Introduction
Atherosclerosis (AS) is the pathological basis of
cardiovascular diseases, and is an important cause of
cardiovascular disease-associated mortality (1,2). The
Report on Cardiovascular Disease in China (2014) revealed that the
prevalence of cardiovascular diseases in China continues to rise;
in 2014, there were ~0.29 billion patients with cardiovascular
disease in China, and one in every five adults was reported to
suffer from a cardiovascular disease (3). Therefore, the effective prevention
and treatment of AS is of great significance to prevent the
occurrence of cardiovascular diseases. The pathogenesis of AS has
been explained by numerous theories, including the lipid
infiltration theory, the endothelial damage theory, the
inflammatory reaction theory and the oxidative stress theory
(4,5). During the occurrence and development
of AS, various inflammatory cells and inflammatory mediators serve
roles in the formation and rupture of fatty streaks, as well as
fibrous, atheromatous and unstable plaques (6). In 1999, Ross proposed the concept
that AS is a chronic inflammatory disease (7). Nuclear factor (NF)-κB and
mitogen-activated protein kinase (MAPK) signaling pathways may be
involved in the formation of AS via the regulation of inflammatory
cytokine-mediated inflammation. Therefore, prevention and treatment
of AS via the regulation of inflammatory signaling pathways may be
considered a novel strategy to control the inflammatory reaction,
and effectively delay plaque formation or prevent plaque rupture by
stabilizing plaques.
Tianxiangdan Granule was developed by Professor
Dongqing An, a doctor in the field of traditional Chinese medicine.
Tianxiangdan Granule was developed according to the special
geographical features and climate of Xinjiang, China, as well as
the diet and lifestyle of the local residents. Tianxiangdan Granule
is composed of Rhodiola, Ziziphora clinopodioides,
Dalbergia and Salvia miltiorrhiza. It has been used
in the clinical prevention and treatment of AS-associated diseases
for >30 years, with good clinical effects. Previous studies have
revealed that Tianxiangdan Granule exerts the following effects:
Regulation of lipid metabolism, improving endothelial function and
energy metabolism of ischemic myocardia, reducing myocardial cell
damage and reducing oxidative stress (8–11).
At present, few studies have been performed regarding the
association between the effects of Tianxiangdan Granule on the
prevention and treatment of AS, and inflammatory factors and
related signaling pathways; therefore, the present study was
conducted. On the basis of the successful replication of the
apolipoprotein E (ApoE)−/− murine model of Huizhuo Tanzu
type AS, the present study investigated the effects of Tianxiangdan
Granule on inflammatory factors and inflammatory signaling
pathways, in order to explore the possible anti-AS mechanism
underlying the effects of Tianxiangdan Granule.
Materials and methods
Animals
A total of 48 ApoE−/− mice [strain
C57BL/6J] in the present study were introduced from the Jackson
laboratory in the United States by the Peking University Medical
Laboratory Animal Center. The mice were purchased as ApoE−/−. All
mice [male; age, 8 weeks; weight, 18±2 g; license no. SCXK
(Beijing) 2011–0012] were maintained in the Medical Animal
Experimental Center of The First Affiliated Hospital of Xinjiang
Medical University (Urumqi, China). They were fed normal diet at
room temperature (relative humidity: 40–70%, 12 h/d light dark
cycle). The present study was approved by the Animal Ethics
Committee of The First Affiliated Hospital of Xinjiang Medical
University.
Drugs and reagents
Tianxiangdan Granule is composed of four types of
Chinese medicine: Rhodiola root, Dalbergia wood,
Ziziphora clinopodioides and Salvia miltiorrhiza. The
drug was prepared by the Traditional Chinese Medicine Hospital
Affiliated to Xinjiang Medical University (batch no. ZO4000820).
Tianxiangdan Granule obtained a national patent in 2009 (patent no.
200910210063.9) (12).
Atorvastatin calcium tablets (batch no. H20051408) were purchased
from Pfizer Inc. (New York, NY, USA). Cholesterol was obtained from
Amresco, LLC (Solon, OH, USA). Propylthiouracil tablets were
purchased from Riemser Pharma GmbH (Greifswald, Germany). TNF-α
(cat. no. 106958008) and IL-1β (cat. no. 92572000) ELISA kits were
purchased from eBioscience; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Radioimmunoprecipitation assay (RIPA) buffer,
Pierce Phosphatase Inhibitor Mini Tablets, Halt Protease Inhibitor
Cocktail and bicinchoninic acid (BCA) Protein Assay kit were
obtained from Thermo Fisher Scientific, Inc. Triglyceride (TG)
assay kit (cat. no. A110-2), total cholesterol (TC) assay kit (cat.
no. A111-2), low-density lipoprotein cholesterol (LDL-C) assay kit
(cat. no. A113-2) and high-density lipoprotein cholesterol (HDL-C)
assay kit (cat. no. A112-2) were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). The following primary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA): Phosphorylated (p)-p38 MAPK (cat. no. 4631S),
p38 MAPK (cat. no. 9212S), p-NF-κB p65 (cat. no. 3031S), NF-κB p65
(cat. no. 8242S) and GAPDH (cat. no. 5174S). Alkaline
phosphatase-conjugated secondary antibody (Anti-Rabbit) (cat. no.
WP0007) and BCIP/NBT Chromogenic Substrate were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Polyvinylidene
fluoride (PVDF) membrane was obtained from Roche Diagnostics
(Basel, Switzerland).
Establishment of the animal model
Of the 48 8-week-old ApoE−/− mice, 12
were assigned to the normal control group, in which mice were
provided ordinary diets, and were maintained at room temperature
(20–24°C) and 40–70% relative humidity. The remaining 36
ApoE−/− mice were assigned to the modeling group, in
which mice were fed a high-fat diet and were maintained in an
artificial climate box, in order to stimulate the climate and
eating habit characteristics of Xinjiang. Every morning,
ApoE−/− mice in the modeling group were placed in the
artificial climate box at 10:00 a.m. and were taken out at 09:00
p.m. when they were placed back in the room temperature
environment. The temperature of the artificial climate box was set
at 6±2°C, relative humidity was controlled between 25 and 32.8%,
and the light-dark cycle was 12 h/day. The purpose of this method
was to establish the Huizhuo Tanzu type AS model (13,14).
After 12 weeks of feeding, 2 ApoE−/− mice from the
normal control group and 6 ApoE−/− mice from the
modeling group were randomly selected and sacrificed, after which,
aortic roots were obtained and observed under a light microscope
following hematoxylin and eosin (H&E) staining. In samples from
the modeling group, an increased number of foam cells, cholesterol
crystals and atheromatous plaques were observed, and lumen stenosis
was clearly demonstrated. In addition, the remaining
ApoE−/− mice in the modeling group appeared lethargic,
their participation in activities was decreased, food and water
intake was reduced, their fur appeared lackluster, weight growth
was not obvious, and they had sticky stools. These signs confirmed
that the Huizhuo Tanzu type AS model had been successfully
established. Furthermore, the remaining 30 ApoE−/− mice
were randomly divided into three groups: Model group, Tianxiangdan
group [2.7 g·(kg·d)−1], and atorvastatin group [6.1 mg
(kg·d)−1]; n=10 mice/group. The aforementioned doses
were selected by equivalent conversion, based on the clinical
routine dose used to treat human adults: Mouse experimental dose [g
(kg·d)−1]=9.1 × human adult clinical dose
[g·(kg·d)−1]. To obtain the experimental doses, drugs
were dissolved in distilled water and administered by gavage once a
day (15). Doses were adjusted
according to body weight each week. Mice in the normal control and
model groups were administered an oral gavage of distilled water
(0.2 ml·d−1). All mice were given free access to food
and water, and their activities were not restricted. Mice in the
normal control group were placed at room temperature (20–24°C) and
40–70% relative humidity, and were given a normal diet. Mice in the
model, Tianxiangdan and atorvastatin groups were administered a
high-fat diet and continued to undergo intermittent maintenance in
the artificial climate box. The high-fat diet comprised: Basal
diet, 72.5%; lard, 10%; sucrose, 10%; pig bile salt, 0.2%;
cholesterol, 2%; propylthiouracil, 0.12%; egg yolk, 5%; and 21
Super-Vita, 0.1%. The temperature of the artificial climate box was
set at 6±2°C, and relative humidity was controlled between 25 and
32.8%.
Experimental specimen collection
A total of 12 weeks following administration of the
first gavage, ApoE−/− mice in all groups were fasted.
After 12 h the following operations were conducted: i) Mice were
anesthetized by 0.1 ml/kg intraperitoneal injection of anaesthetic
composed of 2 ml diazepam, 2 ml ketamin, 1 ml atropine and 5 ml
0.9% sodium chloride, and blood samples were collected from the
eyeballs. The samples were placed in microcentrifuge tubes and were
centrifuged at 3,000 × g for 10 min at 4°C; the serum obtained by
separation was maintained at −80°C for the detection of serological
markers. Repeated freezing and thawing was avoided. ii) Mice were
anesthetized by 0.1 ml/kg intraperitoneal injection of anesthetic
(injection of diazepam, ketamine and atropine), then were
sacrificed by cervical dislocation. Thoraces and abdomens were
opened; arterial tissues from the aortic root to the branches
between the iliac and renal artery were obtained and rinsed in
normal saline. A length of ~0.5 cm from the aortic root was
obtained, and fixed in 10% formalin for H&E staining and plaque
area (PA) analysis. Residual aortic tissues were quickly placed in
microcentrifuge tubes, labeled, and placed into liquid nitrogen and
quickly frozen. The samples were then transferred into an ultra-low
temperature freezer at −80°C, and preserved for detection by
western blotting.
H&E staining PA analysis
Aorta tissues were fixed at 56°C for 12 h with 10%
formaldehyde and dehydrated, prior to conventional paraffin
embedding and dyeing with hematoxylin and eosin. From the aortic
root, uniform slices were obtained (~5 µm); 40 consecutive slices
were obtained from each sample. One slice was obtained from every
five slices; therefore, a total of eight slices were obtained from
each sample. After H&E staining, aortic morphological
alterations were observed under a light microscope; measurement and
analysis of relevant pathological morphological indices were
conducted using the multifunctional image analysis software of
Image-Pro Plus (version 6.0; Media Cybernetics, Inc., Rockville,
MD, USA). Blood vessel lumen area (LA) and PA measurements were
obtained, and the PA/LA ratio was calculated. The mean values of
PA/LA were calculated for statistical analysis.
Triglyceride (TG), total cholesterol
(TC), high-density lipoprotein cholesterol (HDL-C) and low-density
lipoprotein cholesterol (LDL-C) serum levels detected by enzymatic
colorimetry
Prior to detection, serum samples were removed from
the ultra low temperature freezer and were placed in a refrigerator
at 4°C. Subsequently, TG, TC, HDL-C and LDL-C serum levels were
detected according to the manufacturer's protocols an automatic
biochemical analyzer (cat. no. BS200; Mindray Medical International
Co., Ltd., Shenzhen, China).
Serum expression levels of interleukin
(IL)-1β and tumor necrosis factor (TNF)-α detected by ELISA
method
Prior to detection, serum samples were removed from
the ultra low temperature freezer and were placed in a refrigerator
at 4°C. Subsequently, the serum levels of proinflammatory cytokines
IL-1β and TNF-α were detected using an ELISA kit, according to the
manufacturer's protocol. Absorbance values were determined using an
enzymatic microplate reader at a wavelength of 450 nm. A standard
curve was generated, with concentration as the horizontal
coordinate and standard absorbance value as the vertical
coordinate. The concentrations of IL-1β and TNF-α in the samples
were calculated using the standard curve.
Expression levels of NF-κB p65 and p38
MAPK signaling pathways detected by western blotting
Aortic tissue protein was extracted using RIPA with
a protease inhibitor and phosphatase inhibitor, and protein
concentration was determined using a BCA protein assay kit. Protein
samples (20 µg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 80 V and were
transferred onto a PVDF membrane by electroblotting. The membrane
was blocked in TBS-Tween 20 (TBST) supplemented with 5% w/v bovine
serum albumin (Beijing Raghan Biological Technology Co. Ltd.,
Beijing, China) for 1 h at room temperature. Subsequently, the
membrane was washed three times with TBST, and was incubated with
the following primary antibodies: GAPDH (cat. no. 5174S), NF-κB p65
(cat. no. 8242S), p-NF-κB p65 (cat. no. 3031S), p38 MAPK (cat. no.
9212S) and p-p38 MAPK (cat. no. 4631S) (all antibodies: 1:1,000 of
dilution, Cell Signaling Technology, Inc.), at 4°C overnight with
agitation. After washing the membrane with TBST three times, the
alkaline phosphatase-conjugated secondary antibody (cat. no.
WP0007) was added (1:1 of dilution; Thermo Fisher Scientific,
Inc.), and the membrane was further incubated at 37°C for 1 h. The
membrane was then washed with TBST and underwent visualization in
the dark with the BCIP/NBT Chromogenic Substrate. The blots were
scanned and analyzed using Quantity One v4.62 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was conducted using statistical
software SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation of three independent
experiments. Intergroup comparison was performed using one-way
analysis of variance, and the comparison between two groups was
performed using the Least Significant Difference method. P<0.05
was considered to indicate a statistically significant
difference.
Results
Physiological alterations of Huizhuo
Tanzu type AS ApoE−/− mice
Mice in the normal control group were lively,
irritable and restless, had a quick response, bit each other
frequently, ate a lot of food and drank a lot of water, gained
significant amounts of weight, had clean and shiny fur, and had dry
granular stools. Mice in the model group became lethargic, their
participation in activities significantly decreased, their food and
water intake decreased, their fur was lackluster, weight growth was
not obvious, and they had sticky stools. Characteristics of the
mice in the Tianxiangdan and atorvastatin groups were better
compared with those in the model group, their fur was relatively
clean and shiny, and their water and food consumption, and weight
gain, was larger than in the model group, and their stools were
granular but slightly sticky (Table
I).
 | Table I.Biological characteristics of
apolipoprotein E−/− mice in the various groups. |
Table I.
Biological characteristics of
apolipoprotein E−/− mice in the various groups.
| Characteristic | Normal control
group | Model group | Atorvastatin
group | Tianxiangdan
group |
|---|
| Tongue | Pink | Bruising, with
ecchymosis | Purple or dark red,
with ecchymosis | Dark red, with
ecchymosis |
| Coating on the
tongue | Thin white
coating | Thick and greasy
coating | Relatively thick
and greasy coating | Thin and greasy
coating |
| Fur | Clean and
shiny | Dry and
lackluster | Relatively dry and
dull | Relatively clean
and shiny |
| Emotional
state | Irritable and
agitated | Docile | Docile | Relatively
irritable and restless |
| Behavioral
state | Lively,
fighting | Reduced movement,
tired and sleepy | Reduced movement,
tired and sleepy | Relatively lively,
occasionally biting/fighting |
| Response to
stimulus | Agile | Slow | Slow | Relatively
agile |
| Stool state | Dry, granular | Sticky | Slightly
sticky | Relatively dry,
granular |
| Food intake | Normal | Significantly
reduced | Reduced | Slightly
reduced |
| Water intake | Normal | Significantly
reduced | Reduced | Slightly
reduced |
| Weight change | Significant
growth | Growth not
obvious | Slightly increased
growth | Slightly increased
growth |
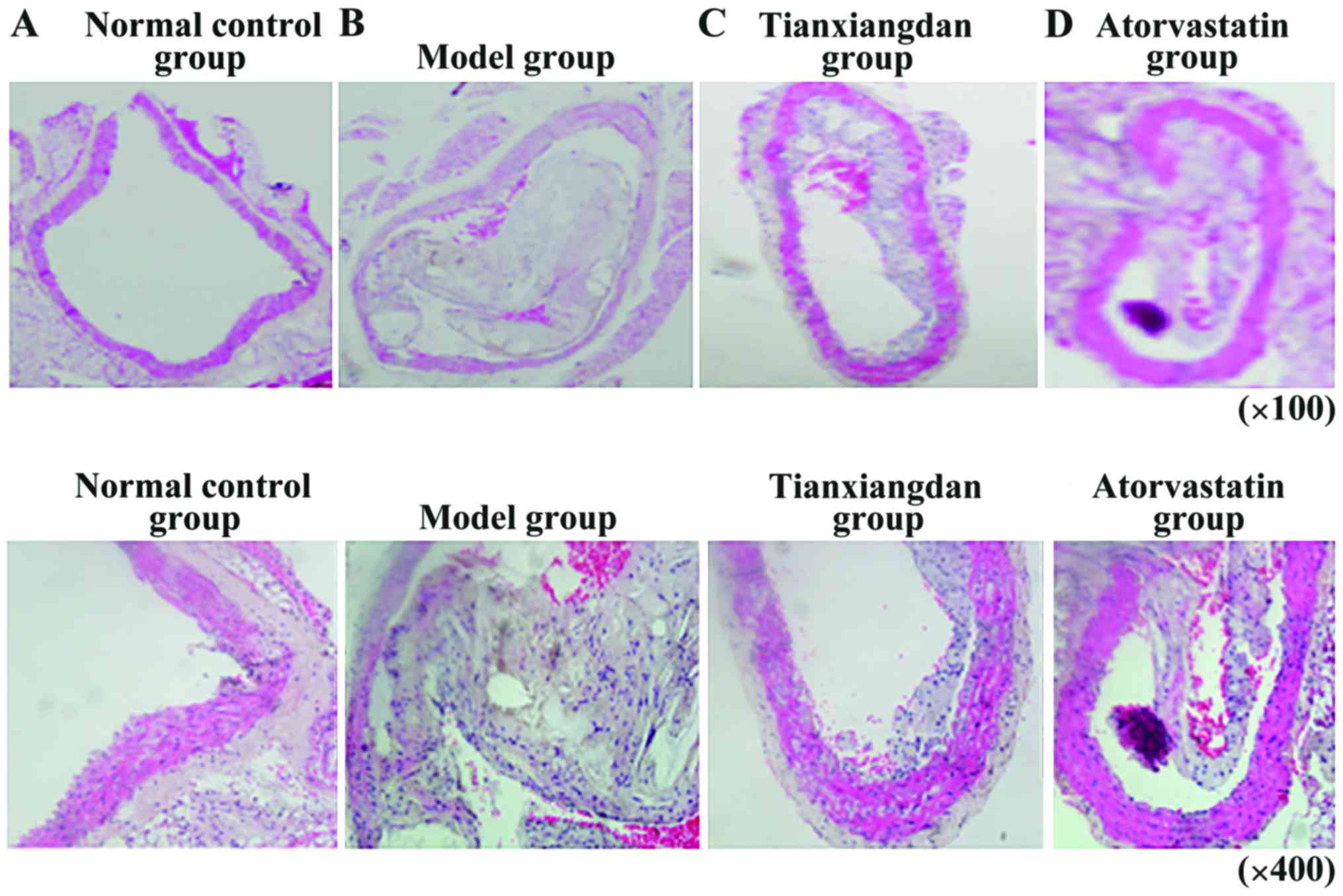
Pathological alterations in the aorta
of Huizhuo Tanzu type AS ApoE−/− mice
In the normal control group, no obvious smooth
muscle cell hyperplasia was detected, slight hyperplasia of the
intima was observed, and no cholesterol crystal formation or
obvious stenosis was detected in the aortic lumen (Fig. 1A). In the model group, numerous
atherosclerotic plaque lesions appeared and the thickness of the
aortic tube wall was obviously uneven. In addition, endothelial
lesions had a larger PA and a thin fibrous cap. Large amounts of
foam cells and cholesterol crystals were also detected, lipid cores
were larger, and the majority of plaques did not closely adhere to
the tube wall and some were detaching. Furthermore, calcification
occurred locally, and considerable inflammatory cell infiltration
was detected in the adventitia (Fig.
1B). In the Tianxiangdan group, atherosclerotic lesions were
mainly fibrous plaque lesions, aortic tube wall thickness was
uneven, the intima was markedly thicker, and fibrous plaques
covered with a fibrous cap were clearly observed. The PA was
markedly smaller than that in the model group. All plaques closely
adhered to the aortic wall, and no detachment was observed. In
addition, fibrous caps were thicker, lipid cores were small, and a
small amount of inflammatory cell infiltration was detected in the
adventitia (Fig. 1C). Pathological
alterations were less severe than in the model group and plaque
stability was improved, thus suggesting that Tianxiangdan increased
plaque stability. In the atorvastatin group, the majority of
alterations were detected in the atherosclerotic fibrous plaque
lesions, the thickness of the aortic wall was uneven, the intima
appeared markedly thicker, and fibrous plaques covered by a fibrous
cap were observed. The PA was markedly smaller compared with in the
model group, and the plaques did not closely adhere to the aortic
wall; however, detachment was not observed. Lipid cores were not
obvious, and adventitial inflammatory cell infiltration was rare
(Fig. 1D). Pathological
alterations were less severe than in the model group, thus
indicating that atorvastatin could increase plaque stability.
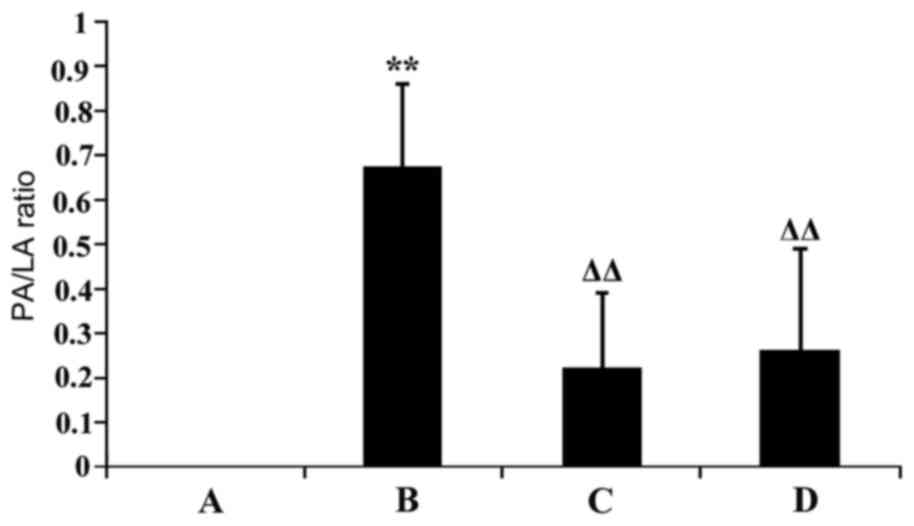
Morphological alterations in aortic
lumen stenosis of Huizhuo Tanzu type AS ApoE−/−
mice
The PA/LA ratio was compared among the groups; PA
and LA were measured using Image-Pro Plus software (version 6.0).
Compared with the normal control group, the aortic PA/LA ratio was
significantly increased in the model group (P<0.01). Compared
with in the model group, aortic PA/LA ratio was significantly
decreased in the Tianxiangdan and atorvastatin groups (P<0.01).
There was no statistically significant difference in aortic PA/LA
ratio between mice in the Tianxiangdan and atorvastatin groups
(P>0.05; Fig. 2).
Alterations in the serum lipid levels
of Huizhuo Tanzu type AS ApoE−/− mice
The results indicated that compared with in the
normal control group, TC, TG, LDL-C and HDL-C content was
significantly higher in the model group (P<0.05). Compared with
in the model group, TC and LDL-C serum content was significantly
lower in the atorvastatin and Tianxiangdan groups (P<0.01).
There was no statistically significant difference in serum lipid
levels between mice in the Tianxiangdan and atorvastatin groups
(P>0.05; Table II).
 | Table II.Comparison of serum lipid levels in
apolipoprotein E−/− mice from the various groups. |
Table II.
Comparison of serum lipid levels in
apolipoprotein E−/− mice from the various groups.
| Group | n | TC (mmol/l) | TG (mmol/l) | LDL-C (mmol/l) | HDL-C (mmol/l) |
|---|
| Normal control | 8 |
11.87±1.78 |
1.26±0.19 |
5.09±1.09 |
2.03±0.86 |
| Model | 8 |
36.66±1.91a |
1.64±0.19b |
33.08±1.61a |
7.64±0.98a |
| Tianxiangdan | 8 |
32.04±0.99c |
1.46±0.16 |
18.76±1.44c |
8.39±1.13 |
| Atorvastatin | 8 |
31.67±2.19c |
1.60±0.15 |
19.12±2.39c |
8.54±1.35 |
Alterations in serum expression levels
of IL-1β and TNF-α in Huizhuo Tanzu type AS ApoE−/−
mice
Compared with in the normal control group, serum
IL-1β and TNF-α levels were significantly higher in the model group
(P<0.01). Compared with in the model group, the serum levels of
IL-1β and TNF-α were significantly lower in the Tianxiangdan and
atorvastatin groups (P<0.01). There was no statistically
significant difference in serum IL-1β and TNF-α levels between mice
in the Tianxiangdan and atorvastatin groups (P>0.05; Table III).
 | Table III.Serum levels of IL-1β and TNF-α in
apolipoprotein E−/− mice. |
Table III.
Serum levels of IL-1β and TNF-α in
apolipoprotein E−/− mice.
| Group | n | IL-1β (pg/ml) | TNF-α (pg/ml) |
|---|
| Normal control | 8 | 12.33±2.09 | 8.26±5.04 |
| Model | 8 |
35.39±1.56a |
61.74±8.06a |
| Tianxiangdan | 8 |
25.16±1.17b |
26.30±5.08b |
| Atorvastatin | 8 |
24.60±1.29b |
24.24±6.74b |
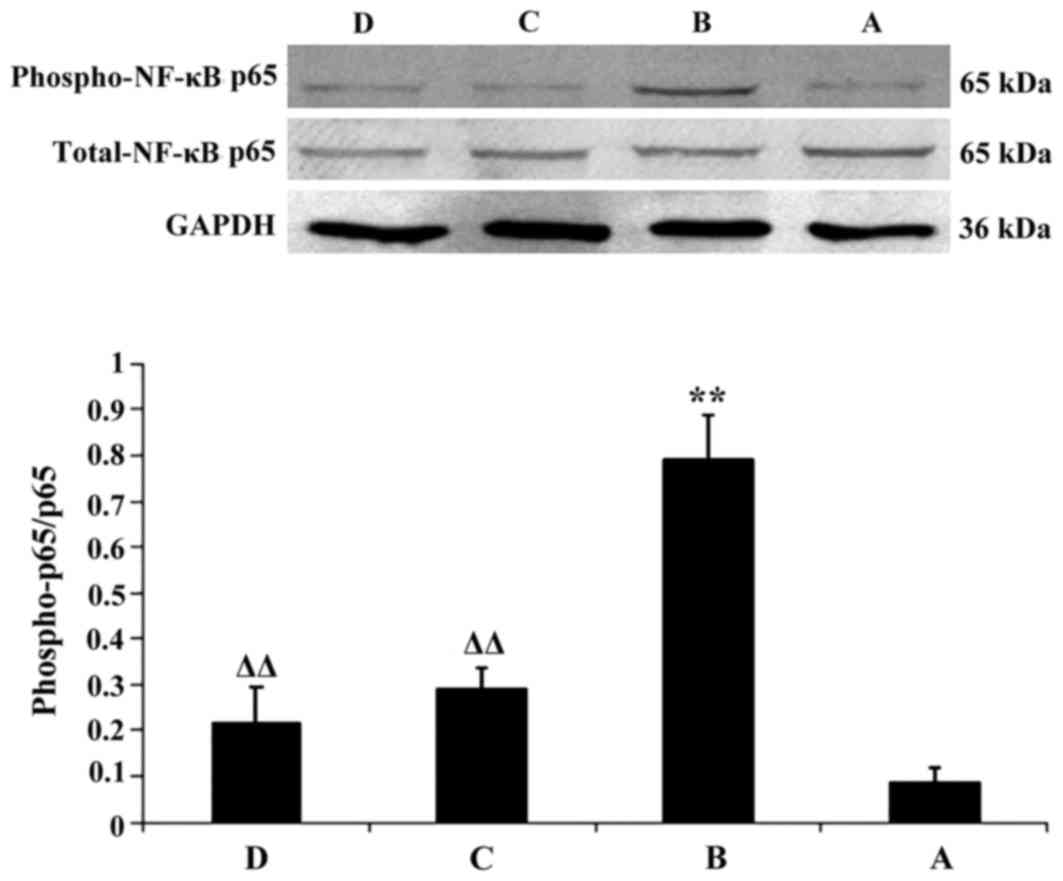
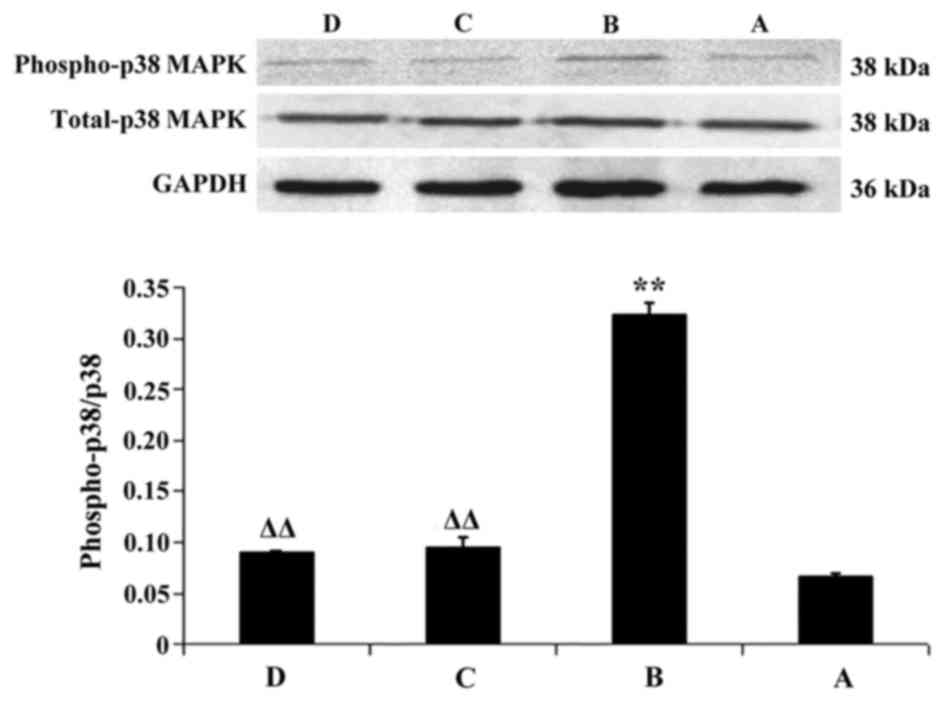
Alterations in the expression levels
of NF-κB p65 and p38 MAPK in aortic tissues of Huizhuo Tanzu type
AS ApoE−/− mice
Compared with the in normal control group, the
protein expression levels of p-NF-κB p65 and p-p38 MAPK were
significantly increased in the aortic tissue of the model group
(P<0.01). Compared with in the model group, the protein
expression levels of p-NF-κB p65 and p38 MAPK were significantly
decreased in the Tianxiangdan and atorvastatin groups (P<0.01).
There was no statistically significant difference in the protein
expression levels of p-NF-κB p65 and p-p38 MAPK between mice in the
Tianxiangdan and atorvastatin groups (P>0.05; Figs. 3 and 4).
Discussion
AS is caused by complex pathological alterations,
which are mediated by numerous factors. The pathogenesis of AS
cannot be completely explained by one or two theories; however, it
has been confirmed that the inflammatory response has a critical
role in the occurrence and development of AS (16). Previous studies regarding signaling
pathways and gene transcription levels have revealed that the
cytokine-mediated inflammatory response is a key factor in the
formation of AS; therefore, regulation of the inflammatory response
may be important in the prevention and treatment of AS (17,18).
NF-κB was initially detected in B lymphocytes in 1986 by Sen and
Baltimore (19). NF-κB is a
homologous or heterologous dimer transcription factor formed from
the Rel family of proteins, and is composed of two subunits, p50
and p65, which can specifically bind with the promoter or enhancer
κB sequence (GGGACTTTCC) of the immunoglobulin κ light chain gene.
Under physiological conditions, NF-κB is located in the cytoplasm,
where the p65 subunit binds to the κB inhibitor protein (IκB)
monomer, covering the nuclear localization signal of the p50
protein; therefore, the NF-κB complex is in the inactive state,
which cannot enter the nucleus and serve a regulatory role
(20). When the body is exposed to
external stimuli, IκB undergoes phosphorylation, dissociates from
the NF-κB dimers, thus exposing the nuclear localization signal of
the p50 protein, resulting in NF-κB activation and nuclear
translocation. Subsequently, NF-κB binds with the specific binding
site on DNA strands and initiates gene transcription and protein
expression; therefore, NF-κB serves an important role in regulation
of the immune response, inflammatory reaction and cell growth
(21). Numerous studies have
revealed that activated NF-κB can be found in atherosclerotic sites
and plaques, whereas in normal blood vessels, little or no
expression of NF-κB is detected (22,23).
Once activated, NF-κB is involved in regulation of the activation
and transcription of AS-associated genes, promoting the occurrence
and development of AS, thus suggesting that activation of the NF-κB
signaling pathway is closely associated with the occurrence and
development of AS (24–27). In AS pathology, NF-κB regulates the
expression of numerous genes, including cytokines (TNF-α, IL-1 and
IL-6), monocyte chemoattractant proteins and adhesion molecules,
and it participates in inflammation, which can lead to the
formation of atherosclerotic plaques, and can result in plaque
instability and rupture (28,29).
It is well known that the proinflammatory cytokines IL-1 and TNF-α
are regulated by NF-κB; at least one κB sequence is present in
their promoters and enhancers. When NF-κB is activated it binds to
the κB binding site, resulting in activation of IL-1 and TNF-α
genes; therefore, the genetic transcripts of IL-1 and TNF-α are
enhanced, and the production and release of IL-1 and TNF-α is
increased. IL-1 and TNF-α serve an important role in the
inflammatory immune response (30–33),
and IL-1 and TNF-α are activators of the NF-κB signaling pathway,
which results in further activation of NF-κB, whereas activated
NF-κB binds to the κB binding site in the TNF-α and IL-1 gene
promoters, resulting in enhancement of the transcription and
expression of TNF-α and IL-1; therefore, the inflammatory response
is strengthened. During this process, the inflammatory factors
TNF-α and IL-1, and associated cytokines, are released from
positive and negative feedback loops, and via NF-κB activation,
resulting in the upregulation of cytokines and persistence of
inflammation. These findings indicated that TNF-α and IL-1 may
participate in the occurrence and development of AS (34,35).
The MAPK signaling pathway is an important pathway
that mediates the information transfer between extracellular
signals, and intracellular and nuclear signals (36). The MAPK family is predominantly
composed of extracellular signal-regulated kinase (ERKI/2),
stress-activated protein kinase/c-Jun N-terminal kinase (JNK) and
p38 MAPK. Each MAPK is activated by a specific MAPK kinase (MAPKK),
which is in turn activated by a MAPKK kinase; through this
conservative tertiary enzymatic cascade, MAPK signaling pathways
activate their downstream transcription factors (37). p38 MAPK, which is a member of the
MAPK family, is an important intracellular signaling pathway
involved in the inflammatory response, which is closely associated
with the mechanisms underlying inflammatory regulation and control
(38). The p38 MAPK signaling
pathway is predominantly involved in cellular inflammation and
apoptosis under stress conditions (39), and activation of the p38 MAPK
signaling pathway is associated with the release of various
inflammatory cytokines. It has previously been reported that
specific blocking of the p38 MAPK pathway can reduce inflammation
(40). When the body is exposed to
long-term inflammation, p38 MAPK and NF-κB may be activated; and
activation of p38 MAPK further activates NF-κB via phosphorylation,
or inflammatory cytokine (TNF-α and IL-1) secretion (41–43).
In addition, when NF-κB is activated, it may in turn activate the
p38 MAPK signaling pathway via its products, including the
inflammatory cytokines TNF-α and IL-1. These findings suggested
that a complex mutual activating network exists between p38 MAPK
and NF-κB, ultimately resulting in NF-κB and p38 MAPK activation,
which mediates the inflammatory response and may participate in the
formation of AS (44,45).
The incidence of AS is higher in the Xinjiang area
compared with in the other provinces of China; this is primarily
due to the specific climate, and the lifestyle and dietary habits
of the local people. Professor Dongqing An, a doctor whose
expertise is in traditional Chinese medicine, initially proposed
that the Huizhuo Tanzu type is the main type of AS present in the
Xinjiang region of China, due to the specific climate, and the diet
and lifestyle characteristics of the local inhabitants (46). Clinical manifestations of this type
of AS include: Chest tightness, chest pain, halitosis, nausea and
vomiting, belching, abdominal distension, gastric distress,
dizziness, drowsiness, poor or smelly stools or constipation, dark
tongue, elevated pulse pressure and thick and greasy hair. A series
of prescriptions, which are clinically effective for the treatment
of Huizhuo Tanzu type AS, have been successfully developed,
including Tianxiangdan, which has been clinically applied for the
treatment of AS in China for >30 years. A previous study used an
artificial climate box to simulate the specific climate of the
Xinjiang region and fed ApoE−/− mice a high-fat diet,
resulting in the successful establishment of Huizhuo Tanzu type AS
animal model (14). Therefore, the
Huizhuo Tanzu type AS model may be used for further experimental
studies on the anti-AS mechanism of Tianxiangdan Granule.
In the present study, ApoE−/− mice were
fed a high-fat diet to simulate the diet characteristics of
Xinjiang residents, and to simulate the specific climate and
environment of the Xinjiang region the mice were placed in an
artificial climate box. Following successful replication of the
Huizhuo Tanzu type AS animal model, each group of mice received a
different intervention. The aortic PA/LA ratio was significantly
higher in the model group compared with in the normal control
group, whereas the PA/LA ratio was reduced in the Tianxiangdan and
atorvastatin groups compared with in the model group. There was no
significant difference in the PA/LA ratio between the Tianxiangdan
and atorvastatin groups, thus suggesting that the Tianxiangdan
Granule can inhibit the formation of atherosclerotic plaques and
may reduce PA. Serum lipid levels were determined by enzymatic
colorimetry, and the results indicated that the serum contents of
TG, TC, HDL-C and LDL-C were significantly higher in the model
group compared with in the normal control group. The increase in
HDL-C levels in the model group may be associated with the murine
stress response. TC and LDL-C levels in the Tianxiangdan and
atorvastatin groups were significantly lower than in the model
group; however, there was no significant difference in TC and LDL-C
levels between the Tianxiangdan and atorvastatin groups, thus
suggesting that Tianxiangdan Granule may regulate blood lipid
levels via reducing TC and LDL-C levels. The serum expression
levels of IL-1β and TNF-α were determined by ELISA; the results
revealed that the expression levels of IL-1β and TNF-α levels were
significantly higher in the model group compared with in the normal
control group, whereas IL-1β and TNF-α expression was reduced in
the Tianxiangdan and atorvastatin groups. These findings suggested
that Tianxiangdan Granule may reduce the serum expression levels of
IL-1β and TNF-α in an ApoE−/− mouse model of Huizhuo
Tanzhu type AS, resulting in inhibition of the inflammatory
response. Aortic tissue protein expression levels were detected in
the ApoE−/− mice by western blotting; the results
revealed that the protein expression levels of p-NF-κB p65 and
p-p38 MAPK were significantly upregulated in the model group.
However, following treatment with Tianxiangdan Granule or
atorvastatin, the phosphorylation levels of NF-κB p65 and p38 MAPK
were reduced. However, there was no significant difference in the
phosphorylation levels of NF-κB p65 and p38 MAPK between the
Tianxiangdan and atorvastatin groups. Previous studies have aimed
to determine the mechanisms underlying the anti-AS effects of
traditional Chinese medicine, and have focused on various
inflammatory signaling pathways, including p38 MAPK, JNK, ERK1/2
and NF-κB. These studies have revealed that Chinese medicine may
suppress the inflammatory response via its effects on the
phosphorylation levels of p38 MAPK and NF-κB p65; this may explain
how these medicines exert their anti-AS effects (47–54).
Since these signaling pathways influence each other, and form
complex and changeable networks, the present study only
investigated the effects of Tianxiangdan Granule on p38 MAPK and
NF-κB signaling pathways, and aimed to determine whether
Tianxiangdan Granule exerts anti-AS effects via related signaling
pathways. Our research group aims to conduct further in-depth
studies in subsequent research.
In conclusion, the present study indicated that
Tianxiangdan Granule is able to reduce AS PA, regulate the level of
serum lipids, and inhibit expression of the inflammatory cytokines,
IL-1β and TNF-α. Therefore, Tianxiangdan Granule may serve an
anti-AS role. These functions may be mediated by inhibition of the
phosphorylation levels of NF-κB and p38 MAPK signaling
pathways.
Acknowledgements
The present study was supported by a grant from the
National Nature Science n of China (grant nos. 81160428 and
81360571). The authors would like to thank Mr. Liang Li
(Experimental center of the First Affiliated Hospital of Xinjiang
Medical University, Urumqi, China) for his technical help in animal
experimentation.
References
|
1
|
Lehrke M and Lebherz C: AAV-mediated gene
therapy for atherosclerosis. Curr Atheroscler Rep. 16:4342014.
View Article : Google Scholar
|
|
2
|
Sidney S, Rosamond WD, Howard VJ and
Luepker RV: National Forum for Heart Disease and Stroke Prevention:
The ‘heart disease and stroke statistics-2013 update’ and the need
for a national cardiovascular surveillance system. Circulation.
127:21–23. 2013. View Article : Google Scholar
|
|
3
|
Chen WW, Gao RL and Liu LS: The Chinese
cardiovascular disease report 2014 Profile. Chin Circulat J.
617–622. 2015.
|
|
4
|
Anogeianaki A, Angelucci D, Cianchetti E,
D'Alessandro M, Maccauro G, Saggini A, Salini V, Caraffa A, Tete S,
Conti F, et al: Atherosclerosis: A classic inflammatory disease.
Int J Immunopathol Pharmacol. 24:817–825. 2011. View Article : Google Scholar
|
|
5
|
Charakida M, O'Neil F, Masi S,
Papageorgiou N and Tousoulis D: Inflammatory disorders and
atherosclerosis: New therapeutic approaches. Curr Pharm Des.
17:4111–4120. 2011. View Article : Google Scholar
|
|
6
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar
|
|
7
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:S419–S420. 1999. View Article : Google Scholar
|
|
8
|
An D, Hu J, Zhao M and Zheng J: Effect of
Tianxiangdan on serum vascular endothelial growth factor and
correlative factors in coronary heart disease. Chin J Integrat Med
Cardio/Cerebrovas Dis. 5:662–664. 2007.(In Chinese).
|
|
9
|
An D, Zhou M, Zhang X and Ling C: Effect
of Tianxiangdan on thrombosis and myocardial ultramicrostructure in
rat model with myocadial ischemia. J Xinjiang Med Uni. 30:434–437.
2007.(In Chinese).
|
|
10
|
Yin J, Zhu J, Liu Q and An Q: The effect
of Tianxiangdan granule on T-SOD, GSH-Px, MDA, LD in serum of
angina of coronary heart disease patients. Chin J Inform Trad Chin
Med. 10:13–15. 2003.(In Chinese).
|
|
11
|
Wang XY, Jiao YJ and An DQ: Effects of
different concentrations of Tianxiangdan on blood lipid and VEGF
levels in ApoE (−/-) gene knockout mice with atherosclerosis. J
Emer Trad Chin Med. 23(400–403): 415S42014.(In Chinese).
|
|
12
|
An DQ: A medicine for treating coronary
heart disease and its preparation method. CN200910210063.9. Filed
Nov 4, 2009; issued May 11. 2011.
|
|
13
|
Gao Z, Ablimiti A and Wufuer H: Thinking
about the establishment of animal model of cold dryness syndrome in
Northwest China. Chin J Inf Tradit Chin Med. 17:6–7. 2010.(In
Chinese).
|
|
14
|
Niyazi G, An DQ and Li M: Study on
Establishing Animal Model of Huizhuo Tanzu Type Atherosclerosis.
Chin J Exp Tradit Med Form. 19:265–269. 2013.(In Chinese).
|
|
15
|
Xu SY, Bian RL and Chen X: The
Experimental Method of Pharmacology. Third edition. Beijing:
People's Med Publishing House; 2013, (In Chinese). View Article : Google Scholar
|
|
16
|
Lucas AR, Korol R and Pepine CJ:
Inflammation in atherosclerosis: Some thoughts about acute coronary
syndromes. Circulation. 113:e728–e732. 2006. View Article : Google Scholar
|
|
17
|
Li YJ, Yang Q and Zhu XX: TCM
anti-inflammation: An effective approach for treating
atherosclerosis. Mod Tradit Chin Med Mater Med-World Sci Technol.
9:22–28. 2007.(In Chinese).
|
|
18
|
Daviglus ML, Talavera GA, Avilés-Santa ML,
Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N,
Kaplan RC, et al: Prevalence of major cardiovascular risk factors
and cardiovascular diseases among hispanic/latino individuals of
diverse backgrounds in the United States. JAMA. 308:1775–1784.
2012. View Article : Google Scholar :
|
|
19
|
Sen R and Baltimore D: Multiple unclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar
|
|
20
|
Gordon JW, Shaw JA and Kirshenbaum LA:
Multiple facets of NF-κB in the heart: To be or not to NF-κB. Circ
Res. 108:1122–1132. 2011. View Article : Google Scholar
|
|
21
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar
|
|
22
|
Misra A, Haudek SB, Knuefermann P, Vallejo
JG, Chen ZJ, Michael LH, Sivasubramanian N, Olson EN, Entman ML and
Mann DL: Nuclear factor-kappaB protects the adult cardiac myocyte
against ischemia-induced apoptosis in a murine model of acute
myocardial infarction. Circulation. 108:3075–3078. 2003. View Article : Google Scholar
|
|
23
|
Morita M, Yano S, Yamaguchi T and Sugimoto
T: Advanced glycation end products-induced reactive oxygen species
generation is partly through NF-kappa B activation in human aortic
endothelial cells. J Diabetes Complications. 27:11–15. 2013.
View Article : Google Scholar
|
|
24
|
Lawrence T, Gilroy DW, Nash Colwille PR
and Willoughby DA: Possible new role for NF-kappa B in the
resolution of inflammation. Nat Med. 7:1291–1297. 2001. View Article : Google Scholar
|
|
25
|
Freund C, Schmidt-Ullrich R, Baurand A,
Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C
and Bergmann MW: Requirement of nuclear factor-kappaB in
angiotensin II- and isoproterenol-induced cardiac hypertrophy in
vivo. Circulation. 111:2319–2325. 2005. View Article : Google Scholar
|
|
26
|
Zelarayan L, Renger A, Noack C, Zafiriou
MP, Gehrke C, van der Nagel R, Dietz R, de Windt L and Bergmann MW:
NF-kappaB activation is required for adaptive cardiac hypertrophy.
Cardiovasc Res. 84:416–424. 2009. View Article : Google Scholar
|
|
27
|
Liuzzo G, Santamaria M, Biasucci LM,
Narducci M, Colafrancesco V, Porto A, Brugaletta S, Pinnelli M,
Rizzello V, Maseri A and Crea F: Persistent activation of nuclear
factor kappa-B signaling pathway in patients with unstable angina
and elevated levels of C-reactive protein evidence for a direct
proinflammatory effect of azide and lipopolysaccharide-free
C-reactive protein on human monocytes via nuclear factor kappa-B
activation. J Am Coll Cardiol. 49:185–194. 2007. View Article : Google Scholar
|
|
28
|
Wu XL, Zheng B, Jin LS, Zhang RN, He M,
Yang Z and Wen JK: Chinese medicine Tongxinluo reduces
atherosclerotic lesion by attenuating oxidative stress and
inflammation in microvascular endothelial cells. Int J Clin Exp
Pathol. 8:6323–6333. 2015.
|
|
29
|
Kang Q, Liu W, Liu H and Zhou M: Effect of
compound chuanxiong capsule on inflammatory reaction and
PI3K/Akt/NF-κB signaling pathway in atherosclerosis. Evid Based
Complement Alternat Med. 2015:5845962015. View Article : Google Scholar :
|
|
30
|
Latkovslds G, Licis N and Kalnms U:
C-reactive protein levels and common polymorphisms of the
interleukin-1 gene cluster and interleukin-6 gene in patients with
coronary heart disease. Eur J Immunogenet. 31:207–213. 2004.
View Article : Google Scholar
|
|
31
|
Zhu T, Zhang L, Ling S, Duan J, Qian F, Li
Y and Xu JW: Scropolioside B inhibits IL-1β and cytokines
expression through NF-κB and inflammasome NLRP3 pathways. Mediators
Inflamm. 2014:8190532014. View Article : Google Scholar :
|
|
32
|
Popa C, Netea MG, van-Riel PL, van der
Meer JW and Stalenhoef AF: The role of TNF-alpha in chronic
inflammatory conditions, intermediary metabolism, and
cardiovascular risk. J Lipid Res. 48:751–762. 2007. View Article : Google Scholar
|
|
33
|
Zhang H, Park Y, Wu J, Chen Xp, Lee S,
Yang J, Dellsperger KC and Zhang C: Role of TNF-alpha in vascular
dysfunction. Clin Sci (Lond). 116:219–230. 2009. View Article : Google Scholar :
|
|
34
|
Novitskiy G, Ravi R, Potter JJ,
Rennie-Tankersley L, Wang L and Mezey E: Effects of acetaldehyde
and TNF alpha on the inhibitory kappa B-alpha protein and nuclear
factor kappa B activation in hepatic stellate cells. Alcohol
Alcohol. 40:96–101. 2005. View Article : Google Scholar
|
|
35
|
Morello S, Ito K, Yamamura S, Lee KY,
Jazrawi E, Desouza P, Barnes P, Cicala C and Adcock IM: IL-1beta
and TNF-alpha regulation of the adenosine receptor (A2A)
expression: Differential requirement for NF-kappa B binding to the
proximal promoter. J Immunol. 177:7173–7183. 2006. View Article : Google Scholar
|
|
36
|
Schramek H: MAP kinases: From
intracellular signals to physiology and disease. News Physiol Sci.
17:62–67. 2002.
|
|
37
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar
|
|
38
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar
|
|
39
|
Ju H, Behm DJ, Nerurkar S, Eybye ME,
Haimbach RE, Olzinski AR, Douglas SA and Willette RN: p38 MAPK
inhibitors ameliorate target organ damage in hypertension: Part1.
p38 MAPK-dependent endothelial dysfunction and hypertension. J
Pharmacol Exp Ther. 307:932–938. 2003. View Article : Google Scholar
|
|
40
|
Goldstein DM and Gabriel T: Pathway to the
clinic: Inhibition of P38 MAP kinase. A review of ten chemotypes
selected for development. Curr Top Med Chem. 5:1017–1029. 2005.
View Article : Google Scholar
|
|
41
|
He X, Shu J, Xu L, Lu C and Lu A:
Inhibitory effect of astragalus polysaccharides on
lipopolysaccharide-induced TNF-a and IL-1β production in THP-1
cells. Molecules. 17:3155–3164. 2012. View Article : Google Scholar
|
|
42
|
Yang K, Qiu BY, Yan J, Yang YX, Zhang T,
Chen X, Zou YP, Gan HT and Huang XL: Blockade of p38
mitogen-activated protein kinase pathway ameliorates delayed
gastric emptying in streptozotocin-induced diabetic rats. Int
Immunopharmacol. 23:696–700. 2014. View Article : Google Scholar
|
|
43
|
El Bekay R, Alvarez M, Monteseirín J, Alba
G, Chacón P, Vega A, Martin-Nieto J, Jiménez J, Pintado E, Bedoya
FJ and Sobrino F: Oxidative stress is a critical mediator of the
angiotensin II signal in human neutrophils: Involvement of
mitogen-activated protein kinase, calcineurin, and the
transcription factor NF-kappaB. Blood. 102:662–671. 2003.
View Article : Google Scholar
|
|
44
|
Lindroos PM, Rice AB, Wang YZ and Boner
JC: Role of nuclear factor-kappaB and mitogen-activated protein
kinase signaling pathways in IL-1beta-mediated induction of
alpha-PDGF receptor expression in rat pulmonary myofibroblasts.
Immunol. 161:3464–8. 1998.
|
|
45
|
Zhang X, Wu M, Jiang H, Hao J, Zhang Q,
Zhu Q, Saren G, Zhang Y, Meng X and Yue X: Angiotensin II
upregulates endothelial lipase expression via the NF-kappa B and
MAPK signaling pathways. PLoS One. 9:e1076342014. View Article : Google Scholar :
|
|
46
|
An DQ, Zhao MF, Zheng J, et al: Xinjiang
obstruction of dirty phlegm syndrome retraces. Xinjiang J Tradit
Chin Med. 25:1–2. 2007.(In Chinese).
|
|
47
|
Xiao Y, Wang YC, Li LL, Jin YC, Sironi L
and Wang Y and Wang Y: Lactones from Ligusticum chuanxiong Hort.
reduces atherosclerotic lesions in apoE-deficient mice via
inhibiting over expression of NF-kB-dependent adhesion molecules.
Fitoterapia. 95:240–246. 2014. View Article : Google Scholar
|
|
48
|
Liang CJ, Lee CW, Sung HC, Chen YH, Wang
SH, Wu PJ, Chiang YC, Tsai JS, Wu CC, Li CY and Chen YL: Magnolol
reduced TNF-α-induced vascular cell adhesion molecule-1 expression
in endothelial cells via JNK/p38 and NF-κB signaling pathways. Am J
Chin Med. 42:619–636. 2014. View Article : Google Scholar
|
|
49
|
Chen Z, Cai Y, Zhang W, Liu X and Liu S:
Astragaloside IV inhibits platelet-derived growth
factor-BB-stimulated proliferation and migration of vascular smooth
muscle cells via the inhibition of p38 MAPK signaling. Exp Ther
Med. 8:1253–1258. 2014. View Article : Google Scholar :
|
|
50
|
Shen DZ, Xin SL, Chen C and Liu T: Effect
of atorvastatin on expression of TLR4 and NF-κB p65 in
atherosclerotic rabbits. Asian Pac J Trop Med. 6:493–496. 2013.
View Article : Google Scholar
|
|
51
|
Bao MH, Zhang YW and Zhou HH: Paeonol
suppresses oxidized low-density lipoprotein induced endothelial
cell apoptosis via activation of LOX-1/p38MAPK/NF-κB pathway. J
Ethnopharmacol. 146:543–551. 2013. View Article : Google Scholar
|
|
52
|
Liang CJ, Lee CW, Sung HC, Chen YH, Wang
SH, Wu PJ, Chiang YC, Tsai JS, Wu CC, Li CY and Chen YL: Magnolol
reduced TNF-α-induced vascular cell adhesion molecule-1 expression
in endothelial cells via JNK/p38 and NF-κB signaling pathways. Am J
Chin Med. 42:619–637. 2014. View Article : Google Scholar
|
|
53
|
Park Haeng S, Sung YY, Nho Jin K and Kim
Kyoung H: Anti-atherosclerotic effects of Polygonum aviculare L.
ethanol extract in ApoE knock-out mice fed a Western diet mediated
via the MAPK pathway. J Ethnopharmacol. 151:1109–1115. 2014.
View Article : Google Scholar
|
|
54
|
Chang YL, Chen CL, Kuo CL, Chen BC and You
JS: Glycyrrhetinic acid inhibits ICAM-1 expression via blocking JNK
and NF-kappaB pathways in TNF-alpha-activated endothelial cells.
Acta Pharmacol Sin. 31:546–553. 2010. View Article : Google Scholar :
|