Introduction
Allergic rhinitis (AR) is an immunoglobulin
E-mediated type of inflammation of the upper airway, which is
induced by allergens and regulated by T cells. AR has an estimated
worldwide incidence rate of 10–20% (1). The authors' previous epidemiological
investigations demonstrated that, in western China, the prevalence
of self-reported AR was 32.30% in Chongqing, 34.3% in Chengdu,
37.9% in Urumqi, and 30.3% in Nanning (2). AR has a major effect on quality of
life by causing symptoms of sneezing, nasal congestion, nasal
pruritus, rhinorrhea and obstruction of the nasal passages.
Furthermore, AR is a known risk factor for comorbid conditions,
including asthma, rhinosinusitis, nasal polyposis and sleep
disorders, resulting in important medical and social problems
(3,4). Over the last 20 years, the
pathogenesis of AR has been widely investigated and the majority of
studies have focused on proinflammatory cytokines. However,
anti-inflammatory cytokines in AR have received less attention.
Interleukin (IL)-37, a novel member of the IL-1
family and originally termed IL-1F7, has been shown to be a natural
suppressor of innate immunity and inflammatory responses (5,6).
IL-1F7b is the largest variant, and is referred to as IL-37 in the
present study. IL-37 interacts with the cell surface receptors,
IL-18Ro and IL-18-binding protein (BP), and has five splice
variants (IL-1F7a-e) (7). It is
expressed in human peripheral blood mononuclear cells (PBMCs) and
various tissues at low levels, and can be induced by inflammatory
stimulation, including Toll-like receptor agonists. A previous
study demonstrated that the expression of IL-37 in dendritic cells
(DCs) promoted the generation of semimature tolerogenic DCs and
suppressed antigen-specific immune responses of the skin, which
revealed IL-37 as an inhibitor of the adaptive immune response
(8). The abnormal expression of
IL-37 has been reported in several inflammation-related diseases,
including Vogt-Koyanagi-Harada disease (9–11),
inflammatory bowel disease (12),
systemic lupus erythematosus (13), Graves' disease (14), ankylosing spondylitis (15) and rheumatoid arthritis (16,17).
In addition, Lunding et al (18) demonstrated that IL-37 ablated a Th2
cell-directed allergic inflammatory response and the hallmarks of
experimental asthma in mice, suggesting that IL-37 may be critical
for the pathogenesis of asthma. It has also been reported that
IL-37 is an important cytokine in the control of asthma by
suppressing the production of inflammatory cytokines, tumor
necrosis factors, IL-β, IL-6 and thymic stromal lymphopoietin
(19,20). These findings indicate that IL-37
may be involved in inflammatory responses, and may have an
immunosuppressive role in allergic disease. However, whether there
are any correlations between IL-37 and AR remain to be elucidated,
and the function of IL-37 in AR warrants further investigation.
The present study aimed to determine the mRNA and
protein levels of IL-37 in PBMCs from patients with AR, in order to
evaluate the expression of IL-37 in AR. This was followed by
examination of the possible immunosuppressive effect of IL-37 on
inflammatory mediators and CD4+ T cells in the
pathogenesis of AR.
Materials and methods
Subjects
Overall, 39 patients (23 women and 16 men) between
12 and 52 years of age were recruited, between April 2015 and
September 2015. All patients were identified by and treated at the
outpatient clinic of the Department of Otolaryngology, Head and
Neck Surgery at the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China). The diagnosis of AR was based on the
patients' medical history, symptoms and the presence of a positive
skin prick test (SPT; Allergopharma, Hamburg, Germany) in response
to a panel of common allergens defined by the Allergic Rhinitis and
its Impact on Asthma 2008 guidelines (21). The SPT results were diagnosed in
accordance with these commendations of the Subcommittee on Allergen
Standardization and Skin Tests of the European Academy of Allergy
and Clinical Immunology (22). A
positive SPT result was defined as the formation of a wheal
measuring ≥50% of the diameter of the histamine control wheal, and
≥3 mm larger than the diameter of the negative control wheal. A
total of 18 inhaled allergens were assessed, including house dust,
grass, tree, mold, food, and cat and dog dander. Patients with
accompanying systemic disease were excluded from the study. An
additional 43 healthy volunteers of the same ethnicity as the
patients were recruited as a control group.
Ethics statement
The local Ethics Committee of the First Affiliated
Hospital of Chongqing Medical University provided permission and
assisted in obtaining informed consent from all participants.
Written informed consent was obtained from all participants.
Informed consent was obtained from the next of kin, caretakers or
guardians of any minors involved in the study.
Cell isolation and culture
The PBMCs were obtained using standard
Ficoll-Hypaque density centrifugation (TBD Science, Tianjin, China)
at 800 × g for 25 min at room temperature within 1 h of collection.
The cells were then washed twice with phosphate-buffered saline
(PBS) and resuspended at 1×106/ml in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, 100 ium
supplemented with 10 mmol/l L-glutamine. To investigate the effect
of IL-37 on the production of proinflammatory cytokines by the
PBMCs, the PBMCs from four controls were stimulated with 100 ng/ml
lipopolysaccharide (LPS; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) in the presence of recombinant IL-37 (rIL-37) at different
concentrations (0, 50, 100 and 200 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) for 72 h at room temperature. Following
incubation, the cells and culture supernatants were collected to
analyze the RNA and protein levels of cytokines. The duration of 72
h was selected according to a previous study; therefore, the
cytokines were not measured at different time-points (10). Subsequently, the PBMCs from seven
patients with AR and six controls were re-suspended at a
concentration of 1×106 cells/ml and stimulated with or
without 100 ng/ml rIL-37 in the presence of 100 ng/ml LPS for 72 h
at room temperature, following which the culture supernatants were
harvested and frozen at −80°C for cytokine analysis using an
enzyme-linked immunosorbent assay (ELISA). The cells in the control
groups were stimulated with the same volume of PBS alone.
To investigate the effect of IL-37 on effector
cytokine production by CD4+ T cells, the CD4+
T cells were separated from the PBMCs obtained the AR patients and
controls using magnetic microbeads (CD4+ cell purity
≥98%; Miltenyi Biotec, Inc., Cambridge, MA, USA). The
CD4+ T cells were re-suspended at a concentration of
1×106 cells/ml and stimulated with anti-CD3/CD28
(eBioscience; Thermo Fisher Scientific, Inc.) in the presence or
absence of 100 ng/ml rIL-37 for 72 h to detect the levels of IL-10
and IL-17 in the supernatants.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA levels of IL-37 and proinflammatory
cytokines from the PBMCs were determined using RT-qPCR analysis.
Total RNA was extracted from the isolated PBMCs using TRIzol
extraction (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions, and was reverse transcribed to
cDNA using random hexamer primers and RNase H-reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). The expression levels
of mRNA were determined using the ABI Prism 7500 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
SYBR Premix Taq (Takara Biotechnology Co., Ltd., Dalian, China).
The PCR primer sequences are summarized in Table I. PRISM samples contained 1X SYBR
Green Master Mix, 1.5 µl, 5 µM primers, and 25 ng synthesized cDNA
in a 25 µl volume. Reaction mixtures were heated to 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 10 sec, and
annealing extension at 60°C for 60 sec. All PCR reactions were
performed in duplicate. The PCR products were verified by melting
curve analysis. Relative mRNA levels of target genes were
calculated by the 2−ΔΔcq method (23).
 | Table I.List of the sequences of human gene
primers. |
Table I.
List of the sequences of human gene
primers.
| Primer, length
(bp) | Sequence (5′-3′) |
|---|
| IL-37, 25 | F:
AGTGCTGCTTAGAAGACCCGG |
|
| R:
AGAGTCCAGGACCAGTACTTTGTGA |
| IL-1, 20 | F:
ACCAAACCTCTTCGAGGCAC |
|
| R:
AGCCATCATTTCACTGGCGA |
| IL-27EBI3, 10 | F:
TTACAAGCGTCAGGGAGCTG |
|
| R:
TTCCCCGTAGTCTGTGAGGT |
| IL-27P28, 22 | F:
CTGGACCAACATGGAGAGGATG |
|
| R:
TAAGACGAGCTCCAAGGAGTG |
| IL-6, 21 | F:
AGCCACTCACCTCTTCAGAAC |
|
| R:
ACATGTCTCCTTTCTCAGGGC |
| IL-10, 20 | F:
TACGGCGCTGTCATCGATTT |
|
| R:
TAGAGTCGCCACCCTGATGT |
| β-actin, 22 | F:
CCTGACTGACTACCTCATGAAG |
|
| R:
GACGTAGCACAGCTTCTCCTTA |
ELISA analysis
The concentrations of IL-1entrationls of and IL-27
in the culture supernatants of the PBMCs were assayed using
specific ELISA kits (R&D Systems, Inc.) according to the
manufacturer's instructions. All assays were performed in
duplicate. The results are expressed in pg/ml. In preliminary
experiments, the serum level of IL-37 was determined using ELISA,
however, the level of IL-37 was too low to be detected. Therefore,
in the serum level of IL-37 was not examined in the present
study.
Flow cytometry
For analysis of the expression of IL-37, the PBMCs
were fixed with BD Cytofix™ fixation buffer (BD Biosciences,
Franklin Lakes, NJ, USA) at 37°C for 10 min and permeabilized in BD
Phosflow™ Perm Buffer III (BD Biosciences) at 4°C for 30 min. The
cells were then incubated with anti-IL-37 antibody (cat. no.
ab116282, 1:200; Abcam, Cambridge, MA, USA) for 30 min at 22°C.
Subsequently, the cells were stained with PE goat anti-mouse
immunoglobulin (cat. no. 1010-09, 1:100; 4A Biotech Co., Ltd.,
Beijing, China), which was incubated with the cells for 30 min at
22°C according to the manufacturer's instructions. The fluorescence
profiles were analyzed using a FACScan cytometer equipped with
CellQuest software 4.0 (BD Biosciences) in terms of the mean
fluorescence intensity (MFI). The results were calculated as
increments relative to the isotypic control (IC) using the
following formula: (MFI of sample – MFI of IC)/MFI of IC. The data
were processed using FlowJo software 10 (Treestar, Inc., Ashland,
OR, USA).
Statistical analysis
The software used for statistical analysis was SPSS
for Windows (version 20.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Differences between the
values were determined using an independent samples t-test or
paired sample t-test. Grouped data were analyzed using a one-way
analysis of variance, followed by the Student-Newman-Keuls test.
When the equal variance test failed, a Mann-Whitney Rank Sum test
was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
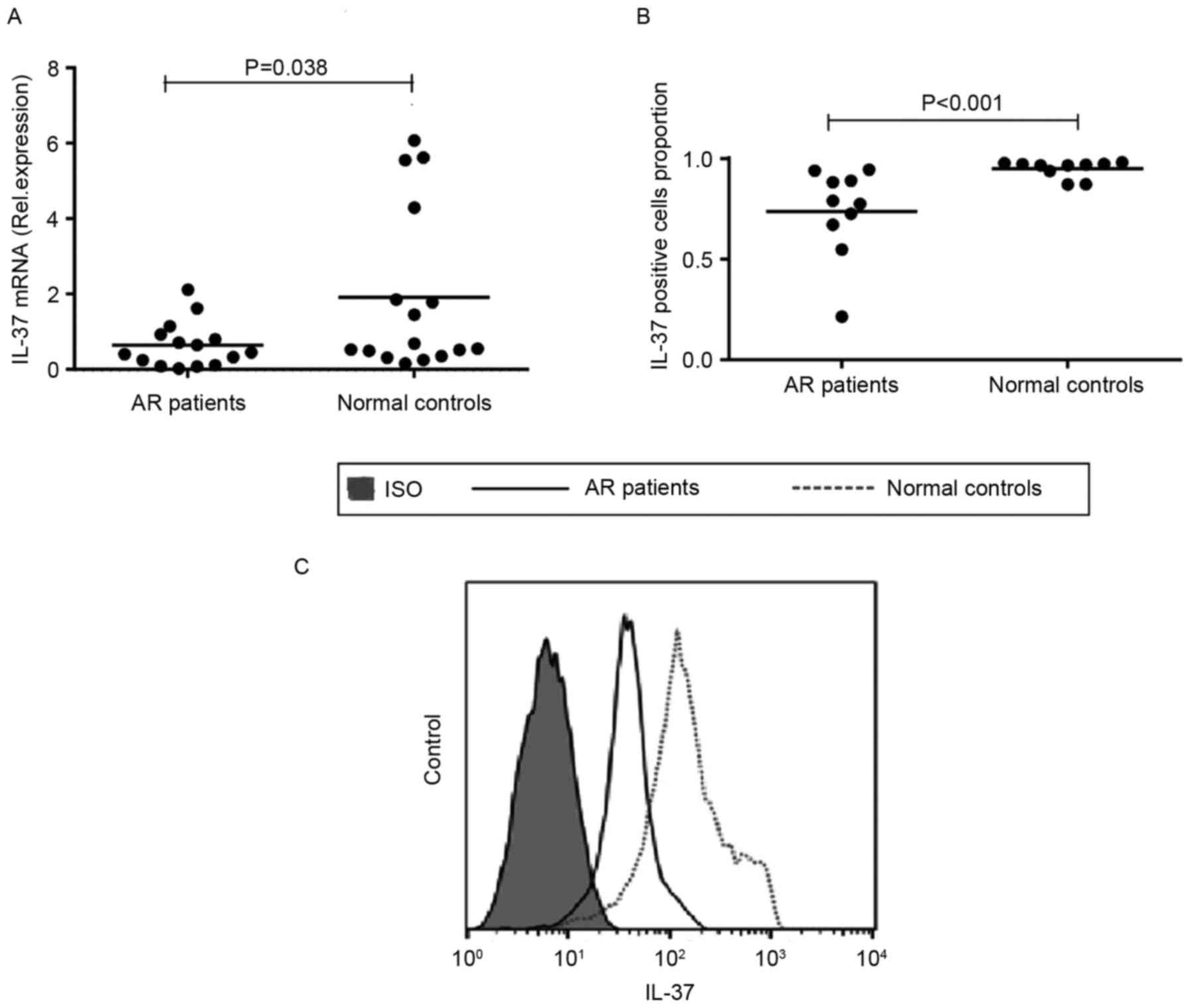
Decreased expression of IL-37 in PBMCs
from patients with AR
To investigate the role of IL-37 in AR, the mRNA
expression levels of IL-37 were determined in PBMCs from patients
with AR and controls using RT-qPCR analysis. The results showed
that the mRNA expression of IL-37 was significantly decreased in
the in the PBMCs from patients with AR, compared with the normal
controls (P=0.038). The protein levels of IL-37 in the PBMCs were
also determined using flow cytometry. The results showed that the
protein level of IL-37 in the PBMCs from patients with AR was
significantly lower, compared with that in the controls
(P<0.001), which was consistent with the obtained mRNA data
(Fig. 1A-C).
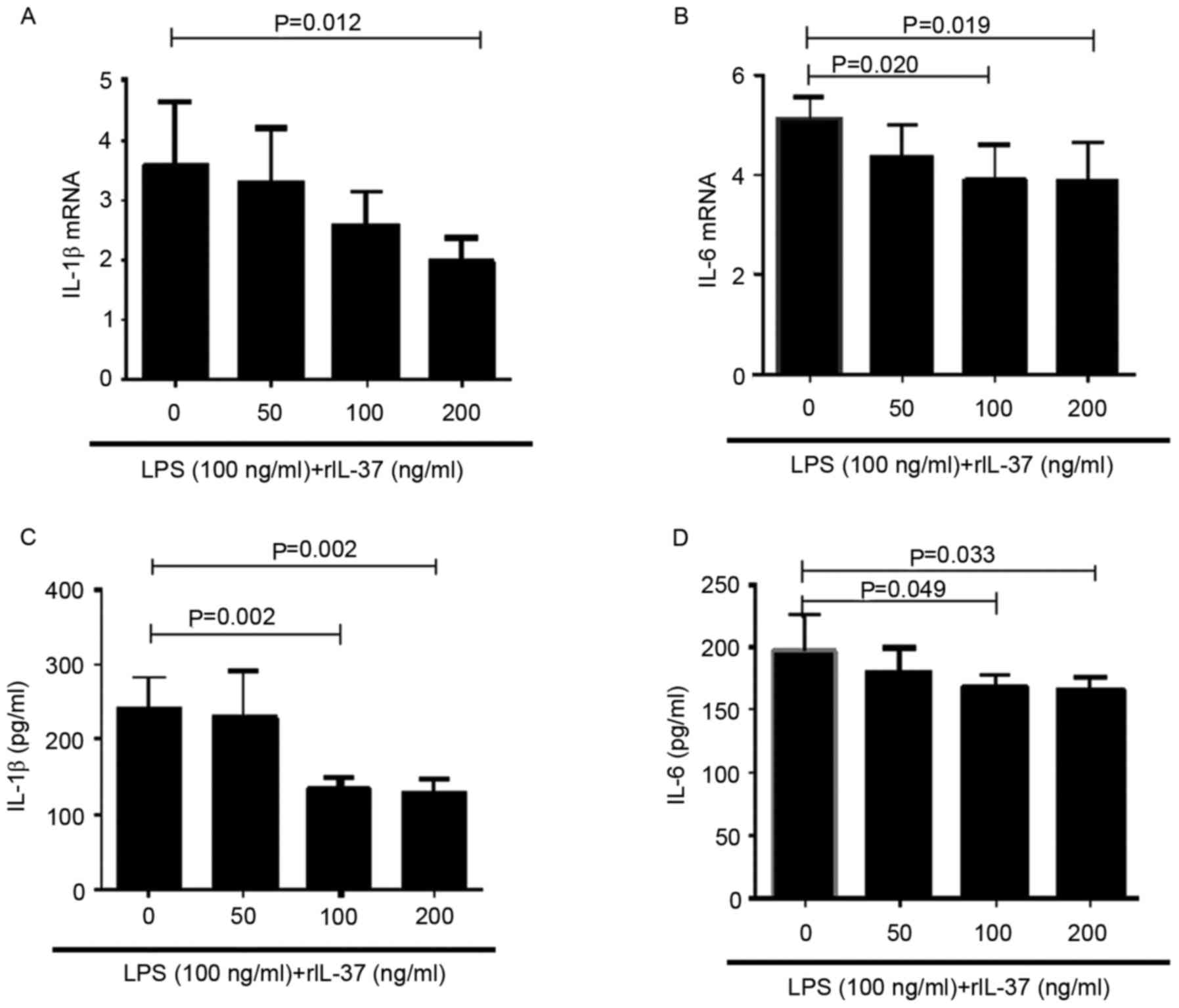
IL-37 inhibits the production of
proinflammatory cytokines by PBMCs from patients with AR and
controls
To investigate the effect of IL-37 on the production
of proinflammatory cytokines in PBMCs, the PBMCs from healthy
controls were stimulated with LPS in the presence of different
concentrations of rIL-37 (0, 10, 100 and 200 ng/ml) for 72 h. The
mRNA expression levels of IL-1β and IL-6 in the PBMCs were measured
using RT-qPCR analysis, and the protein expression levels of these
two cytokines in the supernatants were measured using ELISA. The
results demonstrated that the mRNA and protein levels of IL-1β and
IL-6 were decreased when the PBMCs were stimulated with rIL-37 at
100 and 200 ng/ml (Fig. 2A-D).
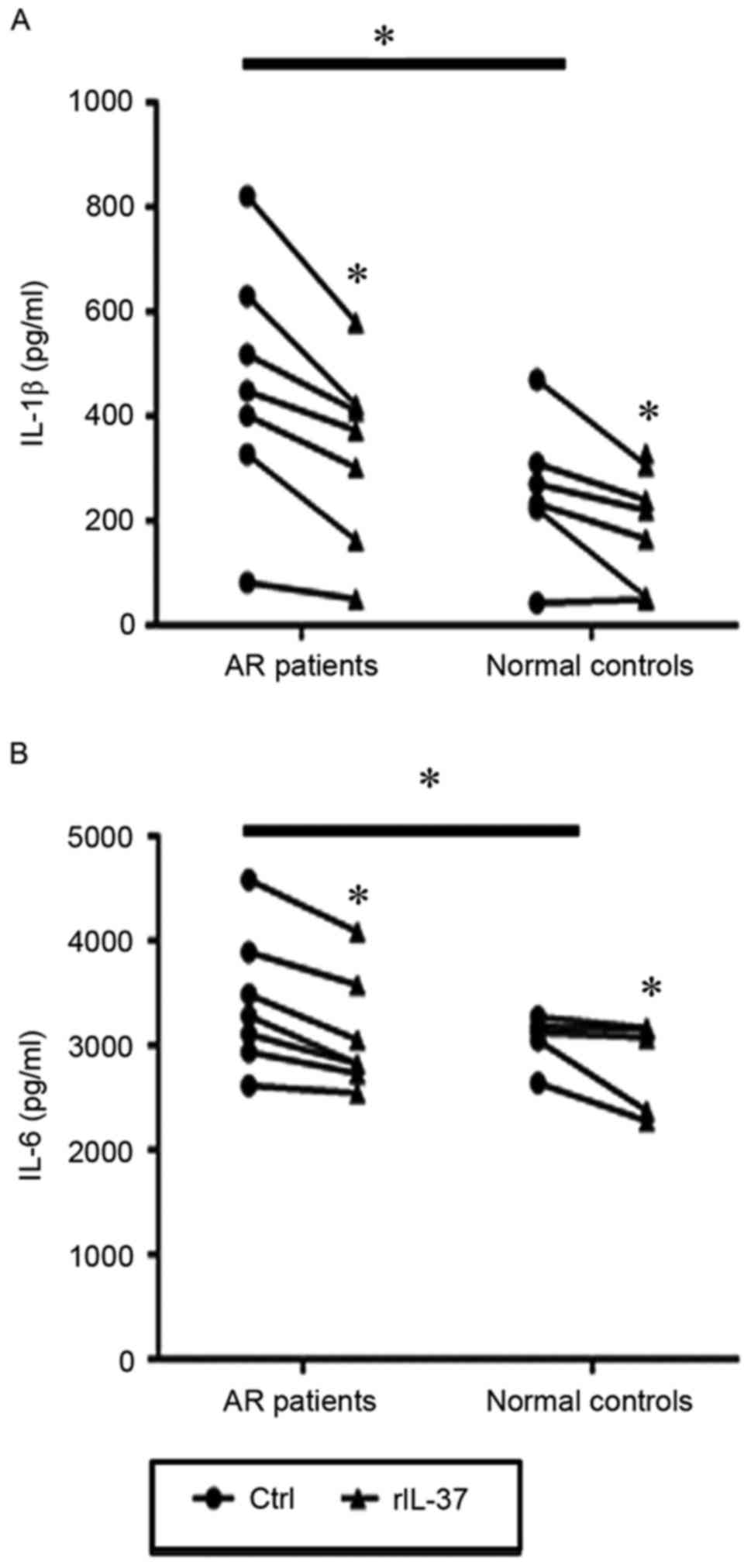
The PBMCs from patients with AR and controls were
then stimulated with LPS in the presence or absence of 100 ng/ml of
rIL-37. The results showed that the expression levels of IL-1l and
IL-6 in culture supernatants from the patients with AR were
significantly higher than those from the controls (P=0.04 and
P=0.031; Fig. 3A and B). In
addition, the levels of IL-1l and IL-6 in the rIL-37-stimulated
PBMC culture supernatants from the patients with AR were
significantly decreased compared with those in the unstimulated
PBMC culture supernatants (P=0.002 and P=0.001). In the healthy
controls, the same significant differences were observed in the
production of IL-1p and IL-6 between the rIL-37-stimulated and
unstimulated PBMCs (P=0.005 and P=0.028; Fig. 3A and B).
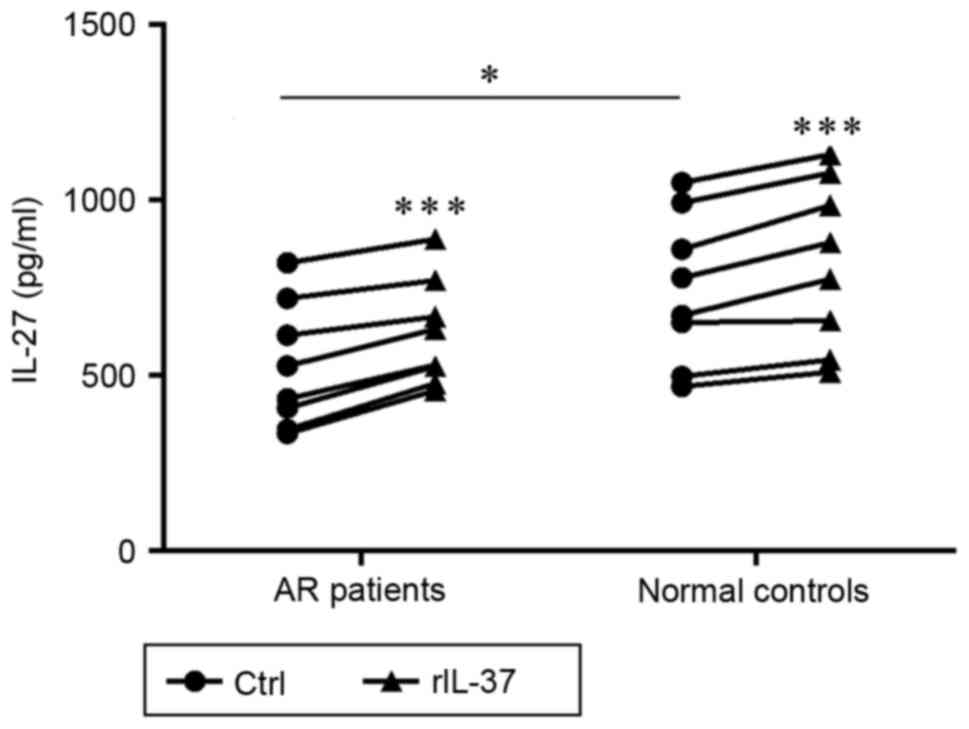
IL-37 enhances the production of IL-27
in PBMCs from patients with AR and normal controls
To investigate the effect of IL-37 on the
immunosuppressive production of IL-27 in PBMCs, the concentrations
of IL-27 in the culture supernatants from the PBMCs were measured
using ELISA. As exhibited in Fig.
4, compared with the control subjects, the level of IL-27 in
the LPS-stimulated supernatants was significantly decreased in the
patients with AR (P=0.042). In addition, the levels of IL-27 in the
AR and control groups were markedly enhanced in the
rIL-37+LPS-stimulated supernatants, compared with the levels in the
respective LPS-stimulated supernatants (P<0.001 and P<0.001;
Fig. 4).
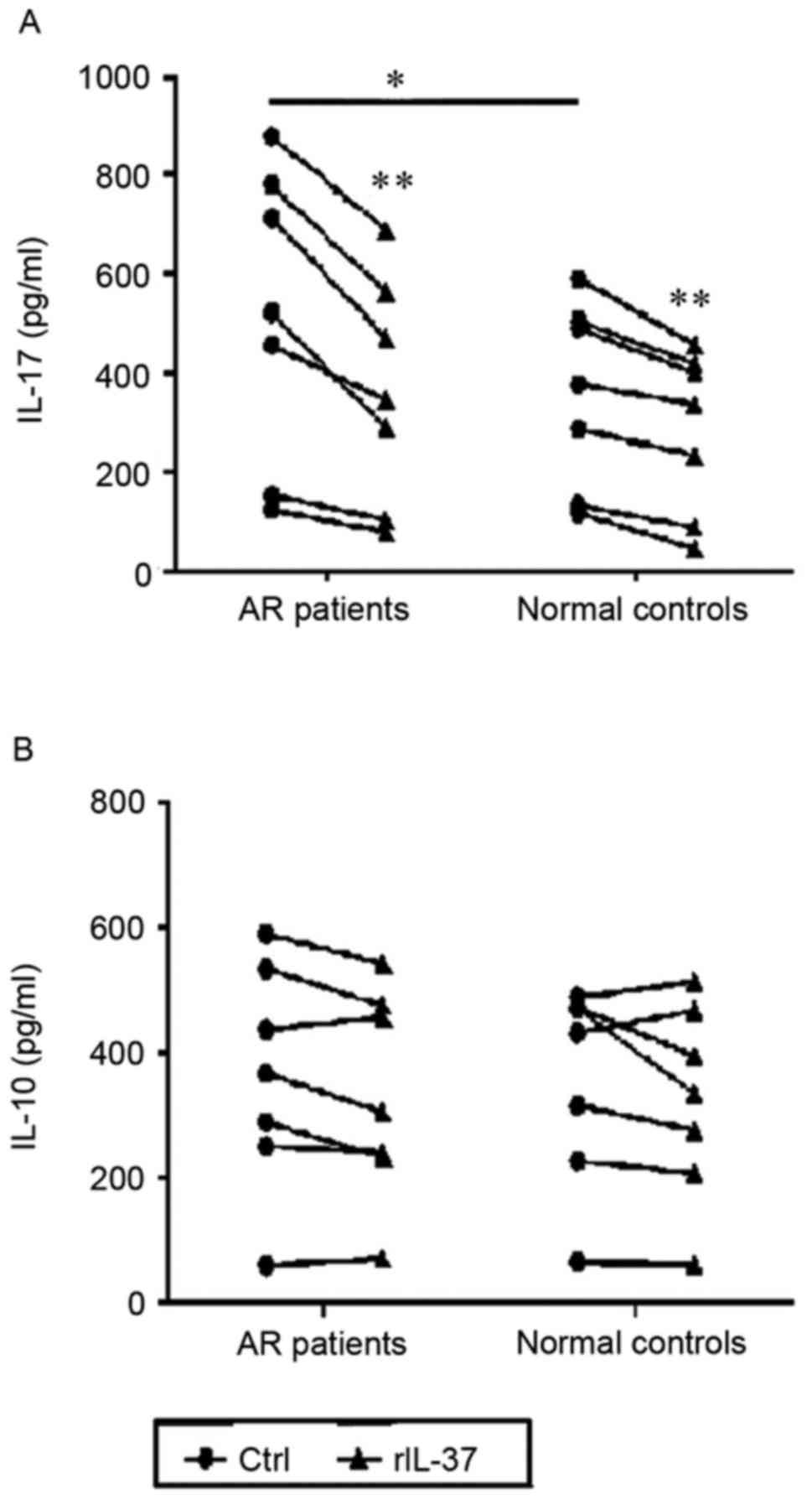
IL-37 reduces the production of IL-17A
by CD4+ T cells from patients with AR and normal
controls
To investigate the effect of IL-37 on
CD4+ T cells, which are important in the pathogenesis of
AR, the effect of IL-37 on cytokine production by CD4+ T
cells was examined. CD4+ T cells from the patients with
AR and controls were stimulated with anti-CD3/CD28 or
anti-CD3/CD28+rIL-37. The levels of effector cytokines in the
supernatants were then determined using ELISA. IL-37 significantly
reduced the production of IL-17A by CD4+ T cells
(P<0.001 and P<0.001). Compared with the control subjects,
the level of IL-17A in the LPS stimulated supernatant was
significantly increased in the patients with AR (P=0.04). However,
IL-37 did not affect the production of IL-10 by stimulated
CD4+ T cells in the AR or healthy control group
(P=0.222, and P=0.070; Fig. 5A and
B).
Discussion
In the present study, significant decreases in the
expression of IL-37 were found at the mRNA and protein levels in
PBMCs from patients with AR. In addition, significantly decreased
levels of IL-1r and IL-6, and an increased level of IL-27 were
found in rIL-37-stimulated PBMC culture supernatants from patients
with AR. Finally, it was demonstrated that IL-37 reduced the
production of IL-17 by stimulated CD4+ T cells in the
patient group. These results indicated that, in AR patients, IL-37
significantly inhibited the production of proinflammtory cytokines
from PBMCs and efficiently suppressed the release of IL-17 by
CD4+ cells. Therefore, it was hypothesized that IL-37
acts not only as a negative regulator, but that it also has
anti-inflammatory properties in an allergic immune response in the
airways.
IL-37 is a novel molecule of the IL-1 family with
anti-inflammatory effects. On examining the role of IL-37 in
allergic respiratory disease, previous data have demonstrated that
IL-37 is able to ablate a Th2 cell-directed allergic inflammatory
response and the hallmarks of experimental asthma in mice,
suggesting that IL-37 may be important in allergic pathogenesis
(18). Decreases in the expression
and production of IL-37 have also been recorded in restimulated
PBMCs of children with allergic bronchial asthma (24), and a decreased level of IL-37 in
induced sputum was found to correlate with disease severity,
indicating that IL-37 may be an important cytokine in the control
of asthma by suppressing the production of inflammatory cytokines
(20). A study by Liu et al
(25) reported significantly
decreased expression of IL-37b in serum and nasal lavage from
children with AR. Consistent with these findings, the experiments
in the present study showed that PBMCs from patients with AR
expressed a lower level of IL-37, compared with those from healthy
controls. Taken together, the above findings indicate that IL-37
may be involved in the development of allergic respiratory
disease.
On the basis of the above findings, it was
hypothesized that a decrease in the production of IL-37 may be
implicated in the pathogenesis of AR by a reduced capacity to
counterbalance an ongoing allergic inflammatory response in the
airways. To confirm this hypothesis, the present study examined the
effect of rIL-37 on the production of inflammatory cytokines in
PBMCs from patients with AR and healthy controls. The results
showed that the expression levels of IL-1e and IL-6 were
significantly increased in the PBMCs from patients with AR, and
IL-37 effectively decreased the production of these proinflammatory
cytokines. These findings indicated that IL-37 may have an
immunosuppressive effect in the pathogenesis of AR through a
potential anti-inflammatory function.
IL-27, a novel member of the IL-12 family, is
reported to prevent the development of Th2 cells and Th17 cells in
various inflammatory settings (26). The authors' previous study
indicated that IL-27 gene polymorphisms were likely to be involved
in susceptibility to AR (27). In
the present study, the potential association between IL-37 and
IL-27 in AR was examined. The results showed that the production of
IL-27 in PBMCs of patients with AR was significantly decreased, and
IL-37 downregulated the expression of IL-27 in PBMCs from patients
with AR and normal controls. This demonstrated that IL-27 acted in
synergy with IL-37 in the anti-inflammatory process, which had an
immunosuppressive effect in the pathogenesis of AR.
As is already known, the function of CD4+
T cells is critical in immune responses. Our previous study
demonstrated that the imbalance of Treg/Th17 cells was important in
the pathogenesis of AR, and allergy may aggravate chronic sinusitis
by promoting the imbalance of Th17/Treg cells (28). In the present study, the results
showed that IL-37 reduced the production of IL-17 by
CD4+ T cells, which indicated that IL-37 had a
suppressive function in the immune regulation of AR and was able to
inhibit Th17 cell responses. However, owing to limitations in time
and funding, the present study included only 39 patients and 43
healthy volunteers. In further investigations, an increased sample
size is planned to confirm this conclusion, and to detect the
expression of IL-6, IL-1β, IL-27, IL-10 and IL-17A in the PBMCs of
patients with AR and controls. Future experiments also aim to
examine the expression of IL-37 receptor, IL-1R8 and additional
detailed investigations are required to clarify the exact
mechanisms involved.
In conclusion, the results of the present study
demonstrated that the expression of IL-37 in PBMCs from patients
with AR was significantly reduced; rIL-37 negatively regulated the
production of proinflammatory cytokines and induced the production
of IL-27 by PBMCs. In addition, IL-37 reduced the production of
IL-17 by CD4+ T cells. These findings indicate that
IL-37 may be an important cytokine involved in the pathogenesis of
AR. It may have a protective effect on AR by inhibiting the
production of proinflammatory cytokines and through suppressive
regulation of the Th17 response. However, further investigations
are required to clarify the exact mechanism and to confirm whether
IL-37 may be a potential target for the prevention and treatment of
AR.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470676). The
authors would like to thank the staff of the First Affiliated
Hospital of Chongqing Medical University for assistance with the
collection of clinical samples, and members of the laboratory for
assistance with sample processing, scientific discussion and
clinical data collection.
References
|
1
|
Togias A: Rhinitis and asthma: Evidence
for respiratory system integration. J Allergy Clin Immunol.
111:1171–1184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen J, Ke X, Hong S, Zeng Q, Liang C, Li
T and Tang A: Epidemiological features of allergic rhinitis in four
major cities in Western China. J Huazhong Univ Sci Technolog Med
Sci. 31:433–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young MC: Rhinitis, sinusitis, and
polyposis. Allergy Asthma Proc. 19:211–218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leynaert B, Neukirch F, Demoly P and
Bousquet J: Epidemiologic evidence for asthma and rhinitis
comorbidity. J Allergy ClinImmunol. 106 Suppl 5:S201–S205. 2000.
View Article : Google Scholar
|
|
5
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: Back to the future. Immunity.
39:1003–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boraschi D, Lucchesi D, Hainzl S, Leitner
M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C,
Posselt G, et al: IL-37: A new anti-inflammatory cytokine of the
IL-1 family. Eur Cytokine Netw. 22:127–147. 2011.PubMed/NCBI
|
|
8
|
Luo Y, Cai X, Liu S, Wang S, Nold-Petry
CA, Nold MF, Bufler P, Norris D, Dinarello CA and Fujita M:
Suppression of antigen-specific adaptive immunity by IL-37 via
induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA.
111:15178–15183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye Z, Wang C, Tang J, Zhou Y, Bai L, Liu
Y, Kijlstra A and Yang P: Decreased interleukin-37 expression in
Vogt-Koyanagi-Harada disease and upregulation following
immunosuppressive treatment. J Interferon Cytokine Res. 35:265–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye Z, Wang C, Kijlstra A, Zhou X and Yang
P: A possible role for interleukin 37 in the pathogenesis of
Behcet's disease. Curr Mol Med. 14:535–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bouali E, Kaabachi W, Hamzaoui A and
Hamzaoui K: Interleukin-37 expression is decreased in Behçet's
disease and is associated with inflammation. Immunol Lett.
167:87–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonseca-Camarillo G, Furuzawa-Carballeda J
and Yamamoto-Furusho JK: Interleukin 35 (IL-35) and IL-37:
Intestinal and peripheral expression by T and B regulatory cells in
patients with inflammatory bowel disease. Cytokine. 75:389–402.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, et al: IL-37 inhibits the production of
inflammatory cytokines in peripheral blood mononuclear cells of
patients with systemic lupus erythematosus: Its correlation with
disease activity. J Transl Med. 12:692014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang
K and Huang Z: Increased expression of IL-37 in patients with
Graves' disease and its contribution to suppression of
proinflammatory cytokines production in peripheral blood
mononuclear cells. PLoS One. 9:e1071832014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B, Huang K, Ye L, Li Y, Zhang J,
Zhang J, Fan X, Liu X, Li L, Sun J, et al: Interleukin-37 is
increased in ankylosing spondylitis patients and associated with
disease activity. J Transl Med. 13:362015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan
YX and Jiang YF: Plasma levels of IL-37 and correlation with TNF-α,
IL-17A, and disease activity during DMARD treatment of rheumatoid
arthritis. PLoS One. 9:e953462014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia T, Zheng XF, Qian BH, Fang H, Wang JJ,
Zhang LL, Pang YF, Zhang J, Wei XQ, Xia ZF and Zhao DB: Plasma
interleukin-37 is elevated in patients with plasma interleukin-37
is elevated in patients with rheumatoid arthritis: Its correlation
with disease activity and Th1/Th2/Th17-related cytokines. Dis
Markers. 2015:7950432015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lunding L, Webering S, Vock C, Schröder A,
Raedler D, Schaub B, Fehrenbach H and Wegmann M: IL-37 requires
IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation
in mice. Allergy. 70:366–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berraïes A, Hamdi B, Ammar J, Hamzaoui K
and Hamzaoui A: Increased expression of thymic stromal
lymphopoietin in induced sputum from asthmatic children. Immunol
Lett. 178:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Charrad R, Berraïes A, Hamdi B, Ammar J,
Hamzaoui K and Hamzaoui A: Anti-inflammatory activity of IL-37 in
asthmatic children: Correlation with inflammatory cytokines TNF-α,
IL-β, IL-6 and IL-17A. Immunobiology. 221:182–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63 Suppl 86:S8–S160.
2008. View Article : Google Scholar
|
|
22
|
No authors listed: Position paper:
Allergen standardization and skin tests. The European Academy of
Allergology and Clinical Immunology. Allergy. 48 Suppl 14:S48–S82.
1993.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raedler D, Ballenberger N, Klucker E, Böck
A, Otto R, da Costa Prazeres O, Holst O, Illig T, Buch T, von
Mutius E and Schaub B: Identification of novel immune phenotypes
for allergic and nonallergic childhood asthma. J Allergy Clin
Immunol. 135:81–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Deng L, Chen Y, Sun C, Wang J, Zhou
L, Li H and Luo R: Anti-inflammatory effect of IL-37b in children
with allergic rhinitis. Mediators Inflamm. 2014:7468462014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wojno ED and Hunter CA: New directions in
the basic and translational biology of interleukin-27. Trends
Immunol. 33:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen Y, Yuan XD, Hu D, Ke X, Wang XQ, Hu
GH, Hong SL and Kang HY: Association between interleukin-27 gene
polymorphisms and susceptibility to allergic rhinitis. Hum Immunol.
75:991–995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Y, Tang XY, Yang YC, Ke X, Kou W, Pan
CK and Hong SL: Impaired balance of Th17/Treg in patients with
nasal polyposis. Scand J Immunol. 74:176–185. 2011. View Article : Google Scholar : PubMed/NCBI
|