Introduction
With improved living conditions, dietary changes and
reduced labor intensity, the incidence of diabetes mellitus is
rapidly increasing worldwide (1–3).
Diabetic patients with poor glucose control can experience multiple
complications, including cardiovascular disease, kidney disease,
blindness and amputation, which not only seriously affects the
quality of life of patients, but also imposes a heavy burden on
family and society (4–6). Cerebral infarction is one of the
major complications and one of the main causes of mortality
associated with diabetes (5).
Previous studies have shown that diabetes is one of the independent
risk factors of stroke; diabetic patients succumb to
cerebrovascular complication-associated mortality more frequently,
compared with non-diabetic patients (3,6,7).
Therefore, the prevention and treatment of diabetes complicated by
cerebral infarction requires investigation to reduce the mortality
and disability rates of patients.
Under physiological conditions, the brain is unable
to store carbohydrates, so the maintenance of brain function is
almost entirely dependent on the constant supply of glucose from
the blood for the production of ATP (8,9).
Therefore, the transport of glucose into the brain is a key step in
maintaining brain metabolism, which is regulated by a glucose
transporter (GLUT) (10). At
present, 13 types of GLUT proteins have been identified in
mammalian cells (11,12). The transporters responsible for
glucose transport in the brain are predominantly GLUT1 and GLUT3
(13). Investigations have shown
that GLUT1 is divided into two types: 55 kDa GLUT1 is located in
the blood-brain barrier (BBB), whereas 45 kDa GLUT1 is present in
the choroid plexus ependymal epithelial cells and glial cells,
which are mainly responsible for glucose transport through the BBB
to the brain (14,15). GLUT3 is predominantly expressed in
neurons, and is rich in neuronal dendrites and axons. This is
possibly due to dendritic and axonal cells lacking high levels of
mitochondria and the anaerobic glycolysis of glucose being
insufficient (16,17). A continuous supply of glucose is
necessary to ensure brain bioelectrical activity. Therefore,
investigating the dynamic changes in the expression of GLUTl and
GLUT3 in the brain is of value for the clinical treatment of
diabetes.
Jiang Huang (Curcuma longa L) is also known
as turmeric, which originates from China, Japan and the United
States (18,19). Jiang Huang has been approved by the
U.S. Food and Drug Administration as a natural food additive
(20). Curcumin is extracted from
turmeric as a soluble phenolic pigment, which is widely used in
food additives (21,22). Multiple studies have shown that
curcumin has several pharmacological effects, including
anti-inflammatory, anti-oxidative, antiviral, antifibrotic and
anticoagulation effects, and lipid regulation (21,22).
Due to its low side effects and its safety, curcumin offers
promising potential for clinical applications and has received
increasing attention in investigations.
In the present study, it was demonstrated for the
first time, to the best of our knowledge, that curcumin
significantly improved diabetes mellitus-associated cerebral
infarction, primarily by increasing the expression of GLUT1 and
GLUT3.
Materials and methods
Establishment of a diabetic rat
model
Adult male Sprague-Dawley rats (220–230 g), provided
by the Animal Facility of the Health Science Center of Peking
University (Beijing, China) were housed in the laboratory animal
room and maintained at 25±1°C with 65±5% humidity on a 12-h
light/dark cycle (lights on between 07:30 a.m. and 19:30 p.m.) for
at least 1 week prior to the experiments. The animals were provided
with food and water ad libitum. The rats were fed for 7 days
and fasted for 12 h. In the experimental group, streptozotocin
dissolved in citric acid sodium citrate buffer (pH 4.5) was
injected into the left lower abdominal cavity at a dose of 60
mg/kg. The control group was injected with the same dose of citrate
buffer. At 72 h post-injection, blood glucose was measured in the
tail vein blood of the rats (One-Touch Johnson Ultra glucose
meter). A blood glucose level >16.7 mmol/l was defined as
diabetic. Blood glucose and body weights were measured once each
week.
All experimental protocols described in the present
study were approved by the Ethics Review Committee for Animal
Experimentation of Xuanwu Hospital Capital Medical University
(Beijing, China).
Transient middle cerebral artery
occlusion (MCAO)
The rats were subjected to transient focal cerebral
ischemia induced by right MCAO with modifications. In brief, the
rats were anesthetized with 10% chloral hydrate (360 mg/kg; i.p.),
following which arterial blood samples were obtained via a femoral
catheter, and measurements of pO2, pCO2 and
pH were recorded using an AVL 998 Blood Gas Analyzer (Roche
Diagnostics, Basel, Switzerland). Rectal temperature was maintained
at 37±0.5°C during MCAO via a temperature-regulated heating lamp. A
fiber-optic probe was attached to the parietal bone overlying the
MCA territory 5-mm posterior and 5-mm lateral to the bregma, and
was connected to a laser-Doppler flowmeter (Perifluxsystem 5000;
Perimend, Stockholm, Sweden) for continuous monitoring of the
cerebral blood flow (CBF). A 40 nylon monofilament suture with a
heat-blunted tip was introduced into the internal carotid artery
through the stump of the external carotid artery, gently advanced
for a distance of 18 mm from the common carotid artery bifurcation
to block the origin of the MCA for 90 min, and then withdrawn to
allow reperfusion. Only animals, which exhibited a reduction in CBF
>85% during right MCAO and a CBF recovery >80% following 10
min of reperfusion were included in the study. Following close of
the wounds, the animals were allowed to recover from anesthesia
prior to being returned to their housing cages. For the curcumin
treatment group, the rats were administered with curcumin by gavage
at a dose of 40 mg/kg, and saline was used as a control (Con).
Assessment of neurological deficit
scores and analysis of survival rates
The neurological deficit scores were assessed prior
to sacrifice of the rats 24 h following reperfusion as described
previously (23,24). Each rat was assessed and scored by
two examiners who remained blinded to the identity of the rat and
the treatment protocol. The following neurological deficit scoring
system was used: 0, no motor deficits (normal); 1, forelimb
weakness and torso turning to the ipsilateral side when held by
tail (mild); 2, circling to the contralateral side but normal
posture at rest (moderate); 3, unable to bear weight on the
affected side at rest (severe); and 4, no spontaneous locomotor
activity or barrel rolling (critical). If no deficit was observed 2
h following recovering from anesthesia, the animal was removed from
further experiments.
Edema measurement
The rats were sacrificed by decapitation under deep
anesthesia with 10% chloral hydrate (360 mg/kg, i.p.) at 6, 12, 24
or 72 h following reperfusion. The ipsilateral and contralateral
hemispheres were dissected, and the wet weight of the tissue was
determined. The tissues were dried at 120°C for 24 h. The
percentage of cerebral water was determined as follows: (wet
weight-dry weight)/dry weight ×100.
Measurement of infarct volume
Following reperfusion, the rats were deeply
anesthetized with 10% chloral hydrate and then sacrificed;
following which the whole brain was rapidly removed. Coronal
sections (n=10 for each group) were cut into 2-mm sections and
stained with standard 2% 2,3,5-triphenyltetrazolium chloride (TTC;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 10 min at
37°C, followed by overnight immersion in 4% formalin. Infarct
volume, expressed as a percentage of whole brain volume, was
measured using an image processing and analysis system (1.25X
objective; Q570IW; Leica Microsystems GmbH, Wetzlar, Germany) and
was calculated by integration of the infarct area on each brain
section along the rostral-caudal axis.
Hematoxylin and eosin (H&E)
staining
The rats were sacrificed 24 and 72 h post-MCAO with
an overdose of 10% chloral hydrate (360 mg/kg, i.p.) and were
transcardially perfused with 0.9% saline solution followed by 4%
ice-cold phosphate-buffered paraformaldehyde. The brains were
removed, fixed overnight, and equilibrated in phosphate-buffered
30% sucrose. Coronal sections of 1.0–2.0 mm from the bregma were
cut on a cryostat (Leica CM3,000; Leica Microsystems GmbH) to a
thickness of 25-µm and used for H&E staining under an inverted
microscope (XDS-500D; Shanghai Caikon Optical Instrument Co., Ltd.,
Shanghai, China).
Transmission electron microscopy
(TEM)
TEM was used to evaluate the ultrastructural changes
of brain sections. Cerebral fragments were fixed with 2.5%
glutaraldehyde solution overnight at 4°C; following which they were
washed with PBS and fixed with 1% osmic acid at 4°C for 2 h. The
tissues were embedded in an epon/araldite mixture. Ultra-thin
sections were cut at 90 nm and stained with uranyl acetate and lead
citrate. The samples were observed under a 1230 type TEM (JEOL,
Ltd., Tokyo, Japan) and images were captured.
TUNEL staining
Nuclear fragmentation was detected using TUNEL
staining with an apoptosis detection kit (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Protein extraction, western blot
analysis and antibodies
Cellular proteins were extracted using RIPA buffer
containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% (v/v) NP-40 and
0.1% (w/v) SDS with 1% (v/v) PMSF (Solarbio Science &
Technology Co., Ltd. Beijing, China), 0.3% (v/v) protease inhibitor
(Sigma; Merck Millipore) and 0.1% (v/v) phosphorylated proteinase
inhibitor (Sigma; Merck Millipore). The lysates were centrifuged at
10,000 × g at 4°C for 15 min and the supernatant was collected for
total protein. A BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) was used to determine the protein concentration.
Equal quantities of protein (15 µg) were separated on an SDS-PAGE
gel (10% v/v) polyacrylamide) and transferred onto a PVDF membrane.
Nonspecific binding was blocked using 8% (w/v) milk in TBS-T for 2
h at room temperature. The membranes were then incubated with
primary antibodies against β-actin (cat. no. 4970; 1:1,000; Cell
Signaling Technology, Inc., Boston, MA, USA), GLUT1 (cat. no.
12939; 1:1,000; Cell Signaling Technology, Inc.), GLUT3 (cat. no.
ab41525; 1:1,000; Abcam, Cambridge, UK), B-cell lymphoma 2 (Bcl-2;
cat. no. ab59348; 1:1,000; Abcam), and Bcl-2-associated X protein
(Bax; cat. no. ab32503; 1:1,000, Abcam) overnight at 4°C. Following
several washes with TBST, the membranes were incubated with
HRP-conjugated goat anti-rabbit IgG (cat. no. ZB-2306; 1:5,000;
OriGene Technologies, Inc., Beijing, China) for 2 h at room
temperature and then washed. The target proteins were visualized
using enhanced chemiluminescence (Merck KGaA, Darmstadt, Germany)
according to the manufacturer's recommendations, quantified using
density analysis normalized against β-actin, according to the
manufacturer's protocol (ImageJ version 1.8.0, National Institutes
of Health, Bethesda, MD, USA), and expressed as the fold-change,
compared with the control.
N2a cell culture
The N2a cell line was obtained from Professor Huaxi
Xu (Biomedical Research, Xiamen University, Xiamen, China).
Briefly, the cells were grown in DMEM with 10% FBS (Invitrogen;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin and
incubated at 37°C with 5% CO2/95% air in a humidified
atmosphere.
Cell transfection
The small interfering (si)RNAs targeting GLUT1 or
GLUT3 or a non-specific siRNA (NC) were purchased from Genepharma
Co., Ltd. (Shanghai, China). Transfection of si-GLUT1 (sense,
5′-GGAATTCAATGCTGATGATGA-3′ and antisense
5′-TCATCATCAGCATTGAATTCC-3′), si-GLUT3 (sense
5′-CTACCTGTCAACAGCGTTTC-3′ and antisense 5′-CAGTATGTGACCAATGTAC-3′)
or NC (sense 5′-TTCTCCGAACGTGTCACGT-3′ and antisense
5′-ACGTGACACGTTCGGAGAA-3′) was performed with HiperFect
transfection reagent (Qiagen, Inc., Valencia, CA, USA) according to
the manufacturer's protocol. In brief, 6×105 cells were
equally seeded in 6-well plates with 2 ml DMEM containing serum and
antibiotics. At the same time, si-GLUT1, si-GLUT3 or NC were mixed
with HiperFect transfection reagent (Qiagen, Inc.) and incubated at
room temperature for 10 min. The complex was then respectively
transfected into the cells for 48 h.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical significance was analyzed using one-way
analysis of variance followed by Tukey's test for multiple
comparisons. Statistical analysis was carried out with Student's
t-test (GraphPad Prism 7; GraphPad Software, Inc., La Jolla, CA,
USA). The two-tailed unpaired Student's t-test was used for
comparing the band density values between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin improves cerebral infarction
in rats
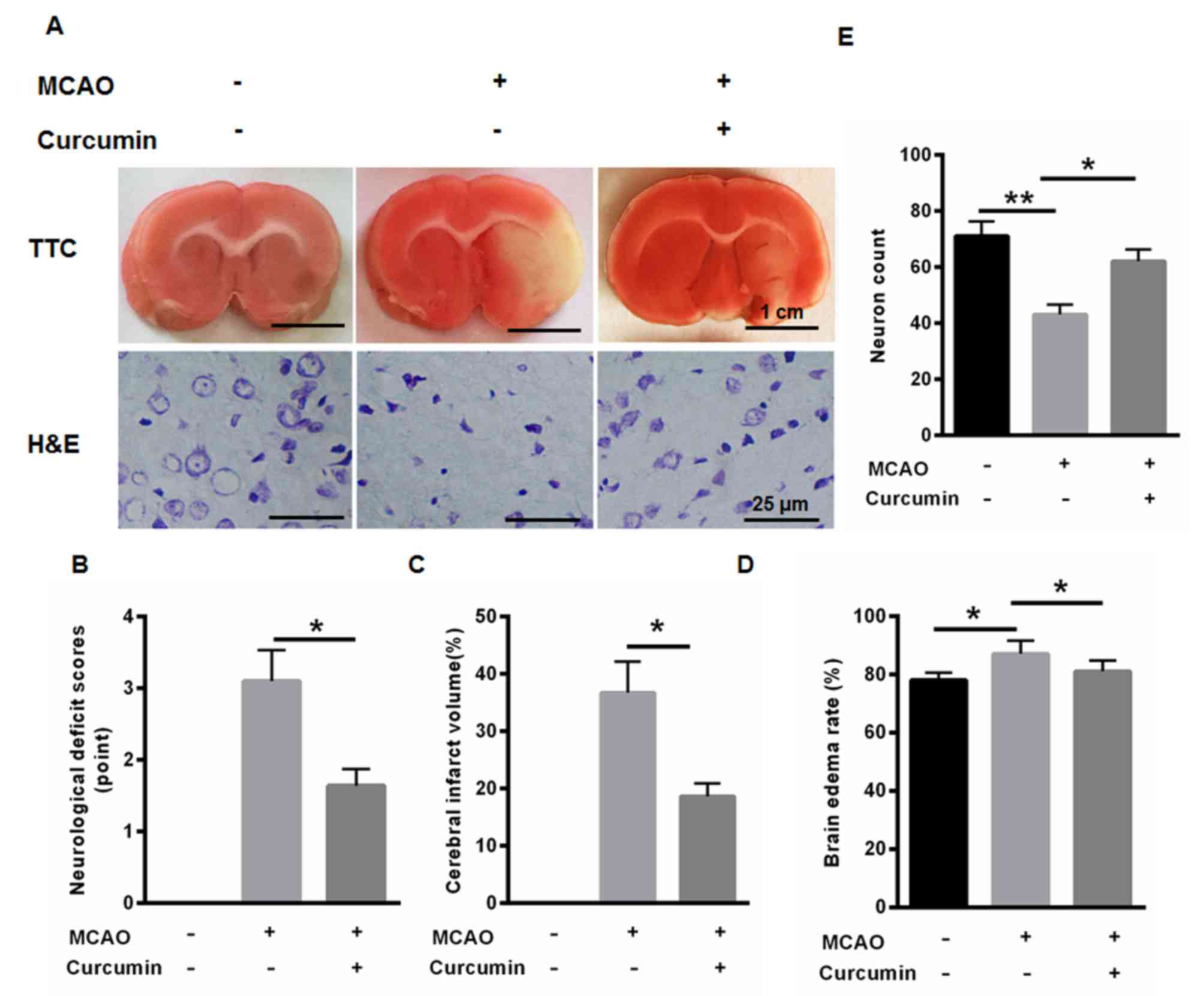
Cerebral infarction was examined using TTC staining.
As shown in Fig. 1A, MCAO induced
cerebral infarction. MCAO treatment also significantly induced
neurological deficits, cerebral infarct volume and brain edema
rate, whereas curcumin markedly improved neurological deficits,
cerebral infarct volume and brain edema rate (Fig. 1B-D). In addition, the neuron count
was markedly reduced following MCAO treatment, whereas neuron count
was enhanced following curcumin treatment (Fig. 1E). These data indicated the
protective role of curcumin in cerebral infarction of diabetic
rats.
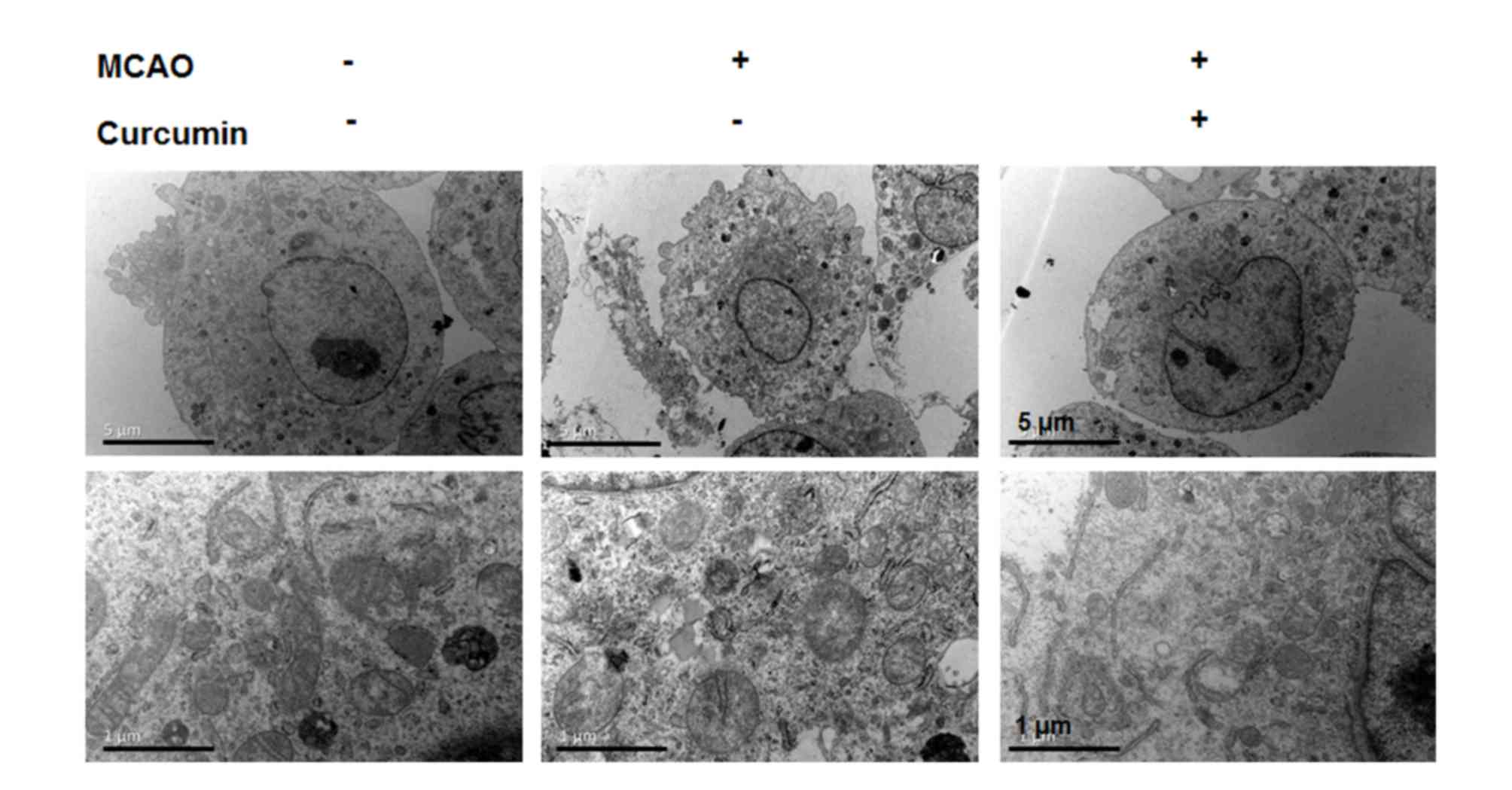
TEM analysis indicates that curcumin
treatment alleviates I/R injury-induced neuron damage
To determine whether curcumin treatment induced the
stabilization of neuronal and ultrastructure in the peri-infarct
cortex, TEM was performed. Normal cortical neurons had large oval
nuclei with a clear nuclear membrane and normal mitochondria
(Fig. 2). In the I/R group,
irregular nuclear membranes, chromatin condensation and numerous
vacuoles were identified in the neurons (Fig. 2). By contrast, the above changes
were alleviated in the curcumin group (Fig. 2). These results indicated that
curcumin treatment alleviated neuron damage.
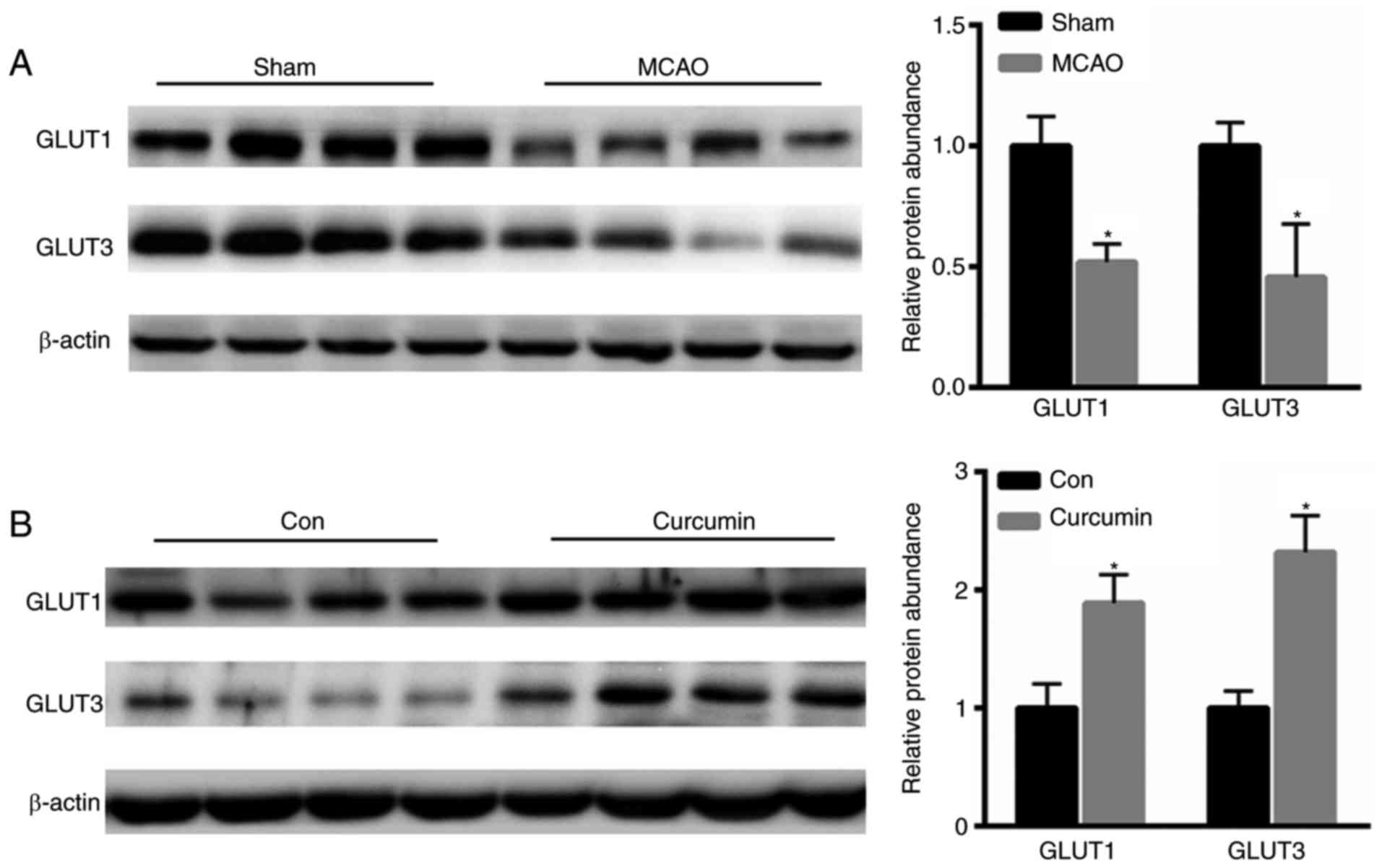
Curcumin enhances cerebral expression
of GLUT1 and GLUT3
Subsequently, the present study examined the
expression of GLUT1 and GLUT3 in the rat brains. Compared with the
control group, reduced expression levels of GLUT1 and GLUT3 were
shown in the MCAO group (Fig. 3A).
However, following curcumin treatment, the levels of GLUT1 and
GLUT3 were markedly increased (Fig.
3B).
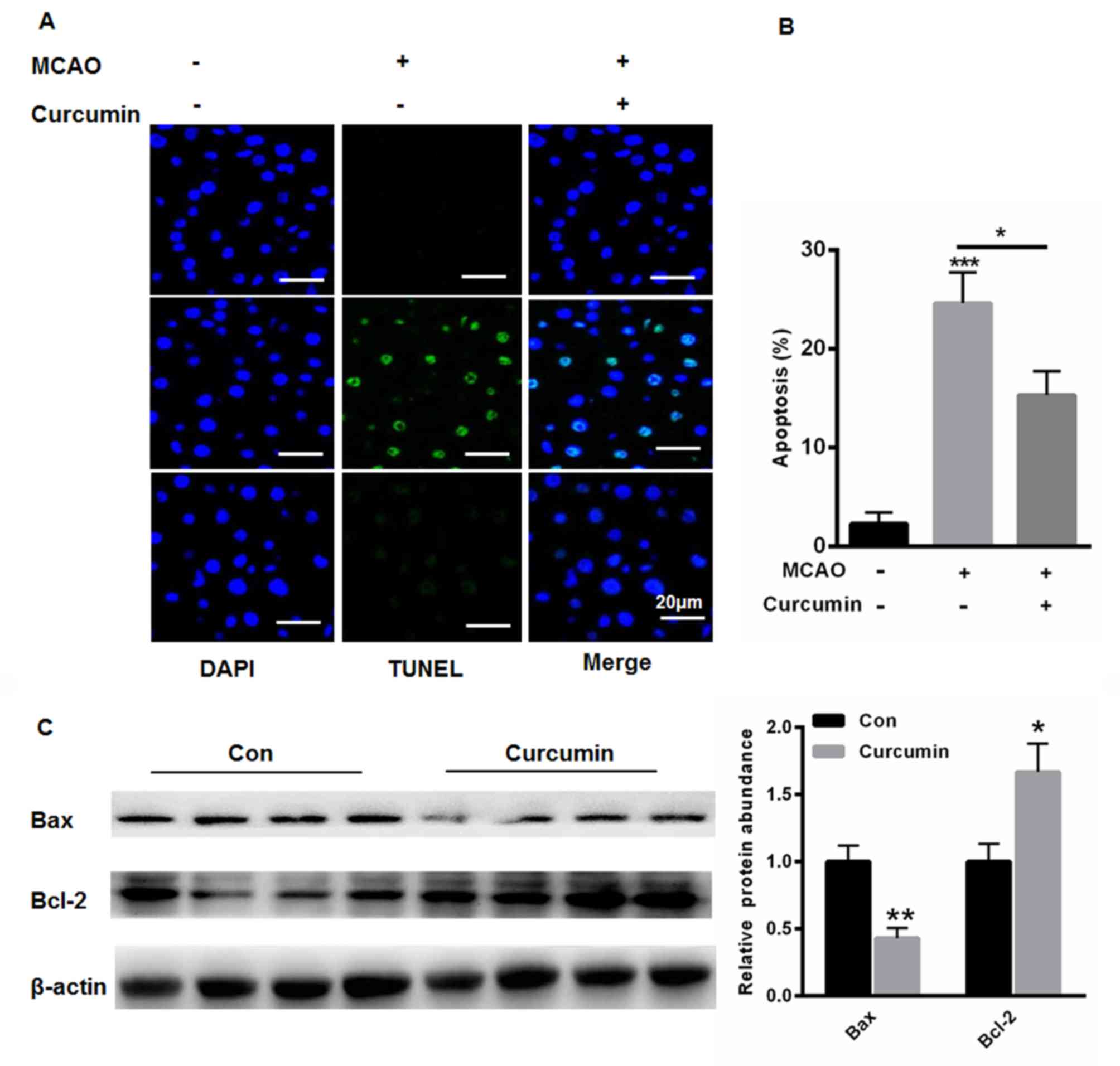
Curcumin reduces cell apoptosis in rat
brains
The present study also evaluated the apoptotic cells
in the rat brains. Compared with the control group, MCAO treatment
significantly increased the number of apoptotic cells (Fig. 4A and B). However, curcumin markedly
reduced cell apoptosis (Fig. 4A and
B). The results of the western blot analysis showed that
curcumin increased the expression of Bcl-2 but reduced the
expression of Bax (Fig. 4C),
indicating an anti-apoptotic role of curcumin in the cerebral
brain.
Curcumin protects brain cells from
apoptosis mainly by upregulating GLUT1 and GLUT3
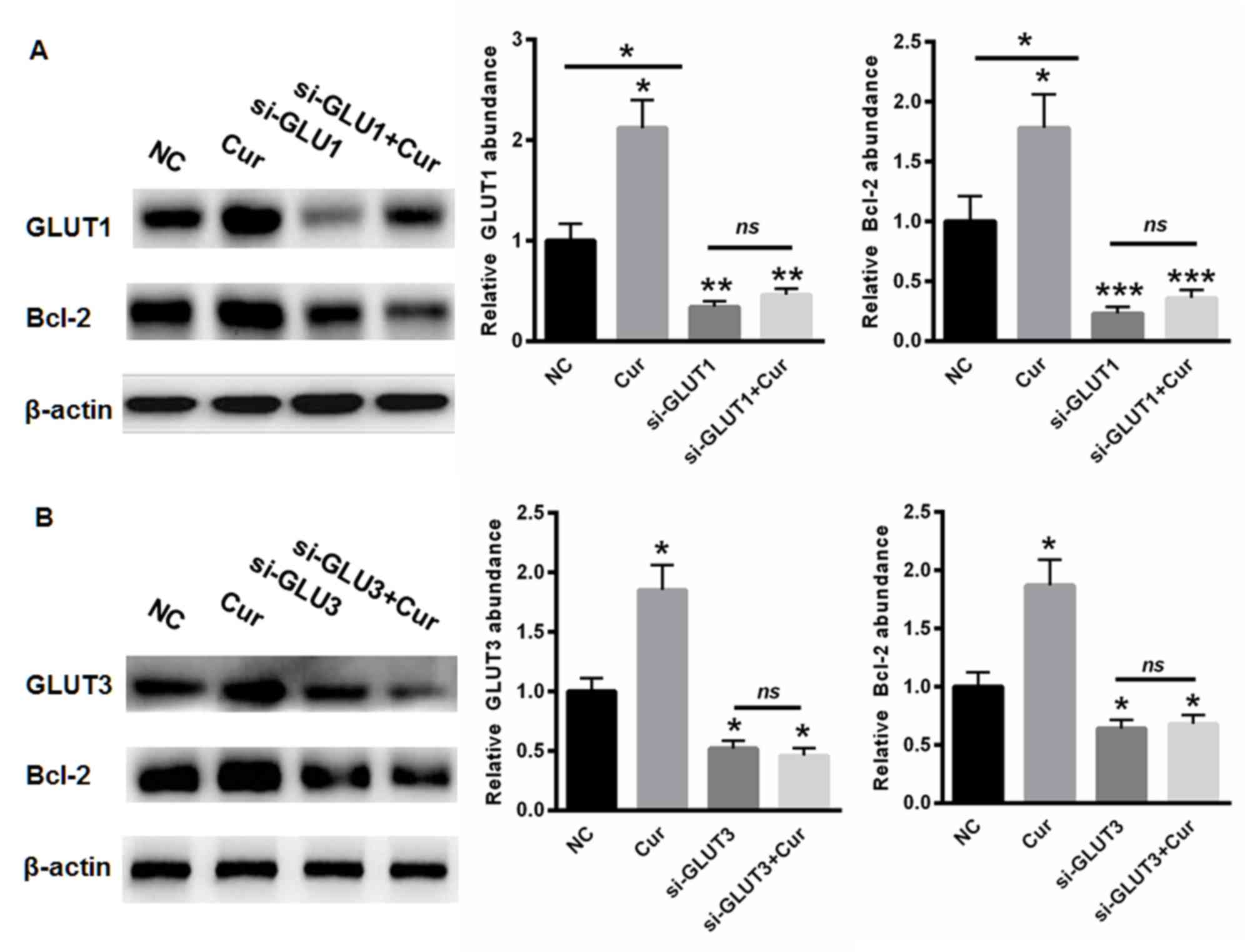
To further evaluate whether curcumin protected
against cell apoptosis by modulating the expression of GLUT1 and
GLUT3, siRNAs targeting GLUT1 and GLUT3 were selected and used to
transfect cells. As shown in Fig.
5A, curcumin treatment enhanced the expression of GLUT1. The
siRNA targeting GLUT1 significantly suppressed the level of GLUT1
and Bcl-2, even following curcumin treatment (Fig. 5A). Similarly, the expression of
GLUT3 was increased in the cells treated with curcumin (Fig. 5B), whereas silencing GLUT3
inhibited the levels of GLUT3 and Bcl-2, even following curcumin
incubation (Fig. 5B). These data
showed that curcumin protected the brain cells from apoptosis,
predominantly by upregulating the expression of GLUT1 and
GLUT3.
Discussion
The main risks of diabetes are chronic complications
caused by chronic hyperglycemia, and some of the most important
complications are vascular complications (3,6). It
is reported that 75% of diabetic patients worldwide succumbed to
diabetic vascular complication-associate mortality, and this
mortality rate ranks as the second highest of total causes of
diabetes-associated mortality (7,23).
There are also regional differences in the mortality and disability
rates of vascular complications. Diabetic patients with
cerebrovascular disease have 2–4 times the mortality rate of
non-diabetic patients (5).
Diabetic cerebrovascular disease is mainly caused by diabetic
cerebral infarction, which accounts for 85% of diabetic
cerebrovascular disease (8,25).
The prevalence of the diabetic vascular disease rate increases with
increasing duration of diabetes, ischemic lesions rather than
hemorrhagic lesions, particularly in lacunar infarction, and due to
repeated relapse of the disease, therefore, the prevention and cure
of cerebral infarction is key to reducing diabetes morbidity rates
(5,6).
Curcumin is reported to significantly inhibit the
growth, metastasis, invasion and colony formation of multiple
tumors (26–28). In the present study, it was
demonstrated that curcumin treatment in rats with diabetes improved
cerebral infarction. The brain is almost entirely dependent on
glucose for energy, and glucose is transported from the blood
circulation into the brain cells through two main processes
(14,15). The first process is through the BBB
by the GLUT1 transporter. The glucose is then transported into the
cells, mainly through the cell membrane (17). In the condition of diabetes,
chronic hyperglycemia can decrease glucose transport (15). As a protective mechanism, the
downregulation of glucose intracellular flow reduces the cytotoxic
effect of high glucose. However, a long-term reduction in glucose
supply may cause a series of disadvantages. Multiple studies have
shown that this downregulation is mediated by glucose transporters
(10,16). Therefore, identifying how to
improve the expression of GLUT1 and GLUT3 glucose transporters in
the presence of chronic hyperglycemia in diabetes is important.
In the present study, the data demonstrated that
curcumin significantly enhanced the expression of GLUT1 and GLUT3,
suggesting an improved glucose supply in the rat brains. The
present study also evaluated whether curcumin reduced the cell
apoptosis induced by cerebral infarction. TUNEL staining and
western blot analysis showed that curcumin reduced the number of
apoptotic cells and increased the expression of Bcl-2, indicating
an anti-apoptotic role of curcumin in the cerebral brain. Of note,
it was found that the siRNA targeting GLUT1 or GLUT3 significantly
suppressed the levels of GLUT1, GLUT3 and Bcl-2, even following
curcumin treatment. These data showed that curcumin protected the
brain cells from apoptosis, mainly by upregulating GLUT1 and
GLUT3.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that curcumin improved
diabetes-induced cerebral infarction, mainly by enhancing the
expression of GLUT1 and GLUT3 in the rat brain.
References
|
1
|
Zhao L and Hu FX: α-Lipoic acid treatment
of aged type 2 diabetes mellitus complicated with acute cerebral
infarction. Eur Rev Med Pharmacol Sci. 18:3715–3719. 2014.
|
|
2
|
Liu X, Zhou Y, Wang J, Liu X, Yang C, Li
J, Chen S and Wu S: Effect of central obesity on the events of
new-onset cerebral infarction among type 2 diabetes mellitus
patients. Zhonghua Liu Xing Bing Xue Za Zhi. 35:390–392. 2014.(In
Chinese).
|
|
3
|
Inagaki K, Nagao M and Oikawa S: Internal
medicine and neurological diseases: Progress in diagnosis and
treatment. Topics: II neurological diseases related to diabetes
mellitus; 2. Cerebral infarction, coma, hypoglycemia. Nihon Naika
Gakkai Zasshi. 101:2180–2187. 2012.(In Japanese). View Article : Google Scholar
|
|
4
|
Cambon H, Derouesne C, Yelnik A,
Duyckaerts C and Hauw JJ: Effect of diabetes mellitus and blood
glucose on the size of cerebral infarction and causes of death.
Neuropathological study of 77 cases of infarction in the sylvian
artery area. Rev Neurol (Paris). 147:727–734. 1991.
|
|
5
|
Du L, Ma J and Zhang X: Higher serum uric
acid may contribute to cerebral infarction in patients with type 2
diabetes mellitus: A meta-analysis. J Mol Neurosci. 61:25–31. 2017.
View Article : Google Scholar
|
|
6
|
Ichikawa H, Shimizu Y, Kuriki A, Murakami
H, Mukai M and Kawamura M: The brainstem is at high risk for
recurrent noncardioembolic cerebral infarction in association with
diabetes mellitus: A hospital-based study. Eur Neurol. 67:26–32.
2012. View Article : Google Scholar
|
|
7
|
Kawamura T, Umemura T, Kanai A, Nagashima
M, Nakamura N, Uno T, Nakayama M, Sano T, Hamada Y, Nakamura J and
Hotta N: Soluble adhesion molecules and C-reactive protein in the
progression of silent cerebral infarction in patients with type 2
diabetes mellitus. Metabolism. 55:461–466. 2006. View Article : Google Scholar
|
|
8
|
Long Y, Zhan Q, Yuan M, Duan X, Zhou J, Lu
J, Li Z, Yu F, Zhou X, Yang Q and Xia J: The expression of
microRNA-223 and FAM5C in cerebral infarction patients with
diabetes mellitus. Cardiovasc Toxicol. 17:42–48. 2017. View Article : Google Scholar
|
|
9
|
Petzold S, Kapellen T, Siekmeyer M, Hirsch
W, Bartelt H, Siekmeyer W and Kiess W: Acute cerebral infarction
and extra pontine myelinolysis in children with new onset type 1
diabetes mellitus. Pediatr Diabetes. 12:513–517. 2011. View Article : Google Scholar
|
|
10
|
Tang HL, Li DD, Zhang JJ, Hsu YH, Wang TS,
Zhai SD and Song YQ: Lack of evidence for a harmful effect of
sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk
among type 2 diabetes patients: A network and cumulative
meta-analysis of randomized controlled trials. Diabetes Obes Metab.
18:1199–1206. 2016. View Article : Google Scholar
|
|
11
|
Mittal N, Mittal R, Kumar H and Medhi B:
Sodium glucose co-transporter 2 inhibitors for glycemic control in
type 2 diabetes mellitus: Quality of reporting of randomized
controlled trials. Perspect Clin Res. 7:21–27. 2016. View Article : Google Scholar :
|
|
12
|
Nunez DJ, Yao X, Lin J, Walker A, Zuo P,
Webster L, Krug-Gourley S, Zamek-Gliszczynski MJ, Gillmor DS and
Johnson SL: Glucose and lipid effects of the ileal apical
sodium-dependent bile acid transporter inhibitor GSK2330672:
Double-blind randomized trials with type 2 diabetes subjects taking
metformin. Diabetes Obes Metab. 18:654–662. 2016. View Article : Google Scholar
|
|
13
|
Ohki T, Isogawa A, Toda N and Tagawa K:
Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2
inhibitor, as a second-line treatment for non-alcoholic fatty liver
disease patients with type 2 diabetes mellitus who do not respond
to incretin-based therapies including glucagon-like peptide-1
analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig.
36:313–319. 2016. View Article : Google Scholar
|
|
14
|
Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH,
Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ and Ko SH: Effect of
sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal
renin-angiotensin system in an animal model of type 2 diabetes.
PLoS One. 11:e01657032016. View Article : Google Scholar :
|
|
15
|
Singh AK and Singh R: Sodium-glucose
co-transporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors
combination therapy in type 2 diabetes: A systematic review of
current evidence. Indian J Endocrinol Metab. 20:245–253. 2016.
View Article : Google Scholar :
|
|
16
|
Tang H, Cui W, Li D, Wang T, Zhang J, Zhai
S and Song Y: Sodium-glucose co-transporter 2 inhibitors in
addition to insulin therapy for management of type 2 diabetes
mellitus: A meta-analysis of randomized controlled trials. Diabetes
Obes Metab. 19:142–147. 2017. View Article : Google Scholar
|
|
17
|
Yabe D, Iwasaki M, Kuwata H, Haraguchi T,
Hamamoto Y, Kurose T, Sumita K, Yamazato H, Kanada S and Seino Y:
Sodium-glucose co-transporter-2 inhibitor use and dietary
carbohydrate intake in Japanese individuals with type 2 diabetes: A
randomized, open-label, 3-arm parallel comparative, exploratory
study. Diabetes Obes Metab. 19:739–743. 2017. View Article : Google Scholar :
|
|
18
|
Aziz MT, El Ibrashy IN, Mikhailidis DP,
Rezq AM, Wassef MA, Fouad HH, Ahmed HH, Sabry DA, Shawky HM and
Hussein RE: Signaling mechanisms of a water soluble curcumin
derivative in experimental type 1 diabetes with cardiomyopathy.
Diabetol Metab Syndr. 5:132013. View Article : Google Scholar :
|
|
19
|
Margina D, Gradinaru D, Manda G, Neagoe I
and Ilie M: Membranar effects exerted in vitro by
polyphenols-quercetin, epigallocatechin gallate and curcumin-on
HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem
Toxicol. 61:86–93. 2013. View Article : Google Scholar
|
|
20
|
Nishiyama T, Mae T, Kishida H, Tsukagawa
M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K
and Kitahara M: Curcuminoids and sesquiterpenoids in turmeric
(Curcuma longa L.) suppress an increase in blood glucose level in
type 2 diabetic KK-Ay mice. J Agric Food Chem. 53:959–963. 2005.
View Article : Google Scholar
|
|
21
|
Bulboacă AD, Bolboacă S and Suci S:
Protective effect of curcumin in fructose-induced metabolic
syndrome and in streptozotocin-induced diabetes in rats. Iran J
Basic Med Sci. 19:585–593. 2016.
|
|
22
|
Castro CN, Tabarrozzi Barcala AE,
Winnewisser J, Gimeno ML, Noguerol Antunica M, Liberman AC, Paz DA,
Dewey RA and Perone MJ: Curcumin ameliorates autoimmune diabetes.
Evidence in accelerated murine models of type 1 diabetes. Clin Exp
Immunol. 177:149–160. 2014. View Article : Google Scholar :
|
|
23
|
Li G, Xu X, Wang D, Wang J, Wang Y and Yu
J: Microglial activation during acute cerebral infarction in the
presence of diabetes mellitus. Neurol Sci. 32:1075–1079. 2011.
View Article : Google Scholar
|
|
24
|
Zhang B, Wang D, Ji TF, Shi L and Yu JL:
Overexpression of lncRNA ANRIL up-regulates VEGF expression and
promotes angiogenesis of diabetes mellitus combined with cerebral
infarction by activating NF-κB signaling pathway in a rat model.
Oncotarget. 8:17347–17359. 2017.
|
|
25
|
Kobayashi S: Progress in diagnosis of and
therapy for cerebral infarction in patients with diabetes mellitus.
Nihon Naika Gakkai Zasshi. 93:1532–1538. 2004.(In Japanese).
View Article : Google Scholar
|
|
26
|
Chiu J, Khan ZA, Farhangkhoee H and
Chakrabarti S: Curcumin prevents diabetes-associated abnormalities
in the kidneys by inhibiting p300 and nuclear factor-kappaB.
Nutrition. 25:964–972. 2009. View Article : Google Scholar
|
|
27
|
Chuengsamarn S, Rattanamongkolgul S,
Luechapudiporn R, Phisalaphong C and Jirawatnotai S: Curcumin
extract for prevention of type 2 diabetes. Diabetes Care.
35:2121–2127. 2012. View Article : Google Scholar :
|
|
28
|
El-Azab MF, Attia FM and El-Mowafy AM:
Novel role of curcumin combined with bone marrow transplantation in
reversing experimental diabetes: Effects on pancreatic islet
regeneration, oxidative stress and inflammatory cytokines. Eur J
Pharmacol. 658:41–48. 2011. View Article : Google Scholar
|