Introduction
When the internal environment of blood vessels is in
disorder, endothelial cells produce a large amount of reactive
oxygen species (ROS) through the NADPH oxidase pathway, there by
inducing the abnormal proliferation of vascular smooth muscle cells
(VSMCs), platelet accumulation and vascular reconstruction
(1). VSMC proliferation and
migration are important in vascular reconstruction; they cause
intimal thickening and consequently atherosclerosis and restenosis,
following percutaneous coronary intervention or venous
transplantation (2,3). Therefore, inhibiting the abnormal
proliferation of VSMCs is instrument altoreversing vascular
reconstruction. However, specific drugs against the proliferation
of VSMCs have not emerged yet. Developing such drugs with minimal
toxicity and side effects could significantly contribute to
improving vascular reconstruction, life quality, and survival of
patients with coronary artery diseases.
Incases of vascular injury, platelet-derived growth
factor-BB (PDGF-BB) is released. Its binding with cell membrane
receptor PDGFR-β activates NAPDH oxidase to produce large amounts
of ROS, which induce the activation of various down stream
signalling pathways and mitogen-activated protein kinase (MAPK)
signalling pathways (4,5). In VSMCs, the activation of MAPK
signalling pathways such as the extracellular signal-related
protein kinases 1 and 2 (ERK1/2) pathway and p38 MAPK pathway can
lead to cell proliferation and migration as well as the change of
VSMCs from the contractile phenotype to the proliferative phenotype
(6). Lee et al (7) demonstrated that reduced ROS inhibits
PDGF-BB-induced proliferation and migration of VSMCs.
Paeoniflorin (PAE) is a monoterpene glycoside from
Paeonia lactiflora that reputedly performs various
biological functions, including anti oxidative, anti-free radical,
anti platelet functions, and improves micro circulation and immune
regulatory effects (8). In studies
on cardio vascular diseases, PAE has been demonstrated to improve
myocardial infarction (9),
LPS-induced myocarditis (10) and
myocardial ischemia/reperfusion injury in rats (11), by inhibiting inflammation. PAE can
also improve pressure overload-induced cardiac remodeling by
inhibiting transforming growth factor (TGF)-β/Smads and NF-κB
signalling pathways (8). Never the
less, whether PAE has a therapeutic effect on VSMC proliferation
and migration in vitro induced by PDGF-BB, as well as its
effect on MAPK signalling pathway, is still unknown. In the present
study, in vitro experiments were performed to examine
whether PAE possessed protective effects, and the potential
mechanism was discussed.
Materials and methods
Materials
PAE (99% as determined by high-performance liquid
chromatography analysis) was ordered from Shanghai Winherb Medical
Technology Co., Ltd. (Shanghai, China). Recombinant human PDGF-BB
was purchased from Pepro Tech, Inc. (Rock Hill, NJ, USA). Cell
Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). A cell proliferation ELISA,
BrdU (colorimetric) kit was purchased from Roche (11647229001;
Roche Diagnostics, Basel, Switzerland). Propidium iodide (PI) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
TRIzol was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Primary anti bodies to recognize the total
and phosphorylated (p-) forms of p38 (p-p38, 4511P; p38, 9212P),
ERK1/2 (p-ERK1/2, 4370p; ERK1/2, 4695), c-Jun N-terminal kinase
(JNK) (p-JNK, 4668P; JNK, 9258) and GAPDH (2118) were ordered from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Membranes were
incubated with the secondary antibody goat anti-rabbit
immunoglobulin G (926-32211; LI-COR Biosciences, Lincoln, NE, USA).
Sprague-Dawley (SD) rats (150–200 g) were ordered from the
Zhengzhou University Center for Animal Experiment (Zhengzhou,
China). For the in vitro study, PAE was dissolved in DMSO
(Sigma-Aldrich; Merck KGaA).
Cell culture
VSMCs were collected from the thoracic artery of
male SD rats aged <8 weeks. All the animal experiment procedures
conformed to the Guidelines for Animal Care and Use, and the
protocol was approved by the Animal Care and Use Center of the
Zhengzhou University People's Hospital (Henan Province People's
Hospital; Zhengzhou, China). The cells were cultured and prepared
at 37°C in a 5% CO2 incubator, as described previously
(12). The culture medium
consisted of Dulbecco's modified Eagle's medium (DMEM) (11330032;
Gibco; Thermo Fisher Scientific, Inc.) supplemented 1:1 with F-12
nutrient mixture F-12 + 10% fetal bovine serum (10099; Gibco;
Thermo Fisher Scientific, Inc.) + 1% penicillin and streptomycin.
When the cells covered 80% of the bottom of the culture flask
(2×105 cells/ml), cell passage was performed by
digestion with 0.25% trypsin. VSMCs of the third to fifth
generation were used. Prior to experiments, the cells were starved
by culturing them in DMEM/F12 containing 0.5% FBS and 0.5%
penicillin and streptomycin (FBS can partially counteract the
activity of antibiotics) for 24 h to synchronize the cells. Cells
were pre treated with different concentrations of PAE for 1 h prior
to stimulation with PDGF-BB (20 ng/ml).
Cell viability
The toxicity of PAE on VSMCs was determined by the
trypan blue exclusion test. After 12, 24 and 48 h incubation with
PAE of different concentrations (50, 100, 200 µM), the VSMCs were
removed from culture and the cells that excluded 0.4% trypan blue
(37°C for 3 min) were counted in an automated Cell Counter
(Invitrogen; Thermo Fisher Scientific, Inc.); 3 slides were counted
in each group, with 3 fields of view from each.
Cell proliferation and DNA
synthesis
Cell proliferation was determined using a CCK-8
assay, according to the manufacturer's instructions. Briefly,
following the indicated treatment, 10 µl of CCK-8 solution was
added to each well, and the plate was incubated at 37°C for 2.5 h.
The color intensity was read at a wave length of 450 nm. VSMC DNA
synthesis was assessed by measuring the incorporation of BrdU. BrdU
incorporation was measured using a cell proliferation ELISA kit
according to the manufacturer's protocol. The effect of PAE on cell
proliferation and DNA synthesis was expressed as the percentage
compared with the control group, which was set at 100%.
Flow cytometry analysis of cell cycle
progression
Cell cycle progression was determined using PI
staining according to the manufacturer's instructions and
fluorescence-activated cellsorting (FACS). Following 24 h treatment
with 200 µM PAE and/or 20 ng/ml PDGF-BB, cells were harvested and
fixed with 70% ethanol over night at 4°C. Fixed cells were
collected by centrifugation (2,067 × g at 4°C for 5 min), washed
once using PBS, and incubated with 1 ml of PI staining buffer (20
µg/ml PI and 50 µg/ml RNaseA) at 4°C for 30 min. Afterward,
cellular fluorescence was measured by flow cytometry analysis with
a FACS Calibur Flow Cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). Cell cycle distributions were analyzed using the Multi cycle
AV software version 3 (Phoenix Flow Systems, San Diego, CA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from VSMCs using TRIzol
reagent, according to the manufacturer's protocol. The RNA yields
and purities were spectrophotometrically analyzed by the A260/A280
and A230/A260 ratios using a Nano Drop 2000c (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). The RNA (2 µg of each
sample) was reverse transcribed into cDNA using oligo (dT) primers
and the Transcript or First Strand cDNA Synthesis kit (cat. no.
04896866001; Roche Diagnostics) under the following conditions:
50°C 60 min, 90°C 5 min and 4°C 90 min. SYBR-Green PCR Master Mix
(cat. no. 04707516001; Roche Diagnostics) was then used to quantify
PCR amplifications with the Light Cycler 480 instrument (software
version 1.5; Roche Diagnostics). The PCR conditions were as
follows: Initial denaturation at 94°C for 2 min, followed by 25–35
amplification cycles consisting of denaturation at 94°C for 40 sec,
annealing at 58°C for 45 sec and elongation at 72°C for 1 min. The
following primer pairs were used for qPCR: OPN, forward
5′-GCTGAAGCCTGACCCATC-3′ and reverse 5′-TCCCGTTGCTGTCCTGAT-3′;
cyclin dependent kinase (CDK) 4, forward 5′-ATGTTGTCCGGCTGATGG-3′
and reverse 5′-CACCAGGGTTACCTTGATCTCC-3′; CDK2, forward
5′-GCTTTCTGCCATTCTCATCG-3′ and reverse 5′-GTCCCCAGAGTCCGAAAGAT-3′;
cyclin D1, forward 5′-CCCGAGGAGTTGCTGCAAATGGA-3′ and reverse
5′-AGGGCCACAAAGGTCTGTGCA-3′; cyclin E, forward
5′-GTCCTGGCTGAATGTATACATGC-3′ and reverse 5′-CCC TAT TTT GTT CAG
ACA ACA TGG C-3′; cyclin dependent kinase inhibitor 1A (CDKN1A),
forward 5′-TGTACCAGCCACAGGCACCATGT-3′ and reverse
5′-TCGCCATGAGCGCATCGCAAT-3′; GAPDH, forward
5′-GACATGCCGCCTGGAGAAAC-3′, and reverse 5′-AGCCCAGGATGCCCTTTAGT-3′.
The 2−ΔΔCq method was used to analyze data from
real-time quantitative PCR experiments (13).
Scratch migration test
VSMCs were plated in 6-well plates, grown to
confluence to forma monolayer and treated with PDGF-BB and/or PAE
for 24 h. Subsequently, the cells were serum-starved for 4 h.
Wounds were created by scraping the cell monolayer with a 200 µl
sterile micropipette tip and rinsing 3 times with PBS. All of the
cells were stimulated with basal medium (DMEM/F12 containing 0.5%
FBS and 0.5% PS). At 0, 6, 12, and 24 h post-injury, migratory
cells were photo graphed using an inverted microscope (IX51;
Olympus Corporation, Tokyo, Japan).
ROS measurement
Intracellular ROS generation was determined by
2′,7′-dichlorofluorescin diacetate (DCFH-DA; cat. no. D6883;
Sigma-Aldrich; Merck KGaA) which is oxidized to fluorescent DCF by
ROS. Following 1 h pre treatment with PAE, cells were incubated
with PDGF-BB for 2 h. Subsequently, VSMC cells were washed twice
and incubated with 5 µM of DCFH-DA solution in serum-free medium at
37°C for 30 min in the dark. Data were collected using a
fluorescence readeratan excitation/emission wave length of 488 and
525 nm. A fluorescence microscope (cat. no. IX51; Olympus
Corporation) was also used to evaluate the DCF fluorescence of
cells on cover slip.
Western blotting analysis
Protein was extracted from VSMCs indifferent groups.
The cells were lysed in RIPA lysis buffer (Wuhan Goodbio Technology
Co. Ltd., Wuhan, China)containing 50 mM Tris-HCl, 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate and 0.1% SDS; the cells were
then scraped into 1.5-ml centrifuge tubes. The cell suspension was
centrifuged at 3,362 × g for 30 min at 4°C and the protein
concentration was measured using a bicinchoninic acid protein assay
kit (23227; Thermo Fisher Scientific, Inc.) using the Synergy HT
microplate reader. The cell lysates (40 µg per lane) were separated
by 10% SDS-PAGE, then proteins were transferred on to a
polyvinylidene fluoride membrane and incubated with the primary
anti bodies diluted 1:1,000 in 5% w/v non-fat dry milk, 1X
Tris-buffered saline, 0.1% Tween-20 at 4°C with gentle agitation,
overnight. The primary antibodies were p-p38, p38, p-JNK, JNK,
p-ERK1/2, ERK1/2 and GAPDH. There after, membranes were incubated
with these condary antibody, goat anti-rabbit immunoglobulin G
(926-32211; 1:100; LI-COR Biosciences) for 60 min. The blots were
scanned by an Odyssey two-color infrared imaging system (version
3.0; LI-COR Biosciences) to quantify protein expression. Protein
expression levels were normalized to the level of GAPDH
expression.
Statistical analysis
All data are expressed as the mean ± standard
deviation of ≥3 replicates and analyzed using SPSS version 19.0
(IBM Corp., Armonk, NY, USA). Comparisons of two groups were
performed using the unpaired Student's t test. Multiple comparison
analyses were performed by one-way analysis of variance, followed
by Student-Newman-Keuls tests. P<0.05 was considered to indicate
a statistically significant difference.
Results
PAE does not affect the viability of
VSMCs
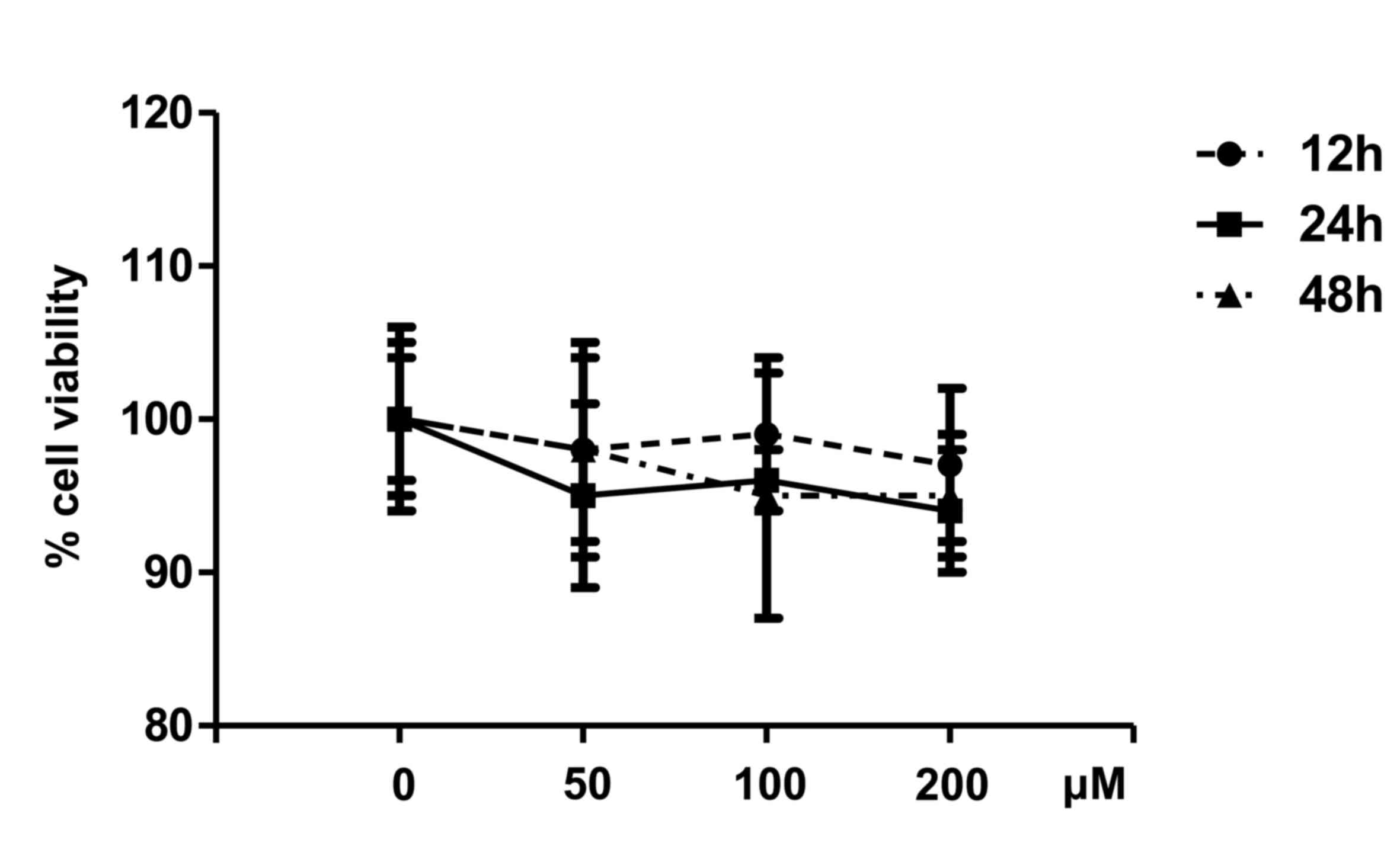
The cytotoxicity of PAE on VSMCs was assessed using
the trypan blue exclusion test. Compared with untreated cells,
concentrations of 50, 100, and 200 µM PAE did not result in
significant cytotoxicity in VSMCs following 12, 24 or 48 h of
incubation (Fig. 1). The cell
viability in different groups was all maintained at ~94%, there by
indicating that PAE had no cytotoxicity at the tested
concentrations.
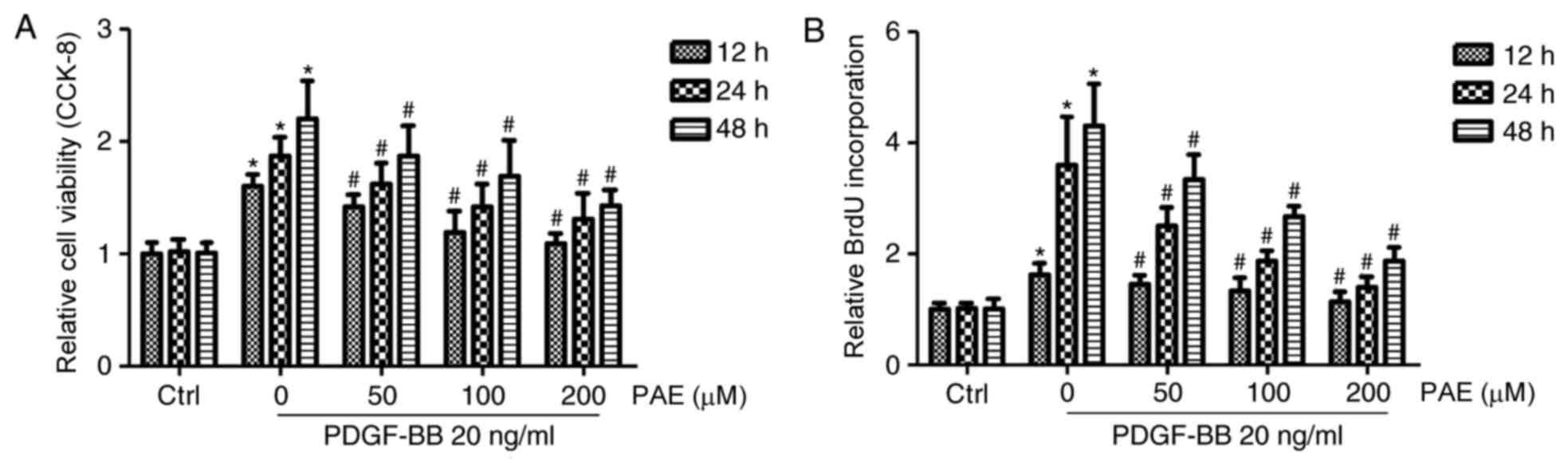
PAE inhibits VSMC proliferation and
DNA synthesis induced by PDGF-BB
To determine the effect of PAE on VSMC
proliferation, CCK-8 cell proliferation assays were performed on
VSMCs treated with different concentrations of PAE (50, 100, and
200 µM) and PDGF-BB (20 ng/ml) for 12, 24 or 48 h. Compared with
the control group, PDGF-BB significantly induced VSMC proliferation
in a time-dependent manner, which was blocked by PAE in a time-and
concentration-dependent manner; the most remarkable suppression of
proliferation compared with PDGF-BB only was obtained at 200 µM
(Fig. 2A). The suppressive effect
of PAE on DNA synthesis was then investigated by measuring the
incorporation of BrdU. Compared with the control group, PDGF-BB
significantly increased DNA synthesis in VSMCs in a time-dependent
manner, which was also blocked by PAE in a concentration-and
time-dependent manner (Fig.
2B).
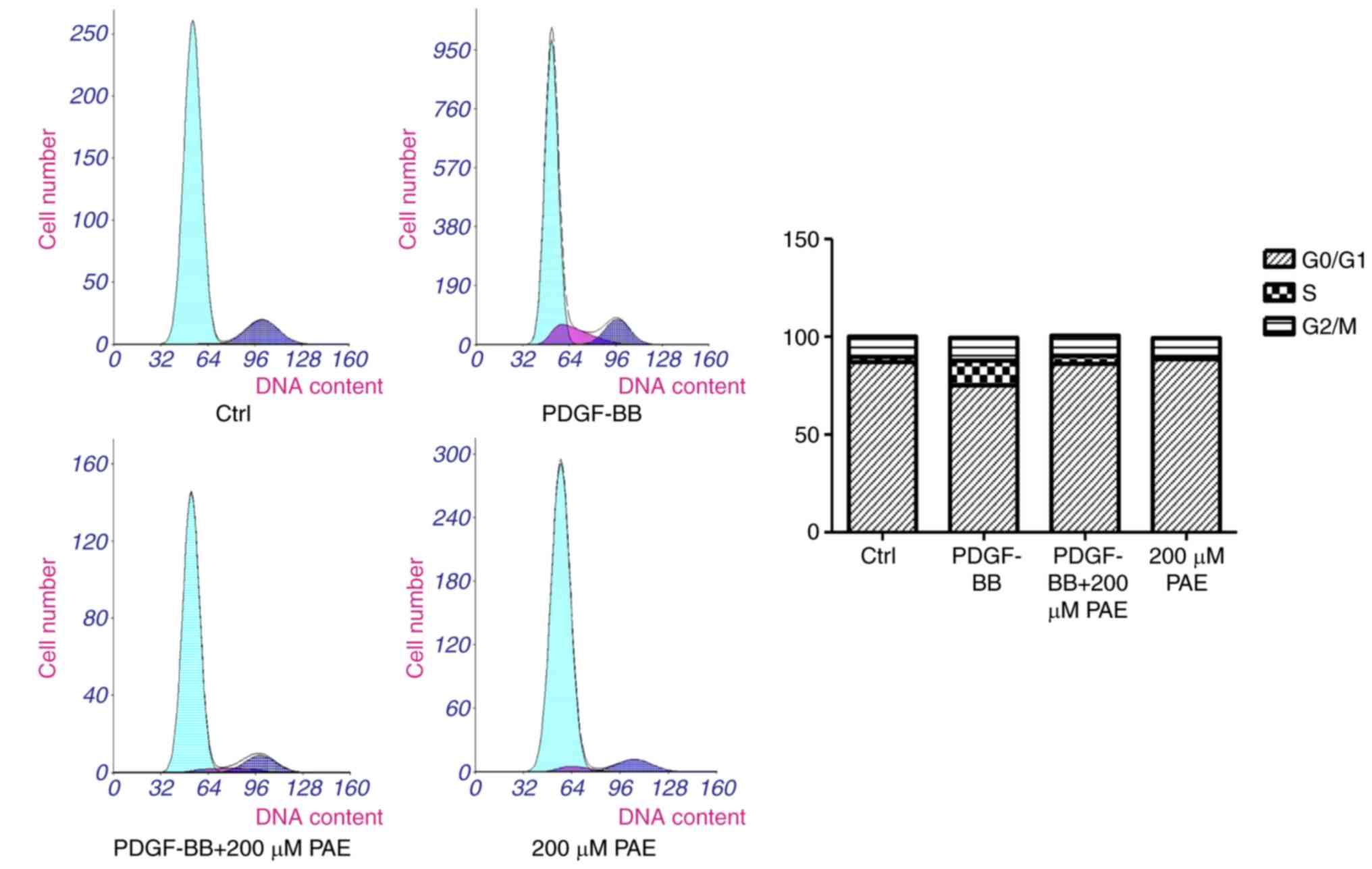
PAE blocks PDGF-BB-induced cell cycle
progression through arrest of G0/G1 to Sphasetransition
Flow cytometry analysis was used to analyze the
effect of PAE on cell cycle progression. PDGF-BB was demonstrated
to increase the percentage of cells in Sphase, where as the
populations of G0/G1 were decreased, compared with control
(Fig. 3). This condition was
reversed when the cells were treated with 200 µM PAE, there by
indicating that PAE suppressed cell cycle progression; in addition,
200 µM PAE increased the G0/G1 populations and depressed the
percentage of cells in Sphase compared with the PDGF-BB group.
These results therefore demonstrated that PAE affected the G0/G1 to
Sphase transition rather than being involved in the S or G2/M phase
(Fig. 3).
Effect of PAE on mRNA expression
levels of cell cycle regulatory and migration genes and cell
migration
To characterize the potential mechanism responsible
for PAE-induced cell cycle arrest and cell migration events,
RT-qPCR was used to examine expression of cyclins, CDKs, CDKN1A and
the cell cycle inhibitory expression and cell migration-related
protein, OPN. Compared with the control group, PDGF-BB treatment
significantly increased the expression levels of genes related to
cell cycle, including cyclin D1, cyclin E, CDK4 and CDK2 (Fig. 4A), and expression of OPN, which is
related to cell migration (Fig.
4B). However, mRNA expression of CDKN1A was down regulated
significantly compared with control (Fig. 4A). PAE down regulated
PDGF-BB-induced expression of CDK4, CDK2, cyclin D1, cyclin E and
OPN genes, but up regulated the expression of CDKN1A (Fig. 4A and B). Never the less, the use of
PAE alone had no significant effect on gene expression (Fig. 4A and B). The effect of PAE on cell
migration was also detected by scratch assay; cells stimulated with
PDGF-BB migrated faster than the basal medium-treated cells, and
PAE visibly inhibited cell migration (Fig. 4C).
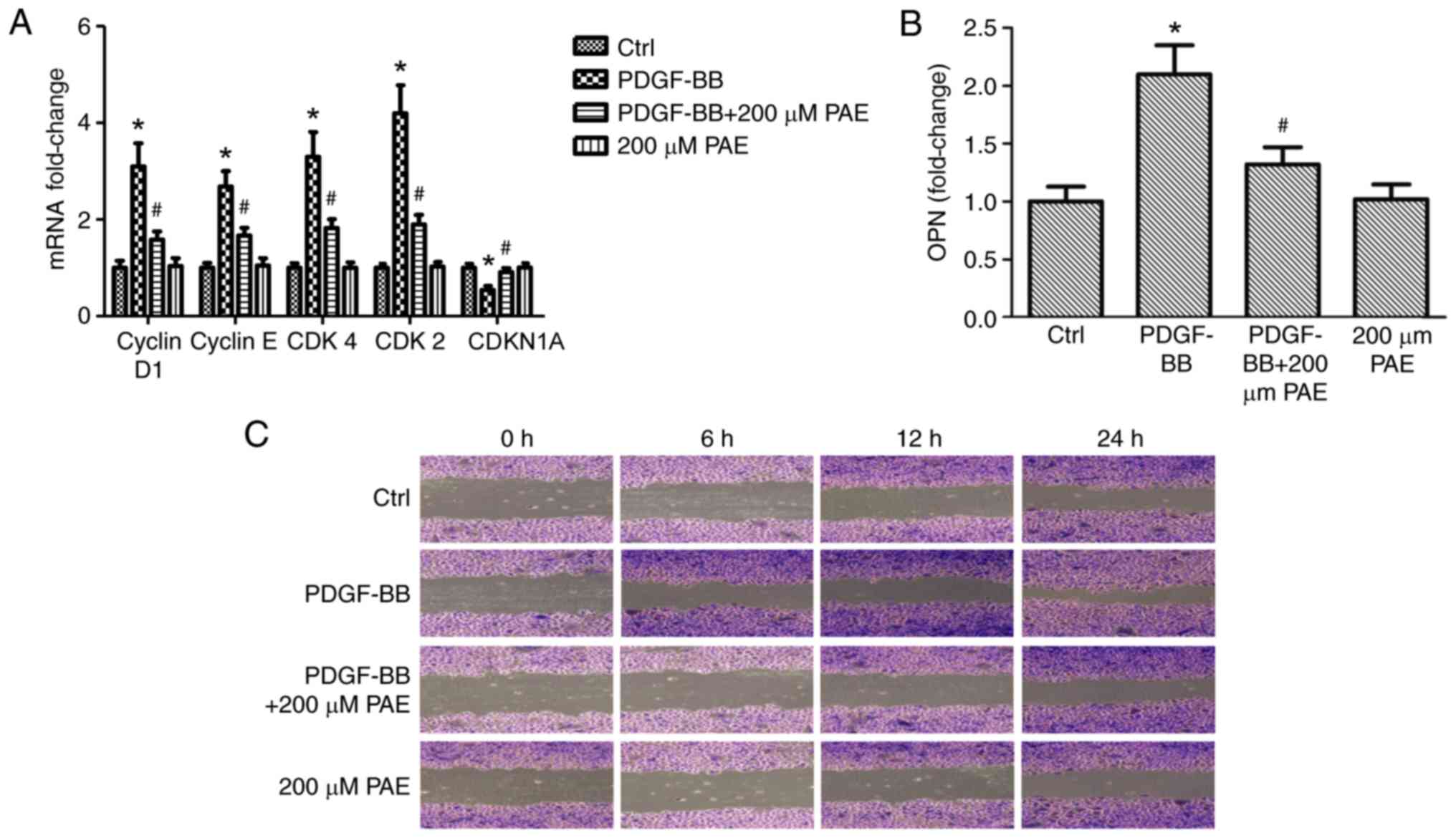 | Figure 4.Effect of PAE on the mRNA expression
levels of cell cycle regulatory and migration genes and cell
migration in VSMCs. VSMCs were grown with PAE (200 µM) in the
absence or presence of PDGF-BB (20 ng/ml) for 24 h, then mRNA
expression levels of (A) cyclin D1, cyclin E, CDK4, CDK2, CDKN1A
and (B) OPN were analyzed by reverse transcription-quantitative
polymerase chain reaction. Data are expressed as the mean +
standard deviation of 3 independent replicates. *P<0.05 vs.
control; #P<0.05 vs. PDGF-BB only. (C) In
vitro scratch migration assay, in which cells were treated with
200 µM PAE in the absence or presence of 20 ng/ml PDGF-BB and
imaged at 0, 6, 12 and 24 h. Each experiment was performed 3
independent times. PAE, paeoniflorin; VSMCs, vascular smooth muscle
cells; PDGF-BB, platelet derived growth factor-BB; CDK, cyclin
dependent kinase; CDKN1A, cyclin dependent kinase inhibitor 1A;
OPN, osteopontin. |
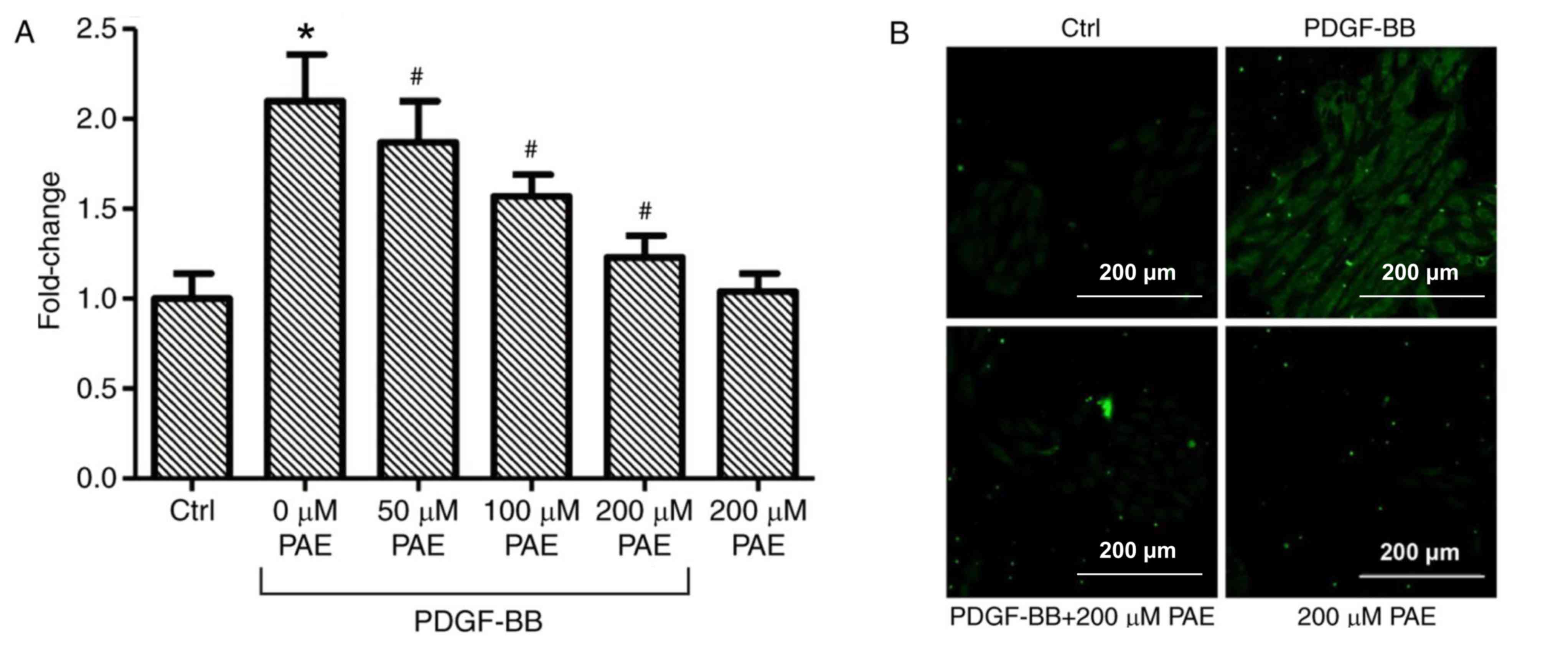
PAE decreases PDGF-BB-induced
production of ROS
VSMCs which were incubated with DCFH-DA demonstrated
increased fluorescence intensity when treated with PDGF-BB compared
with control, which indicated that PDGF-BB induced ROS production
(Fig. 5A and B). PAE treatment
significantly reduced PDGF-BB-induced ROS production in a
concentration-dependent manner compared with PDGF-BB only (Fig. 5A and B). Microscopic examination of
DCF-derived fluorescence, also revealed that that 200 µM PAE
visibly inhibited the accumulation of intra cellular ROS in
PDGF-BB-treated cells compared with PDGF-BB alone (Fig. 5B). In addition, no effect on ROS
generation was observed in cells treated with 200 µM PAE alone
compared with control cells (Fig. 5A
and B).
Molecular mechanisms involved in the
inhibition of VSMC proliferation by PAE
To explore the molecular mechanisms by which PAE
inhibited VSMC proliferation, the effects of PAE on MAPK signalling
pathway activation were examined. Significant activation of ERK1/2,
p38, and JNK was observed following 15 min of PDGF-BB treatment
compared with control, but the total levels of these molecules were
not affected (Fig. 6). Compared
with PDGF-BB only, PAE significantly reduced the phosphorylation of
p38 and ERK1/2 in a concentration-dependent manner, but exhibited
no inhibitory effect on the phosphorylation of JNK (Fig. 6).
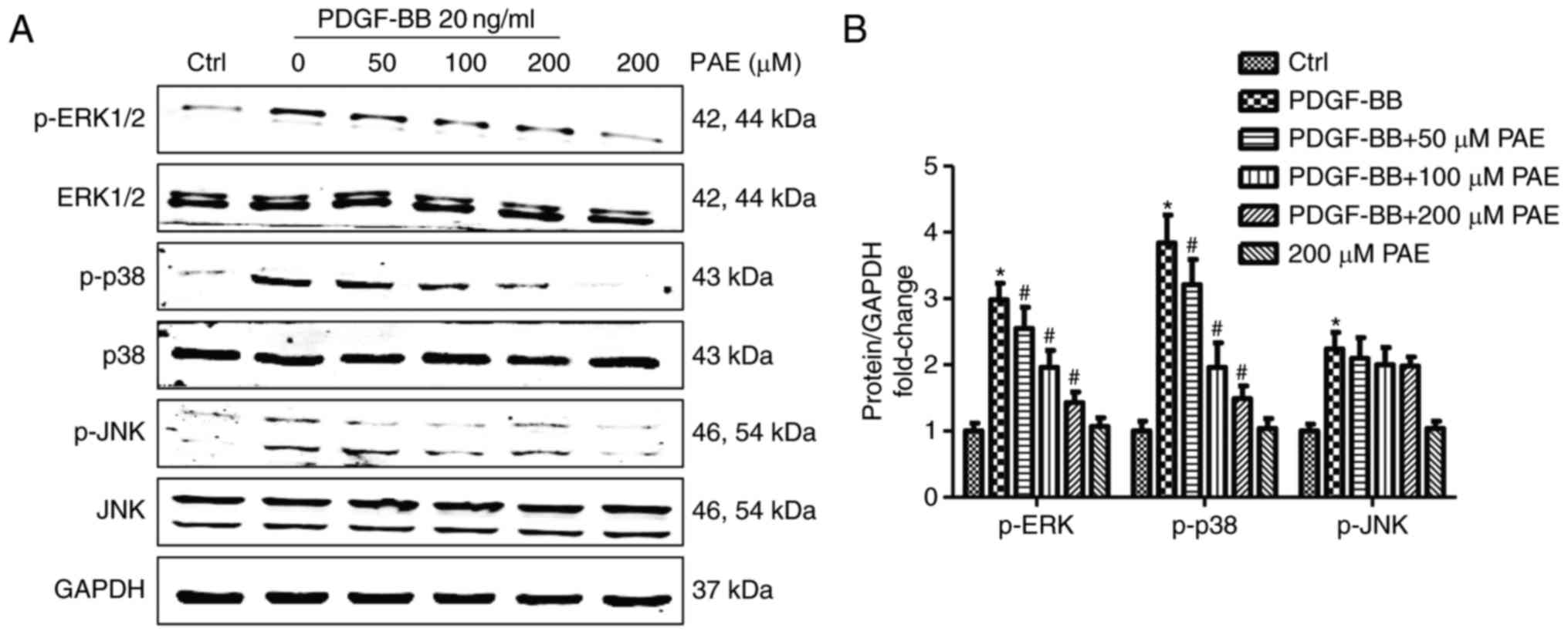 | Figure 6.Effect of PAE on the activation of
mitogen-activated protein kinase signalling pathways in
PDGF-BB-induced VSMCs. VSMCs were pre treated with PAE (50, 100,
200 µM) for 1 h prior to 20 ng/ml PDGF-BB treatment. The
phosphorylation of p38, ERK, JNK induced by 15 min of PDGF-BB
treatments was analyzed by western blotting analysis. (A)
Representative western blots and (B) quantitative analysis. Data
are expressed as the mean + standard deviation of ≥3 independent
replicates. *P<0.05 vs. control; #P<0.05 vs.
PDGF-BB only. PAE, paeoniflorin; PDGF-BB, platelet derived growth
factor-BB; VSMCs, vascular smooth muscle cells; p-, phosphorylated;
ERK1/2, extracellular signal-related protein kinases 1 and 2; JNK,
c-Jun N-terminal kinase. |
Discussion
In the present study, VSMC proliferation and
migration was demonstrated to be enhanced by PDGF-BB compared with
control, and inhibited by PAE compared with PDGF-BB alone in a
concentration-dependent manner, without cell cytotoxicity. PAE
suppressed the cell cycle at the G0/G1 to Sphase by inhibiting them
RNA expression of cyclin D1, cyclin E, CDK4, and CDK2; this
glycoside also increased them RNA expression of CDKN1A in
PDGF-BB-stimulated VSMCs. PDGF-BB-induced expression of OPN was
also prevented by PAE supplementation. These beneficial effects of
PAE on the proliferation of VSMCs were associated with the
inhibition of ROS production, and reduced activation of the ERK1/2
and p38 signaling pathways.
PAE inhibits the proliferation of lung cancer cells
(14,15), HeLa human cervical cancer cells
(16) and human colorectal
carcinoma (HT29) cells by blocking cell cycle progression in the
G0/G1 or Sphase and inducing apoptosis. However, the effect of PAE
on PDGF-BB-induced proliferation of VSMCs remains unclear. The
present study demonstrated for the first time, to the best of our
knowledge, that PAE inhibited PDGF-BB-induced proliferation of
VSMCs. According to previous research, VSMCs change from a
contractile phenotype to proliferative phenotype in the presence of
PDGF-BB (17). Never the less, the
combined use of PAE and PDGF-BB would inhibit such a change in
phenotype. The results of CCK-8 and BrdU assays indicated that PAE
inhibited PDGF-BB-induced cell proliferation. Flow cytometry
demonstrated that PAE arrested the cells in transition from G0/G1
phase to Sphase. Expression of genes that are involved in the check
points of the transition from G0/G1 phase to Sphase were also
examined: PDGF-BB considerably upregulated them RNA expression
levels of cyclin D1, cyclin E, CDK4 and CDK2, but down regulated
them RNA expression of CDKN1A. The combined use of PAE and PDGF-BB
reversed the changes in mRNA expression levels of these genes. Thus
PAE antagonized PDGF-BB-induced cell proliferation by regulating
the cell cycle-related proteins.
In atherosclerosis and restenosis following
percutaneous coronary intervention, the migration of VSMCs is an
important step in intimal formation (18). OPN is the marker gene for the
change of VSMCs from a contractile to proliferative phenotype. The
expression of OPN is closely associated with the migration ability
of VSMCs (19,20). The present study demonstrated that
following stimulation with PDGF-BB for 48 h, OPN mRNA expression
increased, which corresponded to strong cell migration ability.
However, the combined use with PAE reversed this phenomenon.
ROS are important for the proliferation and
migration of VSMCs. Previous cell experiments indicated that a
large amount of ROS are conducive to the proliferation of VSMCs and
to intimal hyperplasia (21). In
the present study, PAE was demonstrated to significantly inhibit
PDGF-BB-induced generation of ROS, there by indicating that the
inhibitory effect of PAE on the proliferation of VSMCs may be
partially related to the inhibition of ROS generation. Further
more, the activation levels of ROS-induced down stream MAPK
signalling pathways were examined. MAPK signalling consists of a
sequence of successively functioning kinases that ultimately result
in the dual phosphorylation and activation of p38, JNK1/2 and
ERK1/2. MAPK signalling pathways are be activated during
PDGF-BB-induced proliferation of VSMCs (4,5). ERK
and p38 MAPK signalling pathways have important regulatory
functions in vascular reconstruction (22). According to the results of the
present study, PAE significantly inhibited PDGF-BB-induced
activation of ERK and p38 MAPK signalling pathways, but had little
impact on JNK signaling. Therefore, PAE was found to inhibit
PDGF-BB-induced proliferation and migration of VSMCs by inhibiting
the ERK and p38 MAPK signalling pathways.
In summary, to the best of our knowledge, the
present study observed for the first time that PAE inhibited
PDGF-BB-induced proliferation and migration of VSMCs. PAE may
regulate cell cycle and expression level of cell migration-related
proteins by inhibiting ROS-mediated ERK1/2 and p38 signaling
pathways. PAE may also serve as a potential drug against
atherosclerosis and restenosis following percutaneous coronary
intervention. However, the findings of the present experiment
should be confirmed by experiments in large animals.
Glossary
Abbreviations
Abbreviations:
|
CCK-8
|
Cell Counting Kit-8
|
|
CDK
|
cyclin-dependent kinase
|
|
CDKN1A
|
cyclin-dependent kinase inhibitor
1A
|
|
ERK
|
extra cellular signal-regulated
kinase
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FBS
|
fetal bovine serum
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
OPN
|
osteopontin
|
|
PAE
|
paeoniflorin
|
|
PDGF-BB
|
platelet derived growth factor-BB
|
|
ROS
|
reactive oxygen species
|
|
VSMCs
|
vascular smooth muscle cells
|
References
|
1
|
Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun
C, Wang Y and Mehta JL: Hemodynamic shear stress via ROS modulates
PCSK9 expression in human vascular endothelial and smooth muscle
cells and along the mouse aorta. Antioxid Redox Signal. 22:760–771.
2015. View Article : Google Scholar :
|
|
2
|
Sadowitz B, Seymour K, Gahtan V and Maier
KG: The role of hyaluronic acid in atherosclerosis and intimal
hyperplasia. J Surg Res. 173:e63–e72. 2012. View Article : Google Scholar
|
|
3
|
Rivard A and Andres V: Vascular smooth
muscle cell proliferation in the pathogenesis of atherosclerotic
cardio vascular diseases. Histol Histopathol. 15:557–571. 2000.
|
|
4
|
Chang Y, Uen YH, Chen CC, Lin SC, Tseng
SY, Wang YH, Sheu JR and Hsieh CY: Platonin inhibited
PDGF-BB-induced proliferation of rat vascular smooth muscle cells
via JNK1/2-dependent signaling. Acta Pharmacol Sin. 32:1337–1344.
2011. View Article : Google Scholar :
|
|
5
|
Wang Y, Wang Y, Liu D, Wang W, Zhao H,
Wang M and Yin H: Cordyceps sinensis polysaccharide inhibits
PDGF-BB-induced inflammation and ROS production in human mesangial
cells. Carbohydr Polym. 125:135–145. 2015. View Article : Google Scholar
|
|
6
|
Lee CK, Lee HM, Kim HJ, Park HJ, Won KJ,
Roh HY, Choi WS, Jeon BH, Park TK and Kim B: Syk contributes to
PDGF-BB-mediated migration of rat aortic smooth muscle cells via
MAPK pathways. Cardiovasc Res. 74:159–168. 2007. View Article : Google Scholar
|
|
7
|
Lee KP, Lee K, Park WH, Kim H and Hong H:
Piperine inhibits platelet-derived growth factor-BB-induced
proliferation and migration in vascular smooth muscle cells. J Med
Food. 18:208–215. 2015. View Article : Google Scholar
|
|
8
|
Zhou H, Yang HX, Yuan Y, Deng W, Zhang JY,
Bian ZY, Zong J, Dai J and Tang QZ: Paeoniflorin attenuates
pressure overload-induced cardiac remodeling via inhibition of
TGFβ/Smads and NF-κB pathways. J Mol Histol. 44:357–367. 2013.
View Article : Google Scholar
|
|
9
|
Chen C, Du P and Wang J: Paeoniflorin
ameliorates acute myocardial infarction of rats by inhibiting
inflammation and inducible nitric oxide synthase signaling
pathways. Mol Med Rep. 12:3937–3943. 2015. View Article : Google Scholar
|
|
10
|
Cao W, Zhang W, Liu J, Wang Y, Peng X, Lu
D, Qi R, Wang Y and Wang H: Paeoniflorin improves survival in
LPS-challenged mice through the suppression of TNF-alpha and IL-1
beta release and augmentation of IL-10 production. Int
Immunopharmacol. 11:172–178. 2011. View Article : Google Scholar
|
|
11
|
Nizamutdinova IT, Jin YC, Kim JS, Yean MH,
Kang SS, Kim YS, Lee JH, Seo HG, Kim HJ and Chang KC: Paeonol and
paeoniflorin, the main active principles of Paeonia
albiflora, protect the heart from myocardial
ischemia/reperfusion injury in rats. Planta Med. 74:14–18. 2008.
View Article : Google Scholar
|
|
12
|
Chen C, Tang Y, Deng W, Huang C and Wu T:
Salidroside blocks the proliferation of pulmonary artery smooth
muscle cells induced by platelet-deried growth factor-BB. Mol Med
Rep. 10:917–922. 2014. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Antiproliferative activity of paeoniflorin is through
cell cycle arrest and the Fas/Faslig and-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar
|
|
15
|
Wu Q, Chen GL, Li YJ, Chen Y and Lin FZ:
Paeoniflorin inhibits macrophage-mediated lung cancer metastasis.
Chin J Nat Med. 13:925–932. 2015.
|
|
16
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar
|
|
17
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar
|
|
18
|
Karki R, Kim SB and Kim DW: Magnolol
inhibits migration of vascular smooth muscle cells via cytoskeletal
remodeling pathway to attenuate neointima formation. Exp Cell Res.
319:3238–3250. 2013. View Article : Google Scholar
|
|
19
|
Jiang H, Lun Y, Wu X, Xia Q, Zhang X, Xin
S and Zhang J: Association between the hypomethylation of
osteopontin and integrin β3 promoters and vascular smooth muscle
cell phenotype switching in great saphenous varicose veins. Int J
Mol Sci. 15:18747–18761. 2014. View Article : Google Scholar :
|
|
20
|
Chen JJ, Zhang J, Cai Y, Zhou YB, Wen GB,
Tang CS, Qi YF and Jiang ZS: C-type natriuretic peptide inhibiting
vascular calcification might involve decreasing bone morphogenic
protein 2 and osteopontin levels. Mol Cell Biochem. 392:65–76.
2014. View Article : Google Scholar
|
|
21
|
Rodrigues E, Mariutti LR and Mercadante
AZ: Scavenging capacity of marine carotenoids against reactive
oxygen and nitrogen species in a membrane-mimicking system. Mar
Drugs. 10:1784–1798. 2012. View Article : Google Scholar :
|
|
22
|
Muslin AJ: MAPK signalling in cardio
vascular health and disease: Molecular mechanisms and therapeutic
targets. Clin Sci (Lond). 115:203–218. 2008. View Article : Google Scholar :
|