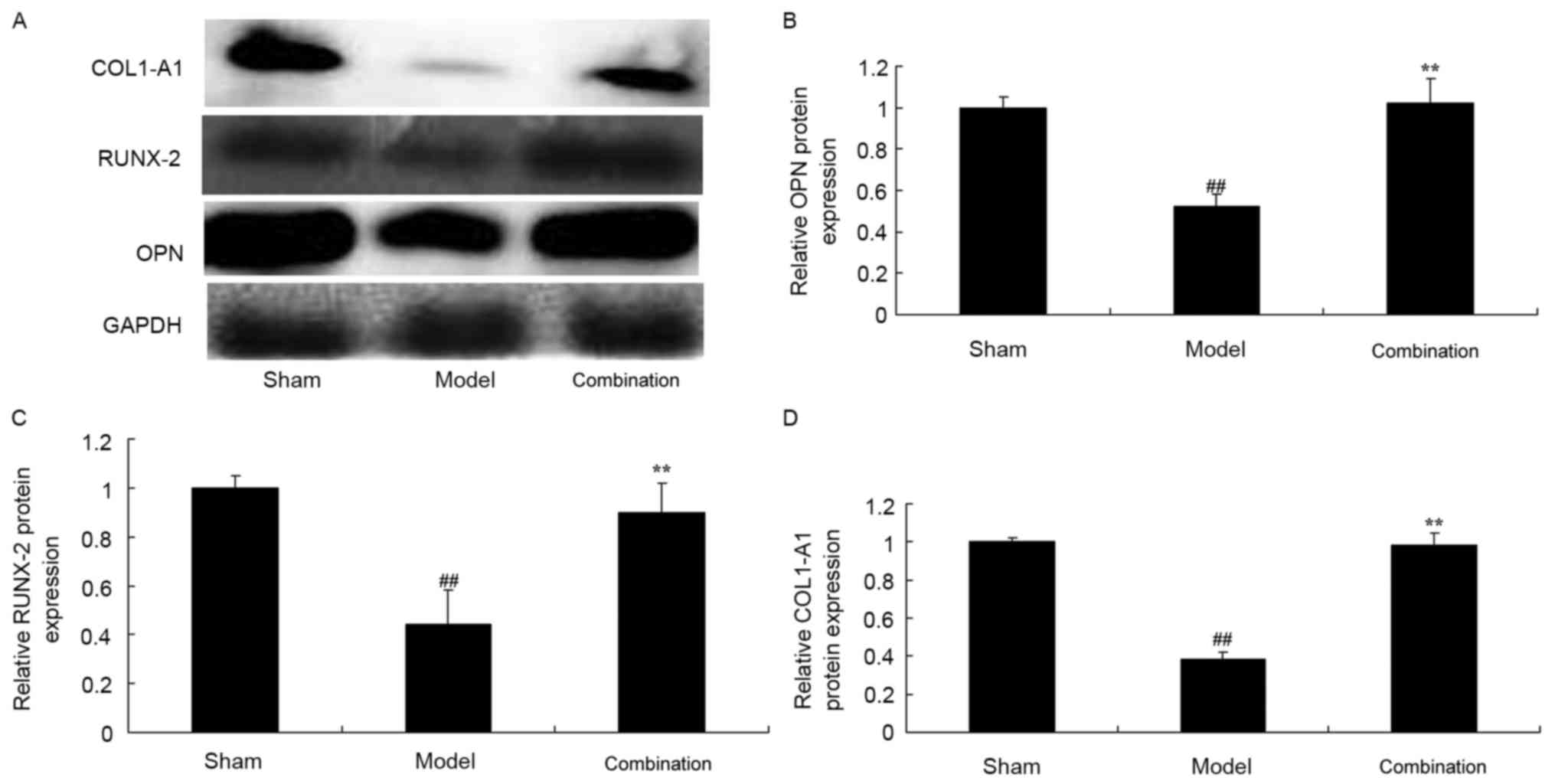 | Figure 3.ESWT combined with BMMSC
transplantation increased OPN, RUNX-2 and COL1-A1 protein
expression levels in a rabbit bone nonunion model. ESWT combined
with BMMSC transplantation enhanced OPN, RUNX-2 and COL1-A1 protein
expression levels. (A) Western blot analysis and statistical
analysis of (B) OPN, (C) RUNX-2 and (D) COL1-A1 protein expression
levels in a rabbit bone nonunion model. Sham, sham group; model,
bone nonunion model group; combination, ESWT combined with BMMSC
transplantation group. ##P<0.01 vs. sham group;
**P<0.01 vs. model group. ESWT, extracorporeal shock-wave
therapy; BMMSC, bone marrow mesenchymal stem cell; OPN,
osteopontin; RUNX-2, anti-runt related transcription factor 2;
COL1-A1, anti-collagen type I a1 chain. |
ESWT combined with BMMSC
transplantation enhances IGF-1, VEGF and TGF-β contents in a rabbit
bone nonunion model
To detect the underlying mechanism of ESWT combined
with BMMSC in bone nonunion, IGF-1, VEGF and TGF-β contents were
measured using ELISA kits. Fig. 4
indicates that the IGF-1, VEGF and TGF-β contents of the rabbit
bone nonunion model group were markedly lower than those of the
sham control group. ESWT combined with BMMSC transplantation
significantly promoted IGF-1, VEGF and TGF-β contents in the rabbit
bone nonunion model (Fig. 4).
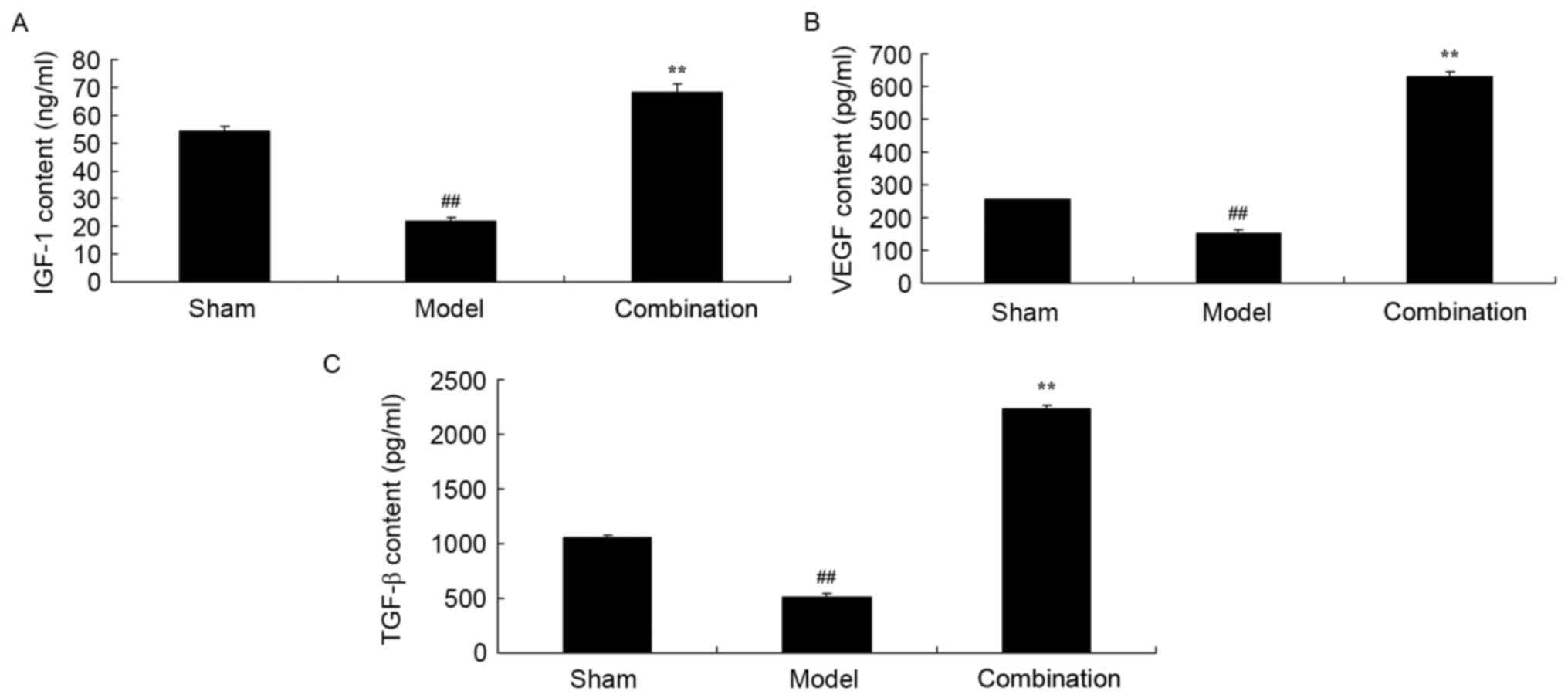 | Figure 4.ESWT combined with BMMSC
transplantation enhanced IGF-1, VEGF and TGF-β contents in a rabbit
bone nonunion model. ESWT combined with BMMSC transplantation
enhanced (A) IGF-1, (B) VEGF and (C) TGF-β contents in a rabbit
bone nonunion model. Sham, sham group; model, bone nonunion model
group; combination, ESWT combined with BMMSC transplantation group.
##P<0.01 vs. sham group; **P<0.01 vs. model group.
ESWT, extracorporeal shock-wave therapy; BMMSC, bone marrow
mesenchymal stem cell; IGF-1, insulin-like growth factor; TGF,
transforming growth factor-β; VEGF, vascular endothelial growth
factor. |
ESWT combined with BMMSC
transplantation enhances P2X7, BMP-2 and BMP-4 protein expression
levels in the rabbit bone nonunion model
In the current study, the inhibition of P2X7, BMP-2
and BMP-4 protein expression levels in a rabbit bone nonunion model
were observed, compared with the sham control group (Fig. 5). After day 15, ESWT combined with
BMMSC transplantation significantly elevated P2X7, BMP-2 and BMP-4
protein expression levels in the rabbit bone nonunion model
(Fig. 5).
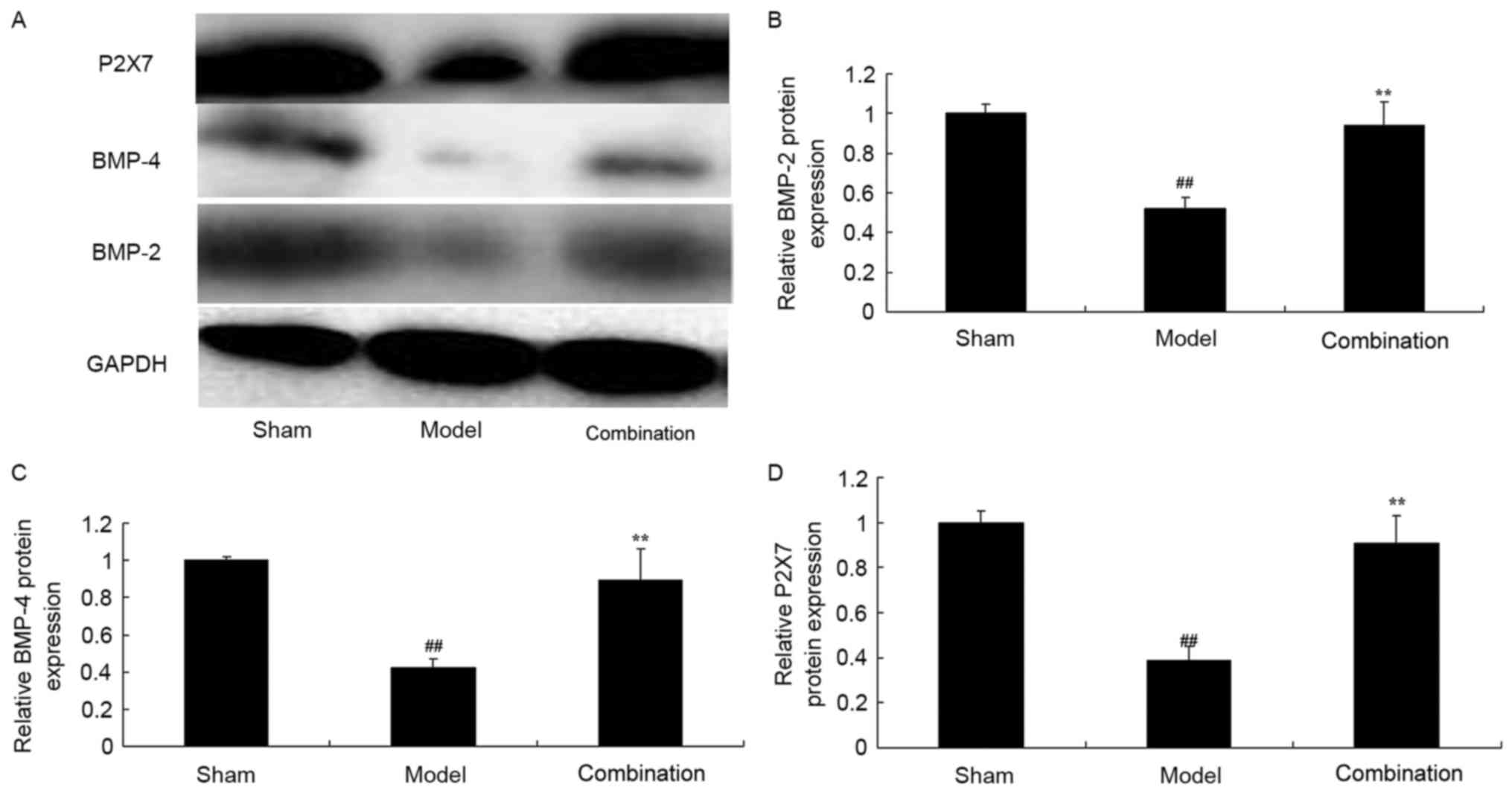 | Figure 5.ESWT combined with BMMSC
transplantation enhanced P2X7, BMP-2 and BMP-4 protein expression
levels in a rabbit bone nonunion model. ESWT combined with BMMSC
transplantation enhanced P2X7, BMP-2 and BMP-4 protein expression
levels. (A) Western blot analysis and statistical analysis of (B)
BMP-2, (C) BMP-4 and (D) P2X7 protein expression levels in a rabbit
bone nonunion model. Sham, sham group; model, bone nonunion model
group; combination, ESW combined with BMMSC group.
##P<0.01 vs. sham group; **P<0.01 vs. model group.
ESWT, extracorporeal shock-wave therapy; BMMSC, bone marrow
mesenchymal stem cell; BMP, bone morphogenetic protein; P2X7,
purinergic receptor P2X 7l. |
Discussion
Fracture and bone tissue damage are common and
serious problems in the clinical setting (6). With the population aging, this type
of problem is becoming increasingly prominent (23). Development of artificial bone with
high biocompatibility, that is completely biodegradable with a
degradation rate that matches bone cell growth rate, with high
biological activity and stable mechanical properties is an
effective approach for treating the above-mentioned bone issues
(5).
In recent years, MSCs have been a particularly
popular research topic, with the most development potential
(6). MSCs differentiate into
various mesodermal cell types, such as fat precursor cells, bone
cells and cartilage cells, and differentiate to bone, heart and
smooth muscle cells (7).
Furthermore, MSCs present morphological characteristics of
non-mesoblast cells, such as neuronal and hepatic cells (7). The present results indicated that
ESWT combined with BMMSC transplantation effectively enhanced
mechanical strength, fracture stiffness and histological scoring,
and increased ALP activity in a rabbit bone nonunion model.
RUNX-2 is a key transcription factor that controls
osteogenic differentiation of human MSCs (24). The cytokine regulates regulatory
proteins jointly and induces the undifferentiated progenitors to
differentiate into osteoblasts (24). Furthermore, Osterix (Osx) and
RUNX-2 regulate the transcription factor of osteogenic
differentiation (25). In
particular, bone cells are essential for regulating the effects of
early- and late-stage osteoblast differentiation (25). RUNX-2 and Osx combination initiates
the differentiation of preosteoblast (which expresses type I
collagen and bone sialoprotein) to osteoblasts. Subsequently, it
induces and activates transcription factors, and induces the
maturity of osteoblasts jointly with Osx and various Wnt/β-link
protein signaling components (25). Collectively, the present study
demonstrated that ESWT combined with BMMSC transplantation promoted
ALP miRNA expression levels and activity, and induced OPN, RUNX-2
and COL1-A1 protein expression in a rabbit bone nonunion model.
It remains controversial as to whether there are
P2X7 receptors in osteoblast cell lines (15). Previously, it was detected that the
MG-63 osteogenesis cell strain contains P2X7 transcriptional
factors (26). However, P2X7
specific receptor adjusts osteoblast differentiation (27). Subsequently, in vitro immune
cell chemical analysis and membrane pore forming reaction
experiments demonstrated P2X7 receptor expression in a human
bone-derived stem cell subgroup (22). In addition, whether ESWT combined
with BMMSC transplantation significantly increased P2X7 protein
expression levels in a rabbit bone nonunion model was investigated
in the present study.
Studies have also indicated that following bone
fracture, TGF-β is activated with extensive expression, and this
increased expression is maintained for the duration of the healing
process; in addition, ectogenic TGF-β stimulates osteoblasts and
accelerates fracture rehabilitation (28). Studies on bone formation and
fracture recovery must not ignore the effects of cytokines and
polypeptide GFs, and must focus on the effects of TGF-β (28,29).
IGF-1 exerts moderate mitosis effects on
osteoblasts. It regulates cell cycle activities and exerts
para-insulin effects. IGF-1 is an essential GF for bone cell
secretion (30). It contributes to
promoting the formation of osteoclasts, stimulating the activity of
osteoclasts, adjusting bone resorption and participating in bony
remodeling (31). Notably, the
current study indicated that ESWT combined with BMMSC
transplantation significantly promoted IGF-1, VEGF and TGF-β
contents in a rabbit bone nonunion model.
BMP are important in the growth and development of
bones, as well as during rehabilitation following trauma. However,
the primary function is to induce the formation of bones (29). BMPs induce specific,
undifferentiated and active ectomesenchymal cells in the muscles
and around blood vessels to differentiate them into cartilage and
osteocytes. The process is irreversible (32). The bone induction ability of BMPs
primarily presents on cartilage, muscles and blood vessels
(32). For liver, spleen, kidney
and other organs, however, it does not exhibit an obvious bone
induction ability. Currently, BMP is caused by different reactions
of mesenchymal cells (33). It
also indicates that the osteoinductive activity of BMP is closely
associated with the surrounding environment. BMPs show a strong
ability to induce bone formation at cartilage muscles and around
blood vessels (34). Studies have
confirmed that BMPs binding to receptors may phosphorylate and
release Smad proteins, and subsequently enter into the nucleus to
activate the transcription and expression of specific genes,
resulting in osteogenic and chondrogenic differentiation (19,21).
BMP-2/4 is vital to cartilage tissue engineering, and is considered
to be associated with MSCs regulating the cell cycle and
differentiation of chondrocytes; thus, BMP-2/4 facilitates the
synthesis and secretion of cartilage matrix (35). In the current study, ESWT combined
with BMMSC transplantation significantly induced BMP-2 and BMP-4
protein expression levels in a rabbit bone nonunion model. Pfaff
et al (36) demonstrated
that ESWT enhances bone healing via BMP-2, BMP-4, IGF-1, VEGF, and
TGF-β expression (36).
In conclusion, the present study demonstrated that
ESWT combined with BMMSC transplantation improves bone repair in a
rabbit bone nonunion model via BMPs and P2X7 expression. ESWT
combined with BMMSC transplantation was presented as a novel and
effective method to improve bone repair in a rabbit bone nonunion
model, which may be useful in clinical applications. However, this
study only employed an in vivo model, which is a limitation,
model study or clinical research are required for further
study.
Acknowledgements
The present study was supported by Southern Medical
University Scientific Research Start-up Program and Guangdong
Province Science and Technology Project (grant no. 2017ZC0121).
References
|
1
|
Yin P, Zhang L, Li T, Zhang L, Wang G, Li
J, Liu J, Zhou J, Zhang Q and Tang P: Infected nonunion of tibia
and femur treated by bone transport. J Orthop Surg Res. 10:492015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vedung T and Vinnars B: Ectopic bone
formation after medial femoral condyle graft to scaphoid nonunion.
J Wrist Surg. 3:46–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malizos KN, Koutalos A, Papatheodorou L,
Varitimidis S, Kontogeorgakos V and Dailiana Z: Vascularized bone
grafting and distal radius osteotomy for scaphoid nonunion advanced
collapse. J Hand Surg Am. 39:872–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zura R, Mehta S, Della Rocca GJ and Steen
RG: Biological risk factors for nonunion of bone fracture. JBJS
Rev. 4:pii: 01874474-201601000-00005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong L, Harhaus L, Heffinger C, Bickert
B, Kremer T, Kneser U and Hirche C: A comparative study on
autologous bone grafting combined with or without posterior
interosseous nerve neurectomy for scaphoid nonunion treatment. J
Plast Reconstr Aesthet Surg. 68:1138–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ismail HD, Phedy P, Kholinne E, Djaja YP,
Kusnadi Y, Merlina M and Yulisa ND: Mesenchymal stem cell
implantation in atrophic nonunion of the long bones: A
translational study. Bone Joint Res. 5:287–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathieu M, Rigutto S, Ingels A, Spruyt D,
Stricwant N, Kharroubi I, Albarani V, Jayankura M, Rasschaert J,
Bastianelli E and Gangji V: Decreased pool of mesenchymal stem
cells is associated with altered chemokines serum levels in
atrophic nonunion fractures. Bone. 53:391–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1543–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koga T, Lee SY, Niikura T, Koh A, Dogaki
Y, Okumachi E, Akisue T, Kuroda R and Kurosaka M: Effect of
low-intensity pulsed ultrasound on bone morphogenetic protein
7-induced osteogenic differentiation of human nonunion
tissue-derived cells in vitro. J Ultrasound Med. 32:915–922. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ismail HD, Phedy P, Kholinne E, Kusnadi Y,
Sandhow L and Merlina M: Existence of mesenchymal stem cellsin
sites of atrophic nonunion. Bone Joint Res. 2:112–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed-Maldonado AB and Lue TF: Re: A
meta-analysis of extracorporeal shock wave therapy for Peyronie's
disease. Eur Urol. 70:895–896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Argüelles-Salido E, Campoy-Martínez P,
Aguilar-García J, Podio-Lora V and Medina-López R: Prediction of
the energy required for extracorporeal shock wave lithotripsy of
certain stones composition using simple radiology and computerized
axial tomography. Actas Urol Esp. 38:115–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai L, Sun N, Zhang B, Liu ST, Zhao Z,
Jin HC, Ma XL and Xing GY: Effects of focused extracorporeal shock
waves on bone marrow mesenchymal stem cells in patients with
avascular necrosis of the femoral head. Ultrasound Med Biol.
42:753–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH and Cho SH: Effect of
extracorporeal shock wave therapy on denervation atrophy and
function caused by sciatic nerve injury. J Phys Ther Sci.
25:1067–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun D, Junger WG, Yuan C, Zhang W, Bao Y,
Qin D, Wang C, Tan L, Qi B, Zhu D, et al: Shockwaves induce
osteogenic differentiation of human mesenchymal stem cells through
ATP release and activation of P2X7 receptors. Stem Cells.
31:1170–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang SW, Walker C, Pennock J, Else K,
Muller W, Daniels MJ, Pellegrini C, Brough D, Lopez-Castejon G and
Cruickshank SM: P2X7 receptor-dependent tuning of gut epithelial
responses to infection. Immunol Cell Biol. 95:178–188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheung WY, Fritton JC, Morgan SA,
Seref-Ferlengez Z, Basta-Pljakic J, Thi MM, Suadicani SO, Spray DC,
Majeska RJ and Schaffler MB: Pannexin-1 and P2X7-receptor are
required for apoptotic osteocytes in fatigued bone to trigger RANKL
production in neighboring bystander osteocytes. J Bone Miner Res.
31:890–899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakaki H, Fujiwaki T, Tsukimoto M, Kawano
A, Harada H and Kojima S: P2X4 receptor regulates P2X7
receptor-dependent IL-1β and IL-18 release in mouse bone
marrow-derived dendritic cells. Biochem Biophys Res Commun.
432:406–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bach FC, Miranda-Bedate A, van Heel FW,
Riemers FM, Müller MC, Creemers LB, Ito K, Benz K, Meij BP and
Tryfonidou MA: Bone morphogenetic protein-2, but not mesenchymal
stromal cells, exert regenerative effects on canine and human
nucleus pulposus cells. Tissue Eng Part A. 23:233–242. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CL, Xiao F, Wang CD, Zhu JF, Shen C,
Zuo B, Wang H, Li, Wang XY, Feng WJ, et al: Gremlin2 suppression
increases the BMP-2-induced osteogenesis of human bone
marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2
signaling pathway. J Cell Biochem. 118:286–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavery K, Swain P, Falb D and
Alaoui-Ismaili MH: BMP-2/4 and BMP-6/7 differentially utilize cell
surface receptors to induce osteoblastic differentiation of human
bone marrow-derived mesenchymal stem cells. J Biol Chem.
283:20948–20958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Falk S, Schwab SD, Frøsig-Jørgensen M,
Clausen RP, Dickenson AH and Heegaard AM: P2X7 receptor-mediated
analgesia in cancer-induced bone pain. Neuroscience. 291:93–105.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller NA, Calcagni M and Giesen T:
Treatment of painful nonunion of the distal phalanx in the finger
with bone graft and dorsal reverse adipofascial flap based on an
exteriorized pedicle. Tech Hand Up Extrem Surg. 19:115–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Ge C and Franceschi RT: MAP
kinase-dependent RUNX2 phosphorylation is necessary for epigenetic
modification of chromatin during osteoblast differentiation. J Cell
Physiol. 232:2427–2435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative effect of Mir-205 on osteogenic differentiation of bone
mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and
ERK/MAPK pathway. Int J Mol Sci. 16:10491–10506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sollazzo V, Palmieri A, Pezzetti F,
Massari L and Carinci F: Effects of pulsed electromagnetic fields
on human osteoblastlike cells (MG-63): A pilot study. Clin Orthop
Relat Res. 468:2260–2277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grol MW, Brooks PJ, Pereverzev A and Dixon
SJ: P2X7 nucleotide receptor signaling potentiates the
Wnt/β-catenin pathway in cells of the osteoblast lineage.
Purinergic Signal. 12:509–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ota K, Quint P, Weivoda MM, Ruan M,
Pederson L, Westendorf JJ, Khosla S and Oursler MJ: Transforming
growth factor beta 1 induces CXCL16 and leukemia inhibitory factor
expression in osteoclasts to modulate migration of osteoblast
progenitors. Bone. 57:68–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau KW, Rundle CH, Zhou XD, Baylink DJ and
Sheng MH: Conditional deletion of IGF-I in osteocytes unexpectedly
accelerates bony union of the fracture gap in mice. Bone. 92:18–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou JM, Chen EY, Lin F, Lin QM, Xue Y, Lan
XH and Wu M: Lactoferrin induces osteoblast growth through IGF-1R.
Int J Endocrinol. 2015:2828062015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peeters M, Detiger SE, Karfeld-Sulzer LS,
Smit TH, Yayon A, Weber FE and Helder MN: BMP-2 and BMP-2/7
heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a
large animal model of mild intervertebral disc degeneration. Biores
Open Access. 4:398–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Visser R, Bodnarova K, Arrabal PM,
Cifuentes M and Becerra J: Combining bone morphogenetic proteins-2
and −6 has additive effects on osteoblastic differentiation in
vitro and accelerates bone formation in vivo. J Biomed Mater Res A.
104:178–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HY, Lee JH, Yun JW, Park JH, Park BW,
Rho GJ, Jang SJ, Park JS, Lee HC, Yoon YM, et al: Development of
porous beads to provide regulated BMP-2 stimulation for varying
durations: In Vitro and In Vivo studies for bone regeneration.
Biomacromolecules. 17:1633–1642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G,
Li Q, Chen X, Ji J, Zhang Y and OuYang HW: The promotion of bone
regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by
effects on integrin-BMP/Smad signaling pathway in BMSCs.
Biomaterials. 34:4404–4417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pfaff JA, Boelck B, Bloch W and Nentwig
GH: Growth factors in bone marrow blood of the mandible with
application of extracorporeal shock wave therapy. Implant Dent.
25:606–612. 2016. View Article : Google Scholar : PubMed/NCBI
|