Introduction
Sepsis and its complications are still serious
clinical problems (1). Lung injury
(ALI) remains the common complication of sepsis (2). Unfortunately, development of
effective pharmacologic and ventilatory treatment strategies for
sepsis-induced lung injury has not made significant progress over
the past several decades. Pulmonary microvascular endothelial cells
play an important role in pulmonary blood barrier. They are
involved in the transportation of nutrients and metabolites in the
lung tissue, inflammatory response and endothelial regulation
(3). Many studies have shown that
PMVECs were destroyed during sepsis-induced lung injury, thus
leading to increased permeability of pulmonary blood barrier and
then activation of the coagulation system and inflammatory response
system (4). Therefore, PMVECs is
essential to reduce lung injury in sepsis.
HSPA12B, a newly discovered member of HSP70
superfamily, is predominately expressed in vascular endothelial
cells and participates in vascular generation and migration
(5). Zhou et al reported
that upregulation of HSPA12B could attenuate sepsis-induced cardiac
dysfunction (6). HSPA12B was also
demonstrated to improve cardiac function and remodeling after
myocardial infarction via eNOS-dependent mechanism (7). In vitro, Wu et al
(8) found that HSPA12B could
inhibit LPS-induced inflammatory response in HUVECs. Our previous
study showed that HSPA12B was upregulated in LPS-stimulated
endothelial cells and it was defensive against LPS induced
impairment of endothelial permeability (9). Therefore, HSPA12B may be an intrinsic
protein mediating endothelial protection against inflammatory
insult, but it remains unclear how HSPA12B was upregulated.
Post-transcriptional regulation may be involved in the regulation
of HSPA12B gene expression; however, the underlying mechanism has
not been well understood.
miRNA-mRNA interaction is one of the most common
mechanisms of post-transcriptional regulation (10,11).
One miRNA can act on a variety of mRNAs and an mRNA may also be
affected by multiple miRNAs (12,13).
Currently there are limited data that investigate the role of miRNA
in the regulation of HSPA12B and the function of vascular
endothelial cells. In the present study, we screened several miRNAs
potentially correlated with HSPA12B by bioinformatics analysis and
performed in vitro experiments to identify the candidate
miRNAs targeting at the 3′UTR of HSPA12B mRNA.
Materials and methods
Reagents
Escherichia coli LPS was purchased from
Sigma-Aldrich (St. Louis, MO, USA), and the NF-κB inhibitor were
obtained from Calbiochem (catalog number 481406, EMD Chemicals, San
Diego, CA, USA). Liposome Lipofectamine 2000 was purchased from
Invitrogen (Carlsbad, CA, USA). Human anti-HSPA12B antibody was
from Abcam (Cambridge, UK); anti-β-actin antibody, goat anti-rabbit
IgG-HRP secondary antibodies, and rabbit anti-mouse IgG-HRP
secondary antibodies were from Cell Signaling Technology (Danvers,
MA, USA). VE-cadherin antibody was obtained from R&D Systems
Inc (Minneapolis, MN, USA).
Bioinformatics analysis
The miRNAs potentially related to human HSPA12B were
searched in the TargetScan (http://www.targetscan.org/), miRbase (http://www.mirbase.org/) and PicTar (http://pictar.mdc-berlin.de/) databases.
Cell culture and transfection
HUVEC was cultured in DMEM high glucose medium
(Hyclone, Logan, UT, USA), supplemented with 10% fetal bovine serum
at 37°C with 5% CO2. Transfection was performed using
Lipofectamine 2000 (Invitrogen, CA, USA) according to the
manufacturer's instruction. After the cells reached 80% confluency,
10 nM miRNA mimic or negative control (Genepharma, Shanghai, China)
were transfected into HUVECs by using Lipofectamine 2000. The
transfection mixture was incubated for 6 h. Negative control mimics
were transfected as matched controls.
Real-time PCR
Total RNA containing miRNA was extracted from HUVECs
using Trizol reagent (Invitrogen). Primers used for the polymerase
chain reaction (PCR) were designed to be isoform specific (Table I). Following instructions of the
Rever Tra Ace qPCR RT Kit (Toyobo, Osaka, Japan), cDNA was obtained
by reverse transcription (30 min at 37°C and 5 min at 98°C) from 1
µg purified total RNA. To quantify the expression level of mRNA of
HSPA12B, semi-quantitative PCR system was set up using 2 µl of 10×
PCR Buffer, 1 µl of dNTP at 2.5 mM, 1 µl of Mgcl2 at 25
mM, 1 µl of forward and reverse primers at 10 µmol/l, 1 µl cDNA,
Taq enzyme and 0.25 µl deionized water to constitute a total volume
of 20 µl. The thermal cycle for amplification were as follows:
(1) 94°C for 3 min; (2) 28 cycles of denaturation, each
consists of 30 sec at 94°C, 45 sec at 58°C and 20 sec at 72°C
followed by (3) a final extension
for 7 min at 70°C. GAPDH were used as internal control. PCR
products were semi-quantified by electrophoresis in 1.5% agarose
gel.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Gene | Forward 5′-3′ | Stem-Loop primer
5′-3′ |
|---|
| miRNA universal
reverse primer | TGGTGTCGTGGAGTCG |
|
| hsa-miR-1224-3p |
ACACTCCAGCTGGGCCCCACCTCCTCT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTGAGGAG |
| hsa-miR-3127-5p |
ACACTCCAGCTGGGATCAGGGCTTGTGGA |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTTCCCAT |
| hsa-miR-3657 |
ACACTCCAGCTGGGTTAGTGGTTATTA |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACACAGGG |
| hsa-miR-4505 |
ACACTCCAGCTGGGAGGCAGGGTC |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCGACCC |
| hsa-miR-3665 |
ACACTCCAGCTGGGGCGGCGGGGC |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCGTCCAC |
| hsa-miR-4736 |
ACACTCCAGCTGGGGTCGGGTCTAT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCGTCCA |
| hsa-miR-940 |
ACACTCCAGCTGGGCCCCTCGCCCCCG |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTTCCGTCC |
| hsa-miR-4692 |
ACACTCCAGCTGGGTAGACTATGGGTGT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTCCGTC |
| hsa-miR-4514 |
ACACTCCAGCTGGGAAGGGGTTAG |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGTCCGTC |
| hsa-miR-4742-5p |
ACACTCCAGCTGGGAGACATTTATAGGGA |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTCCGTT |
| hsa-miR-3664-3p |
ACACTCCAGCTGGGTTGAGACAGAAATG |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGAGTCCT |
| U6 Forward
primer |
CTCGCTTCGGCAGCACA |
|
| U6 Reverse
primer |
AACGCTTCACGAATTTGCGT |
|
Western blot
The cells were washed twice with ice cold PBS and
lysed with cell lysis buffer RIPA. Protein concentrations of the
cell lysate extracts were measured with BCA assay (Pierce,
Rockford, IL, USA) and equalized with the extraction reagent. Equal
amount of the extracts were loaded and subjected to SDS-PAGE,
transferred onto PVDF membranes, and blocked with 5% non-fat milk
in TBST, blotted with the antibody against HSPA12B (1:1,000), and
subsequently secondary antibody (1:2,000). The bands on the
membrane were visualized with an enhanced chemiluminescence (ECL)
kit (GE, Amersham, UK). β-actin was used as an internal loading
control.
Luciferase activity assay
293 cells were seeded in 24-well plates. After the
cells reached 60% confluency, the mixture with 0.5 µg
pGL3-HSPA12B-3′UTR plasmid (provided by Obio, Shanghai, China), 0.5
µg mutant pGL3-HSPA12B-3′UTR plasmid (provided by Obio, Shanghai,
China) and 50 pmol miRNA mimic or negative control (Genepharma,
Shanghai, China) were incubated with Lipofectamine 2000
(Invitrogen, CA, USA). After 24 h, cells were collected and lysed
with 10 µl Luciferase Assay Substrate (Invitrogen, CA, USA), and
then 10 µl cell lysate supernatant was added. Luciferase assays
were measured using Luminometer (E1910, Promega). And the
luciferase activity was normalized by Renilla luciferase
intensity.
Flow cytometry
Cells were removed from culture dish with trypsin
and harvested by centrifugation. Antibody for flow cytometry was
added and reaction was conducted in the dark for 30 min. After
centrifuging, the supernatant was discarded and 1xPBS was added.
Total VE-cadherin expression was detected using a flow cytometer
(BD FACSCalibur, Franklin Lakes, NJ, USA).
Transendothelial electrical resistance
(TEER)
Transendothelial electrical resistance (TEER) of
HUVEC monolayer was determined using MERSSTx01 Electrode according
to the instruction manual of the manufacturer (EMD Millipore
Corporation, Billerica MA, USA). 0.4 µm fibronectin-coated
Transwell filters was used and 1×105 HUVECs were seeded
on each filter until full confluence. After cell culturing of 72 h,
MERSSTx01 electrode was used to detect the TEER value of HUVECs
sequentially and the mean was expressed in the common unit (V
cm2) after subtraction of the value of a blank cell-free
filter.
Scratch test
HUVECs (5×105 cells per well) were
cultured 24 h in a 6-well plates. A wound was made by craping
monolayer cells with a 200 µl pipette tip. Then the wounded cell
monolayers were washed with serum-free medium. The medium was
replaced with serum-free DMEM. The migration path of cell was
tracked at 0 and 24 h using Olympus microscope and representative
scratch zones were photographed.
Statistical analysis
The results were presented as the mean ± SD.
Comparison of mean between groups was performed using one-way
analysis of variance (ANOVA). P<0.05 was considered to be
significant. GraphPad Prism5 (La Jolla, USA) was used for all
statistical analysis.
Results
miRNA expression features in HUVECs
stimulated with LPS
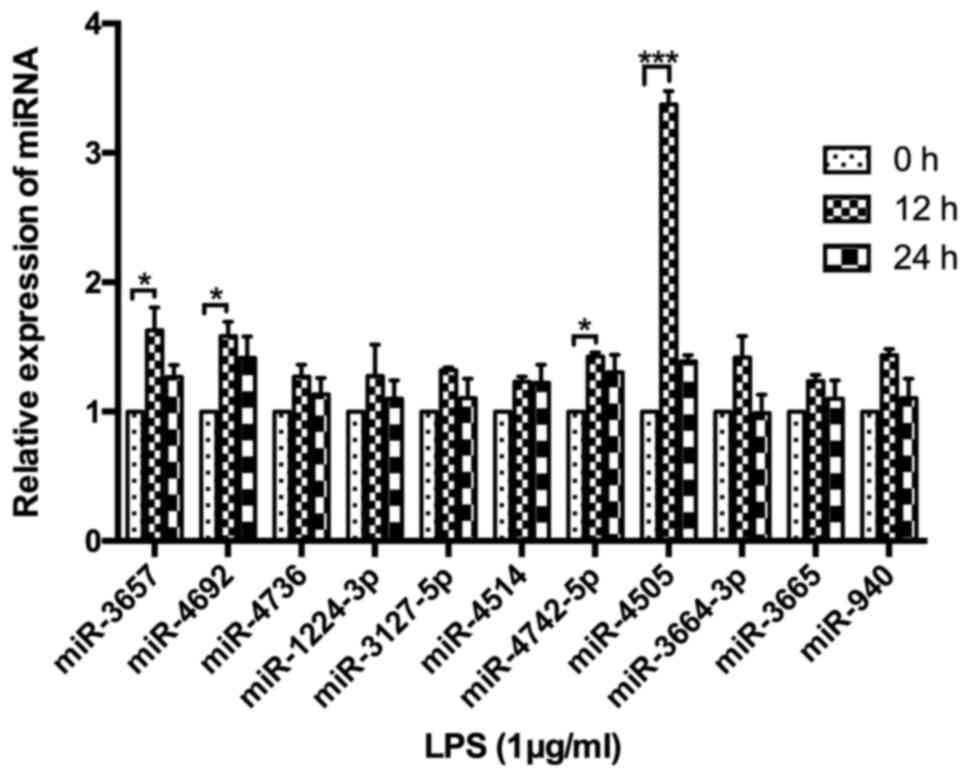
A total of 11 miRNAs were identified to potentially
target the 3′UTR region of HSPA12B mRNA, including miR-3657,
miR-642a, miR-4505, miR-3665, miR-4736, miR-940, miR-4692,
miR-4514, miR-4742-5p, miR-4269 and miR-3664-3p. In order to
further establish the relationship between HSPA12B and the miRNAs
screened by the bioinformatics analysis, the miRNA expression
levels were determined by RT-PCR in the LPS-stimulated HUVECs. As a
result, miR-3657, miR-4692, miR-4742 and miR-4505 were all
upregulated in HUVECs at 12 h after LPS stimulation (P<0.05)
(Fig. 1). The expression level of
miR-4505 was highest, with a changing level more than 3 compared
with 0 h.
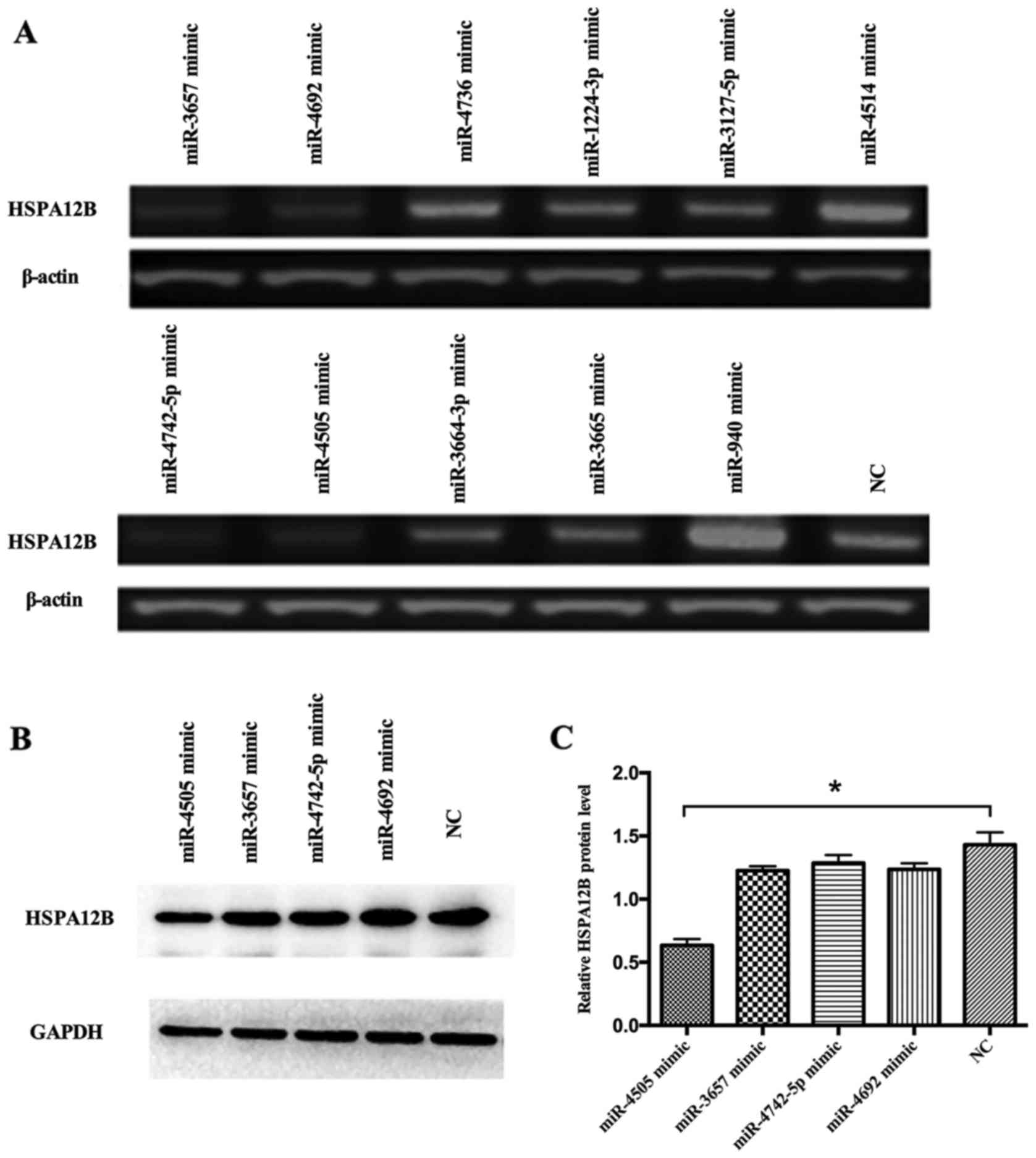
miRNA-4505 inhibits HSPA12B expression
in LPS-challenged HUVECs
The HUVECs were transfected with the miRNA mimics of
the 4 miRNA candidates as well as the negative control (NC). The
RT-PCR and western blot assays showed that the expression levels of
HSPA12B in the HUVECs transfected with the mimics of miR-3657,
miR-4692, miR-4742-5p and miR-4505 were lower than those
transfected with the negative control (Fig. 2A-C). In addition, in our previous
research, HSPA12B was elevated in 12 h upon the LPS stimulation
(5). We suspected that miR-4505
may negatively regulate the expression of HSPA12B. Therefore,
miR-4505 was screened for further investigation as a potential
candidate regulating the expression of HSPA12B in LPS-induced
HUVECs.
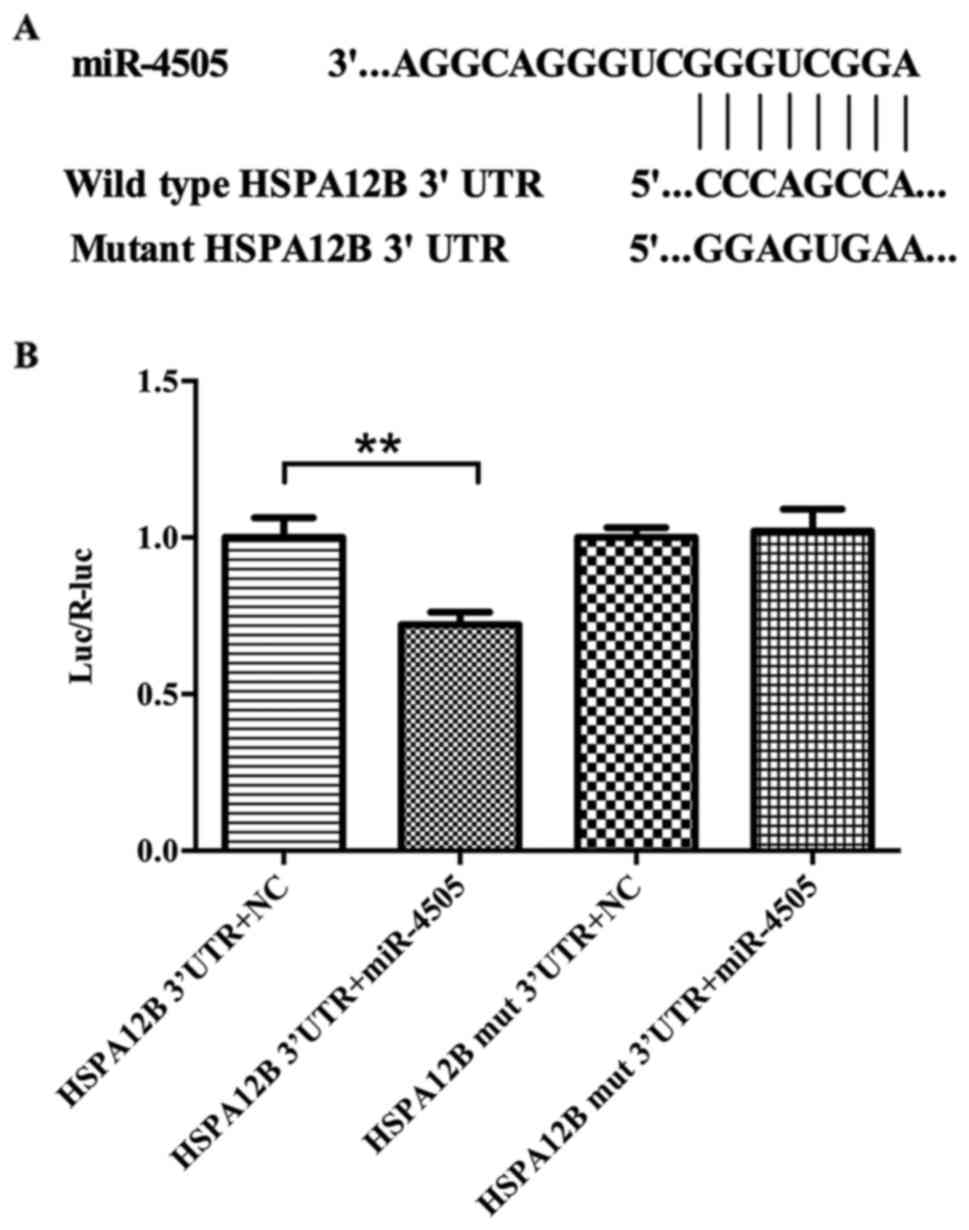
miR-4505 directly targets at 3′UTR of
HSPA12B mRNA
Luciferase analysis was performed to investigate the
relationship between HSPA12B and miR-4505. To obtain direct
evidence that miR-4505 targets HSPA12B mRNA, we designed a mutant
in the HSPA12B mRNA 3′UTR that mutated eight common nucleotides in
HSPA12B target site. The wild-type and mutant 3′UTR sequences of
HSPA12B were shown in Fig. 3. The
fluorescence ratio in HUVECs co-transfected with pGL3-HSPA12B-3′UTR
plasmid and hsa-miR-4505 mimics was significant lower than in those
transfected with pGL3-HSPA12B-3′UTR plasmid and negative control
(P<0.05), but there was no significant difference between the
two groups of cells co-transfected with mutant pGL3-HSPA12B-3′UTR
and miR-4505 mimics or negative control (Fig. 3).
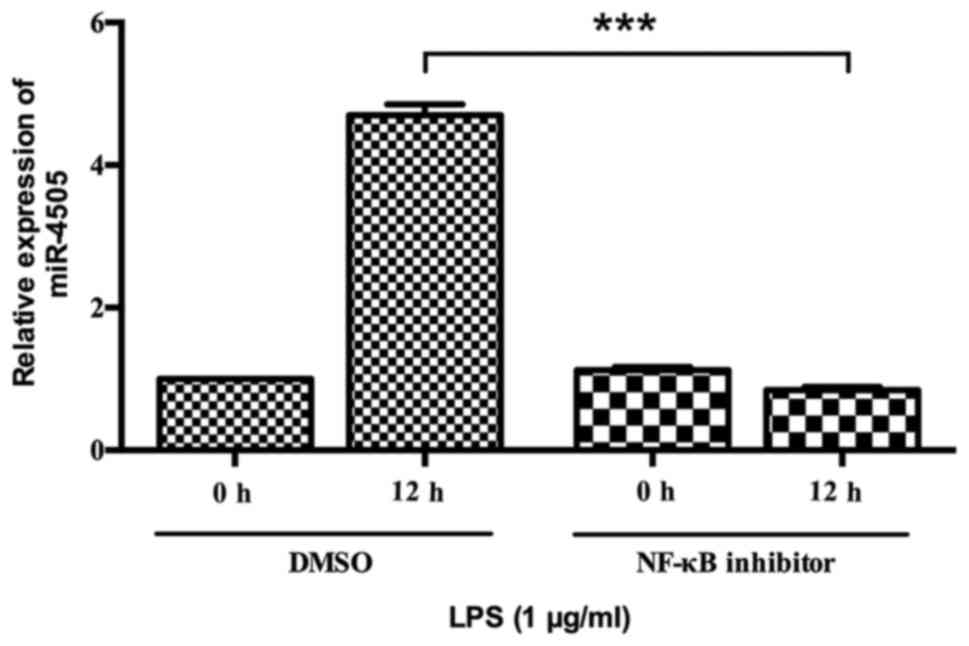
The role of NF-κB in miR-4505
upregulation induced by LPS
Since miR-4505 was upregulated by LPS stimulation,
we studied whether NF-κB was involved in regulating the expression
of miR-4505. Cells were pretreated with NF-κB inhibitor and
subsequently stimulated with LPS. The expression of miR-4505 was
detected at 0 and 12 h after LPS treatment. It was shown that
miR-4505 was significantly downregulated at 12 h after LPS
stimulation in inhibitor group compared with in control group
(P<0.05) (Fig. 4).
miR-4505 aggravates LPS-induced HUVEC
injury by targeting HSPA12B
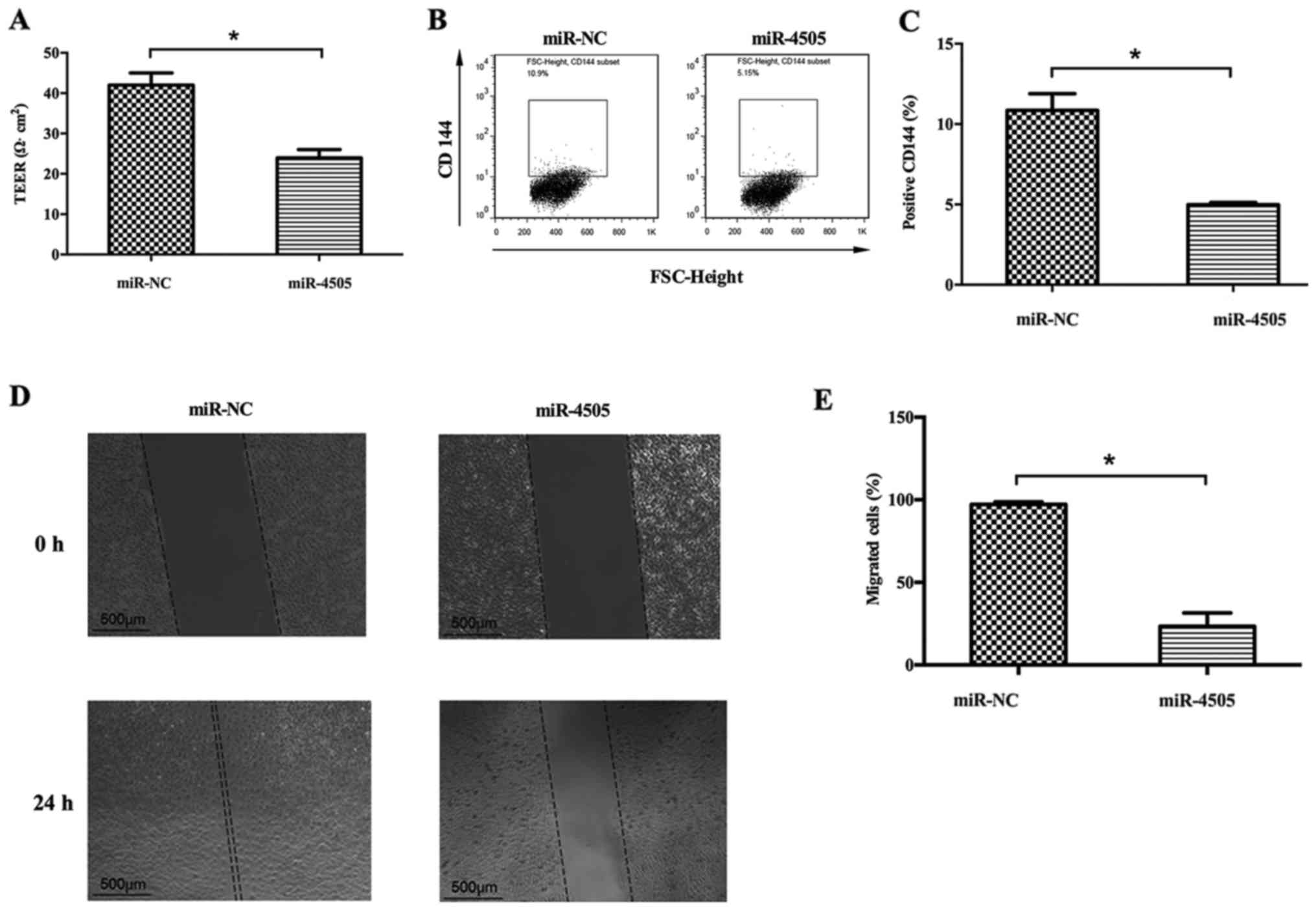
Transendothelial electrical resistance (TEER),
VE-cadherin (CD144) expression and migration capacity were
determined in HUVEC in order to study the effect of miR-4505 on
HUVEC function. TEER assay showed that TEER was significantly lower
in miR-4505 group than in the NC group (P<0.05) (Fig. 5A). Furthermore, flow cytometry
suggested that VE-cadherin was significantly downregulated after
miR-4505 transfection (P<0.05) (Fig. 5B and C). The scratch test showed
that area of miR-4505 mimic transfection group at 0 h was
1.77±0.005 mm2, 24 h was 1.38±0.1 mm2; NC
group at 0 h was 1.77±0.003 mm2, 24 h was 0.04±0.02
mm2. The migration area in the miR-4505 group
(23.4±5.7%) was significantly reduced compared with the NC group
(97.2±1.1%) (P<0.05) (Fig. 5D and
E).
Discussion
To date, our present study demonstrated that
miR-4505 negatively regulated the expression of HSPA12B by
targeting the 3′UTR of HSPA12B mRNA. The expression level of
hsa-miR-4505 increases after LPS stimulation in HUVECs, the
mechanism may involve NF-κB activation. miR-4505 induces high
permeability and reduces migration in LPS-induced HUVECs.
HSPA12B, as a member of the HSP70 family, is mainly
expressed in endothelial cells. Our previous study showed that
depletion of HSPA12B was harmful to LPS induced endothelial injury,
as manifested by TEER and VE-Cadherin expression. HSPA12B protein
was upregulated since 9 h after LPS stimulation, but returned to
normal level at 24 h. Therefore, we speculated that HSPA12B was one
of the intrinsic negative-feedback mechanisms inhibiting
endothelial inflammation, but some unknown reasons were inhibiting
the expression of HSPA12B after the onset of inflammation. Both
translational and post-translational factors might be involved in
the downregulation of HSPA12B, among which miRNAs were critical for
silencing protein expression. Therefore, we screened several miRNAs
potentially correlated with HSPA12B by the bioinformatics
analysis.
Endothelial dysfunction is a hallmark of
inflammatory organ dysfunction, such as acute respiratory distress
syndrome (14,15). Some endothelium-derived molecules
have been identified as biomarker for sepsis prognosis. For
example, angiopoietin-2 was reported to be associated with
respiratory failure or septic shock in several studies (16,17).
Our previous study also demonstrated that HSPA12B was a good
predictor for death in patients with severe sepsis (18). Collapse of endothelial cells might
be one of the causes of the secretion of these proteins. Thus,
protecting endothelial cells might be a promising direction for
improving sepsis or acute respiratory distress syndrome.
Endothelial function was partially manifested by the
permeability and migration. During inflammation response, increased
endothelial permeability triggers the body fluids accumulating,
leads to pulmonary edema and organ dysfunction (19). Vascular endothelial cells and
extracellular components (such as glycocalyx) are important
regulators of pulmonary vascular barrier (20). VE-cadherin is a component of
endothelial junctions, and plays a key role in the maintenance of
vascular integrity (21). Several
studies showed that monolayer endothelial cell could increase the
endothelium permeability via VE-Cadherin antibody (22). VE-cadherin antibody administration
can cause pulmonary and cardiac edema in mice (23,24).
In sepsis, high expression of cytokines (TNF-α, vascular
endothelial growth factor) could redistribute VE-Cadherin focally
and increase the endothelium permeability. Therefore, our study
demonstrated that miR-4505 aggravated endothelial injury by
targeting at HSPA12B.
In summary, miR-4505 negatively regulates HSPA12B
expression via direct interaction with the 3′UTR of HSPA12B mRNA.
Upregulation of miR-4505 by LPS challenge was dependent on NF-κB.
Overexpression of miR-4505 aggravated endothelial injury as shown
by the increased permeability and reduce migration capacity.
However, there are several limitations in our present study. First,
the study was carried out in HUVECs, we have no data in vivo
about miRNA-4505. To evaluate the efficacy of miR-4505 in
HSPA12B-mediated protective effect on endothelial cells following
LPS challenge, a more comprehensive study in vivo may be
necessary to validate our findings. Second, the expression of
miR-4505 in plasma of sepsis patients remains to be measured.
Further experiments need to be performed to determine whether the
miR-4505 negatively regulates HSPA12B expression in sepsis
patients.
Acknowledgements
This work was supported by the grants from the
National Natural Science Foundation of China (No. 81270128). The
manuscript partly presented in the 10th National Assembly severe
medical National Medical Conference 2016 as the form of poster
presentation (http://www.medmeeting.org/Upload/user/186151/file/20160520/20160520095931_8385.pdf).
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernu R, Wallet F, Thiollière F, Martin O,
Richard JC, Schmitt Z, Wallon G, Delannoy B, Rimmelé T, Démaret C,
et al: An attempt to validate the modification of the
American-European consensus definition of acute lung injury/acute
respiratory distress syndrome by the Berlin definition in a
university hospital. Intensive Care Med. 39:2161–2170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parikh SM, Mammoto T, Schultz A, Yuan HT,
Christiani D, Karumanchi SA and Sukhatme VP: Excess circulating
angiopoietin-2 may contribute to pulmonary vascular leak in sepsis
in humans. PLoS Med. 3:e462006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stevens T: Functional and molecular
heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc.
8:453–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steagall RJ, Rusiñol AE, Truong QA and Han
Z: HSPA12B is predominantly expressed in endothelial cells and
required for angiogenesis. Arterioscler Thromb Vasc Biol.
26:2012–2018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Qian J, Li C, Li J, Zhang X, Ding
Z, Gao X, Han Z, Cheng Y and Liu L: Attenuation of cardiac
dysfunction by HSPA12B in endotoxin-induced sepsis in mice through
a PI3K-dependent mechanism. Cardiovasc Res. 89:109–118. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Zhang Y, Li C, Xie J, Liu Y, Zhu W,
Zhang X, Jiang S, Liu L and Ding Z: HSPA12B attenuates cardiac
dysfunction and remodelling after myocardial infarction through an
eNOS-dependent mechanism. Cardiovasc Res. 99:674–684. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Li X, Huang L, Jiang S, Tu F, Zhang
X, Ma H, Li R, Li C, Li Y, et al: HSPA12B inhibits
lipopolysaccharide-induced inflammatory response in human umbilical
vein endothelial cells. J Cell Mol Med. 19:544–554. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang Q, Chen Y, Zhang X, Yu G, Wan X, Wang
J, Bo L and Zhu K: Heat shock protein A12B protects against
sepsis-induced impairment in vascular endothelial permeability. J
Surg Res. 202:87–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meltzer PS: Cancer genomics: Small RNAs
with big impacts. Nature. 435:745–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee Y, Jeon K, Lee JT, Kim S and Kim VN:
MicroRNA maturation: Stepwise processing and subcellular
localization. EMBO J. 21:4663–4670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babiarz JE, Ruby JG, Wang Y, Bartel DP and
Blelloch R: Mouse ES cells express endogenous shRNAs, siRNAs, and
other microproces-sor-independent, Dicer-dependent small RNAs.
Genes Dev. 22:2773–2785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coletta C, Módis K, Oláh G, Brunyánszki A,
Herzig DS, Sherwood ER, Ungvári Z and Szabo C: Endothelial
dysfunction is a potential contributor to multiple organ failure
and mortality in aged mice subjected to septic shock: Preclinical
studies in a murine model of cecal ligation and puncture. Crit
Care. 18:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee WL and Liles WC: Endothelial
activation, dysfunction and permeability during severe infections.
Curr Opin Hematol. 18:191–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palud A, Parmentier-Decrucq E, Pastre J,
De Freitas Caires N, Lassalle P and Mathieu D: Evaluation of
endothelial biomarkers as predictors of organ failures in septic
shock patients. Cytokine. 73:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Li C, Shao R, Yu H, Zhang Q and
Zhao L: Prognostic significance of the
angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for
early sepsis in an emergency department. Crit Care. 19:3672015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang R, Wan XJ, Zhang X, Kang QX, Bian
JJ, Yu GF, Wang JF and Zhu KM: Plasma HSPA12B is a potential
predictor for poor outcome in severe sepsis. PLoS One.
9:e1012152014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Birukov KG, Zebda N and Birukova AA:
Barrier enhancing signals in pulmonary edema. Compr Physiol.
3:429–484. 2013.PubMed/NCBI
|
|
20
|
Yang Y and Schmidt EP: The endothelial
glycocalyx: An important regulator of the pulmonary vascular
barrier. Tissue Barriers. 1:pii: 23494. 2013. View Article : Google Scholar
|
|
21
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Breviario F, Caveda L, Corada M,
Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D,
Lampugnani MG and Dejana E: Functional properties of human vascular
endothelial cadherin (7B4/cadherin-5), an endothelium-specific
cadherin. Arterioscler Thromb Vasc Biol. 15:1229–1239. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gotsch U, Borges E, Bosse R, Böggemeyer E,
Simon M, Mossmann H and Vestweber D: VE-cadherin antibody
accelerates neutrophil recruiment in vivo. J Cell Sci. 110:583–588.
1997.PubMed/NCBI
|
|
24
|
Corada M, Mariotti M, Thurston G, Smith K,
Kunkel R and Brockhaus M: Vascular endothelial-cadherin is an
important determinant of microvascular integrity in vivo.
Proc Natl Acad Sci USA. 96:9815–9820. 1999. View Article : Google Scholar : PubMed/NCBI
|