Introduction
α-Naphthylisothiocyanate (ANIT) as a well known
hepatotoxicant is usually administered to experimental animals to
mimick the drug-induced cholestasis with liver injury in humans
(1). It has been demonstrated that
ANIT produced a cholangiolitic hepatitis characterized by
intrahepatic cholestasis, hepatocellular and biliary epithelial
cell necrosis, and bile duct obstruction (1–4).
Cholestasis is always tightly associated with a wide range of
diseases including obstructive jaundice (5), acute hepatitis (6), cystic fibrosis (7), primary sclerosing cholangitis (PSC)
(8), and primary biliary cirrhosis
(PBC) (9). However, up to date,
there are very few effective therapies for drug-induced cholestasis
except ursodeoxycholic acid which is the only approved drug by Food
and Drug Administration (10).
Farnesoid X receptor (FXR) as a nuclear receptor for
bile acids (BAs) plays a key role in regulation of BA levels in
enterohepatic circulation (11–13).
It is expressed in several tissues, including liver, intestine,
adipose tissue, the vascular wall, pancreas, and kidney (14). In the liver, BAs bind to FXR, which
transcriptionally upregulates an atypical orphan nuclear receptor
small heterodimer partner (Shp; NR0B2) to inhibit
trans-activation activity of hepatic nuclear factor 4α
(HNF4α) and liver receptor homologue-1 (LRH-1; NR5A2) that bind to
the BA response element in the cholesterol 7α hydroxylase (Cyp7a1)
and sterol 12α-hydroxylase (Cyp8b1) gene promoters (15). In the intestine, BAs binds to FXR
to induces the secretion of fibroblast growth factor 15 (Fgf15)
(Fgf19 in humans), which activates FGF receptor 4 (FGFR4) and its
downstream intracellular signaling pathways on the hepatocytes,
such as extracellular signal-regulated kinase (ERK), protein kinase
Cz (PKCz), and c-Jun N-terminal kinase (JNK) to repress gene
transcription of Cyp7a1 (16,17).
FXR as a BA sensor may be an effective strategy to treat
cholestasis.
Several natural products activate FXR and are thus
the potential therapeutic agents for cholestasis. It has been
reported that the natural product, such as Alisol B 23-acetate and
geniposidic acid exerted the protective effects of ANIT-induced
cholestasis with liver injury through regulation of FXR pathway
(18,19). In the present study, we identified
that resveratrol, an important ingredient from grape skins and
Chinese medicine Polygonum cuspidatum, significantly
activated FXR signaling by screening a library of natural products.
Then we evaluated whether resveratrol played a role in protection
of ANIT-induced cholestasis with liver injury and explored its
underlying molecular mechanism. Here, we reported that resveratrol
may rescue ANIT-induced cholestasis through the regulation of FXR
pathway.
Materials and methods
Reagents and plasmids
6α-Ethylchenodeoxycholic acid (6ECDCA) and
lipopolysaccharides (LPS) were both purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). The HEK293 cell line, phFXR and
phRXR expression vectors, FXR dependent reporter (EcRE-Luc), the
p65 expression vector, the phRL-TK vector, and the NF-κB dependent
reporter plasmid were kindly provided by Dr. Wendong Huang (City of
Hope, Duarte, CA, USA).
Transfection and and reporter gene
assays
HEK293 cells were grown in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% FBS were cotransfected
with EcRE-Luc, phFXR, phRXR and β-galactosidase at the ratio of
10:2.5:2.5:1 by the Lipofectamine 2000 procedure (Invitrogen Life
Technologies, Carlsbad, CA, USA). HepG2 cells were co-transfected
with the NF-κB reporter plasmids, or control plasmid phRL-TK,
β-galactosidase and FXR/RXR plasmids at 1:1 ratios with the p65
expression plasmid by the Lipofectamine 2000 procedure (Invitrogen
Life Technologies). After 24 h, cells were treated with resveratrol
(10 µm) or 6ECDCA (3 µm) for 18 h. Then HepG2 cells were treated
with/without LPS (2 µg/ml). Following 6 h incubation, cells were
harvested and the luciferase activity was determined by using a
luciferase reporter assay system in accordance with the
manufacturer's instructions (Promega, Madison, WI, USA). The
luciferase activity was normalized by β-galactosidase activity.
Each transfection was performed in triplicate and repeated at least
three times.
Cell viability assay
HEK293 cells were seeded in a 96-well plate at a
density of 1×104 cells per well. When confluent, the
cells were incubated individually with resveratrol
(C14H12O3, MW 228.24, HPLC ≥98%)
obtained from Shanghai R&D Center for Standardization of
Traditional Chinese Medicine (Shanghai, China) at the concentration
of 0.1, 1, 3, 10 and 30 µm, respectively. After incubation for 24
h, 10 µl of CCK-8 solution for each well was used to test the cell
viability. The absorbance of the solutions was detected at 450 nm
by a microplate reader (Varioskan Flash; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The cell viability rate was calculated as
the percentage of CCK-8 absorption as follows: (absorbance of drug
– treated sample/absorbance of control sample) × 100%.
Primary mouse hepatocyte culture
Primary hepatocytes from 8-week-old wild-type (WT)
and FXR knockout (KO) mice were isolated by two-step perfusion
using liver perfusion and liver digest Media (collagenase type I
0.8 mg/ml; Worthington Biochemical Corporation, Lakewood, NJ, USA)
as previously described (20).
Purity of live hepatocytes was routinely ≥90% by trypan blue
exclusion. Hepatocytes were cultured in 10% fetal bovine serum in
DMEM supplemented with L-glutamine, antibiotics,
insulin-transferrin-selenium, and 4-(2-hydroxyethyl)-1-piperazine
ethanesulfonic acid (HEPES) (Mediatech, Manassas, VA, USA). Cells
were treated with 6ECDCA (3 µm) and resveratrol (10 µm). Eighteen
hours after treatment, the cells were treated with LPS (2 µg/ml),
and then collected for RNA isolation after a 6 h incubation.
Animal model and experimental
design
WT C57/BL mice were purchased from the Laboratory
Animal Center of Shanghai University of Traditional Chinese
Medicine. FXR KO mice were transferred from UC Davis Medical Center
(Sacramento, CA, USA) and reproduced in SHUTCM animal room. The
mice were housed at 20±2°C with relative humidity at 60–70%. The
animal welfare strictly complied with the Guide for the Care and
Use of Laboratory Animals, and the instructions for the Animal
Experiments were approved by the Institutional Animal Committee of
Shanghai University of Traditional Chinese Medicine [permit no.
SCXK (Hu) 2012–0002]. WT and KO mice were randomly assigned into
four groups (control, ANIT, resveratrol+ANIT and 6ECDCA+ANIT),
respectively. Resveratrol+ANIT group and 6ECDCA+ANIT group mice
were treated with resveratrol (60 mg/kg), or 6ECDCA (20 mg/kg)
respectively for 5 days while control and ANIT (Sigma-Aldrich)
group mice were given normal saline. 4 h after the treatment of
resveratrol or 6ECDCA at the 3rd day, ANIT (dissolved in oil, 60
mg/kg) were administered intragastrically to each group mice except
the control group mice which were administered with olive oil
intragastrically. 48 h after ANIT administration, mice were
anesthetized with isoflurane and blood samples were collected. Then
mice were euthanized by CO2. Livers and ileums were
frozen in liquid nitrogen immediately after collection and stored
in −80°C freezer for further assays.
Biochemical assays and histological
analysis
The levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), alkaline phosphatase (ALP), total
bilirubin (TBIL), direct bilirubin (DBIL) and total bile acid (TBA)
were measured using an automated biochemistry analyzer (2700;
Olympus, Tokyo, Japan). Liver tissues were fixed in 10%
formaldehyde and then paraffin-embedded for hematoxylin and eosin
(H&E) staining.
RNA isolation and quantitative
real-time polymerase chain reaction
HepG2 or primary mouse hepatocytes were seeded into
6-well plates (1×106 cells/well) overnight prior to
treatment. Then the cells were treated with 6ECDCA (3 µm) and
resveratrol (10 µm). Eighteen hours after treatment, the cells were
treated with LPS (2 µg/ml) and then collected for RNA isolation
after a 6 h incubation.
Total RNA from HepG2 cells, primary mouse
hepatocytes, and mouse livers or ileums were extracted by using
spin columns (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. RNA concentrations were equalized and
converted to cDNA using an A3500 reverse transcription system
(Promega). Gene expressions were measured by qRT-PCR (ABI 7500,
Applied Biosystems, Warrington, UK), via SYBR-Green methodology.
The primers used in the experiments are listed in Tables I and II. 36B4 or β-actin was used as an
internal control to normalize all the mRNA levels.
 | Table I.The primers of Mus musculus
for RT-PCR. |
Table I.
The primers of Mus musculus
for RT-PCR.
| Genes | Sequence of forward
and reverse primers (5′-3′) | GeneBank accession
no. |
|---|
| Shp |
TCTGCAGGTCGTCCGACTATTC | NM_011850 |
|
|
AGGCAGTGGCTGTGAGATGC |
|
| Cyp7a1 |
GACGAATTCATGATGAGCATTTCTTTGATC | NM_007824 |
|
|
CATGCGGCCGCCCTCTTCTTCCAACCACATAT |
|
| Cyp27a1 |
CCAGGCACAGGAGAGTACG | NM_024264 |
|
|
GGGCAAGTGCAGCACATAG |
|
| Cyp8b1 |
CCTCTGGACAAGGGTTTTGTG | NM_010012 |
|
|
GCACCGTGAAGACATCCCC |
|
| Fgf15 |
GCCATCAAGGACGTCAGCA | NM_008003 |
|
|
CTTCCTCCGAGTAGCGAATCAG |
|
| Bsep |
GGATGGTTTGACTGCACTTCTG | NM_021022 |
|
|
AGAGGACTGACAGCGAGAATCA |
|
| I-Babp |
CTTCCAGGAGACGTGATTGAAA | NM_008375 |
|
|
CCTCCGAAGTCTGGTGATAGTTG |
|
| Mrp2 |
CCAGTGCACGGTCATCACTATC | NM_013806 |
|
|
GGACCCATATTGGACAGCAGTT |
|
| Mrp3 |
ACCATCCGTACCCAGTTTGAAC | NM_029600 |
|
|
GGCCATCCCATAGAAGATGC |
|
| Ostβ |
AGATGCGGCTCCTTGGAATTA | NM_178933 |
|
|
TGGCTGCTTCTTTCGATTTCTG |
|
| iNOS |
GGAGTGACGGCAAACATGACT | NM_010927 |
|
|
TCGATGCACAACTGGGTGAAC |
|
| COX-2 |
TGCACTATGGTTACAAAAGCTGG | NM_011198 |
|
|
TCAGGAAGCTCCTTATTTCCCTT |
|
| TNF-α |
CGCCCTTCCAGAACTCCAGGCG | NM_013693 |
|
|
TGCTACGACGTGGGCTACAG |
|
| IL-6 |
CAAAGCCAGAGTCCTTCAGAG | NM_031168 |
|
|
TGGTCCTTAGCCACTCCTTC |
|
| IL-1β |
GCAACTGTTCCTGAACTCAACT | NM_008361 |
|
|
ATCTTTTGGGGTCCGTCAACT |
|
| iCAM-1 |
TGCCTCTGAAGCTCGGATATAC | NM_010493 |
|
|
TCTGTCGAACTCCTCAGTCAC |
|
| MCP-1 |
TTAAAAACCTGGATCGGAACCAA | NM_011333 |
|
|
GCATTAGCTTCAGATTTACGGGT |
|
| 36B4 |
GCCCTGCACTCTCGCTTTCT | NM_007475 |
|
|
CAACTGGGCACCGAGGCAACAGTTG |
|
 | Table II.The primers of Homo sapiens
for RT-PCR. |
Table II.
The primers of Homo sapiens
for RT-PCR.
| Genes | Sequence of forward
and reverse primers (5′-3′) | GeneBank accession
no. |
|---|
| Shp |
GTGCCCAGCATACTCAAGAAG | NM_021969 |
|
|
TGGGGTCTGTCTGGCAGTT |
|
| Cyp7a1 |
GAGAAGGCAAACGGGTGAAC | NM_000780 |
|
|
GGATTGGCACCAAATTGCAGA |
|
| Cyp27a1 |
CAGCACGACCTGACCTATGG | NM_000784 |
|
|
TGGTCCAGTCGAGTCATAAAGT |
|
| Cyp8b1 |
CTTGTTCGGCTACACGAAGGA | NM_004391 |
|
|
GCAGGGAGTAGACAAACCTTG |
|
| iNOS |
AGGGACAAGCCTACCCCTC | NM_000625 |
|
|
CTCATCTCCCGTCAGTTGGT |
|
| COX-2 |
ATGCTGACTATGGCTACAAAAGC | NM_000963 |
|
|
TCGGGCAATCATCAGGCAC |
|
| TNF-α |
GAGGCCAAGCCCTGGTATG | NM_000594 |
|
|
CGGGCCGATTGATCTCAGC |
|
| IL-6 |
CCTGAACCTTCCAAAGATGGC | NM_000600 |
|
|
TTCACCAGGCAAGTCTCCTCA |
|
| β-actin |
CATGTACGTTGCTATCCAGGC | NM_001101 |
|
|
CTCCTTAATGTCACGCACGAT |
|
Statistical analysis
Results are expressed as mean ± standard deviation
(SD). Statistical analyses were performed using SPSS 9.0 (SPSS,
Inc., Chicago, IL, USA). Group differences were assessed by
Student's t-test or one-way analysis of variance with Dunnett's
post-test. P<0.05, P<0.01 was considered to indicate a
statistically significant difference.
Results
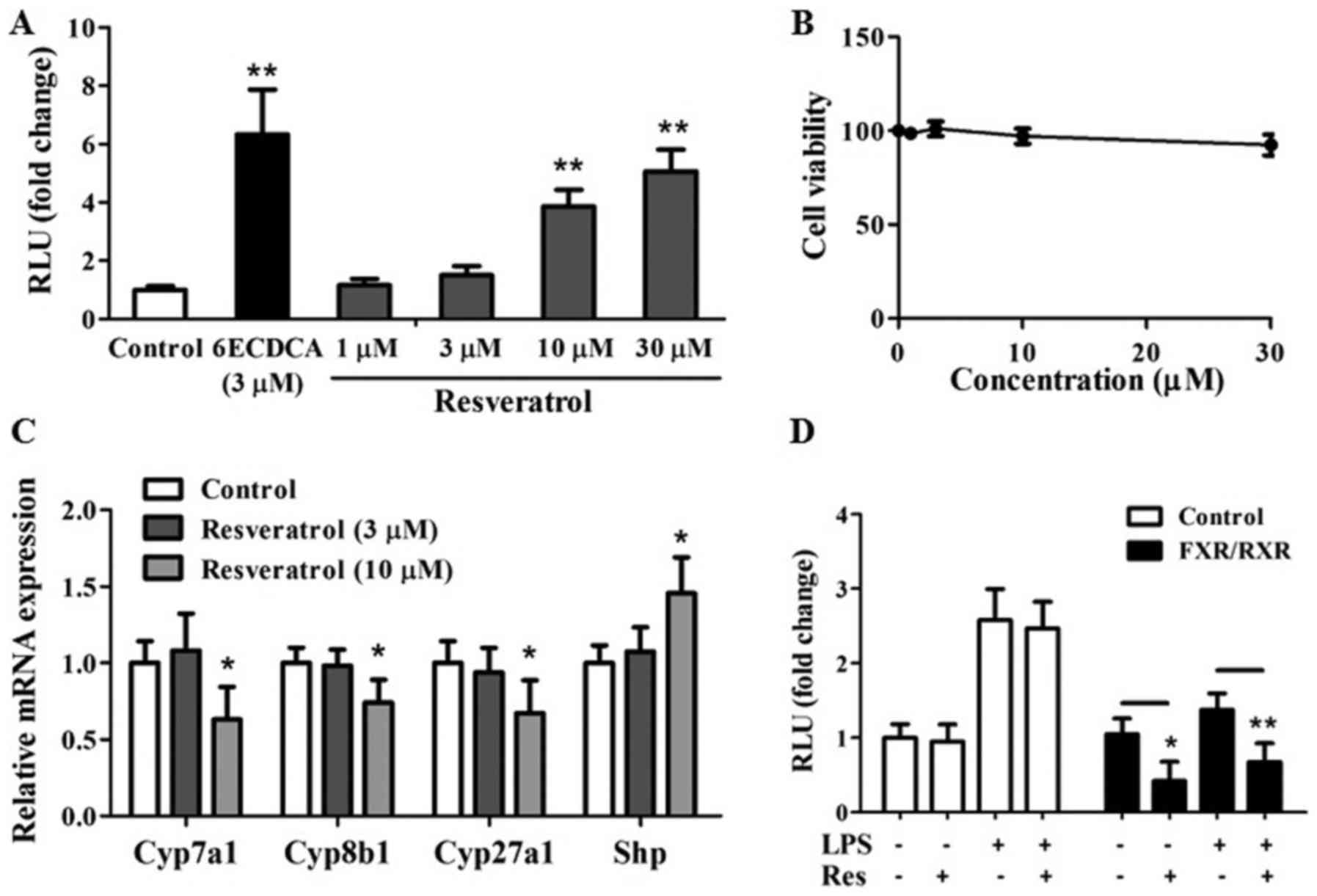
Resveratrol as a FXR agonist
antagonizes the NF-κB transactivity
Resveratrol promotes transcriptional activation of
human FXR in a cell-based co-transfection assay with a dose
dependent manner (Fig. 1A), cell
viability results did not show significant cytotoxicity of
resveratrol on the growth of transit transfected HEK293 cells at a
concentration of 1–30 µm (Fig.
1B). Consistent with FXR transactivation results, resveratrol
at a concentration of 10 µm remarkably downregulated mRNA
expressions of FXR target genes including BA synthesis genes
[Cyp7a1, Cyp8b1, and sterol 27-hydroxylase (Cyp27a1)] (21) and upregulated of Shp which is an
atypical orphan nuclear receptor and directly influenced by FXR
(Fig. 1C) (21) in primary hepatocytes. Collectively,
it is apparent that resveratrol, can activate FXR in
vitro.
We next tested whether resveratrol as a FXR agonist
inhibited NF-κB activity at the level of gene transcription. HepG2
cells were co-transfected with NF-κB reporter plasmid and control
plasmid phRL-TK. Then we assessed the effects of resveratrol on the
regulation of NF-κB reporter activity. The treatment with LPS as a
known NF-κB pathway activator increased NF-κB reporter activity
with 2.5-folds (Fig. 1D). It is
worth noting that NF-κB activity induced by LPS was inhibited by
resveratrol treatment (Fig. 1D).
Transfection of these cells with FXR/RXR inhibited NF-κB activity
in the absence of ligand (Fig.
1D), revealing that FXR may repress NF-κB activity without
addition of exogenous ligand due to the fact that the synthesized
BA in HepG2 cells may induce the transcription activity of FXR as
previous report (22). However,
addition of resveratrol further enhanced the repression of NF-κB
activity (Fig. 1D), suggesting
that resveratrol induced repression of NF-κB activity may through
FXR pathway.
Resveratrol suppressed the LPS-induced
elevation of inflammatory genes in primary hepatocytes and HepG2
cell line
To investigate whether activation of FXR by
resveratrol has effects on the NF-κB pathway, we tested the
influence of resveratrol as a FXR agonist on the expressions of
tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2),
inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) in
HepG2 cells. Cells that were pretreated with the FXR agonists
6ECDCA and resveratrol showed greatly less LPS-induced mRNA
expression of IL-6 than did non-pretreated cells (Fig. 2A). A similar inhibition of
expressions of COX-2, iNOS and TNF-α by 6ECDCA and resveratrol were
observed in response to stimulation with LPS (Fig. 2B-D).
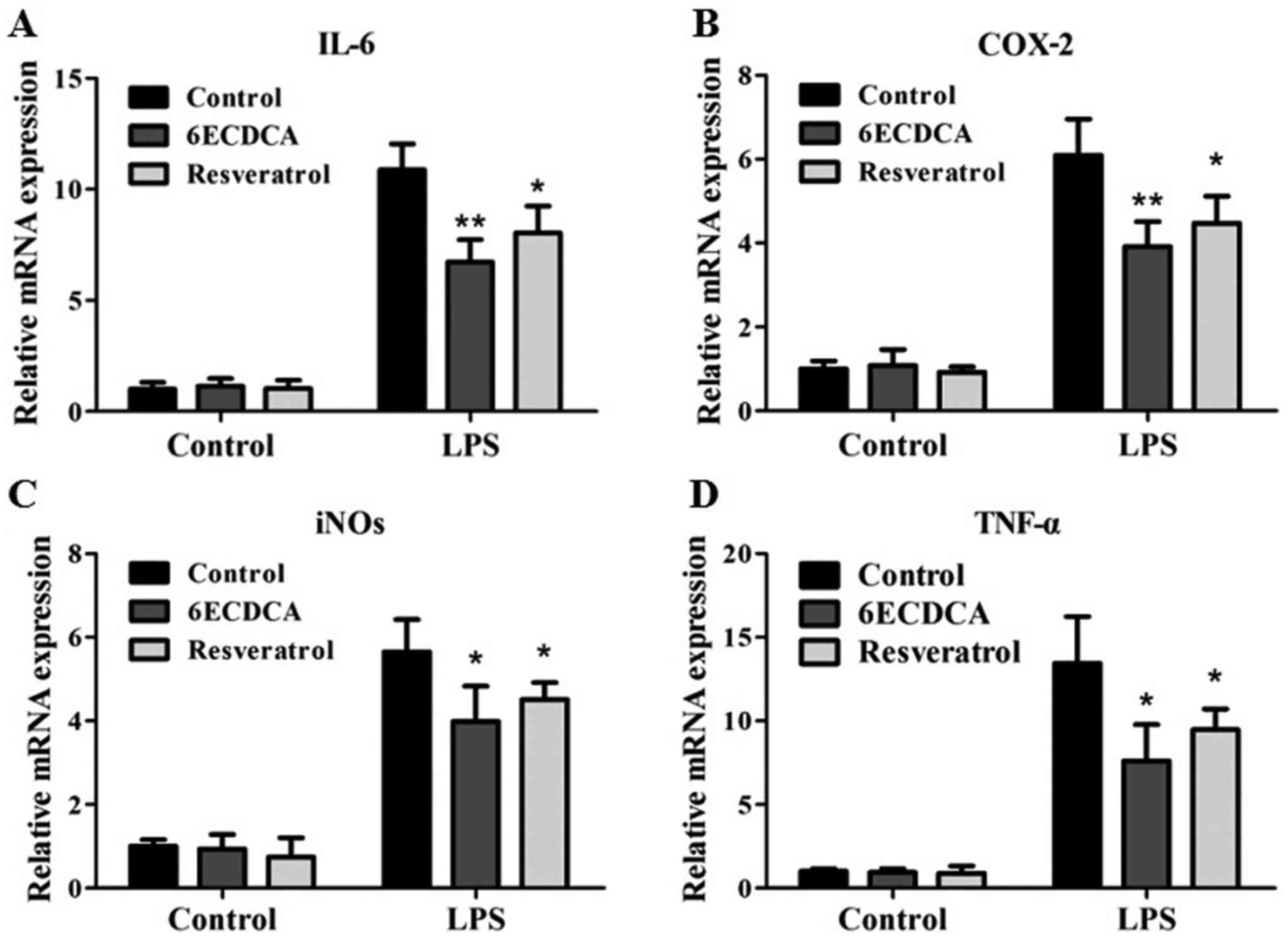 | Figure 2.Resveratrol suppresses the
inflammatory related genes induced by LPS in HepG2 cells.
Quantitative real-time PCR analysis of the levels of inflammatory
related genes (A) IL-6, (B) COX-2, (C) iNOs, (D) TNF-α in HepG2
cells that were pretreated with vehicle (DMSO), 6ECDCA (3 µm) or
resveratrol (10 µm) for 18 h before treatment with LPS (2 µg/ml)
for 6 h. Each bar represents the mean ± SD of triplicates of three
independent experiments. Statistical analyses were done with the
Student's t-test. *P<0.05, **P<0.01 vs. LPS alone
treatment groups. LPS, lipopolysaccharide; IL-6, interleukin-6;
COX-2, cyclooxygenase-2; iNOs, inducible nitric oxide synthase;
TNF-α, tumor necrosis factor-α. |
To confirm that these effects of resveratrol were
mediated by FXR, we also tested the influence of 6ECDCA and
resveratrol on the expressions of inflammatory related genes in
response to NF-κB activation in primary hepatocytes from WT and KO
mice. Inhibition of LPS-induced expressions of IL-6, iNOS and TNF-α
by the FXR agonists 6ECDCA and resveratrol were preserved in
primary hepatocytes from WT mice, but were abolished in primary
hepatocytes from KO mice (Fig.
3A-C). 6ECDCA and resveratrol also repressed the LPS-induced
expression of the NF-κB target gene monocyte chemoattractant
protein-1 (MCP-1) in hepatocytes from WT mice, but not in
hepatocytes from KO mice (Fig.
3D). Therefore, our results indicate that resveratrol represses
the expressions of NF-κB-regulated genes in HepG2 cells and mouse
primary hepatocytes through FXR activation.
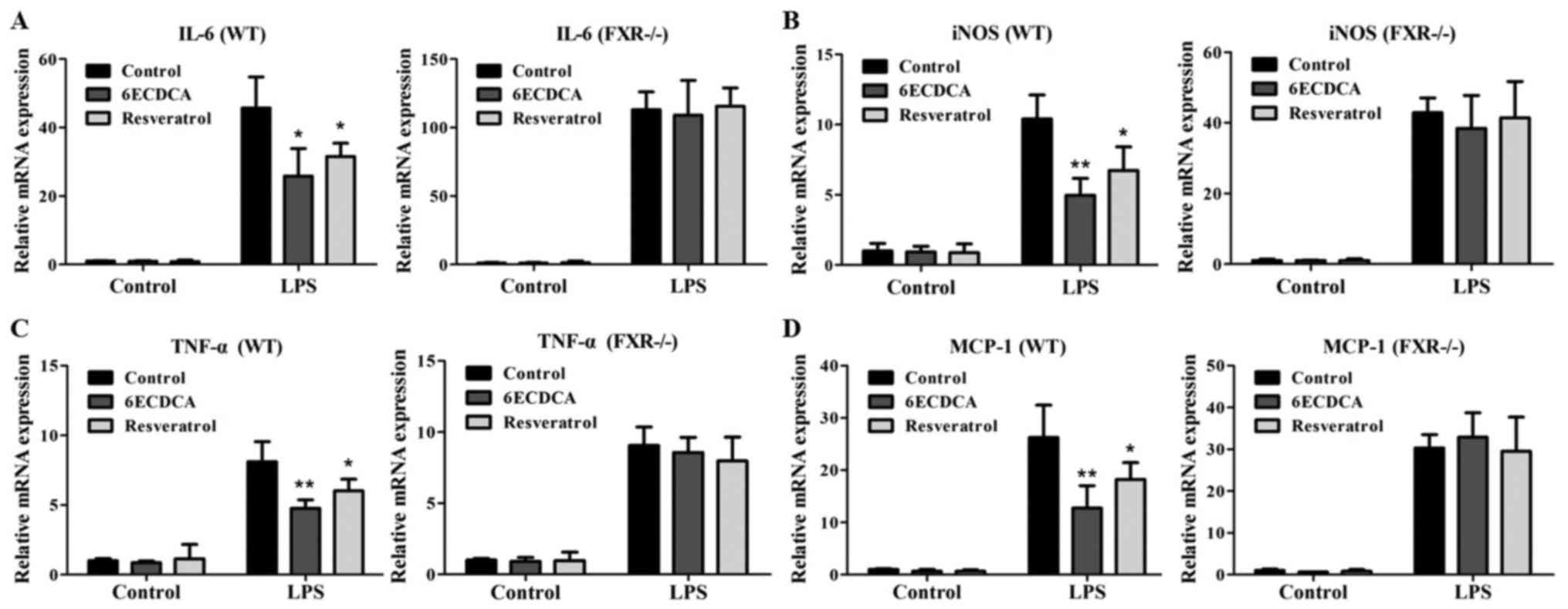 | Figure 3.Resveratrol inhibits the expression
levels of IL-6, iNOS, TNF-α and MCP-1 induced by LPS in primary
hepatocytes dependent on FXR. Quantitative real-time PCR analysis
of the expression of (A) IL-6, (B) iNOs, (C) TNF-α and (D) MCP-1 in
primary hepatocytes from WT and KO mice that were pretreated with
vehicle (DMSO), 6ECDCA (3 µm) or resveratrol (10 µm) for 18 h
before treatment with LPS (2 µg/ml) for 6 h. Each bar represents
the mean ± SD of triplicates of three independent experiments.
Statistical analyses were done with the Student's t-test.
*P<0.05, **P<0.01 vs. LPS alone treatment group (n=3).
LPS, lipopolysaccharide; IL-6, interleukin-6; iNOs, inducible
nitric oxide synthase; TNF-α, tumor necrosis factor-α; MCP-1,
monocyte chemoattractant protein-1; FXR, farnesoid X receptor; WT,
wild-type; KO, knockout. |
Resveratrol exerted protective effect
on ANIT-induced cholestasis with liver injury through regulating
FXR pathway
Then, we explored whether the resveratrol induced
FXR activation against ANIT-induced liver injury with cholestasis.
Serum ALT, AST, ALP, TBA, TBIL, and DBIL levels were assayed as
measures of liver injury with cholestasis. ANIT significantly
induced serum ALT, AST, ALP, TBA, TBIL, and DBIL levels in both WT
and KO mice at 48 hrs after treatment (Fig. 4A and B). However, the pretreatment
of resveratrol at dose of 60 mg/kg B.W. or 6ECDCA at dose of 20
mg/kg B.W. significantly reversed ANIT-induced elevation of ALT,
AST, ALP as well as TBIL, DBIL and TBA at 48 h after ANIT treatment
in WT mice but not in KO mice (Fig. 4A
and B).
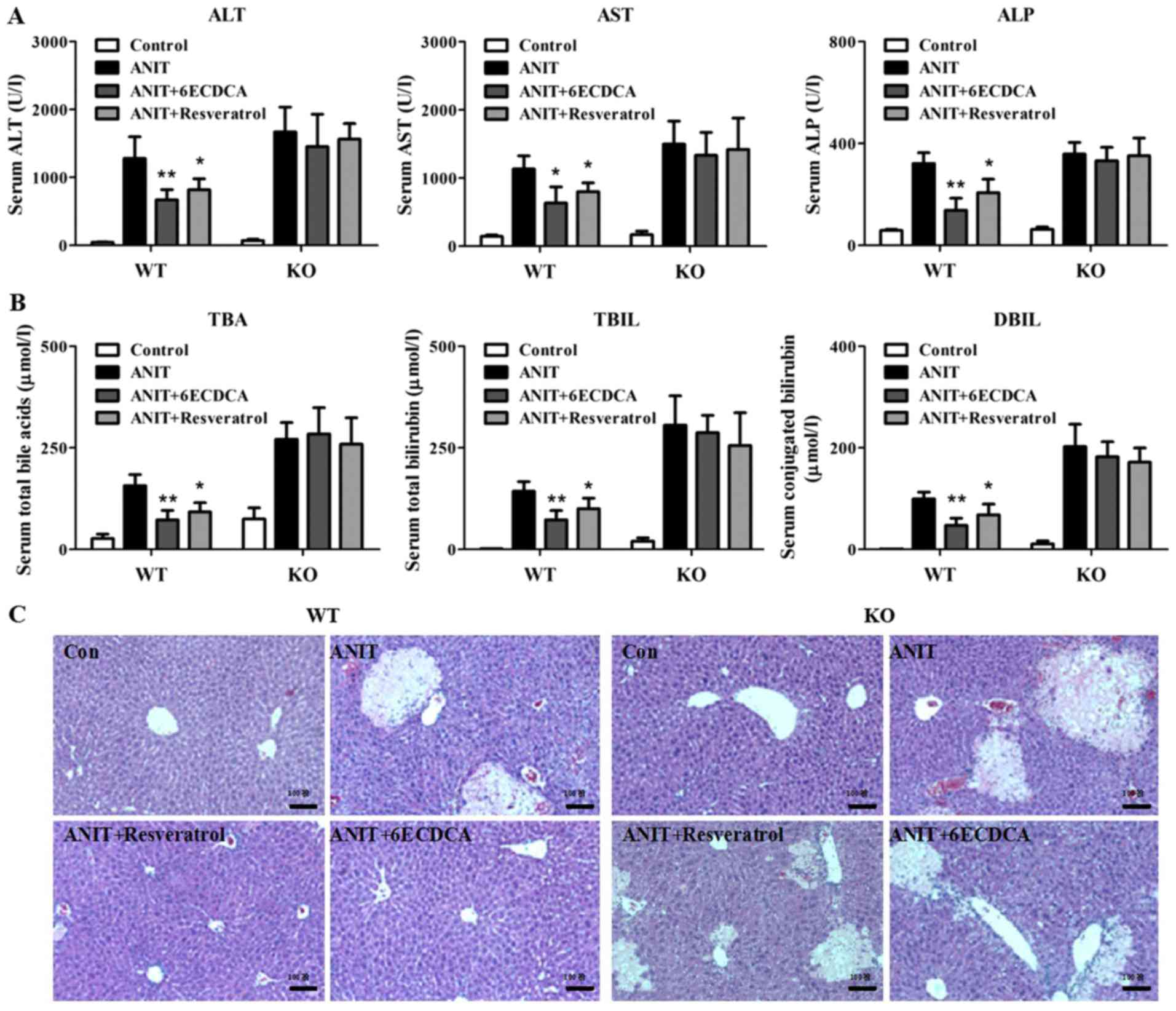 | Figure 4.Resveratrol exerts protective effect
on ANIT-induced cholestasis with liver injury dependent on FXR by
biochemical and histological analysis. (A) Serum ALT, AST, ALP and
(B) TBA, TBIL and DBIL were detected in both WT and KO mice. (C)
Liver tissues from all groups in both WT and KO mice were fixed and
followed by H&E staining. Data are presented as mean ± SD.
Statistical analyses were done with one-way ANOVA (Dunnett's post
test). *P<0.05, **P<0.01 vs. ANIT treatment group
(n=8). ANIT, α-naphthylisothiocyanate; FXR, farnesoid X
receptor; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; ALP, alkaline phosphatase; TBA, total bile acid;
TBIL, total bilirubin; DBIL, direct bilirubin; WT, wild-type; KO,
knockout. |
Histological analysis displayed that both WT and KO
mice demonstrated greater inflammatory infiltration and parenchymal
necrosis after ANIT treatment (Fig.
4C). In contrast, with resveratrol or 6ECDCA pretreatment,
liver injury was attenuated in WT mice but not in KO mice after
ANIT exposure (Fig. 4C). The above
data indicated that FXR served as an important therapeutic target
for resveratrol to exert its protective effect against ANIT-induced
liver injury with cholestasis.
Resveratrol regulate BA-related genes
through FXR pathway
To explore the mechanism of the protective effect of
resveratrol against ANIT-induced cholestasis, the mRNA levels of
FXR target genes were determined (Fig.
5). First, the mRNA levels of BA transporters were detected in
all groups, including bile salt export pump (Bsep), organic solute
transporter β (Ostβ), multidrug resistance-associated protein 2 and
3 (Mrp2 and Mrp3) (Fig. 5A and B).
ANIT treatment significantly downregulated the mRNA expressions of
Bsep in WT mice and upregulated mRNA levels of Ostβ and Mrp3 in
both WT and KO mice (Fig. 5A).
With resveratrol and 6ECDCA treatment, Besp levels increased in WT
mice when compared with the ANIT group, but not in the KO mice. In
contrast, Ostβ and Mrp3 levels decreased in WT mice when compared
with the ANIT group, but not in the KO mice (Fig. 5A). Furthermore, resveratrol and
6ECDCA treatment had no effect on Mrp2 levels (Fig. 5B). Next, the BA synthesis related
genes were assayed. ANIT significantly repressed the mRNA levels of
Cyp7a1 and Cyp8b1 in both WT and KO mice (Fig. 5B). Whereas with resveratrol and
6ECDCA treatment, the mRNA expressions of Cyp7a1 and Cyp8b1
increased when compared with the ANIT group in WT mice, but not in
KO mice (Fig. 5B). Intestine FXR
also play a key role in regulation of BAs homeostasis in
enterohepatic circulation. Therefore, mRNA levels of ileal BA
binding protein (I-Babp), Ostβ and Fgf15 as FXR target genes in
ileum were also investigated. ANIT significantly induced I-Babp
levels in the ileums of both types of mice (Fig. 5C). With resveratrol and 6ECDCA
treatment, I-Babp expression reduced in WT mice but not in KO mice.
ANIT significantly upregulated Ostβ levels in both WT and KO mice,
but had no effect on Fgf15 expression. With resveratrol treatment,
Ostβ was restored to control level while Fgf15 enhanced when
compared with the ANIT group in WT mice, but not in KO mice
(Fig. 5C). Collectively,
resveratrol attenuated ANIT-induced cholestasis through FXR
signaling pathway, resulting in regulation of BA homeostasis in
enterohepatic circulation system.
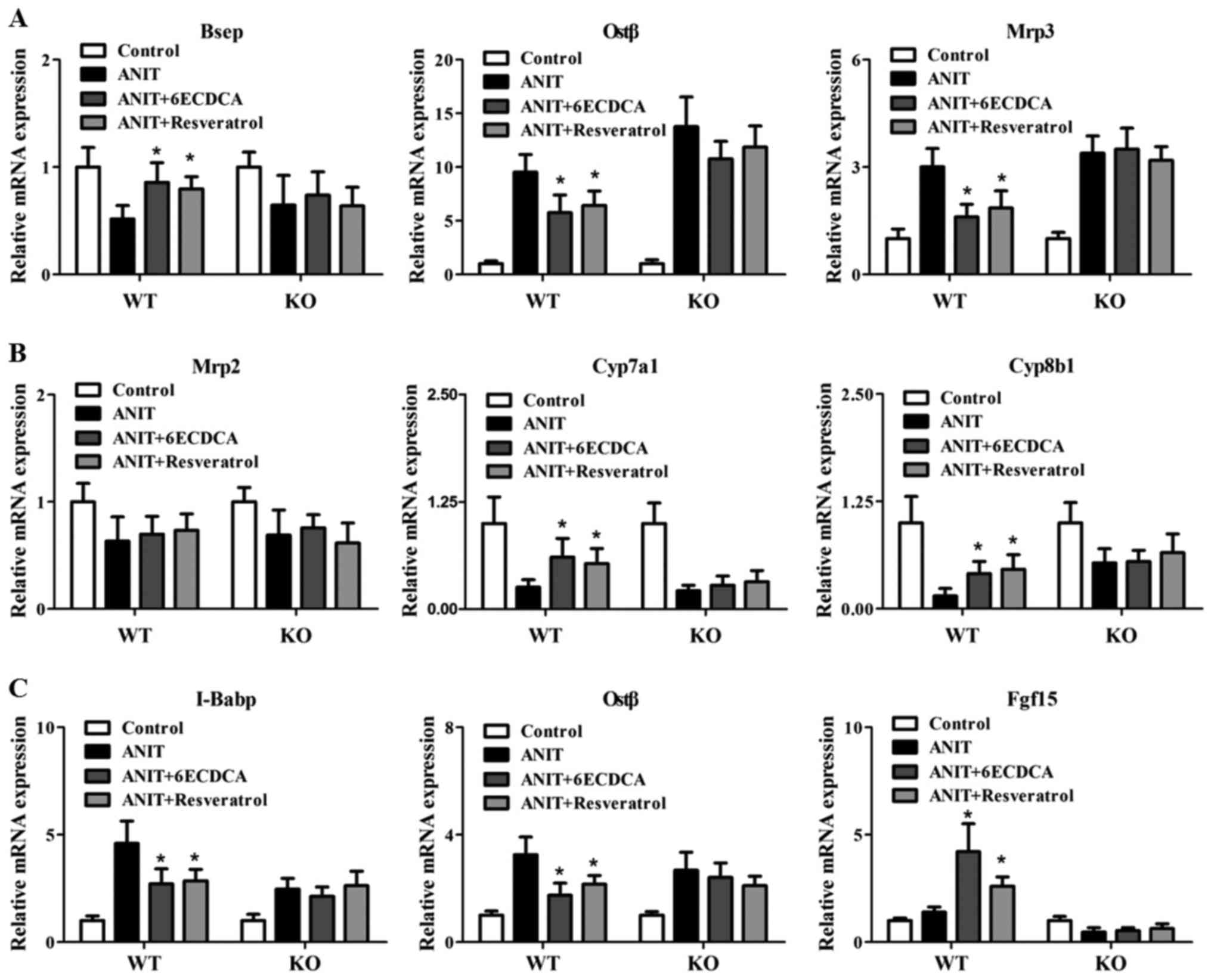 | Figure 5.The effects of resveratrol on bile
acid signaling related genes in WT and KO mice with ANIT treatment.
Liver and ileum tissues from both mice were used for gene
expression test. Hepatic bile acid transport and biosynthesis
related genes including: (A) Bsep, Ostβ, Mrp3; (B) Mrp2, Cyp7a1,
Cyp8b1, and ileum FXR target genes; and (C) I-Babp, Ostβ and Fgf15
were detected by real-time PCR. Data are presented as mean ± SD.
Statistical analyses were done with one-way ANOVA (Dunnett's post
test). *P<0.05 vs. ANIT treatment group (n=8). WT,
wild-type; KO, knockout; ANIT, α-naphthylisothiocyanate; Bsep, bile
salt export pump; Ostβ, organic solute transporter β; Mrp,
multidrug resistance-associated protein; Cyp7a1, cholesterol 7α
hydroxylase; Cyp8b1, sterol 12α-hydroxylase; FXR, farnesoid X
receptor; I-Babp, ileal bile acid binding protein; Ostβ, organic
solute transporter β; Fgf15, fibroblast growth factor 15. |
Resveratrol regulate inflammatory
genes dependent FXR pathway
FXR regulated inflammatory signaling also
contributes to liver injury. In our investigation, inflammatory
genes were assayed in each group (Fig.
6A-F). ANIT treatment significantly induced hepatic
intercellular adhesion molecule-1 (iCAM-1), IL-6, TNF-α, COX-2,
iNOS and IL-1β levels in both of WT and KO mice (Fig. 6A-F). However, the resveratrol or
6ECDCA treatment significantlty decrease the elevation of iCAM-1,
IL-6, TNFα, COX-2, iNOS and IL-1β expression levels induced by ANIT
in the liver of WT mice, but not in KO mice (Fig. 6A-F), indicating that the ANIT
administration induced liver inflammation was not rescued in KO
mice after the pretreatment of resveratrol or 6ECDCA. All the above
results suggested that resveratrol could attenuated ANIT-induced
liver inflammation dependent on FXR-regulated signal pathway.
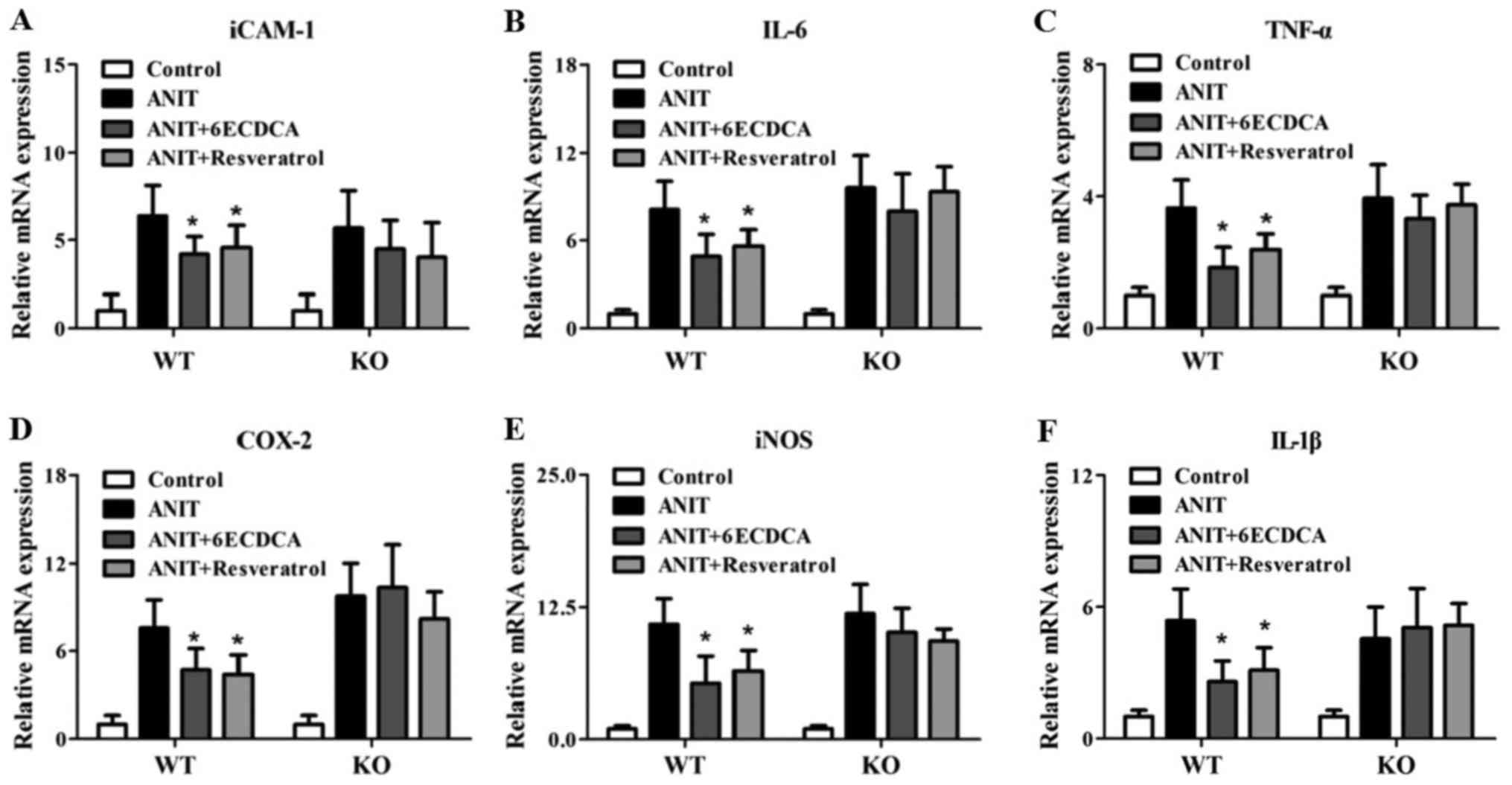 | Figure 6.The effects of resveratrol on
inflammatory signaling related genes in WT and KO mice with ANIT
treatment. Inflammation related genes levels, including: (A)
iCAM-1, (B) IL-6, (C) TNF-α, (D) COX-2, (E) iNOS and (F) IL-1β in
WT and KO mice were detected by real-time PCR. Data are presented
as mean ± SD. Statistical analyses were done with one-way ANOVA
(Dunnett's post test). *P<0.05 vs. ANIT treatment group
(n=8). WT, wild-type; KO, knockout; ANIT,
α-naphthylisothiocyanate; iCAM-1, intercellular adhesion
molecule-1; IL, interleukin; TNF-α, tumor necrosis factor-α; COX-2,
cyclooxygenase-2; iNOS, inducible nitric oxide synthase. |
Discussion
A growing number of natural products have been
discovered for their protective effects against drug-induced
cholestasis with liver injury (18,19).
ANIT as a well-known hepatotoxin has been used in rodents to induce
intrahepatic cholestasis that mimics the disease in human (23). Previously, it has been reported
that resveratrol exhibited a protective effect on ANIT-induced
cholestasis with liver injuries in rats in a dose-dependent manner
(24). However, the molecular
players governing the positive effects of resveratrol on
ANIT-induced cholestasis have remained largely elusive.
In the present study, we examined the in vivo
effect of resveratrol (60 mg/kg B.W.) in the mouse model of
ANIT-induced cholestasis. In agreement with previous reports, the
pretreatment with resveratrol markedly reversed ANIT-induced
elevation of the serum levels of ALT, AST, ALP, TBIL, DBIL and TBA
as well as pathological damages (24). However, in the absence of FXR, the
beneficial effects of resveratrol on ANIT-induced cholestasis in
mice are lost. The above results indicate that the protective
effect of resveratrol on ANIT-induced cholestasis with liver injury
may due to the regulation of FXR pathway.
In order to gain insight into the underlying
mechanism of the protective effect of resveratrol against
ANIT-induced cholestasis, the mRNA expressions of FXR target genes
were investigated. Here, our results demonstrated that resveratrol
regulated FXR target genes Bsep in liver and Fgf15 in ileum of WT
mice with ANIT treatment, but not in the KO mice. Our in
vitro data also showed resveratrol inhibited the mRNA levels of
Cyp7a1, Cyp8b1 and Cyp27a1 which were negatively regulated by FXR
(25). Whereas the mRNA levels of
Shp which was positively regulated by FXR (25) increased after the incubation of
resveratrol in primary hepatocytes. FXR plays a key role in
regulation of BAs homeostasis. The activation of FXR inhibits the
expressions of BAs synthesis genes such as Cyp7a1, Cyp8b1 and
Cyp27a1 and induces the levels BAs related transporters including
Bsep and Ostβ to reduce the BAs accumulation in liver (26). Our data showed that the elevation
of mRNA levels of Cyp7a1, Cyp8b1 and the reduction of Ostβ and
I-Babp levels in WT mice with resveratrol pretreatment were due to
the adaptive response of the lower BAs level at 48 h after ANIT
treatment. However, in KO mice, we observed the high level of serum
total BAs without any changes of mRNA levels of Cyp7a1, Cyp8b1,
Ostβ and I-Babp in resveratrol pretreatment group when compared
with ANIT group. Collectively, the activation of FXR stimulated by
resveratrol attenuated ANIT-induced cholestasis with liver injury,
suggesting that resveratrol may be a potential compound for the
treatment of drug-induced cholestasis.
Furthermore, the key role of FXR in regulating BA
levels after ANIT treatment was confirmed by FXR deficiency mice
that displayed severe cholestasis and exaggerated liver injury.
Previous studies indicate that high levels of BAs can stimulate
cell death and augment inflammatory cytokine production such as
TNF-α (27). Therefore, the
uncontrolled inflammatory cytokine production due to high levels of
BAs may contribute to the overall liver injury. Interestingly, it
has been reported that FXR is a negative mediator of liver
inflammation and there is reciprocal suppression between FXR and
NF-κB signaling pathways (27).
Previous data reported that the p65 subunit of NF-κB directly
interacts with the DNA-binding domain of RXRα and may prevent its
binding to the consensus DNA sequences. Because RXR is a
dimerization partner of FXR, it is possible that NF-κB suppresses
FXR activity by reducing the number of FXR/RXR complexes (28). It also has been demonstrated that
the activation of FXR repressed specific sets of NF-κB target genes
in response to the NF-κB activators (LPS and TNF-α) (29). In our present data, resveratrol
induced the activation of FXR suppresses the activity of NF-κB in
cell culture experiments in vitro. We also identify a
specific effect of resveratrol on ANIT-induced liver inflammation
through FXR activation in vivo. Taken together, with the
activation of the transcriptional factor FXR stimulated by
resveratrol attenuated the ANIT-induced BAs accumulation in liver.
The low level BAs actually improve the liver inflammation which is
induced by BAs accumulation after ANIT treatment. Another point is
that the activation of FXR still directly attenuated the liver
inflammation.
In conclusion, resveratrol exhibit the protective
effect on ANIT-induced cholestasis in mice by activation of nuclear
receptors FXR, which in turn prevents BAs accumulation and
attenuates the inflammation in liver, suggesting that resveratrol
may be a potential compound for the treatment of drug-induced
cholestasis.
Acknowledgements
This study is financially supported by the Natural
Science Foundations of China (81303186 and ZYX-NSFC-016), the
National S&T Major Special Project (2014ZX09301306-007), China
Postdoctoral Science Foundation (2013M531202) and the International
Postdoctoral Exchange Fellowship Program.
Glossary
Abbreviations
Abbreviations:
|
Abcb11
|
bile salt export pump, Besp
|
|
Abcc2/3
|
ATP-binding cassette sub-family C
member 2/3, Mrp2/3
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
ALP
|
alkaline phosphatase
|
|
ANIT
|
α-naphthylisothiocyanate
|
|
BAs
|
bile acids
|
|
COX-2
|
cyclooxygenase-2
|
|
Cyp7a1
|
cholesterol 7α hydroxylase
|
|
Cyp8b1
|
sterol 12α-hydroxylase
|
|
Cyp27a1
|
sterol 27-hydroxylase
|
|
DBIL
|
direct bilirubin
|
|
Fgf15
|
fibroblast growth factor 15
|
|
FXR
|
farnesoid X receptor
|
|
I-Babp
|
ileal bile acid binding protein
|
|
iCAM-1
|
intercellular adhesion molecule-1
|
|
IL-6/1β
|
interleukin-6/1β
|
|
iNOS
|
inducible nitric oxide synthase
|
|
KO
|
knockout
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
Ostβ
|
organic solute transporter β
|
|
Shp
|
small heterod imer partner
|
|
TBA
|
total bile acid
|
|
TBIL
|
total bilirubin
|
|
TNF-α
|
tumor necrosis factor-α
|
|
WT
|
wild-type
|
|
6ECDCA
|
6α-ethylchenodeoxycholic acid
|
References
|
1
|
Plaa GL and Priestly BG: Intrahepatic
cholestasis induced by drugs and chemicals. Pharmacol Rev.
28:207–273. 1976.
|
|
2
|
Zimmerman HJ: Drug-induced liver disease.
Drugs. 16:25–45. 1978. View Article : Google Scholar
|
|
3
|
Kossor DC, Meunier PC, Handler JA, Sozio
RS and Goldstein RS: Temporal relationship of changes in
hepatobiliary function and morphology in rats following
alpha-naphthylisothiocyanate (ANIT) administration. Toxicol Appl
Pharmacol. 119:108–114. 1993. View Article : Google Scholar
|
|
4
|
Roth RA and Dahm LJ: Neutrophil- and
glutathione-mediated hepatotoxicity of
alpha-naphthylisothiocyanate. Drug Metab Rev. 29:153–165. 1997.
View Article : Google Scholar
|
|
5
|
Yoshidome H, Miyazaki M, Shimizu H, Ito H,
Nakagawa K, Ambiru S, Nakajima N, Edwards MJ and Lentsch AB:
Obstructive jaundice impairs hepatic sinusoidal endothelial cell
function and renders liver susceptible to hepatic
ischemia/reperfusion. J Hepatol. 33:59–67. 2000. View Article : Google Scholar
|
|
6
|
Tekin R, Yolbas I, Dal T, Demirpençe Ö,
Kaya S, Bozkurt F, Deveci Ö, Çelen MK and Tekin A: Evaluation of
adults with acute viral hepatitis a and review of the literature.
Clin Ter. 164:537–541. 2013.
|
|
7
|
Flora KD and Benner KG: Liver disease in
cystic fibrosis. Clin Liver Dis. 2:51–61. 1998. View Article : Google Scholar
|
|
8
|
Eaton JE, Talwalkar JA, Lazaridis KN,
Gores GJ and Lindor KD: Pathogenesis of primary sclerosing
cholangitis and advances in diagnosis and management.
Gastroenterology. 145:521–536. 2013. View Article : Google Scholar
|
|
9
|
Hirschfield GM, Chapman RW, Karlsen TH,
Lammert F, Lazaridis KN and Mason AL: The genetics of complex
cholestatic disorders. Gastroenterology. 144:1357–1374. 2013.
View Article : Google Scholar :
|
|
10
|
Fickert P, Pollheimer MJ, Silbert D,
Moustafa T, Halilbasic E, Krones E, Durchschein F, Thüringer A,
Zollner G, Denk H and Trauner M: Differential effects of norUDCA
and UDCA in obstructive cholestasis in mice. J Hepatol.
58:1201–1208. 2013. View Article : Google Scholar :
|
|
11
|
Makishima M, Okamoto AY, Repa JJ, Tu H,
Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ and Shan B:
Identification of a nuclear receptor for bile acids. Science.
284:1362–1365. 1999. View Article : Google Scholar
|
|
12
|
Parks DJ, Blanchard SG, Bledsoe RK,
Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki
AM, Moore DD and Lehmann JM: Bile acids: Natural ligands for an
orphan nuclear receptor. Science. 284:1365–1368. 1999. View Article : Google Scholar
|
|
13
|
Wang H, Chen J, Hollister K, Sowers LC and
Forman BM: Endogenous bile acids are ligands for the nuclear
receptor FXR/BAR. Mol Cell. 3:543–553. 1999. View Article : Google Scholar
|
|
14
|
Huber RM, Murphy K, Miao B, Link JR,
Cunningham MR, Rupar MJ, Gunyuzlu PL, Haws TF, Kassam A, Powell F,
et al: Generation of multiple farnesoid-X-receptor isoforms through
the use of alternative promoters. Gene. 290:35–43. 2002. View Article : Google Scholar
|
|
15
|
Goodwin B, Jones SA, Price RR, Watson MA,
McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al:
A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1
represses bile acid biosynthesis. Mol Cell. 6:517–526. 2000.
View Article : Google Scholar
|
|
16
|
Xie MH, Holcomb I, Deuel B, Dowd P, Huang
A, Vagts A, Foster J, Liang J, Brush J, Gu Q, et al: FGF-19, a
novel fibroblast growth factor with unique specificity for FGFR4.
Cytokine. 11:729–735. 1999. View Article : Google Scholar
|
|
17
|
Kong B, Wang L, Chiang JY, Zhang Y,
Klaassen CD and Guo GL: Mechanism of tissue-specific farnesoid X
receptor in suppressing the expression of genes in bile-acid
synthesis in mice. Hepatology. 56:1034–1043. 2012. View Article : Google Scholar :
|
|
18
|
Meng Q, Chen XL, Wang CY, Liu Q, Sun HJ,
Sun PY, Huo XK, Liu ZH, Yao JH and Liu KX: Alisol B 23-acetate
protects against ANIT-induced hepatotoxity and cholestasis, due to
FXR-mediated regulation of transporters and enzymes involved in
bile acid homeostasis. Toxicol Appl Pharmacol. 283:178–186. 2015.
View Article : Google Scholar
|
|
19
|
Chen H, Huang X, Min J, Li W, Zhang R,
Zhao W, Liu C, Yi L, Mi S, Wang N, et al: Geniposidic acid
protected against ANIT-induced hepatotoxity and acute intrahepatic
cholestasis, due to Fxr-mediated regulation of Bsep and Mrp2. J
Ethnopharmacol. 179:197–207. 2016. View Article : Google Scholar
|
|
20
|
Severgnini M, Sherman J, Sehgal A,
Jayaprakash NK, Aubin J, Wang G, Zhang L, Peng CG, Yucius K, Butler
J and Fitzgerald K: A rapid two-step method for isolation of
functional primary mouse hepatocytes: Cell characterization and
asialoglycoprotein receptor based assay development.
Cytotechnology. 64:187–195. 2012. View Article : Google Scholar
|
|
21
|
Li T and Chiang JY: Bile acid signaling in
metabolic disease and drug therapy. Pharmacol Rev. 66:948–983.
2014. View Article : Google Scholar :
|
|
22
|
Schuetz EG, Strom S, Yasuda K, Lecureur V,
Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ,
et al: Disrupted bile acid homeostasis reveals an unexpected
interaction among nuclear hormone receptors, transporters, and
cytochrome P450. J Biol Chem. 276:39411–39418. 2001. View Article : Google Scholar
|
|
23
|
Tanaka Y, Aleksunes LM, Cui YJ and
Klaassen CD: ANIT-induced intrahepatic cholestasis alters
hepatobiliary transporter expression via Nrf2-dependent and
independent signaling. Toxicol Sci. 108:247–257. 2009. View Article : Google Scholar :
|
|
24
|
Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen
M, Zhao GL, Jiang ZZ and Zhang LY: Resveratrol effectively
attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and
liver injurythrough choleretic and anti-inflammatory mechanisms.
Acta Pharmacol Sin. 35:1527–1536. 2014. View Article : Google Scholar :
|
|
25
|
Ding L, Yang L, Wang Z and Huang W: Bile
acid nuclear receptor FXR and digestive system diseases. Acta Pharm
Sin B. 5:135–144. 2015. View Article : Google Scholar :
|
|
26
|
Matsubara T, Li F and Gonzalez FJ: FXR
signaling in the enterohepatic system. Mol Cell Endocrinol.
368:17–29. 2013. View Article : Google Scholar
|
|
27
|
Greve JW, Gouma DJ and Buurman WA: Bile
acids inhibit endotoxin-induced release of tumor necrosis factor by
monocytes: An in vitro study. Hepatology. 10:454–458. 1989.
View Article : Google Scholar
|
|
28
|
Gu X, Ke S, Liu D, Sheng T, Thomas PE,
Rabson AB, Gallo MA, Xie W and Tian Y: Role of NF-kappaB in
regulation of PXR-mediated gene expression: A mechanism for the
suppression of cytochrome P-450 3A4 by proinflammatory agents. J
Biol Chem. 281:17882–17889. 2006. View Article : Google Scholar
|
|
29
|
Wang YD, Chen WD, Wang M, Yu D, Forman BM
and Huang W: Farnesoid X receptor antagonizes nuclear factor kappaB
in hepatic inflammatory response. Hepatology. 48:1632–1643. 2008.
View Article : Google Scholar :
|