Introduction
Inflammatory bowel disease (IBD) is a type of
chronic inflammation of the gastrointestinal tract (1). It is generally believed that IBD is
related to genetic factors, environmental factors, and imbalance of
intestinal flora (2,3). In the clinical treatment, the vast
majority of patients can be alleviated through the immunomodulatory
therapy, especially the immune inhibitors and biological agents.
However, most patients will relapse while the specific mechanism is
still unclear. Investigation of the relapse mechanism can provide
suggestion to explore the potential prediction biomarker for IBD
recurrence and search for new drug targets to prevent recurrence
and maintain remission. At present, the markers used to predict IBD
recurrence include C-reactive protein (CRP), erythrocyte
sedimentation rate (ESR), prostaglandin E2 protein, and fecalcal
protectin, etc. (4,5). However, most of them are only the
early signs of intestinal mucosal inflammation, which cannot be
applied to predict recurrence in patients at long-term remission or
mucosal healing stage. The number of Fos related antigen-1 (Fra-1)
positive intestinal mucosal epithelial cells in active and
remittent IBD patients are obviously higher than the healthy people
(6–10). Vaishnava et al (11) followed up 32 IBD patients in active
or remittent stage for 10 years and found that Fra-1 expression in
intestinal mucosa epithelial cells were closely associated with the
length of active or remittent stage. it suggested that Fra-1 level
detection may facilitate to predict recurrence in IBD patients
without inflammation in intestinal mucosa. Meanwhile, Fra-1 high
expression in intestinal mucosa epithelial cells may also be one of
the reasons of IBD relapse. However, the specific mechanism of
Fra-1 in promoting IBD recurrence is still unclear. The exploration
on it may provide new strategy for the maintenance treatment of IBD
in remittent stage. This study aims to discuss the mechanism of
Fra-1 in promoting IBD recurrence, thus to provide new idea for IBD
treatment.
Materials and methods
Materials
Human intestinal epithelial cell line HCT-116 was
purchased from ATCC. RPMI-1640 medium, FBS, Trypsin-EDTA (0.25%),
penicillin-streptomycin, and PBS were from Gibco. DMSO was from Sky
Biological Technology Co., Ltd. (Jining, China).
Lipofectamine® 2000 was from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8)
was from Dojindo Molecular Technologies, Inc. (Rockville, MD, USA).
RNA extraction kit was from Takam Biotechnology Co., Ltd (Japan).
Absolute methanol, ethanol, isopropanol, chloroform, and methanal
were from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Primers were designed and synthetized by Sangon Biotech Co., Ltd.
(Shanghai, China).
Instruments
Cell incubator was from Baimei. Biosafety cabinet
was from Baker Co. (Sanford, ME, USA). DK22 electric heating water
bath was from Shanghai Laboratory Equipment Co., Ltd. (Shanghai,
China). Electronic analytical balance was from Beijing Biological
Technology Co., Ltd. (Beijing, China). High-speed centrifuge was
from Eppendorf (Hamburg, Germany). Millipore-Q ultrapure water
system was from EMD Millipore (Billerica, MA, USA).
Human Fra-1 expression vector
construction
The specific recognition sequences for XhoI
and BamHI sites were added to the two ends of the
amplification primer of Fra-1 coding sequence (Fig. 1). cDNA from CACO-2 cells were
selected as template for PCR amplification (95°C 1 min, 35 circles
consisting of 95°C 30 sec, 55°C 30 sec, 72°C 1 min, and 72°C 5 min)
in a total volume of 20 µl (0.1 µl Takara Taq, 2 µl 10X Taq buffer,
1.6 µl DNTP mixture, 1 µl cDNA, 1 µl forward and 1 µl reverse
primer, 15.1 µl ddH2O). The primers sequences were as
follows: Forward 5-TGT TCG CCG TCC TGG AA-3 and reverse 5-CGC CAT
AGG CGT AGT AAT CGA-3. After product purification and double enzyme
digestion, the sequence was connected to the pcDNA3.1(−) vector.
The plasmid was sequenced (ABI3130 Sanger) after amplification and
the results showed that all of the 6 vectors contained Fra-1 coding
sequence, which were completely consistent with Fra-1 sequence in
NCBI database. It suggested the Fra-1 expression vector was
successfully constructed.
Fra-1 transfection
HCT-116 cells were cultured in high-glucose DMEM
medium and digested by enzyme. Then the cells were suspended in 10%
FBS and seeded in 24-well plate at the density of 70–80%. pGPU6/GFP
Neo Fra-1, pGPU6/GFP Neo Con, or pGPU6/GFP Neo GAPDH were diluted
in 50 µl Opti-MEM, while 1.5 µl Lipofectamine® 2000 was
diluted in 50 µl Opti-MEM for 5 min, respectively. Then they were
mixed at room temperature for 20 min. Next, the mixture was added
to the cells for transfection for 48–72 h.
CCK-8 assay
The cells were digested and centrifuged at 600–800
rpm for 5 min. After washed by PBS, the cells were seeded in
96-well plate at 5,000 cells/cell. CCK-8 was used to test the cell
viability every 24 h for 3 continuous days to draw the
proliferation curve.
Damage repair assay
The cells were seeded in six-well plate at
1×105 cells/well. After the cell density reached 100%, a
200 µl tip was adopted to scratch three lines on the bottom. Eight
fields were randomly selected under the microscope to record the
width every 24 h. The reside width percentage was calculated to
draw the curve.
Western blotting
The cells were treated by lysis buffer to extract
the protein. After isolation, the protein was quantified by BCA
method and boiled for 5 min. The protein (30 µg per each sample)
was separated by 10% SDS-PAGE and transferred to PVDF membrane.
After blocked at room temperature for 1 h, the membrane was
incubated in primary antibody (1:1,000) at 4°C overnight. After
washed by PBST for three times, the membrane was further incubated
in secondary antibody at room temperature. At last, the membrane
was treated by ECL reagent for 5 min to obtain the image. For
measurement of caspase-3 activation, anti-caspase-3 antibody was
used to measure the full length and cleaved form of caspase-3 by
western blot.
Immunohistochemistry staining
13 patients with IBD in active phase from June 2015
to January 2016 from the Department of Anus and Intestine Surgery,
The Second Hospital of Shandong University (Jinan, China) were
included in this study. All patients were diagnosed and confirmed
as IBD according to examinations. Meanwhile, 13 individuals from
car accidents were included as a control group. The intestinal
mucosa were collected from patients and controls for
immunohistochemical staining and were dehydrated and embedded in
paraffin following routine methods. The paraffin sections were
removed paraffin, and then immersed in the distilled water
following routine methods. Afterwards, rinsing the paraffin
sections in PBS-T and then blocked with Ultra V Block followed by
washing and addition of primary antibody against Fra-1 (Santa Cruz
Biotechnology, Inc., Dallas, Texas, USA) and incubated for 1–2 h.
Then wash and add primary antibody enhancer with incubation for 30
min followed by addition of HRP-conjugated secondary antibody
(anti-mouse HRP secondary antibody; Abcam, Cambridge, MA, USA). At
last, DAB Plus Substrate (DAB substrate kit; Abcam) was added for
developing. Five random fields were imaged per slide and positive
cells were counted manually by observation, then the average number
of positive cells/field was calculated. Examples of optical fields
that exhibited negative or strong positive staining for Fra-1 were
shown.
Statistical analysis
At least three independent experiments were
performed for each assay. All data analysis was performed on IBM
SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Measurement data
was presented as mean ± standard deviation and compared by t-test,
Spearman rank correlation analysis, or logistic regression analysis
when necessary. P<0.05 was considered to indicate a
statistically significant difference.
Results
Fra-1 overexpression HCT-116 cell line
construction
HCT-116 cells were transfected with Fra-1 expression
vector and screened by G418 (600 µg/ml) to select the monoclonal
cell line. qPCR was applied to verify the cell line with highest
expression, named 116-Fra-1. The cells transfected with pcDNA3.1
(−) empty vector and screened by G418 which was named as 116-neo
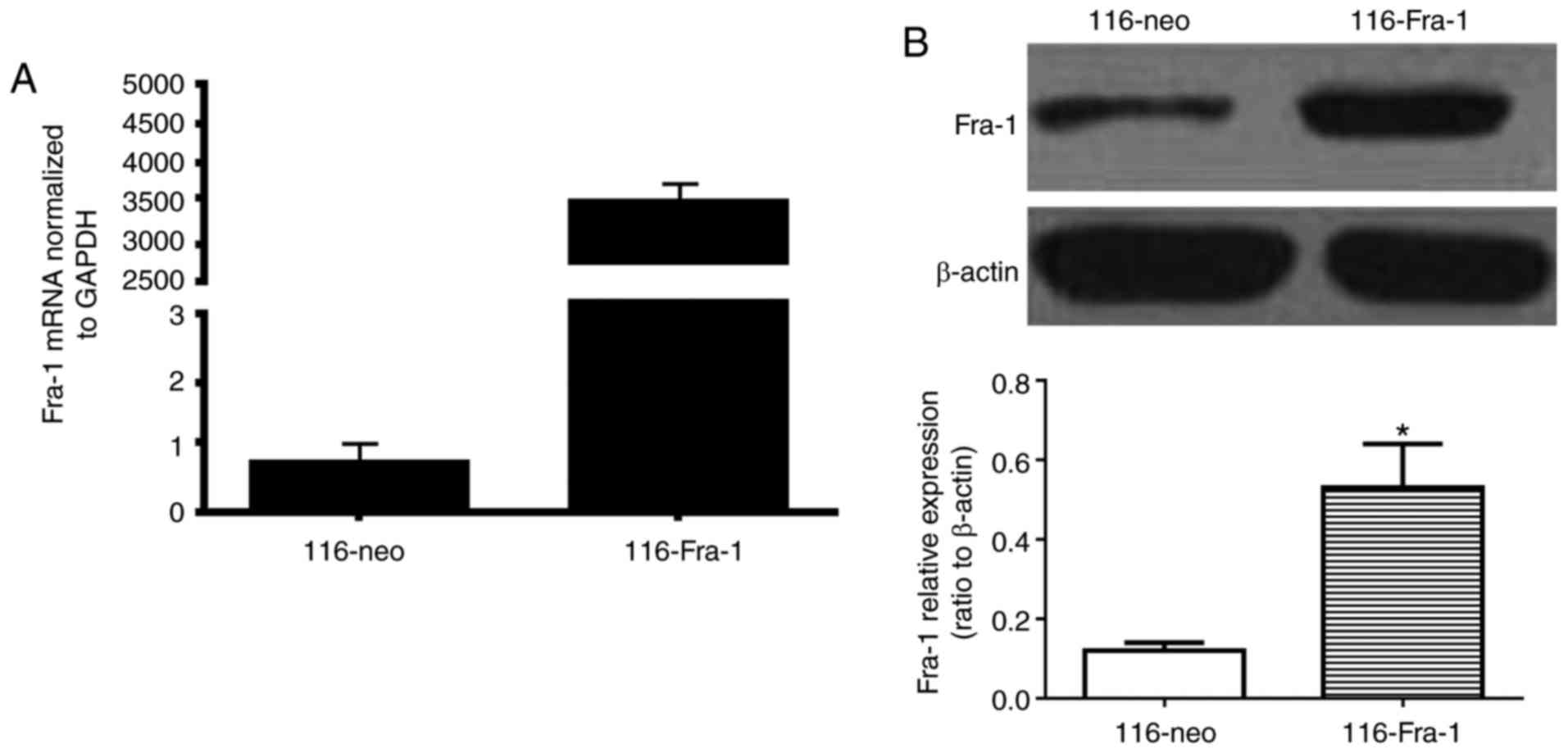
group. qPCR and western blotting both confirmed that Fra-1
expression level in 116-Fra-1 cells (3,500±123 for PCR and
0.53±0.11 for western (ratio to β-actin)) was obviously higher than
that in 116-neo cells (0.7±0.2 for PCR and 0.12±0.02 for western)
(P<0.0001 for PCR and P<0.05 for western) (Fig. 2).
Fra-1 suppressed intestinal mucosal
epithelial cell proliferation and damage repair
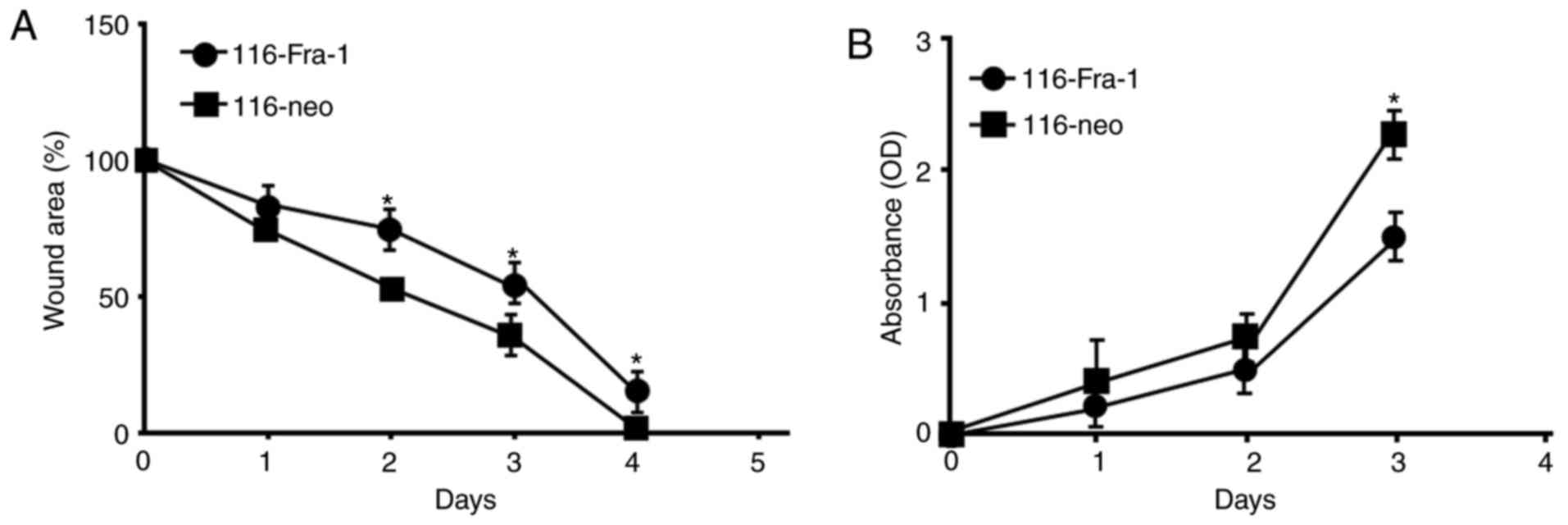
Damage repair model was applied to investigate the
impact of Fra-1 on intestinal epithelial cell damage. It was showed
that at 24 h after damage, the repair effect of 116-Fra-1 cells
were lower than 116-neo cells. The repair schedule exhibited
significant difference at 48 h after damage (P<0.05; Fig. 3A). CCK-8 assay revealed that
116-Fra-1 cell proliferative rate was obviously lower than 116-neo
cells (Fig. 3B), suggesting that
Fra-1 may interfere intestinal epithelial mucosa barrier repair
through affecting cell proliferation and migration.
Fra-1 overexpression regulated
apoptosis related protein expression
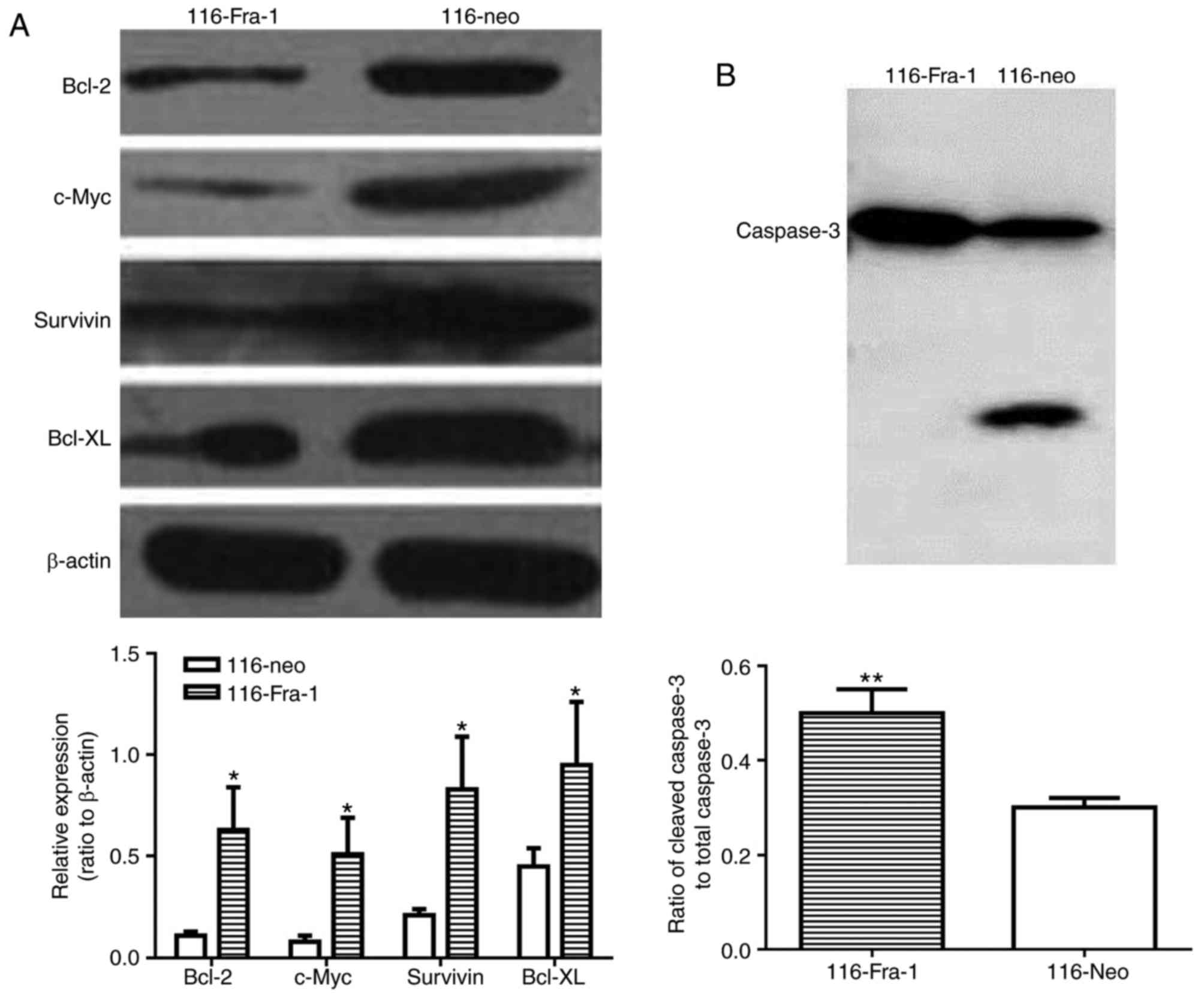
Western blotting demonstrated that Fra-1
overexpression markedly suppressed Bcl-2 (0.11±0.02 vs. 0.63±0.21,
P<0.05), Survivin (0.21±0.03 vs. 0.83±0.26, P<0.05), Bcl-xL
(0.45±0.09 vs. 0.95±0.31, P<0.05), and c-Myc expression
(0.08±0.03 vs. 0.51±0.18, P<0.05) in HCT-116 cells compared with
116-neo group (Fig. 4A).
Consistently, increased active of caspase-3 was also observed in
HCT-116 cells (0.5±0.05) compared with 116-neo group (0.3±0.02)
(P<0.01) (Fig. 4B).
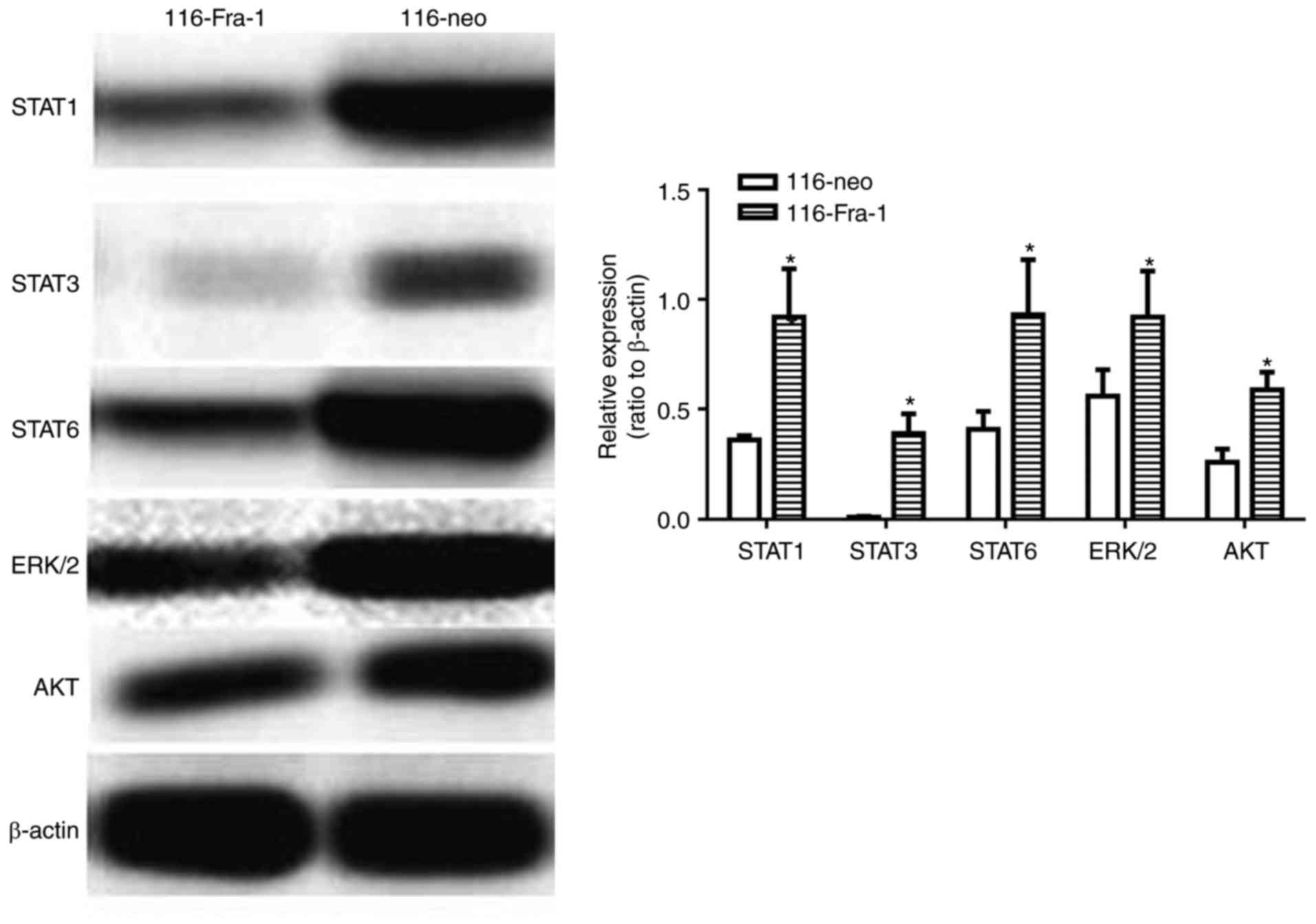
Fra-1 overexpression restrained STAT1,
STAT3, STAT6, ERK1/2, and AKT expression
To further investigate the specific mechanism of
Fra-1 on influencing Bcl-2, Survivin, Bcl-xL, and c-Myc expression,
western blotting was adopted to evaluate the impact of Fra-1
overexpression on a variety of signaling pathways. It was presented
that Fra-1 overexpression inhibited STAT1 (0.36±0.02 vs. 0.92±0.22,
P<0.05), STAT3 (0.01±0.003 vs. 0.39±0.09, P<0.05), STAT6
(0.41±0.08 vs. 0.93±0.25, P<0.05), ERK1/2 (0.56±0.12 vs.
0.92±0.21, P<0.05), and AKT (0.26±0.06 vs. 0.59±0.08, P<0.05)
signaling pathway, of which it showed the most significant effect
on STAT3 signaling pathway (Fig.
5).
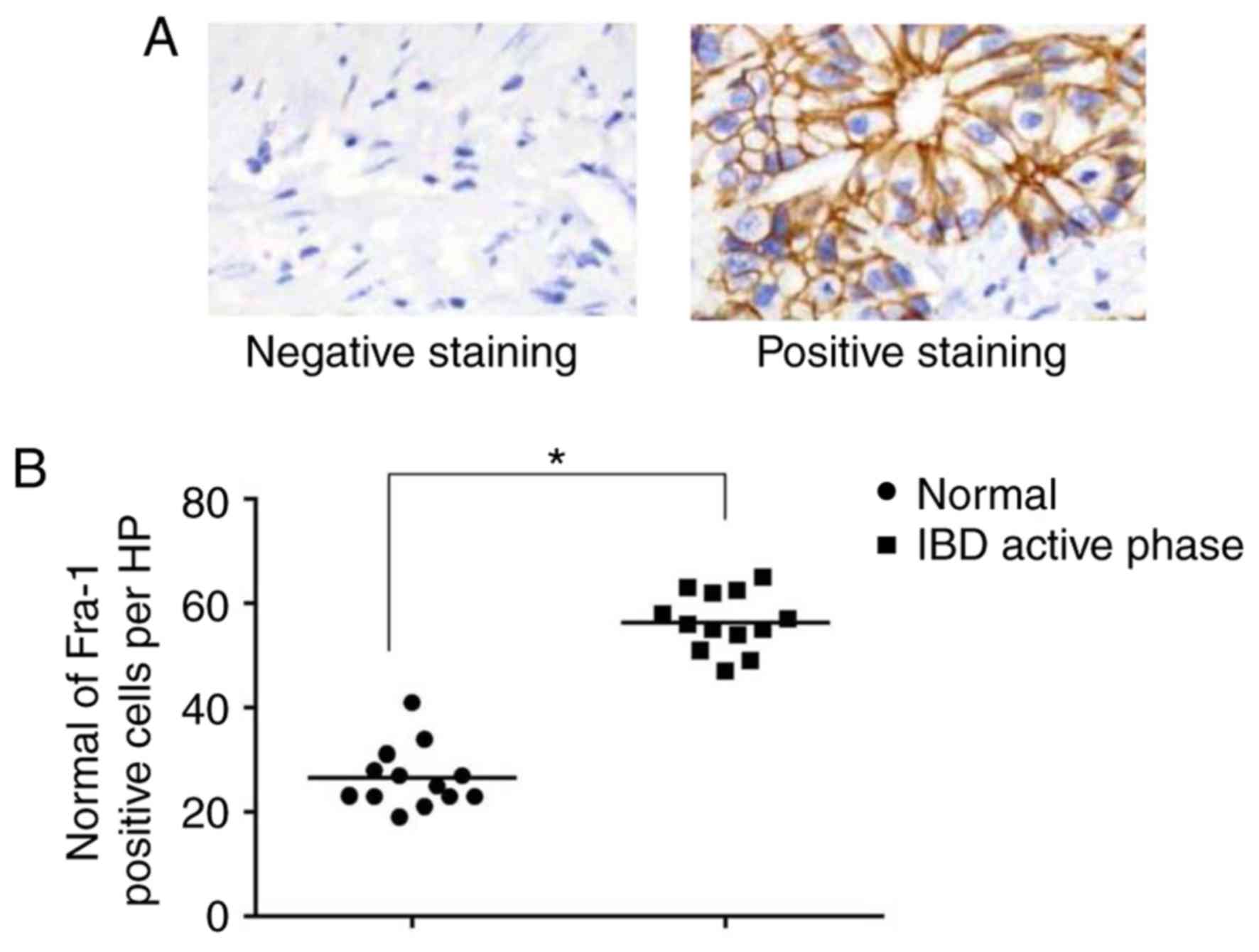
Fra-1 level elevation in intestinal
mucosa epithelial cells in active stage of IBD
Immunohistochemistry was applied to evaluate the
specific role of Fra-1 in active stage of IBD. It was found that
the number of Fra-1 positive inflammatory cells in intestinal
epithelial cells was obviously increased in active stage
(P<0.05). It indicated there may exist factors to induce Fra-1
expression in active stage of IBD, thus Fra-1 played a critical
role in this stage (Fig. 6).
Discussion
IBD, including Crohn's disease and ulcerative
colitis, is a group of chronic inflammatory diseases in the
gastrointestinal tract. Its pathogenesis is still unclear, which is
generally thought to be associated with genetic factors,
environmental factors, and the imbalance of intestinal flora. IBD
is not life-threatening, but the patients may appear recurrent
abdominal pain, diarrhea, and vomiting, seriously affecting the
quality of life.
In clinic, most patients obtain relief through the
immunomodulatory therapy, especially the immune inhibitors and
biological agents. However, a variety of patients are easy to
relapse and difficult to heal. Thus, the role of inflammatory
reaction in IBD remission and relapse attracts more and more
attention.
Fra-1 is one of the members of the Fos family that
contains seven members in mammals. Central SH2 domain and SOCS
module composed of 40 amino acids exist in the protein structure of
each member of the family. JAK/STAT signaling pathway can quickly
activate Fra-1 gene transcription and expression, while Fra-1
protein can inhibit STAT continuous activation, thus forming
negative feedback. Earlier study found that Fra-1 expression in
intestinal mucosal epithelial cells significantly upregulated in
IBD patients at remittent stage (11). Higher Fra-1 expression, shorter IBD
recurrence time, suggesting Fra-1 may be a risk factor of the IBD
deterioration (12,13). Cell proliferation and damage repair
assays demonstrated that Fra-1 overexpression suppressed intestinal
mucosal epithelial cell proliferation and damage repair. Therefore,
Fra-1 upregulation might be one of the important mechanisms of IBD
relapse. Downregulating Fra-1 expression or inhibiting its
upregulation may be effective means to prevent or delay IBD
relapse.
It was reported that various proinflammatory
signaling pathways may be related to Fra-1 upregulation in
intestinal mucosal epithelial cells of IBD patients at remittent
stage, such as STAT1, STAT6, PKA-Cγ, and CREB (14–17).
p-STAT1, p-STAT6, p-PKA-Cγ, and p-CREB levels in intestinal mucosal
epithelial cells also increased compared with healthy people and
showed significant positive correlation with Fra-1. It further
supported the hypothesis that Fra-1 was induced by multiple
proinflammatory signaling pathways. Therefore, the strategy of
inhibiting Fra-1 expression in intestinal mucosal epithelial cells
could be used to explore broad-spectrum anti-inflammatory drugs.
Animal models and clinical trials revealed that intestinal worms
can modify the inflammation in intestinal mucosa epithelial cells,
thus to play a treatment role on IBD. Lin et al (18) reported a case of applying worms
(Trichuris trichiura) to induce and maintain intractable IBD
at remittent stage that cannot be relieved by mesalazine,
6-mercaptopurine, and high dose hormone. Studies reported the
characteristics of intestinal mucosal epithelial cells gene
expression profile, cellular immunity, and histopathology in IBD
under active and remittent stages, while application of
Trichuris trichiura induced IBD relief may be related to
Fra-1 downregulation in intestinal mucosal epithelial cells
(19,20). Trichuris trichiura
engraftment in the intestine may induce immune response produced by
IBD patients aiming to discharge the worm, which is named
IL-22+ T cell-mediated immune response. As an additional
effect, IL-22 induces antibacterial peptides expression and
promotes the repair of the epithelial barrier to induce IBD into
remission, thus to play a treatment role.
Apoptosis related proteins play critical roles in
the inflammatory reaction of IBD. It was revealed that Bcl-2,
Survivin, Bcl-xL, and c-Myc are the members of
inhibitor-of-apoptosis (IAP) family. The protein structure of each
member contained a BIR module composed of 70 amino acids. IAP can
inhibit apoptosis and regulate cell cycle. IAP increased in the
G2/M phase of cell cycle. Once survivin was damaged during this
period, caspase-3 activity enhanced to induce cell apoptosis,
indicating that survivin played an important role in cell
apoptosis. Bcl-2, Survivin, Bcl-xL, and c-Myc levels obviously
declined in the intestinal mucosal epithelial cells of IBD at
remittent stage, suggesting they may participate in intestinal
mucosa epithelium repair to alleviate IBD. Our results exhibited
that Fra-1 overexpression markedly inhibited Bcl-2, Survivin,
Bcl-xL, and c-Myc expression, revealing Fra-1 weakened the
protective effect of intestine mucosa through suppressing their
expression.
Fra-1 overexpression in intestinal mucosal
epithelial cells elevated the risk of IBD relapse by weakening the
protect effect and restraining the damage repair of intestinal
mucosa in IBD at remittent stage. Thus, inhibiting Fra-1 expression
facilitates to IBD remittent maintenance and recurrence delay.
References
|
1
|
Li Y, de Haar C, Chen M, Deuring J,
Gerrits MM, Smits R, Xia B, Kuipers EJ and van der Woude CJ:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar
|
|
2
|
Li Y, Nuij VJ, Baars JE, Biermann K,
Kuipers EJ, Peppelenbosch MP, de Haar C and van der Woude Janneke
C: Increased suppressor of cytokine signaling-3 expression predicts
mucosal relapse in ulcerative colitis. Inflamm Bowel Dis.
19:132–140. 2013. View Article : Google Scholar
|
|
3
|
Li Y, de Haar C, Peppelenbosch MP and van
der Woude CJ: SOCS3 in immune regulation of inflammatory bowel
disease and inflammatory bowel disease-related cancer. Cytokine
Growth Factor Rev. 23:127–138. 2012. View Article : Google Scholar
|
|
4
|
Fang K, Bruce M, Pattillo CB, Zhang S,
Stone R II, Clifford J and Kevil CG: Temporal genomewide expression
profiling of DSS colitis reveals novel inflammatory and
angiogenesis genes similar to ulcerative colitis. Physiol Genomics.
43:43–56. 2011. View Article : Google Scholar
|
|
5
|
Linke A, Goren I, Bösl MR, Pfeilschifter J
and Frank S: Epithelial overexpression of SOCS-3 in transgenic mice
exacerbates wound inflammation in the presence of elevated
TGF-beta1. J Invest Dermatol. 130:866–875. 2010. View Article : Google Scholar
|
|
6
|
Linke A, Goren I, Bosl MR, Pfeilschifter J
and Frank S: The suppressor of cytokine signaling (SOCS)-3
determines keratinocyte proliferative and migratory potential
during skin repair. J Invest Dermatol. 130:876–885. 2010.
View Article : Google Scholar
|
|
7
|
Planell N, Lozano JJ, Mora-Buch R,
Masamunt MC, Jimeno M, Ordás I, Esteller M, Ricart E, Piqué JM,
Panes J and Salas A: Transcriptional analysis of the intestinal
mucosa of patients with ulcerative colitis in remission reveals
lasting epithelial cell alterations. Gut. 62:967–976. 2013.
View Article : Google Scholar
|
|
8
|
Broadhurst MJ, Leung JM, Kashyap V, McCune
JM, Mahadevan U, McKerrow JH and Loke P: IL-22+ CD4+ T cells are
associated with therapeutic Trichuris trichiura infection in
an ulcerative colitis patient. Sci Transl Med. 2:60ra882010.
View Article : Google Scholar
|
|
9
|
Fukui H, Sekikawa A, Tanaka H, Fujimori Y,
Katake Y, Fujii S, Ichikawa K, Tomita S, Imura J, Chiba T and
Fujimori T: DMBT1 is a novel gene induced by IL-22 in ulcerative
colitis. Inflamm Bowel Dis. 17:1177–1188. 2011. View Article : Google Scholar
|
|
10
|
Goldsmith JR, Uronis JM and Jobin C: Mu
opioid signaling protects against acute murine intestinal injury in
a manner involving Stat3 signaling. Am J Pathol. 179:673–683. 2011.
View Article : Google Scholar :
|
|
11
|
Vaishnava S, Yamamoto M, Severson KM, Ruhn
KA, Yu X, Koren O, Ley R, Wakeland EK and Hooper LV: The
antibacterial lectin RegIIIgamma promotes the spatial segregation
of microbiota and host in the intestine. Science. 334:255–258.
2011. View Article : Google Scholar :
|
|
12
|
Mukherjee S, Zheng H, Derebe MG,
Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M,
Jiang QX and Hooper LV: Antibacterial membrane attack by a
pore-forming intestinal C-type lectin. Nature. 505:103–107. 2014.
View Article : Google Scholar
|
|
13
|
Murano T, Okamoto R, Ito G, Nakata T,
Hibiya S, Shimizu H, Fujii S, Kano Y, Mizutani T, Yui S, et al:
Hes1 promotes the IL-22-mediated antimicrobial response by
enhancing STAT3-dependent transcription in human intestinal
epithelial cells. Biochem Biophys Res Commun. 443:840–846. 2014.
View Article : Google Scholar
|
|
14
|
Ahmad R, Chaturvedi R, Olivares-Villagómez
D, Habib T, Asim M, Shivesh P, Polk DB, Wilson KT, Washington MK,
Van Kaer L, et al: Targeted colonic claudin-2 expression renders
resistance to epithelial injury, induces immune suppression, and
protects from colitis. Mucosal Immunol. 7:1340–1353. 2014.
View Article : Google Scholar :
|
|
15
|
Bian Z, Li L, Cui J, Zhang H, Liu Y, Zhang
CY and Zen K: Role of miR-150-targeting c-Myb in colonic epithelial
disruption during dextran sulphate sodium-induced murine
experimental colitis and human ulcerative colitis. J Pathol.
225:544–553. 2011. View Article : Google Scholar
|
|
16
|
Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao
Y, Wang X and Sun B: Interleukin-22 promotes human hepatocellular
carcinoma by activation of STAT3. Hepatology. 54:900–909. 2011.
View Article : Google Scholar
|
|
17
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar :
|
|
18
|
Lin CY, Loven J, Rahl PB, Paranal RM,
Burge CB, Bradner JE, Lee TI and Young RA: Transcriptional
amplification in tumor cells with elevated c-Myc. Cell. 151:56–67.
2012. View Article : Google Scholar :
|
|
19
|
Naher L, Kiyoshima T, Kobayashi I, Wada H,
Nagata K, Fujiwara H, Ookuma YF, Ozeki S, Nakamura S and Sakai H:
STAT3 signal transduction through interleukin-22 in oral squamous
cell carcinoma. Int J Oncol. 41:1577–1586. 2012. View Article : Google Scholar :
|
|
20
|
Dunn ET, Taylor ES, Stebbings S, Schultz
M, Butt AG and Kemp RA: Distinct immune signatures in the colon of
Crohn's disease and ankylosing spondylitis patients in the absence
of inflammation. Immunol Cell Biol. 94:421–429. 2016. View Article : Google Scholar
|