Introduction
Cardiovascular complications are among the most
common complications associated with diabetes mellitus and are a
major cause of hospital admission and mortality among patients with
diabetes (1,2). Atherosclerosis is a cardiovascular
condition experienced by many patients with diabetes and can lead
to severe consequences, such as myocardial infarction, ischemic
stroke and ischemia of peripheral tissues (3,4).
Endothelial dysfunction, which results in the disruption of the
balance between vasodilation and vasoconstriction, is an initiating
factor in the development of atherosclerosis (5,6).
Thus, a therapeutic strategy for restoring endothelial function
would be valuable in preventing serious cardiovascular
complications in patients with diabetes.
Previous studies on NADPH oxidase 4 (NOX4), an
isoform of the NOX family of enzyme complexes, reported conflicting
harmful and beneficial results. The main function of NOX4 results
in the production of hydrogen peroxide (7), which indicated that overactivation
and induction of NOX4 expression may lead to the development of
vascular diseases. Indeed, several studies have demonstrated that
NOX4 activity can be harmful; for example, one study investigated
the effects of NOX4 knockout in a mouse model of stroke and
revealed that NOX4-knockout mice exhibited less oxidative stress,
blood-brain barrier leakage and neuronal apoptosis compared with
control mice (8). Another study
reported that cardiac-specific deletion of NOX4 inhibited cardiac
hypertrophy, fibrosis and apoptosis in response to pressure
overload (9). By contrast, several
studies have demonstrated that NOX4 activity is beneficial in
cardiovascular diseases. For example, overexpression of NOX4 was
reported to enhance vasodilatation and reduce blood pressure
(10). In addition, NOX4
expression was revealed to inhibit the development of endothelial
dysfunction and atherosclerosis in mice deficient in low-density
lipoprotein receptor (7). These
inconsistent results regarding the role of NOX4 in cardiovascular
disease indicated that a more comprehensive understanding of NOX4
signaling is required for the development of treatments to correct
endothelial dysfunction through the regulation of NOX4
activity.
In the present study, a promoter-binding
transcription factor activation profiling plate array and chromatin
immunoprecipitation (ChIP) were used to identify GATA-binding
protein 4 (GATA4) as the transcription factor that may be
responsible for regulating NOX4 expression in human umbilical vein
endothelial cells (HUVECs). Subsequently, the expression levels of
GATA4 and NOX4 in response to hyperglycemia were investigated, as
well as the effects of GATA4 overexpression in HUVECs. Finally,
whether simvastatin treatment was able to improve endothelial
function by upregulating GATA4 was examined. The results of the
present study may provide a novel mechanism of NOX4 regulation as
well as a new target for treating endothelial dysfunction in
patients with diabetes.
Materials and methods
Cell culture
HUVECs were purchased from ScienCell Research
Laboratories, Inc. (San Diego, CA, USA) and grown in Endothelial
Cell Medium (ECM; 5.5 mM glucose; ScienCell) at 37°C. Hyperglycemia
was induced by growing cells in DMEM (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C for 12 h (serum starvation),
followed by exposure to 33 mM glucose for 72 h. Control cells were
cultured in normal ECM containing 5.5 mM glucose.
Mouse diabetes model
Male C57BL/6 mice were purchased from the Shanghai
Laboratory Animal Center of the Chinese Academy of Sciences
(Shanghai, China). All mice were 8 weeks old and weighed 25–30 g.
Mice were housed in the animal facility, which was maintained at
20–25°C with 55% relative humidity and an automatic 12 h light/dark
cycle. All mice received a standard laboratory diet and tap water
ad libitum, and were acclimated for 1 week prior to
experimentation. A total of 40 mice were used in this experiment;
20 in the control group and 20 in the diabetes group. Mice received
an intraperitoneal injection of 110 mg/kg streptozotocin (STZ;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to induce the
diabetes model. Blood glucose concentration was measured at 72 h
following STZ injection. Mice with a glucose level ≥16.7 mmol/l
were considered diabetic. The mice in the control group received an
intraperitoneal injection of saline. This study was approved by the
Ethics Committee of The First Affiliated Hospital, College of
Medicine, Zhejiang University (Hangzhou, Chin).
Promoter-binding transcription factor
profiling plate array
The promoter-binding Transcription Factor Profiling
Plate Array (Signosis, Inc., Santa Clara, CA, USA) was performed
according to the manufacturer's protocol. Briefly, nuclear proteins
were extracted using the nuclear protein extraction kit provided
with the array, according to the manufacturer's instructions.
Biotin-labeled transcription factor probes (Signosis, Inc.) were
mixed with 10 µl nuclear extract with or without NOX4 promoter
(1.15 µg), and the complex was incubated at 25°C for 30 min.
Subsequently, transcription factor-DNA complexes were separated
from unbound probes using the isolation columns provided in the
kit. The bound probes were eluted from the columns with elution
buffer, and hybridization of the eluted probes was performed with
the hybridization plates. Finally, the bound probes were detected
by streptavidin-horseradish peroxidase conjugation using a
microplate fluorometer. In this assay, if the promoter sequence
contains a transcription factor-binding site, it will compete with
the biotin-labeled probe, leading to detection of a lower signal.
The promoter sequence was kindly provided by Professor Adrian Manea
(Institute of Cellular Biology and Pathology ‘Nicolae Simionescu’,
Bucharest, Romania) as described previously (11,12).
The promoter sequence was as follows:
5′-ATATCCTATGGCCTGTGTTGTAAGATTTTTTATTTTAAGAGTTTAAAAGCCATTCGATTAAGCAACTAGTAGTAAACTTTTCTGTCTCTCATTAGTTGTTAAATCTAGAAAAGTTATTGGTTTAACAAACCTTTATTGGATATCTATTTTGCATCAGATTTTTTTCTACATGAATGACATTTTTTTCTCTCTCTTTTCTAATACTGATTTGTTGCAGTGTGATTGAGAGGATTCCTATTACTTACAAACAGACTAGGTATTAAAACAAAAAGATTCCCGAATAGAGTGAACCTAACCTAGAGCCCCTAAGAATTACATCAGCTTTAACCATTAATTCCCTGGGTTCTTCTTATTCAGTAATGTCTTAAAACCTTTTTATCAGTATGGGATGGGCGGGCAAGGGGATAAAGAAACTGGCGGCTGGTGGGAAGCCTTAGGTAAGGCTGTCAGGGGGGCTTTAGTTTGGGAGTGGGATAATTTGCTGAGGAAGGGTGGGAGAAACGTGAACTAGCACACAAAAGGCTGTTTCCGGCTCAAATTTCGTTACCAAGGCTCTTTGAGATGAACTTTTTTGGGGGAAAACAATCAGTCTAAAAGAGCTGTGTCTTCTTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTACAAGGGGGCGGCGAGGGTCCCCACTTTTAGTATGAGTAGCATTGTTCACATGTTTGCCAGTATTTTGGAGCCTGGCAGGCCTGGGTAGAGGCTGCGGGGGACGCCTCCAAGTTCCCACCCGGGACATCCTGAACAGCAGCAGCCACAACAACAGGCTCGCCCCTAGACAAAGGGGCCGGCGCGGCGGAGCAGACTGGTGCAGCCTGGGCCGCGCGCTCAGAGCGCTGGGCGTCTGGGCAGCTGAGTGGGCAGAGCTGACCCGGTGCGGGTGGGAGTCAGGGCGCCCGGAAAACCCGGCTCTGGGTAGCAGACCCCGCCCGGGCTGGCTCGGCGCCGGGCCTTCGGGCTTCCACTCAGTCTTTGACCCTCGGTCCTCGCTCAGCGGCCCGGCAGGCCGCACAACTGTAACCGCTGCCCCGGCCGCCGCCCGCTCCTTCTCGGTCCGGCGGGCACAGAGCGCAGCGCGGCGGGGCCGGCGGCATGGCTGTGTCCTGGAGGAGCTGGCTCGCCAACGAAGGGGTTAAACACCT-3′.
ChIP
ChIP was performed using the SimpleChIP kit from
Cell Signaling Technology, Inc. (Danvers, MA, USA), according to
the manufacturer's protocol. Briefly, owing to the lack of a
ChIP-grade GATA4 antibody, HUVECs were cultured in 6-well plates
until they reached 60–80% confluence and transfected with
GATA4-Flag (2 µg) or Flag-only overexpression plasmid (2 µg; Vigene
Biosciences, Inc., Rockville, MD, USA). Transfection was performed
using jetPEI-HUVEC transfection reagent (Polyplus-Transfection SA,
Illkirch, France) according to the manufacturer's protocol. Cells
were transfected for 4 h at 37°C, after which the transfection
medium was replaced with growth medium containing 30% fetal bovine
serum (Sigma-Aldrich; Merck KGaA) and antibiotics. Following
transfection, HUVECs were fixed in 1% formaldehyde in PBS at 37°C
for 10 min. Chromatin was digested with the addition of 5 µl
nuclease at 37°C for 20 min, and DNA fragments of 200–800 bp were
obtained. Protein A/G agarose was used for preclearing at 4°C for 1
h prior to incubation with 10 µg of anti-Flag (Abcam, Cambridge,
MA, USA) or the immunoglobulin G (included in kit) antibody at 4°C
overnight with rotation. The resultant complex was incubated in 5 M
NaCl and 20 mg/ml proteinase K solution (Cell Signaling Technology,
Inc.) at 65°C for 2 h for the reversal of crosslinking. Finally,
the DNA was purified using spin columns and subjected to real-time
polymerase chain reaction (PCR) analyses using SYBRPremix Ex TaqII
(Tli RNase H Plus) from Takara Biotechnology Co., Ltd. (Dalian,
China). The primers were as follows: forward TAAAAGCCATTCGATTAA,
reverse GTCTGTTTGTAAGTAATAGGAA. The thermocycling conditions
involved an initial denaturation at 95°C for 30 sec, followed by 34
cycles of 95°C for 5 sec and 60°C for 30 sec. PCR products were
separated by 1% gel electrophoresis using agarose gels prestained
with ethidium bromide. Bands were analyzed using Image J1.5.1
(National Institutes of Health, Bethesda, MD, USA).
Western blotting
Western blotting was performed as previously
described (13). Briefly, protein
was extracted from 1.5×106 cells, and the following
antibodies were used: Anti-endothelial nitric oxide synthase (eNOS;
ab76198; 1:1,000; Abcam), anti-phosphorylated (p)-eNOS (ab76199;
1:1,000; Abcam), anti-GATA4 (ab84593; 1:1,000; Abcam), anti-NOX4
(ab133303; 1:1,000; Abcam) and anti-GAPDH (ab8345; 1:1,000; Abcam)
primary antibodies, and HRP-conjugated secondary antibodies [goat
anti-rabbit (ab6721; 1:2,000; Abcam), goat anti-mouse (ab6789;
1:2,000; Abcam;)]. Image J version 1.5.1 (National Institutes of
Health, Bethesda, MD, USA) was used to perform densitometric
analysis.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis of NOX4 expression
RT-qPCR was conducted as previously described
(13). Briefly, RNA was extracted
from 1.5×106 cells using a total RNA purification kit
(Beyotime Institute of Biotechnology, Haimen, China) and reverse
transcription was performed using a PrimeScript 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. The primers used were as follows:
β-actin, forward ATTGGCAATGAGCGGTTC, reverse GGATGC CACAGGACTCCAT;
NOX4, forward CAGATGTTGGG GCTAGGATTG, reverse GAGTGTTCGGCACATGGGTA;
GATA1, forward CTGTCCCCAATAGTGCTTATGG, reverse
GAATAGGCTGCTGAATTGAGGG; GATA2, forward GCA ACCCCTACTATGCCAACC,
reverse CAGTGGCGTCTT GGAGAAG; GATA3, forward GCCCCTCATTAAGCC CAAG,
reverse TTGTGGTGGTCTGACAGTTCG; GATA4, forward
CGACACCCCAATCTCGATATG, reverse GTT GCACAGATAGTGACCCGT; GATA5,
forward CTTCGT GTCCGACTTCTTGGA, reverse CCGAGGCATTCCTTG TGGA;
GATA6, forward CTCAGTTCCTACGCTTCGCAT, reverse
GTCGAGGTCAGTGAACAGCA.
Immunohistochemistry
Immunohistochemical staining for GATA4 expression
was performed as previously described (13,14),
using a primary antibody against GATA4 (ab84593; 1:100; Abcam). The
endothelial layer of the thoracic aorta was sliced into 6–8 µm
thick sections. Primary antibodies were detected using a HRP
conjugated goat anti-rabbit secondary antibody was from (ab6721;
1:2,000; Abcam). Positive expression was assessed by determining
the % of positive GATA4 staining in the entire visual field.
Nitric oxide (NO) measurement
NO levels in the supernatant of HUVEC cultures were
measured using a NO Assay kit (Beyotime Institute of Biotechnology)
as previously described (15).
Briefly, the supernatant of endothelial cell medium was collected
with a pipette. A standard curve was prepared from standard
solutions of 2–50 µmol/l nitrite in ECM. Samples of standard
solutions or cell supernatants were reacted with nitrate reductase
in a 96-well plate for 40 min prior to the addition of Griess
Reagents I and II. Samples were incubated at room temperature for
10 min, and the absorbance in each well was measured at 540 nm,
using a microplate reader, to determine the nitrite
concentrations.
Simvastatin treatment
HUVECs were incubated with 5.5 mM (control) or 33 mM
(high) glucose with or without 1 µmol/l simvastatin (Sigma-Aldrich;
Merck KGaA) for 72 h at 37°C.
GATA4 knockdown
HUVECs were cultured in 6-well plates until they
reached 60–80% confluence. Cells were transfected with GATA4 small
interfering (si)RNA (sc-35455) or negative control (sc-37007) siRNA
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) using
Lipofectamine® RNAiMAX Transfection Reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The cells were incubated at 37°C for 2 days. Experiments were
performed 48 h following transfection.
Migration assay
The migration assay was performed in a Transwell
chamber that was fitted with an 8 µm pore size membrane filter
(Corning Inc., Corning, NY, USA). Cells (2×104) were
plated in the upper chamber in DMEM without FBS and incubated at
37°C for 24 h. The lower chamber contained DMEM with 10% FBS.
Following incubation, cells on the bottom surface of the filter
were fixed and stained with Hoechst 33258 (Beyotime Institute of
Biotechnology). The number of migrating cells was quantified by
counting the total number of cells in 10 fields per chamber using a
fluorescence microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All in vitro cellular preparations, experiments and
measurements were repeated at least three times. Data from in
vivo experiments represent the averages of at least eight
measurements. Significant differences between groups were evaluated
with an unpaired Student's t-test after the homogeneity of variance
was confirmed with an F-test or one-way analysis of variance for
more than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
GATA4 is a transcription factor for
NOX4
The promoter-binding transcription factor profiling
plate array was used to identify the transcription factor that may
be responsible for controlling NOX4 expression. The relative light
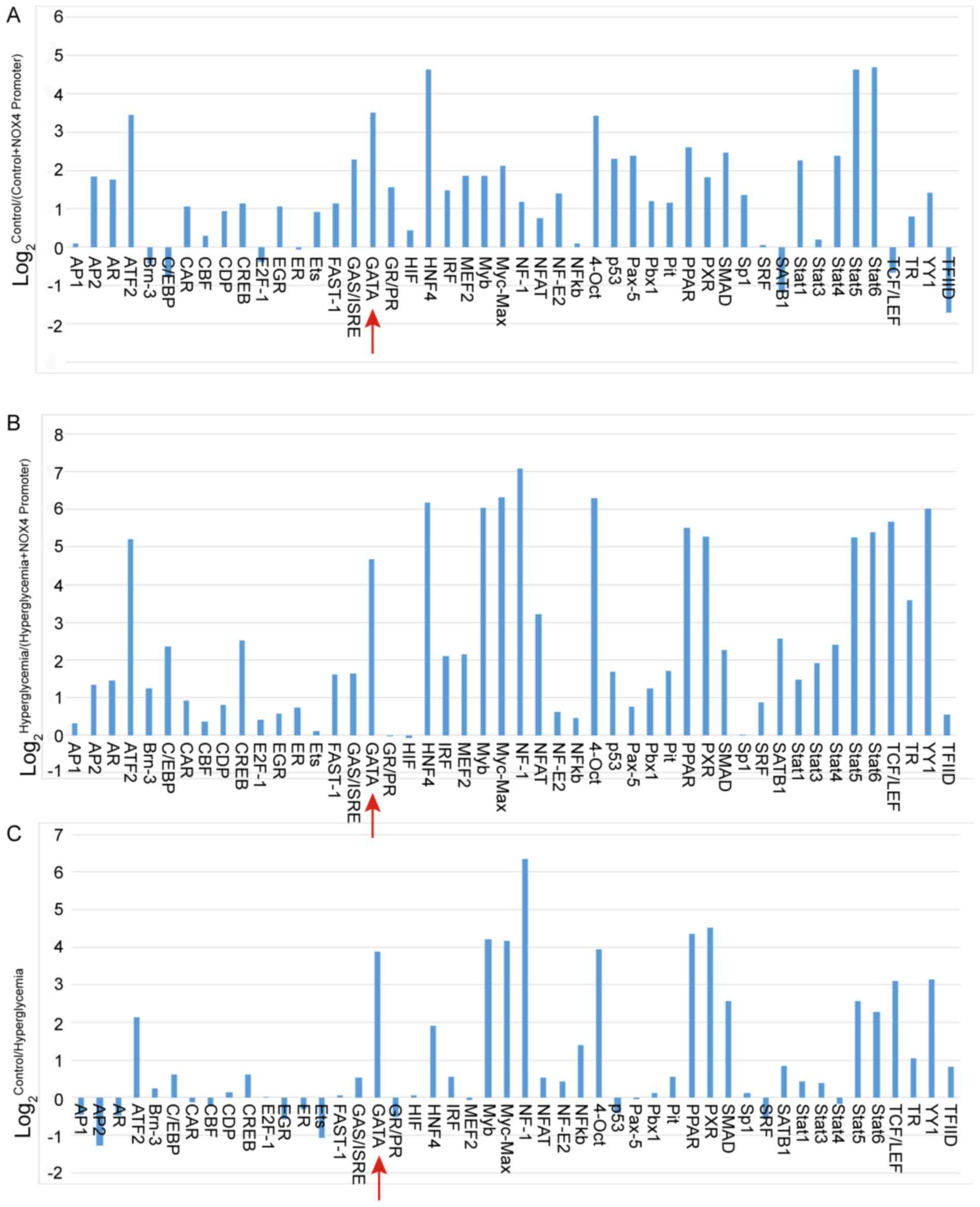
units (RLU) for the GATA probe were increased for both the control
HUVECs/(control HUVECs + NOX4 promoter) and hyperglycemia
HUVECs/(hyperglycemia HUVECs + NOX4 promoter) treatments (Fig. 1A and B, respectively), indicating
that GATA protein can bind with the NOX4 promoter. Furthermore, the
RLUs for the GATA probe were greater in HUVECs cultured in normal
glucose medium compared with that in HUVECs cultured in
high-glucose medium, indicating that GATA activity was inhibited by
hyperglycemia treatment (Fig. 1C).
As this assay is not able to differentiate between the GATA
proteins, the mRNA expression levels of GATA1-6 were measured in
control or hyperglycemia-stimulated HUVECs.
As shown in Fig.
2A, hyperglycemia inhibited GATA4 transcription, whereas the
mRNA expression of other GATA genes remained unchanged. Therefore,
it was concluded that GATA4 may be a transcription factor for
NOX4.
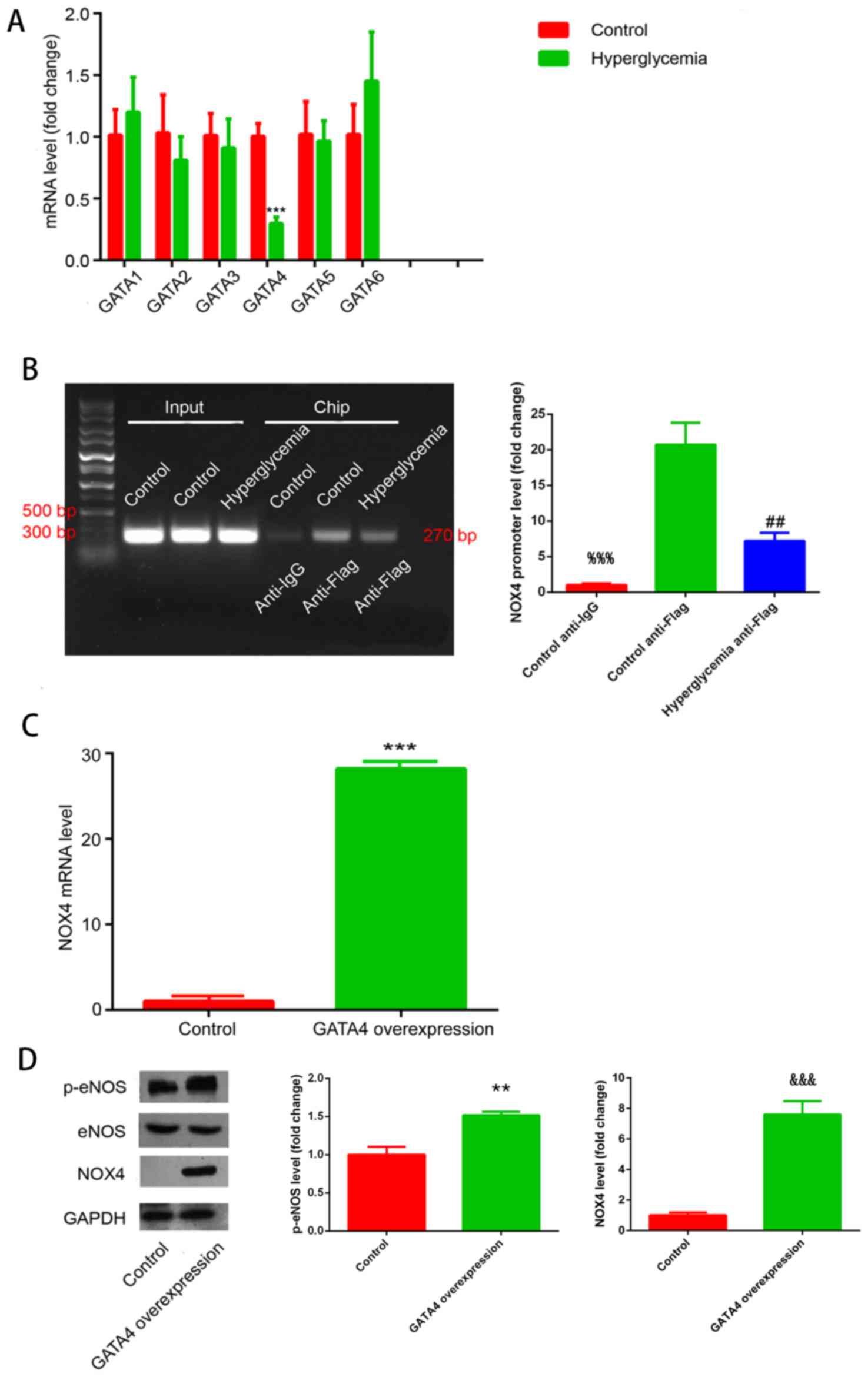 | Figure 2.GATA4 mediates NOX4 expression. (A)
GATA1-6 mRNA expression in HUVECs exposed to control or
hyperglycemic conditions; ***P<0.001, hyperglycemia vs. control
group. (B) Chromatin immunoprecipitation results for binding of
GATA4 to the NOX4 promoter under normoglycemic control conditions
and hyperglycemic conditions; %%%P<0.001, control
anti-Flag vs. control anti-IgG group; ##P<0.01,
hyperglycemia anti-Flag vs. control anti-Flag group. (C) NOX4 mRNA
expression upon GATA4 overexpression; ***P<0.001, GATA4
overexpression group vs. control. (D) NOX4 and p-eNOS protein
expression in HUVECs overexpressing GATA4 were measured by western
blot analysis; **P<0.01, GATA4 overexpression group vs. control;
&&&P<0.001, GATA4 overexpression group
vs. control. eNOS, endothelial nitric oxide synthase; GATA,
GATA-binding protein; IgG, immunoglobulin G; NOX4, NADPH oxidase 4;
p, phosphorylated. |
These results were further confirmed by ChIP
analysis (Fig. 2B). The ChIP band
for the anti-Flag group exhibited increased intensity compared with
the control group, confirming that GATA4 may be able to induce
transcription of NOX4. In addition, the anti-Flag ChIP band
corresponding to HUVECs exposed to hyperglycemia exhibited a lower
intensity compared with the control group, indicating that
hyperglycemia inhibited the binding of GATA4 to the NOX4
promoter.
NOX4 mRNA and protein expression levels were also
analyzed in HUVECs that overexpressed GATA4 (Fig. 2C and D, respectively). GATA4
overexpression induced an increase in NOX4 mRNA and protein
expression under normal glucose condition compared with the control
cells. These experimental results confirmed that GATA4 is a
transcription factor for NOX4.
GATA4 expression is downregulated in
HUVECs exposed to hyperglycemic conditions
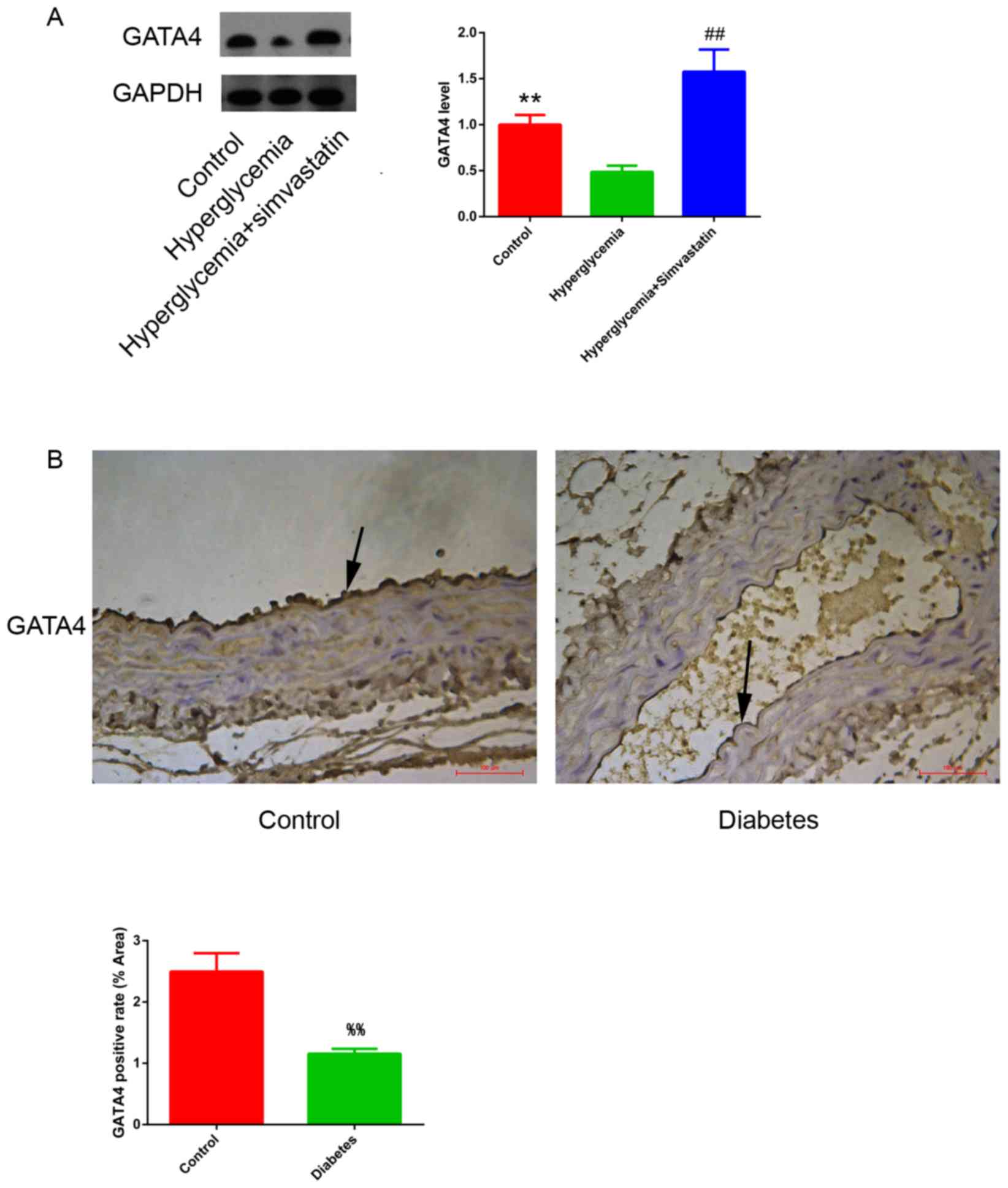
The expression of GATA4 protein in HUVECs cultured
in control vs. high-glucose medium was measured by western blot.
Hyperglycemia resulted in a decrease in GATA4 expression in HUVECs
compared with untreated control cells (Fig. 3A). This finding was further
confirmed in vivo using a mouse diabetes model, which
exhibited lower expression of GATA4 in the endothelium of the
thoracic aorta compared with healthy control mice (Fig. 3B).
GATA4 overexpression improves
endothelial function by increasing NO levels
The role of GATA4 in endothelial function under
hyperglycemic conditions was also investigated using HUVECs that
overexpressed GATA4. GATA4overexpression resulted in an increase in
the level of NO (Fig. 4A) as well
as an increase in p-eNOS protein expression (Fig. 2D) in HUVECs stimulated with
high-glucose medium, which indicated that GATA4 expression improved
endothelial function under hyperglycemia.
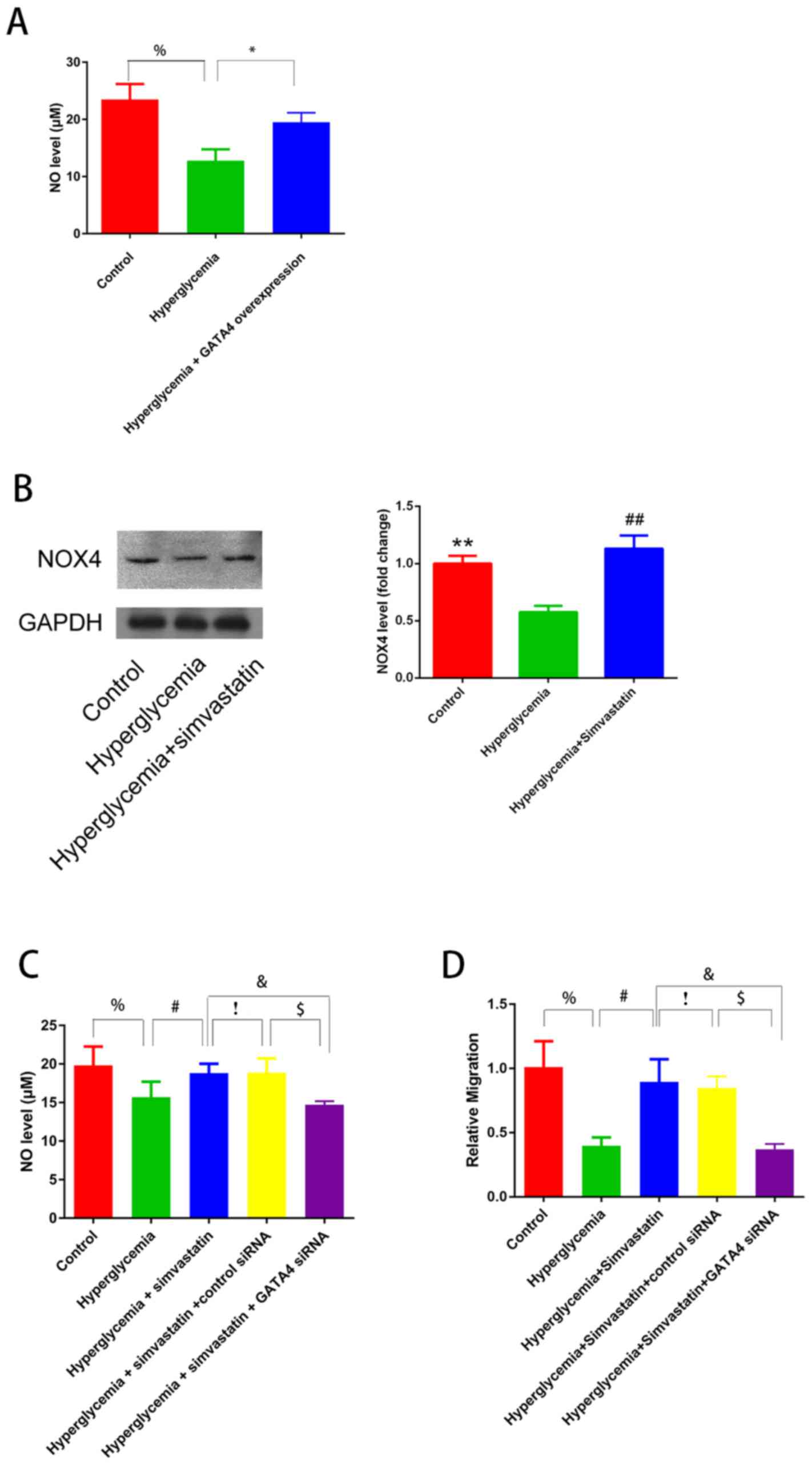 | Figure 4.GATA4 improves endothelial function
and is involved in protection of endothelial function by
simvastatin. (A) NO levels in HUVECs with and without GATA4
overexpression. %P<0.05 control vs. hyperglycemia;
*P<0.01 hyperglycaemia vs. hyperglycaemia + GATA4
overexpression. (B) Western blotting results demonstrating that
NOX4 expression in HUVECs exposed to normoglycemic conditions as
well as hyperglycemic conditions with or without simvastatin
treatment; **P<0.01, hyperglycemia vs. control group;
##P<0.01, hyperglycemia + simvastatin vs.
hyperglycemia group. (C) NO levels in the supernatant of HUVECs
exposed to control culture, hyperglycemia, hyperglycemia +
simvastatin and hyperglycemia + simvastatin + GATA4-siRNA. (D)
Migration analysis in HUVECs exposed to control culture,
hyperglycemia, hyperglycemia + simvastatin and hyperglycemia +
simvastatin + GATA4-siRNA. (C and D) %P<0.05,
hyperglycemia vs. control group; #P<0.05,
hyperglycemia + simvastatin vs. hyperglycemia group;
!P>0.05 hyperglycemia + simvastatin vs. hyperglycemia
+ simvastatin + control siRNA; $P<0.05 hyperglycemia
+ simvastatin + control siRNA vs. hyperglycemia + simvastatin +
GATA4-siRNA; &P<0.05, hyperglycemia + simvastatin
+ GATA4-siRNA vs. hyperglycemia + simvastatin group. GATA4,
GATA-binding protein 4; HUVECs, human umbilical vein endothelial
cells; NO, nitric oxide; NOX4, NADPH oxidase 4; siRNA, small
interfering RNA. |
GATA4 contributes to the protection
against endothelial dysfunction achieved by simvastatin
A previous study has demonstrated that simvastatin
treatment functions to protect endothelial function in diabetes
(16). Therefore, whether this
effect was achieved through GATA4 expression was investigated in
HUVECs. Cells treated with simvastatin exhibited increased protein
expression levels of GATA4 (Fig.
3A) and NOX4 (Fig. 4B), as
well as increased levels NO (Fig.
4C) in hyperglycemia-stimulated HUVECs. Conversely, GATA4-siRNA
treatment decreased the protective effects of simvastatin by
inhibiting NO production (Fig.
4C).
To further confirm the protective role of GATA4 in
endothelial dysfunction, a migration assay was performed.
Simvastatin treatment resulted in increased cell migration in
hyperglycemia-stimulated HUVECs, whereas GATA4 knockdown abolished
this effect (Fig. 4D). These
findings demonstrated the role of GATA4 in protecting endothelial
function in hyperglycemia and indicated that the effects of
simvastatin may be achieved through GATA4.
Discussion
The results of the present study indicated that
GATA4 was able to regulate the transcription of NOX4. This result
is supported by previous studies that demonstrated an association
between GATA4 and NOX4. For example, Murray et al observed
that activation of GATA4 transcription was dependent on NOX4
activation (17). The present
results indicated that GATA4 was able to induce transcription of
NOX4, which indicated that positive feedback occurs between GATA4
and NOX4. Another study reported that CCAAT enhancer-binding
protein (C/EBP) induces transcription of NOX4 (11), whereas other studies demonstrated
an interaction between GATA4 and C/EBP (18,19),
which indicated that GATA4 may serve a role in the regulation of
NOX4 expression.
The present study demonstrated GATA4 had a
protective effect against endothelial dysfunction induced by
hyperglycemia. First, GATA4 expression was inhibited in HUVECs
exposed to high-glucose treatment. This result was consistent with
the results of previous reports that demonstrated that cardiac GATA
expression is downregulated in diabetic mice (20,21).
The decreased GATA4 expression observed in the present study
further indicates that GATA4 may be associated with the molecular
mechanisms underlying diabetes. In addition, genetics studies have
reported that GATA4 mutations may cause neonatal and
childhood-onset diabetes (22),
and that GATA4 polymorphism is associated with diabetes in adults
(23). Second, as NOX4 was
revealed to protect against endothelial dysfunction in diabetes,
GATA4 presumably should have a similar effect given that GATA4
induces NOX4 transcription. Third, GATA4 has previously been
reported to have the ability to inhibit apoptosis (24,25).
As endothelial cell apoptosis is a cause of endothelial dysfunction
(26), the present study proposed
that the protective effect of GATA4 overexpression may be due to
the inhibition of endothelial cell apoptosis stimulated by
hyperglycemia. Fourth, in the presence of diabetes, GATA4 has been
revealed to serve a protective role in many other cardiovascular
diseases. For example, GATA4 overexpression was reported to support
cardiac adaptive responses and survival, whereas GATA4 ablation
induced cardiomyocyte apoptosis and heart dysfunction (27). In addition, GATA4 was demonstrated
to protect cardiomyocytes from doxorubicin-induced cell death
(28).
The present study had several limitations. First,
the co-factors required for GATA4-induced NOX4 transcription have
yet to be identified. Second, although GATA4 protected HUVECs
against hyperglycemia-induced endothelial dysfunction by enhancing
NO secretion, this effect requires confirmation through in
vivo experiments aimed at determining whether GATA4
overexpression leads to vascular relaxation.
In conclusion, the results of the present study
indicated that GATA4 may be a transcription factor that induces
NOX4 expression, which results in increased production of NO.
Additionally, GATA4 inhibited hyperglycemia-induced endothelial
dysfunction in both HUVECs and a mouse model of diabetes. This
protective effect of GATA4 was experimentally linked to increased
production of NO and p-eNOS. Finally, the protection of endothelial
function by simvastatin treatment was demonstrated to be achieved
through GATA4 expression. Overall, the present findings revealed a
novel molecular mechanism in endothelial dysfunction and identified
GATA4 as a potential therapeutic target for preventing endothelial
dysfunction in diabetes patients.
Acknowledgements
This study was supported by The Natural Science
Foundation of Zhejiang Province (grant no. LZ16H020001) and The
National Natural Science Foundation of China (grant no.
81170167).
References
|
1
|
Mellbin LG, Anselmino M and Rydén L:
Diabetes, prediabetes and cardiovascular risk. Eur J Cardiovasc
Prev Rehabil. 17 Suppl 1:S9–S14. 2010. View Article : Google Scholar
|
|
2
|
Riddle MC: Glycemic control and
cardiovascular mortality. Curr Opin Endocrinol Diabetes Obes.
18:104–109. 2011. View Article : Google Scholar
|
|
3
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014. View Article : Google Scholar
|
|
4
|
Sun Z: Atherosclerosis and atheroma plaque
rupture: Normal anatomy of vasa vasorum and their role associated
with atherosclerosis. ScientificWorldJournal. 2014:2850582014.
|
|
5
|
Polovina MM and Potpara TS: Endothelial
dysfunction in metabolic and vascular disorders. Postgrad Med.
126:38–53. 2014. View Article : Google Scholar
|
|
6
|
Bondarenko PG and Sil'Chenko TS: Status of
the immune system in peptic ulcer patients before and after
vagotomy. Klin Khir. 27–29. 1983.(In Russian).
|
|
7
|
Langbein H, Brunssen C, Hofmann A, Cimalla
P, Brux M, Bornstein SR, Deussen A, Koch E and Morawietz H: NADPH
oxidase 4 protects against development of endothelial dysfunction
and atherosclerosis in LDL receptor deficient mice. Eur Heart J.
37:1753–1761. 2016. View Article : Google Scholar
|
|
8
|
Kleinschnitz C, Grund H, Wingler K,
Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft
P, et al: Post-stroke inhibition of induced NADPH oxidase type 4
prevents oxidative stress and neurodegeneration. PLoS Biol. 8:pii:
e1000479. 2010. View Article : Google Scholar :
|
|
9
|
Kuroda J, Ago T, Matsushima S, Zhai P,
Schneider MD and Sadoshima J: NADPH oxidase 4 (Nox4) is a major
source of oxidative stress in the failing heart. Proc Natl Acad Sci
USA. 107:15565–15570. 2010. View Article : Google Scholar :
|
|
10
|
Ray R, Murdoch CE, Wang M, Santos CX,
Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ,
et al: Endothelial Nox4 NADPH oxidase enhances vasodilatation and
reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol.
31:1368–1376. 2011. View Article : Google Scholar
|
|
11
|
Manea SA, Todirita A, Raicu M and Manea A:
C/EBP transcription factors regulate NADPH oxidase in human aortic
smooth muscle cells. J Cell Mol Med. 18:1467–1477. 2014. View Article : Google Scholar :
|
|
12
|
Manea A, Tanase LI, Raicu M and Simionescu
M: Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH
oxidase in human aortic smooth muscle cells. Arterioscler Thromb
Vasc Biol. 30:105–112. 2010. View Article : Google Scholar
|
|
13
|
Sun Z, Han J, Zhao W, Zhang Y, Wang S, Ye
L, Liu T and Zheng L: TRPV1 activation exacerbates
hypoxia/reoxygenation-induced apoptosis in H9C2 cells via calcium
overload and mitochondrial dysfunction. Int J Mol Sci.
15:18362–18380. 2014. View Article : Google Scholar :
|
|
14
|
Zhao C, Guo H, Li J, Myint T, Pittman W,
Yang L, Zhong W, Schwartz RJ, Schwarz JJ, Singer HA, et al: Numb
family proteins are essential for cardiac morphogenesis and
progenitor differentiation. Development. 141:281–295. 2014.
View Article : Google Scholar :
|
|
15
|
Zhong X, Xiu LL, Wei GH, Liu YY, Su L, Cao
XP, Li YB and Xiao HP: Bezafibrate enhances proliferation and
differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS
activation. Acta Pharmacol Sin. 32:591–600. 2011. View Article : Google Scholar :
|
|
16
|
Hou HH, Liao YJ, Hsiao SH, Shyue SK and
Lee TS: Role of phosphatase activity of soluble epoxide hydrolase
in regulating simvastatin-activated endothelial nitric oxide
synthase. Sci Rep. 5:135242015. View Article : Google Scholar :
|
|
17
|
Murray TV, Smyrnias I, Shah AM and Brewer
AC: NADPH oxidase 4 regulates cardiomyocyte differentiation via
redox activation of c-Jun protein and the cis-regulation of GATA-4
gene transcription. J Biol Chem. 288:15745–15759. 2013. View Article : Google Scholar :
|
|
18
|
Tremblay JJ, Hamel F and Viger RS: Protein
kinase A-dependent cooperation between GATA and
CCAAT/enhancer-binding protein transcription factors regulates
steroidogenic acute regulatory protein promoter activity.
Endocrinology. 143:3935–3945. 2002. View Article : Google Scholar
|
|
19
|
LaVoie HA, Singh D and Hui YY: Concerted
regulation of the porcine steroidogenic acute regulatory protein
gene promoter activity by follicle-stimulating hormone and
insulin-like growth factor I in granulosa cells involves GATA-4 and
CCAAT/enhancer binding protein beta. Endocrinology. 145:3122–3134.
2004. View Article : Google Scholar
|
|
20
|
Broderick TL, Parrott CR, Wang D,
Jankowski M and Gutkowska J: Expression of cardiac GATA4 and
downstream genes after exercise training in the db/db mouse.
Pathophysiology. 19:193–203. 2012. View Article : Google Scholar
|
|
21
|
Broderick TL, Jankowski M, Wang D,
Danalache BA, Parrott CR and Gutkowska J: Downregulation in GATA4
and downstream structural and contractile genes in the db/db mouse
heart. ISRN Endocrinol. 2012:7368602012. View Article : Google Scholar :
|
|
22
|
Shaw-Smith C, De Franco E, Allen Lango H,
Batlle M, Flanagan SE, Borowiec M, Taplin CE, van Alfen-van der
Velden J, Cruz-Rojo J, de Nanclares Perez G, et al: GATA4 mutations
are a cause of neonatal and childhood-onset diabetes. Diabetes.
63:2888–2894. 2014. View Article : Google Scholar
|
|
23
|
Muiya NP, Wakil SM, Tahir AI, Hagos S,
Najai M, Gueco D, Al-Tassan N, Andres E, Mazher N, Meyer BF and
Dzimiri N: A study of the role of GATA4 polymorphism in
cardiovascular metabolic disorders. Hum Genomics. 7:252013.
View Article : Google Scholar :
|
|
24
|
Vaskivuo TE, Anttonen M, Herva R, Billig
H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M and Tapanainen
JS: Survival of human ovarian follicles from fetal to adult life:
Apoptosis, apoptosis-related proteins, and transcription factor
GATA-4. J Clin Endocrinol Metab. 86:3421–3429. 2001. View Article : Google Scholar
|
|
25
|
Li HX, Zhou YF, Zhao X, Jiang B and Yang
XJ: GATA-4 protects against hypoxia-induced cardiomyocyte injury:
Effects on mitochondrial membrane potential. Can J Physiol
Pharmacol. 92:669–678. 2014. View Article : Google Scholar
|
|
26
|
Bielli A, Scioli MG, Mazzaglia D, Doldo E
and Orlandi A: Antioxidants and vascular health. Life Sci.
143:209–216. 2015. View Article : Google Scholar
|
|
27
|
Aries A, Paradis P, Lefebvre C, Schwartz
RJ and Nemer M: Essential role of GATA-4 in cell survival and
drug-induced cardiotoxicity. Proc Natl Acad Sci USA. 101:6975–6980.
2004. View Article : Google Scholar :
|
|
28
|
Kobayashi S, Lackey T, Huang Y, Bisping E,
Pu WT, Boxer LM and Liang Q: Transcription factor gata4 regulates
cardiac BCL2 gene expression in vitro and in vivo. FASEB J.
20:800–802. 2006.
|