Introduction
Cranioplasty is the predominant method for the
treatment of a variety of skull defects. Seizure is a common
complication of skull injury and cranioplasty, with the incidence
ranging between 14.8 and 33.0% (1,2).
Seizures pose a serious threat to human health, with patients
subsequently presenting with varying degrees of mental disorders
and physical damage, which greatly impacts upon their normal
learning and quality of life, and may bring serious mental and
economic burdens to society and the patient's family. Seizures are
a frequent neurological problem during the neonatal period,
occurring in 1.8–5 out of 1,000 live births in Canada and The
United States (3). The most common
cause of neonatal seizures is hypoxic/ischemic encephalopathy,
affecting 1–2 out of 1,000 live neonatal births, therefore
contributing to ~2/3 of seizure cases in neonatal babies (3). Clinically, it can be challenging to
diagnose neonatal seizures, which may be exclusively electrographic
(4). In addition, currently
available antiepileptic drugs (AEDs) are often not effective for
neonatal seizures (5). The
frequency of seizure has been demonstrated to be significantly
elevated following cranioplasty, and previous research has
indicated that valproic acid could decrease the risk of
post-operative seizures (6).
Recently, microRNAs (miRNAs) have been identified as
pivotal regulators of gene function, and this has been a
significant step forward in our understanding of gene regulatory
mechanisms (7,8). Although there are only a few hundred
known miRNAs, it is estimated that miRNAs may regulate >1/3 of
all human genes, as each miRNA is likely to regulate hundreds of
target genes (9,10). miRNAs are involved in several
intrinsic cellular processes, including cellular metabolism, immune
responses, hematopoietic differentiation and cell-cycle regulation
(11–14), and their expression patterns have
been investigated in schizophrenia, Down's syndrome and a variety
of tumors (15–17). Previous research demonstrated that
valproic acid (VPA) was able to modulate miRNA expression profiles
in rats (18). The interaction
between miRNAs and their target genes is closely modulated, and our
recent report investigated this using in silico methods to
predict the network of miRNAs that interacted with and interrupted
protein complexes following VPA administration (19). Furthermore, a previous study
revealed that VPA was able to affect expression of miRNAs in the
brain (18).
Based on these observations, the present study
compared patients who underwent cranioplasty from March 2010 to
March 2015 and used AEDs to control preoperative or postoperative
cranioplasty-related seizures, with patients who did not use AED
drugs across the same period. The role of miR155 and its potential
target SCN1A in the control of post-operative seizure were
explored. Downregulation of microRNA-155 by preoperative
administration of VPA prevents postoperative seizure by
upregulating SCN1A.
Materials and methods
Subjects and inclusion/exclusion
criteria
All patients who came to the Department of
Neurosurgery, the Affiliated Yangming Hospital of Ningbo University
(Ningbo, China) for cranioplasty from March 2010 to March 2015, and
were self reliant in their ability to take care of themselves and
to perform basic self-care were suitable for inclusion in the
present study. A total of 85 cases were recruited into the study; 2
patients were lost to follow-up and 3 were excluded due to
rejection reaction and infection, leaving 80 cases [57 males, 23
females; age 36.2±2.6 (range 17–72) years]. A total of 69 cases had
subdural (or intracerebral) hematoma clearance + decompressive
craniectomy due to brain trauma, and 11 had intracranial hematoma
clearance + decompressive craniectomy due to intracranial
spontaneous bleeding. Patients underwent craniotomies in the
previous 3–25 months (average, 4.4±0.7 months) prior to undergoing
the present cranioplasty. The patients presented with several skull
defect sites: 52 cases of unilateral frontotemporal and parietal,
15 cases of bilateral frontotemporal and parietal, 9 cases of
bilateral frontal, 3 cases of unilateral occipital and 1 case of
bilateral occipital. Amongst all subjects, tissue samples were
available for 48 participants (VPA treated, n=23; Control, n=25).
The study protocol was approved by the research ethics committee at
The Affiliated Yangming Hospital of Ningbo University (Ningbo,
China), and written informed consent was obtained from each
participant.
Patients who presented with the following were
excluded: Patients who had hydrocephalus shunt under anesthesia;
patients who had seizures prior to being hospitalized for the
present cranioplasty, including those who had or had not used drug
control; patients who had their implant titanium plate and nail for
cranioplasty removed due to infection or rejection reaction
following cranioplasty; and patients who had severe heart, liver or
kidney dysfunctions or had routine blood abnormalities prior to
surgery.
All enrolled patients were randomly divided into
experimental and control groups. A few patients were not included
in the statistics: 2 cases in the control group were lost to
follow-up and 2 cases underwent reoperation due to postoperative
rejection; 1 case in the experimental group developed an infection.
There were 41 cases remaining in the VPA-treated group with 30 male
cases and 11 female cases. SCN1A expression was detected in 23 of
them. Patients received craniotomies in the previous 3–20 months
(average, 4.2±0.5 months) prior to undergoing the present
cranioplasty. The experimental patients presented with the
following skull defects: 26 cases of unilateral frontotemporal and
parietal, 9 cases of bilateral frontotemporal and parietal, 4 cases
of bilateral frontal, 2 cases of other sites. The defect area of
each bone window was between 4×4 and 12×16 cm (average, 9.5×10.2
cm). There were 39 cases remaining in the control group, with 28
male and 11 female cases. SCN1A expression was detected in 25 of
them. Patients received craniotomies in the previous 3.3–25 months
(average, 4.6±0.8 months) prior to undergoing the present
cranioplasty. The control patients presented with the following
skull defects: 26 cases of unilateral frontotemporal and parietal,
6 cases of bilateral frontotemporal and parietal, 5 cases of
bilateral frontal, 2 cases of other sites. The defect area of each
bone window was between 4×5 and 13×15 cm (average, 9.4×10.5 cm).
The two groups of patients had no significant difference in
clinical features (data not shown).
Surgical approach
Patients underwent clinical examination following
admission, including liver and kidney function analyses, routine
blood tests and head computed tomography scan. Patients were fitted
for custom titanium mesh plates, and underwent operation ~5 days
later. Under general anesthesia, the scalp was cut open along the
original surgical incision line to the skull, and the scalp and
temporal muscle were carefully peeled to expose the endocranium and
fully expose the bone window surface. The custom titanium (titanium
mesh) plate was placed on the skull window and was fixed with a few
titanium nails. Outside the plate a drainage tube was connected
with negative pressure (to be removed 48 h later). All patients
were started on medicine 4 days prior to surgery (following
admission and whilst the custom titanium mesh plate was prepared)
until 1 month following surgery. The control group took oral 20 mg
ATP tablet (tid). The experimental group took ATP (20 mg) and
Compound Sodium Valproate and Valproic Acid Sustained Release
tablets [500 mg (bid); Sanofi-Aventis (Hangzhou) Pharmaceuticals
Co., Ltd., Hangzhou, China].
Efficacy evaluation
The following clinical parameters were observed and
compared between the two groups of patients: Incidence of seizures
following admission (4 days prior to surgery) up to 1 month
following surgery; abnormal rate of liver function; routine blood
tests or other indicators 2 weeks post-surgery (serum aspartate
aminotransferase >2-fold of the preoperative level was
considered abnormal; white blood cell or platelet count reduced by
≥50% of the preoperative level was considered abnormal).
Cell culture and transfection
U251 cells (American Type Culture Collection,
Manassas, VA, USA) were selected for the analysis due to low levels
of expression of miR-155. U251 cells were grown in Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 1%
penicillin/streptomycin, 2 mM glutamine and 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2/95% air. When confluence reached 80%, Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect U251 cells with miRNA (miR)-155 mimics (5UUA AUG CUA AUC
GUG AUA GGG G3), negative control (NC; 5CAG UAC UUU UGU GUA GUA
CAA3) and miR-155 inhibitors (5CCC CUA UCA CGA UUA GCA UUA A3), in
accordance with the manufacturers protocol (GenePharma, Shanghai,
China). The final concentration of miR-155, NC and inhibitor is 100
nM. Three independent experiments were performed.
RNA isolation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to purify total RNA from U251 cells
(5×106 cells) and cerebrospinal fluid (CSF) samples (2
ml) in accordance with the manufacturer's protocol. CSF was sampled
via epidural during surgery. All CSF samples were put on ice
immediately and spun within 15 min at 500 × g for 10 min; the
supernatant was stored at −80°C until further use. DNaseI treatment
(Takara Bio Inc., Otsu, Japan) was used to treat the total RNA,
according to the manufacturer's protocol. A NanoDrop (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) was used to assess the
concentration and quality of RNA. QuantiTect_SYBR_Green RT-PCR kit
(Qiagen GmbH, Hilden, Germany) was used to synthesize cDNA from 200
ng total RNA, in accordance with the manufacturer's protocol. An
Applied Biosystems 7500 Sequence Detection System (Thermo Fisher
Scientific, Inc., USA) was used to perform qPCR. The upstream
primers (5–3) used in the study were: TTA ATG CTA ATC
GTG ATA GG for miR-155; AATTGCACTCGTCCCGGCCTCC for miR-92b;
CCTCTGGGCCCTTCCTCCAG for miR-326; CTGGCCCTCTCTGCCCTTCCGT for
miR-328; TAT GAC TGA TGT GTG CGT GTG TCT G for miR-468; AGG GAT CGC
GGG CGG GTG GCG GCC T for miR-638; AGG CGG GGC GCC GCG GGA CCG C
for miR-663; GTG CGG AAC GCT GGC CGG GGC G for miR-685. Downstream
primer was the universal primer supplied by Qiagen GmbH. The
thermocycling reaction involved 95°C for 10 min (initial
denaturation), followed by 40 cycles of 95°C for 15 sec and 60°C
for 60 sec. Small nuclear RNA U6 (5′CTCGCTTCGGCAGCACA3′ and
5′AACGCTTCACGAATTTGCGT3′) was used as an internal control to
normalize the expression of microRNAs.
For SCN1A detection, each sample was reverse
transcribed into cDNA and analyzed by SYBR-Green Real-Time PCR kit
(Bio-Rad, America). Quantitative real-time PCR was performed using
Applied Biosystems 1900 system (Thermo Fisher Scientific, Inc). The
primers for SCN1A were 5′GCATCCGTGGCTCCCTATTTT3′ and
5′CTCATTGCTCGTTGCCTTTGG3′. GAPDH (5′CCACTCCTCCACCTTTGAC3′ and
5′ACCCTGTTGCTGTAGCCA3′) was used as an internal control for-SCN1A.
The 2−ΔΔCq method (20)
was used to analyze the relative quantification of miR-155 and
SCN1A mRNA. All reactions were performed in triplicate.
Luciferase assay
The University of California Santa Cruz genome
browser (http://genome.ucsc.edu) was used to
examine the downstream region of miR-155. The predicted miR-155
binding site in the 3′-untranslated region (UTR) of SCN1A was
amplified using PCR (5CGCTCGAGA TGA AAA TAA ATA AAA ATA ATT GG3 and
5ATAAGCTTG CTA AAA TAA AAA ATG TAA T3, underlined are XhoI
and HindIII sites respectively, 2103 bp). The reaction
condition was 95°C 2 min, followed by 30 cycles of 95°C 30 sec,
53°C 30 sec and 72°C 2 min. 3′UTR mutants were created using a site
directed mutagenesis kit (Takara Bio Inc.) (Fig. 1). The PCR products and the
site-directed mutagenesis products were inserted into the
XhoI/HindIII restriction sites of a pGL3-Basic vector
(Promega Corporation, Madison, WI, USA) to create the wild-type or
mutant luciferase/reporter constructs. Sequencing was performed to
confirm all the constructs. Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to co-transfect the U251 cells
with wild-type SCN1A 3′UTR or mutant SCN1A 3′UTR luciferase
reporter constructs at a final concentration of 100 nM per well,
together with miR-155 mimic or miR-NC with a final concentration of
100 nM. The Dual-Luciferase Reporter Assay System (Promega
Corporation) was used to measure the activities of firefly and
Renilla luciferase 48 h following transfection. Three
independent experiments were performed.
Western blot analysis
The expression of SCN1A protein was assessed using
western blot analysis. PBS was used to wash the U251 cells and
tissue samples twice, and U251 cells (5×106 cells) were
lysed using radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA) containing Halt Protease Inhibitor
Cocktail (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cellular lysates were centrifuged for 15
min at 1,500 × g at 4°C to obtain the supernatants. A DC Protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to
determine the concentration of protein. Proteins were
heat-denatured in boiling water. Proteins (50 µg/lane) were
separated by 10% SDS-PAGE and transferred to an Immobilon-P
membrane (EMD Millipore, Billerica, MA, USA) according to the
manufacturer's protocol. PBS containing 5% skimmed milk and 0.1%
Tween-20 was used to block the membrane to avoid non-specific
binding. Membranes were incubated with primary rabbit monoclonal
antibodies against SCN1A (cat. no. 14380; 1:5,000) and β-actin
(cat. no. 4970; 1:15,000), both from Cell Signaling Technology
(Danvers, MA, USA), at 4°C for 12 h. PBS containing 0.1% Tween-20
was subsequently used to wash the membranes three times for 10 min
each and the membranes were incubated with the secondary antibody
(cat. no. 14708; 1:8,000; Cell Signaling Technology, Danvers, MA,
USA) at 37°C for 2 h. The blots were subsequently washed with PBS
containing 0.1% Tween-20. Enhanced Chemiluminescence Detection
Reagent (GE Healthcare Life Sciences, Little Chalfont, UK) was used
to detect the bound antibody in accordance with the manufacturer's
protocol.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was tested using either one-way analysis
of variance followed by a Bonferroni post-hoc test, chi-square
statistic or paired Student's t-test (two-tailed). Relationships
between two variables from the same test were evaluated by the
linear Pearson correlation coefficient (r) and multiple regression
analyses. JMP Pro 12 (SAS Institute, Cary, NC, USA) was used to
analyze the data. P<0.05 was considered to indicate a
significant difference.
Results
Evaluation of the preventive effects
of VPA on post-operative seizure
There were 11 cases of seizures in the control
group, with an incidence of ~28.2% (Table I), including 4 cases of
preoperative seizures and 7 cases within 1 month of surgery (6
cases within 2 weeks of surgery; one case 2 weeks post-surgery).
There were 3 cases of seizures in the experimental group, with an
incidence of ~7.3%, including one case of preoperative seizure and
two cases within 1 month of surgery (one case within 2 weeks of
surgery, one case 2 weeks post-surgery). The difference in the
incidence rates of seizures between the groups was statistically
significant (P=0.019; Table I).
Following surgery, there were 2 cases of abnormal liver function in
the control group, and 3 cases in the experimental group. There was
one case of abnormal routine blood analysis (reduced white blood
cells) in each of the two groups. There were no significant
differences identified for liver function or routine blood analysis
between the two groups (Table
I).
 | Table I.Comparisons of clinical data of
experimental and control groups. |
Table I.
Comparisons of clinical data of
experimental and control groups.
| Clinical data | Experimental group
(n=41) | Control group
(n=39) | χ2 | P-value |
|---|
| Seizure, n (%) | 3 (7.3) | 11 (28.2) | 6.04 | 0.019 |
| Abnormal liver
function, n (%) | 3 (7.3) | 2 (5.1) | 1.61 | 1.00 |
| Abnormal routine
blood, n (%) | 1 (2.4) | 1 (2.6) | 0.01 | 1.00 |
Deregulation of miR-155 in
seizure
RT-qPCR was used to investigate differentially
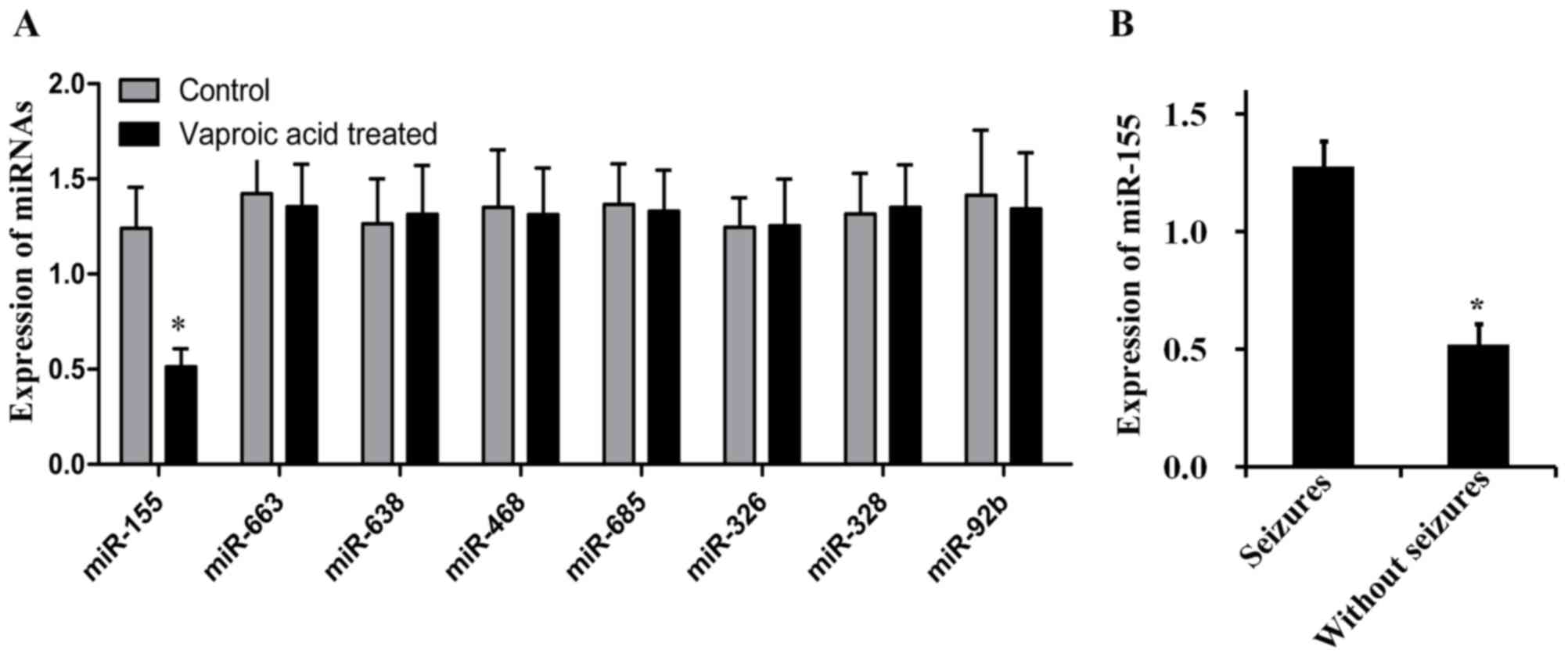
expressed miRNAs in subjects of control and VPA-treated patients. A
total of 8 miRNAs (miRNA-155, miRNA-633, miRNA-638, miRNA-468,
miRNA-685, miRNA-326, miRNA-328 and miRNA-92b) closely associated
with seizure were analyzed by RT-qPCR (Fig. 2). miR-155 was the only
significantly different miRNA between control and VPA-treated
groups and the expression of miR-55 decreased 2.5 fold in the
VPA-treated group when compared with it in the control group. The
remaining 7 miRNAs demonstrated similar expression levels between
the two groups. These results suggested that miR-155 may be a
potential biomarker for seizure development.
miR-155 downregulation may be
associated with seizure occurrence
The expression of miR-155 in patients who
experienced seizures was 2.45 folds higher compared with patients
who did not experience seizures among all patients (Fig. 2B). Pearson correlation analysis
among all patients revealed a positive association between miR-155
expression and seizure occurrence (r=0.503, P=0.018). Furthermore,
a multivariate linear model indicated the relationship between
miR-155 and seizure was persistent in the control (r=0.490,
P=0.029) and experimental groups (r=0.513, P=0.014), indicating
that increased miR-155 expression may be associated with seizure
occurrence.
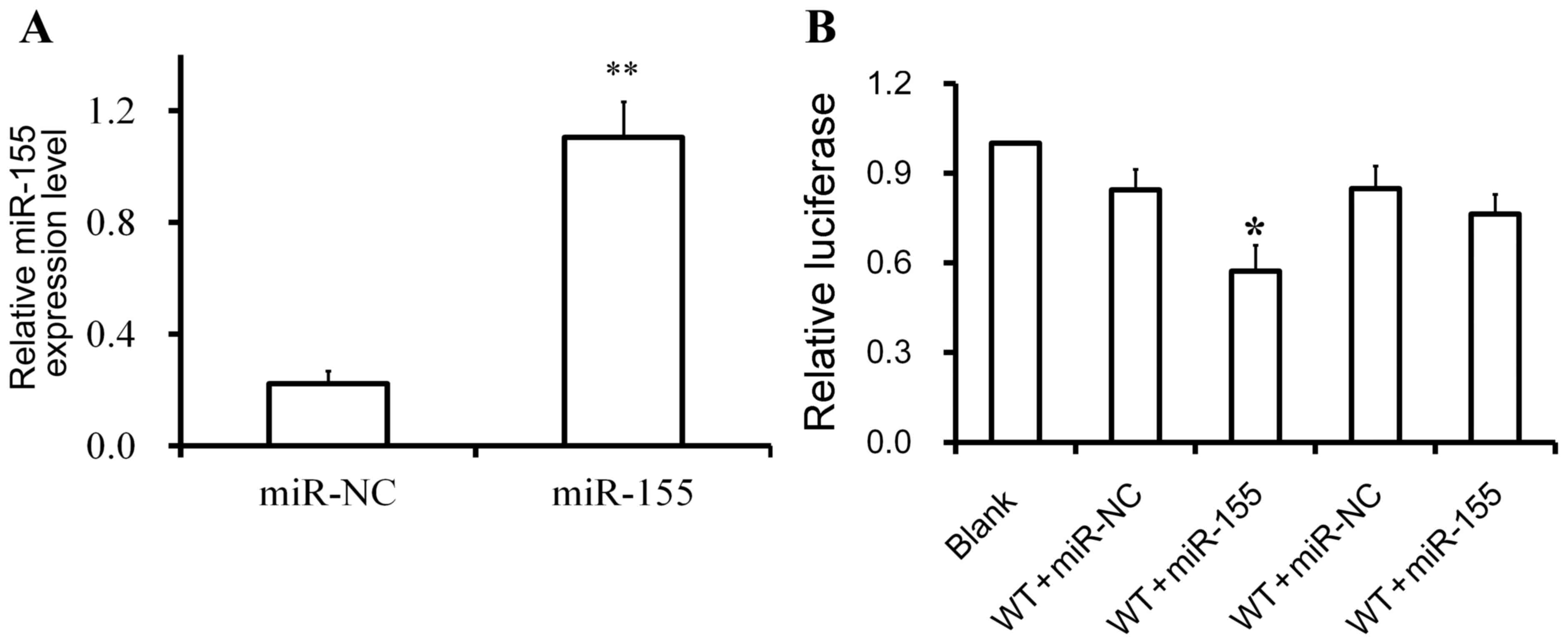
SCN1A may be a target of miR-155
Online miRNA target prediction tools were used to
search for target genes of miR-155, and SCN1A was identified as a
candidate target gene of miR-155, as it contained the appropriate
seed sequence in the 3′UTR (Fig.
1). In addition, it was confirmed that the miR-155 mimics or
miR-NC were successfully transfected into U251 cells (Fig. 3A). A luciferase reporter assay was
used to investigate the regulatory relationship between miR-155 and
SCN1A. The luciferase activity of cells transfected with wild-type
SCN1A 3′UTR and miR-155 mimics was lower compared with cells
transfected with wild-type SCN1A 3′UTR and miR-NC (Fig. 3B). By contrast, the luciferase
activity of cells carrying the mutant SCN1A 3′UTR was comparable
with the scramble control and the blank control. These data
indicated that SCN1A may be a target of miR-155 in U251 cells with
the binding sites located on the SCN1A 3′UTR.
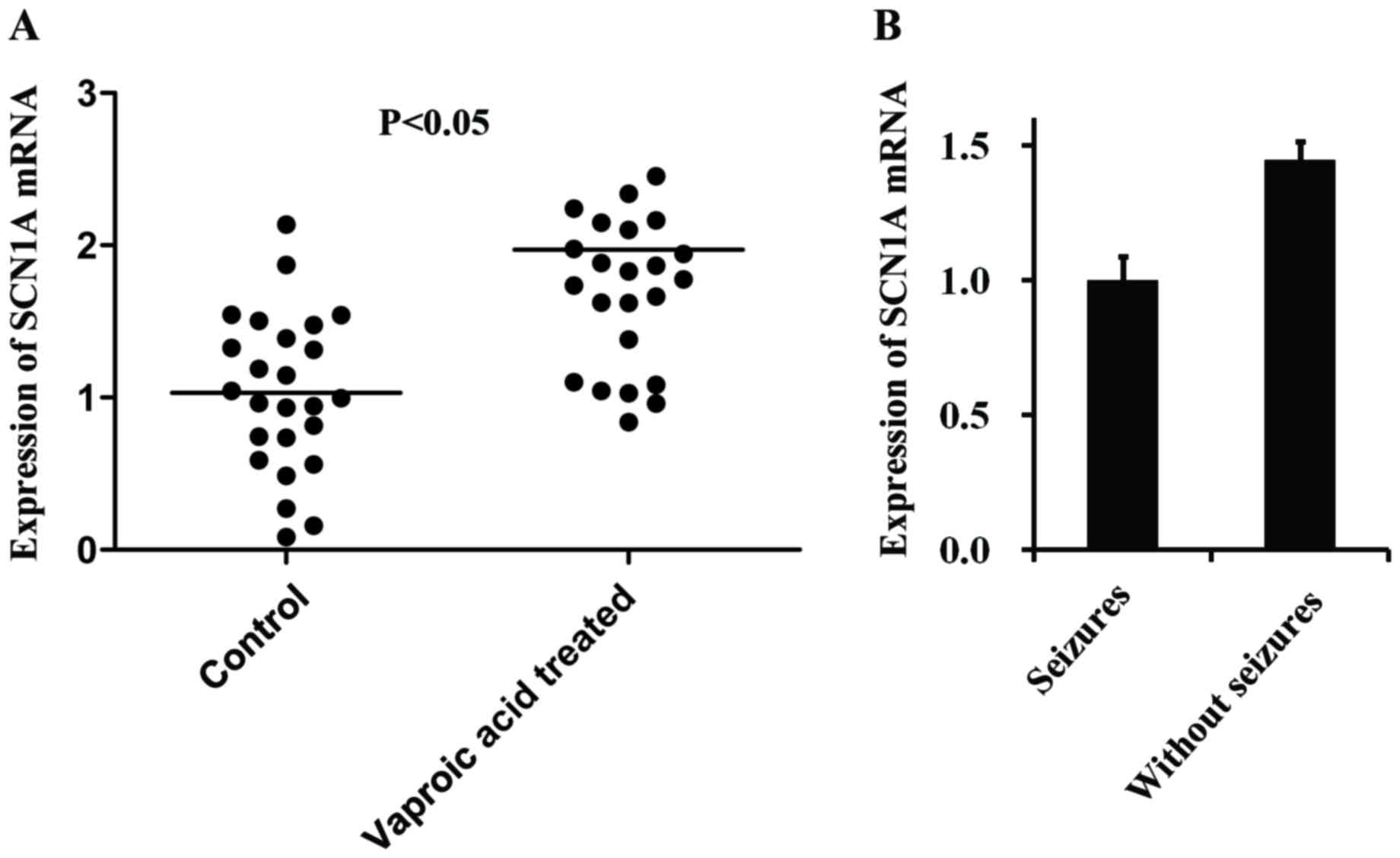
Expression level of SCN1A mRNA varies
in VPA-treated and control groups
The SCN1A mRNA expression level was determined in
the two treatment groups using RT-qPCR (Fig. 4A). The expression of SCN1A mRNA in
the VPA-treated group was higher (r=0.226; P=0.032) compared with
the expression level in the control group. SCN1A exhibits no
difference between patients who experienced seizures and patients
who did not experience seizures (Fig.
4B).
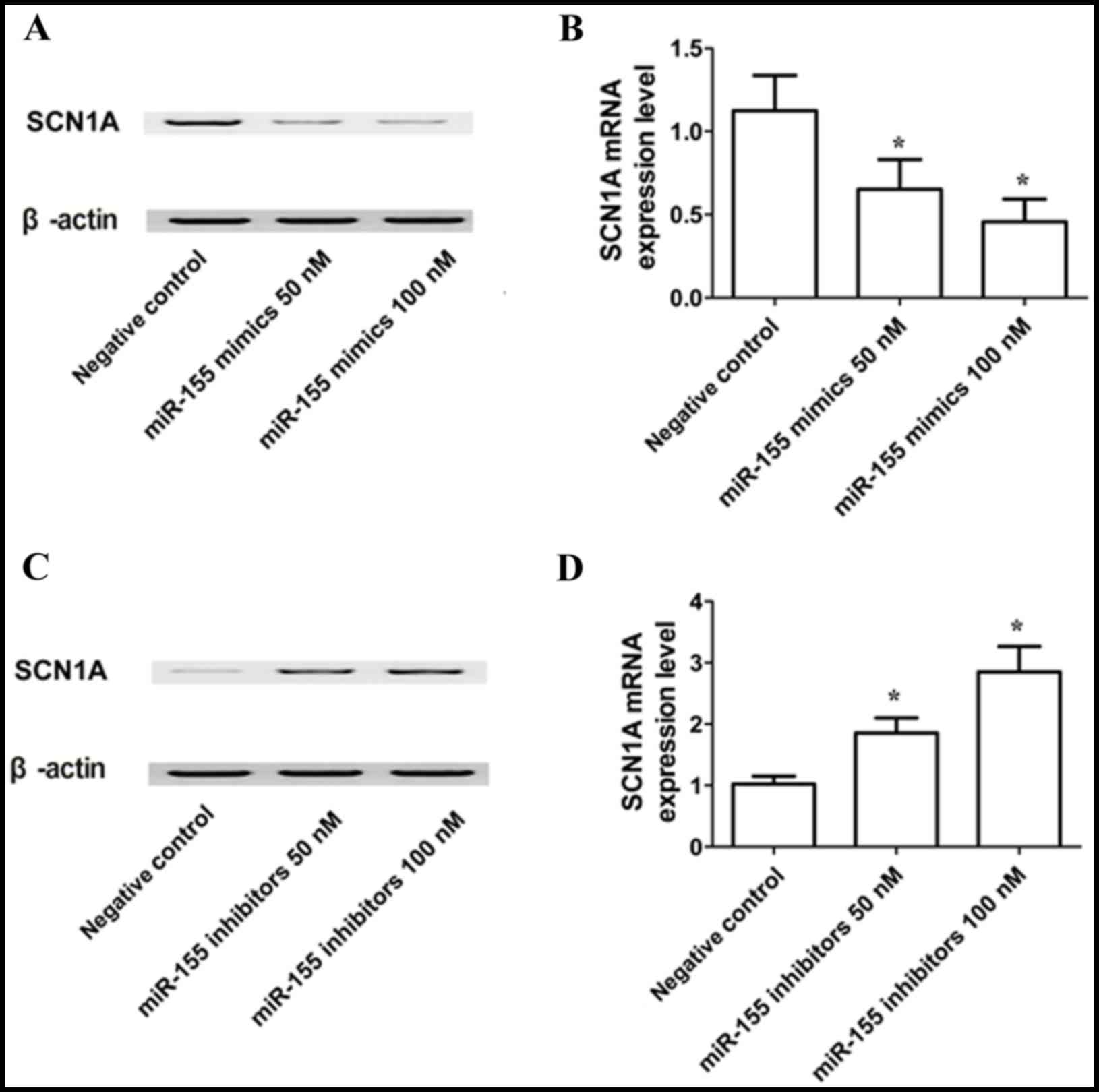
Varying expression levels of SCN1A
mRNA and protein in different in vitro treatment groups
RT-qPCR and western blot analyses were used to
determine the expression of SCN1A mRNA and protein in U251 cells
exposed to miR-155 mimics, miR-155 inhibitors or scramble controls.
The SCN1A protein (Fig. 5A) and
mRNA (Fig. 5B) expression levels
were reduced in U251 cells transfected with 50 or 100 nM miR-155
mimics. The results of RT-qPCR showed the same trends. By contrast,
cells treated with 50 or 100 nM miR-155 inhibitor exhibited an
elevated expression levels of SCN1A protein (Fig. 5C) and mRNA (Fig. 5D) compared with cells treated with
the scrambled negative control, and there was no difference between
the cells treated with 50 and 100 nM miR-155 inhibitor. These
results support a negative regulatory relationship between miR-155
and SCN1A, and suggested a concentration-dependent effect of
miR-155 on the expression of SCN1A.
Discussion
A seizure is an abnormal discharge of neurons in the
brain that may follow brain injury that causes transient central
nervous system disorders, with sudden and recurrent characteristics
that are related to the degree of the brain injury (21). Seizure incidence rate following a
severe head injury is 14.8–33.0%; patients who need craniotomy and
decompressive craniotomy often have cerebral hernia; therefore,
their incidence of seizure will be higher (1,2).
Clinically, many nerve trauma doctors may not be able to easily
diagnose a patient with epilepsy owing to various patient-centric
considerations (including schooling, employment, marriage, driving
and applying for loans, amongst others); in these situations they
will diagnose the patient as suffering from seizures. Furthermore,
whether the prophylactic use of AEDs is necessary following
neurosurgical surgery has been controversial (22,23).
In the present study, 14 cases of seizures following
cranioplasty were observed across the experimental and control
groups, with an incidence of 17.5%. Possible associated clinical
factors include: i) The cranial cavity volume changes following
cranioplasty, resulting in changes to the brain tissue and
cerebrospinal fluid dynamics and breaking the relative equilibrium
that has existed for several months prior to surgery; ii) some
patients may have presented with collapsed bone window tissues
prior to surgery, as subdural stitches are often suspended on
titanium during surgery to reduce the epidural space, which may
cause the arachnoid fibrous band on the brain surface to move the
brain tissue and induce changes to brain tissue function; iii)
cranioplasty changes the oppression of atmospheric pressure on the
brain tissue, bringing alterations to hemodynamics or the external
environment of the brain tissue; iv) when patients underwent
isolation of the endocranium, 15 cases had damage to the
endocranium, of which 12 cases exhibited arachnoid damage and
cerebrospinal fluid outflow, although they received a tight suture
of the endocranium, non-visible brain damage cannot be excluded; v)
the use of some nerve stimulant drugs following surgery, including
xingnaojing and naloxone, may also induce seizures (24,25).
The incidence rate of cranioplasty-related seizures is relatively
high and should be prevented by the use of AEDs (26). In the present study, only 3/41
cases among the experimental group experienced seizures. One of
these cases occurred prior to surgery, and was later indicated to
be caused by an insufficient plasma concentration of valproic acid;
following an increase in dosage, no more seizures were recorded. In
two cases, following surgery, the plasma concentration of valproic
acid was within expected levels, but could not control seizures.
Therefore, the treatment was changed to oxcarbazepine or
levetiracetam tablets for seizure control. In the control group,
11/39 patients experienced seizures; all of these were controlled
well with VPA. In the experimental and control groups there were no
cases that required surgery as a result of a failure to control the
seizures. The difference in the incidence rates of seizures between
VPA-treated group (7.3%) and control group (28.2%) was statically
significant (P<0.05), indicating that VPA may have a preventive
effect for cranioplasty-related seizures.
SCN1A is important for the initiation of action
potentials in the central nervous system (27). This subunit contains 4 homologous
domains (D1-D4) and each contains six transmembrane segments
(S1-S6) (28).
The present study revealed that the expression of
miR-155 was lower in VPA-treated patients, compared with patients
in the untreated control group. Furthermore, computational analysis
revealed that SCN1A was a potential target gene of miR-155, as it
contains a potential seed region in the 3′UTR of the gene, and this
was experimentally supported through the use of a luciferase assay.
Furthermore, the expression of SCN1A mRNA and protein were
estimated in different cell culture treatment groups, which
demonstrated that SCN1A protein and mRNA expression levels were
reduced in U251 cells in a dose-dependent manner, whereas cells
exposed to a miR-155 inhibitor exhibited increased expression
levels of SCN1A protein and mRNA. miR-155 has been reported to be
involved with several diseases, including seizures. Previous study
showed that upregulation of miR-155 was closely associated with
ischemic stroke, intracerebral hemorrhage, and kainate seizures
(29). Moreover, the expressions
of miR-155 were significantly upregulated in the seizure-related
acute and chronic stages of mesial temporal lobe epilepsy (MTLE) in
the immature rat model and also in children with MTLE (30). Antagonist targeting microRNA-155
protects against lithium-pilocarpine-induced Status Epilepticus in
C57BL/6 Mice by activating brain-derived neurotrophic factor
(31). And a report also showed
that VPA downregulated the expression of miR-155 which had
potential protection against cerebral ischemia (6), indicating inhibition of miR-155 is a
potential target for seizure and VPA can effectively inhibit the
epression of miR-155.
In summary, the incidence of cranioplasty-related
seizures is high and is associated with patients' morbidity.
Preventative use of VPA in the early postsurgical stages may reduce
the incidence of seizures.
References
|
1
|
Lee L, Ker J, Quah BL, Chou N, Choy D and
Yeo TT: A retrospective analysis and review of an institution's
experience with the complications of cranioplasty. Br J Neurosurg.
27:629–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pechmann A, Anastasopoulos C,
Korinthenberg R, van Velthoven-Wurster V and Kirschner J:
Decompressive craniectomy after severe traumatic brain injury in
children: Complications and outcome. Neuropediatrics. 46:5–12.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronen GM, Buckley D, Penney S and Streiner
DL: Long-term prognosis in children with neonatal seizures: A
population-based study. Neurology. 69:1816–1822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizrahi EM: Neonatal seizures: Problems in
diagnosis and classification. Epilepsia. 28 Suppl 1:S46–S55. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sankar R and Painter MJ: Neonatal
seizures: After all these years we still love what doesn't work.
Neurology. 64:776–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunsberger JG, Fessler EB, Wang Z,
Elkahloun AG and Chuang DM: Post-insult valproic acid-regulated
microRNAs: Potential targets for cerebral ischemia. Am J Transl
Res. 4:316–332. 2012.PubMed/NCBI
|
|
7
|
Filip A: MiRNA-new mechanisms of gene
expression control. Postepy Biochem. 53:413–419. 2007.(In Polish).
PubMed/NCBI
|
|
8
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guarnieri DJ and DiLeone RJ: MicroRNAs: A
new class of gene regulators. Ann Med. 40:197–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gauthier BR and Wollheim CB: MicroRNAs:
‘Ribo-regulators’ of glucose homeostasis. Nat Med. 12:36–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bi Y, Liu G and Yang R: MicroRNAs: Novel
regulators during the immune response. J Cell Physiol. 218:467–472.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hatfield S and Ruohola-Baker H: microRNA
and stem cell function. Cell Tissue Res. 331:57–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsubara H, Takeuchi T, Nishikawa E,
Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M,
Nimura Y, et al: Apoptosis induction by antisense oligonucleotides
against miR-17-5p and miR-20a in lung cancers overexpressing
miR-17-92. Oncogene. 26:6099–6105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beveridge NJ, Tooney PA, Carroll AP,
Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I and Cairns MJ:
Dysregulation of miRNA 181b in the temporal cortex in
schizophrenia. Hum Mol Genet. 17:1156–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuhn DE, Nuovo GJ, Martin MM, Malana GE,
Pleister AP, Jiang J, Schmittgen TD, Terry AV Jr, Gardiner K, Head
E, et al: Human chromosome 21-derived miRNAs are overexpressed in
down syndrome brains and hearts. Biochem Biophys Res Commun.
370:473–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicoloso MS and Calin GA: MicroRNA
involvement in brain tumors: From bench to bedside. Brain Pathol.
18:122–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou R, Yuan P, Wang Y, Hunsberger JG,
Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G and Manji
HK: Evidence for selective microRNAs and their effectors as common
long-term targets for the actions of mood stabilizers.
Neuropsychopharmacology. 34:1395–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goh WW, Oikawa H, Sng JC, Sergot M and
Wong L: The role of miRNAs in complex formation and control.
Bioinformatics. 28:453–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Wu G, Qin X, Ma Q, Zhou Y, Liu S
and Tan Y: Expression of Nodal on bronchial epithelial cells
influenced by lung microbes through DNA methylation modulates the
differentiation of T-Helper cells. Cell Physiol Biochem.
37:2012–2022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyer FB: Calcium, neuronal
hyperexcitability and ischemic injury. Brain Res Brain Res Rev.
14:227–243. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cranley MR, Craner M and McGilloway E:
Antiepileptic prophylaxis following severe traumatic brain injury
within a military cohort. J R Army Med Corps. 162:109–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klimek M and Dammers R: Antiepileptic drug
therapy in the perioperative course of neurosurgical patients. Curr
Opin Anaesthesiol. 23:564–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Creutzfeldt CJ, Tirschwell DL, Kim LJ,
Schubert GB, Longstreth WT Jr and Becker KJ: Seizures after
decompressive hemicraniectomy for ischaemic stroke. J Neurol
Neurosurg Psychiatry. 85:721–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krause-Titz UR, Warneke N, Freitag-Wolf S,
Barth H and Mehdorn HM: Factors influencing the outcome (GOS) in
reconstructive cranioplasty. Neurosurg Rev. 39:133–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hesdorffer DC, Benn EK, Cascino GD and
Hauser WA: Is a first acute symptomatic seizure epilepsy? Mortality
and risk for recurrent seizure. Epilepsia. 50:1102–1108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martina M, Vida I and Jonas P: Distal
initiation and active propagation of action potentials in
interneuron dendrites. Science. 287:295–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Escayg A, MacDonald BT, Meisler MH, Baulac
S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B,
Chaigne D, et al: Mutations of SCN1A, encoding a neuronal sodium
channel, in two families with GEFS+2. Nat Genet. 24:343–345. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu DZ, Tian Y, Ander BP, Xu H, Stamova
BS, Zhan X, Turner RJ, Jickling G and Sharp FR: Brain and blood
microRNA expression profiling of ischemic stroke, intracerebral
hemorrhage, and kainate seizures. J Cereb Blood Flow Metab.
30:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashhab MU, Omran A, Kong H, Gan N, He F,
Peng J and Yin F: Expressions of tumor necrosis factor alpha and
microRNA-155 in immature rat model of status epilepticus and
children with mesial temporal lobe epilepsy. J Mol Neurosci.
51:950–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai Z, Li S, Li S, Song F, Zhang Z, Qi G,
Li T, Qiu J, Wan J, Sui H and Guo H: Antagonist targeting
microRNA-155 protects against lithium-pilocarpine-induced status
epilepticus in C57BL/6 mice by activating brain-derived
neurotrophic factor. Front Pharmacol. 7:1292016. View Article : Google Scholar : PubMed/NCBI
|