Introduction
The Wnt/β-catenin pathway serves critical functions
in the sequential development of the neoplastic process from
initiation, proliferation and transformation in a broad range of
types of cancer. Aberrant activation of the Wnt pathway may lead to
β-catenin stabilization and accumulation in the cytoplasm, allowing
its translocation into the nucleus to act as a transcriptional
co-activator of members of the T-cell factor (TCF)/lymphoid
enhancing factor (LEF) family of transcription factors. It is known
that the β-catenin/TCF-LEF complex induces the transcription of
genes involved in carcinogenesis including c-myc, matrix
metalloproteinase-7 (MMP-7) and the vascular endothelial growth
factor (VEGF) (1). The tumor
suppressor adenomatous polyposis coli (APC), an essential part of a
multi-protein complex together with protein phosphatase 2A (PP2A),
axin, glycogen synthase kinase 3β (GSK-3β), and casein kinase I
(CK1), serves an important function in turning off the β-catenin
signaling pathway. When this complex is active, GSK-3β and CK1
phosphorylate the amino terminal serine and threonine residues of
β-catenin, an initial step which targets it for degradation by the
proteasomal machinery (2).
Cervical carcinoma, one of the most common
malignancies in women worldwide (3), is associated with persistent
infection with oncogenic or high-risk human papillomavirus
(HR-HPV). Although HR-HPV infection is required, it is insufficient
on its own to cause cervical cancer (4). Several lines of evidence have
indicated a potential association between activation of the Wnt
signaling pathway and HPV-mediated cervical cancer. For instance,
Uren et al (5) and Bulut
et al (6) generated two
models that mimicked the effects of HPV infection on the human
cervical epithelium for cancer progression: i) HPV-immortalized
human keratinocytes, either expressing mutated S37A β-catenin or
overexpressing Wnt1; and ii) double-transgenic mice overexpressing
β-catenin and HPV16-E7 oncogenes. In addition, β-catenin has been
observed to be localized and expressed in the cytoplasm and nucleus
in human cervical carcinoma samples (7,8) and
cell lines (9), indicating the
activation of the Wnt pathway. The results of these previous
studies suggested that activation of the canonical Wnt pathway may
represent one of the key events required for the malignant
transformation of HPV-infected epithelial cells.
Previous studies have demonstrated that β-catenin
deregulation is frequently associated with alterations in the APC
gene, and ~80 to 90% of all APC gene mutations are confined to
codons 1,286 to 1,513, a region known as the mutation cluster
region (MCR), these mutations typically lead to a truncated protein
lacking the β-catenin binding and regulatory sites, as is
frequently observed in colon cancer (10,11).
In addition, transcriptional silencing via promoter
hypermethylation may impair APC function, a phenomenon of
epigenetic inactivation observed in tumors in the absence of APC
gene mutations. For example, in gastric cancer, a decrease in or
loss of APC expression has been identified to be associated with
the APC gene promoter A1 hypermethylation (12,13).
According to these observations, it has been
suggested that the stabilization and activation of β-catenin in
cervical cancer-derived cells are a consequence of alterations in
APC gene expression. To test this hypothesis, the present study
first sought to determine the transcriptional activity of
β-catenin/TCF among a group of cervical cancer cell lines.
Subsequently, MCR-region mutational and methylation status analyses
of the APC gene and its promoter 1A, respectively, were performed
and the impact of APC gene mutations and/or its epigenetic
silencing on two known Wnt target genes in cervical carcinoma cell
lines was analyzed.
Materials and methods
Cell cultures and clinical
samples
Human cervical cancer-derived cell lines CaSki, SiHa
and HeLa, in addition to C33A and the colon cancer cell line SW480
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The gastric cancer cell line KATOIII was provided by Dr
Angel Zarain-Herzberg (Facultad de Medicina-UNAM, CDMX, México).
Human foreskin fibroblasts (HFF) were provided by Dr. María Luisa
Villarreal Ortega (Centro de Investigaciones en Biotecnología-UAEM,
Cuernavaca, Morelos, México). HFF and CaSki cells were grown in
RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); SiHa, HeLa, C33A, SW480 and KATOIII cells were grown in
Dulbecco's modified Eagle's medium/F-12 (Invitrogen; Thermo Fisher
Scientific, Inc.), and all the cultured cells were supplemented
with 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 2 mM L-glutamine (Invitrogen; Thermo Fisher Scientific,
Inc.). Cells were cultured at 37°C in a humidified atmosphere
containing 5% CO2. Normal tissue samples and tumors from
the uterine cervix were obtained from archives of the Department of
Gynecology at the Hospital General Manuel Gea González and the
Department of Pathology at the Instituto Nacional de
Cancerología-SSA (CDMX, México). This set of samples has been
characterized previously for clinical pathological parameters, type
of HPV and β-catenin protein status (7). All the samples were frozen in liquid
nitrogen immediately following resection and stored at −70°C until
processing.
Reporter assay
The TOPflash/FOPflash TCF reporter system (Upstate
Biotechnology, Inc., Lake Placid, NY, USA) was used to directly
measure the levels of Wnt signaling in human cervical cancer cell
lines. A total of 4,000 cells were cultured in 96-well plate and
transfected, with either TOPflash or FOPflash (100 ng) and the
internal control plasmid pRL-Renilla (5 ng; Promega Corporation,
Madison, WI, USA) expressing Renilla luciferase, using
Lipofectamine reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The TOPflash and FOPflash reporters contain two sets of three
copies of wild-type (TOP) or mutant (FOP) β-catenin/TCF binding
sites, respectively, in addition to the thymidine kinase minimal
promoter upstream of the firefly luciferase open reading frame.
Luciferase activity was measured with Dual-Glo™
Luciferase Assay System (Promega Corporation) 48 h following
transfection, according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity. All results are expressed as TOPflash/FOPflash
correlation mean ± standard deviation for independent triplicate
cultures.
Mutational analysis of APC
Genomic DNA was isolated from cells using the
standard Proteinase K treatment followed by phenol/chloroform
extraction (14). Briefly, the
cells were incubated in the lysis buffer (Tris 10 mM, EDTA 20 mM,
SDS 0.5% and Proteinase K 100 µM/ml) for 20 min at 45°C. The lysate
was extracted by phenol/chloroform (1:1, v/v), DNA was precipitated
with isopropanol (1:1, v/v), then washed with 75% ethanol and the
pellet DNA was dissolved in water. The mutation cluster region of
the APC gene was amplified as three overlapping fragments (codons
1032-1703) in a nested polymerase chain reaction (PCR) strategy. In
addition to the previously described primers (15), new primers were used to adapt the
size of different amplicons (Table
I). PCR was performed in a 50-µl volume containing 100 ng
genomic DNA and 0.25 µM each primer using Taq DNA
Polymerase-recombinant (Fermentas; Thermo Fisher Scientific, Inc.).
The PCR reactions were run at 95°C for 5 min followed by 35 cycles
at 95°C for 40 sec, followed by 54°C, 63°C and 60°C for 60 sec as
the annealing step of the three overlapping fragments,
respectively, and finally at 72°C for 60 sec. The PCR products were
purified using the Qiaquick PCR purification kit (Qiagen GmbH,
Hilden, Germany). Mutational analysis by direct sequencing was
performed using the BigDye Terminator version 3.1 Cycle Sequencing
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). All
sequencing analyses were performed at least twice on two
independent PCR products.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene and
method | Sense 5′-3′ | Antisense
5′-3′ | Product length
(bp) |
|---|
| Mutational
analysis |
|
Fragment A |
CCCCTCGAGTCAGATGAGCAGTTG |
CCGGATCCCTGCTTCCTGTGTCG | 796 |
|
Fragment B |
CCCCTCGAGCAGCTCCATCCAAG |
CCGGATCCCCATCTGGAGTAC | 809 |
|
Fragment C |
CCCCTCGAGCCAGATAGCCCTGG |
CCGGATCCCCTCCTTGAGCCTC | 839 |
| MSP |
|
APCprom1AUNMet |
GTGTTTTATTGTGGAGTGTGGGTT |
CCAATCAACAAACTCCCAACAA | 108 |
|
APCprom1AMet |
TATTGCGGAGTGCGGGTC |
TCGACGAACTCCCGACGAA | 98 |
| RT-PCR |
|
MMP7 |
TGTTAAACTCCCGCGTCATA |
GCGTTCATCCTCATCGAAGT | 379 |
|
VEGF |
GAGGGCAGAATCATCACGAA |
AACGCTCCAGGACTTATACC | 395 |
|
β-globin |
CAACTTCATCCACGTTCACC |
GAAGAGCCAAGGACAGGTAC | 260 |
|
APC-exon 15 |
CCCCTCGAGTCAGATGAGCAGTTG |
CCGGATCCCTGCTTCCTGTGTCG | 796 |
Methylation analysis
DNA methylation patterns in the CpG islands of the
APC gene promoter A1 were determined by methylation-specific PCR
(MSP), according to protocols described previously (16). Genomic DNA (1 µg) was treated with
sodium bisulfite using the EZ DNA methylation kit (Zymo Research
Corp., Irvine, CA, USA). For the MSP analysis of APC gene promoter
1A, the primers for the unmethylated reaction amplify a 108-bp PCR
product, and the primers for the methylated reaction amplify a
98-bp product; all primer sequences used are listed in Table I. PCR amplification was carried out
at 95°C for 5 min followed by 35 cycles at 95°C for 60 sec, 60°C
for 40 sec and 72°C for 60 sec. PCR was run in a 50-µl volume
containing 100 ng genomic DNA, 0.25 µM each primer using Taq DNA
Polymerase-recombinant (Fermentas; Thermo Fisher Scientific, Inc.).
Each experiment was repeated twice.
Treatment with 5-aza-2′-deoxycytidine
(Aza) and trichostatin-A (TSA)
Cells were cultured at 60% confluence and incubated
for 48 h in a medium containing 3 µM DNA methyltransferase (DNMT)
inhibitor Aza (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
medium was refreshed every 24 h. The cells were treated with the
histone deacetylase (HDAC) inhibitor TSA (Sigma-Aldrich; Merck
KGaA) at 0.5 and 3 µM of decitabine for further 24 h to complete 72
h of treatment. The medium was removed and the cells were processed
to perform assays by reverse transcription (RT)-PCR and western
blot analysis.
RNA isolation and RT-PCR
Total RNA was extracted from cells by using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. For RT-PCR, total RNA (5 µg) was used
for cDNA synthesis by reverse transcription using a RevertAid H.
Minus First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, in a
total volume of 20 µl. The PCR reactions of APC, VEGF, MMP7 and
β-globin genes were performed using the specific primers presented
in Table I, and standard PCR
conditions were used: 95°C for 5 min, followed by 35 cycles at 95°C
for 60 sec, annealing at 60°C, 62°C, 58°C and 55°C for each gene,
respectively, for 60 sec, and extension at 72°C for 60 sec. The PCR
products were visualized by electrophoresis in 2% agarose gels.
Western blot analysis
Cells were homogenized in lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate and 0.1%
SDS), containing protease inhibitors cocktail (Roche Diagnostics
GmbH, Mannheim, Germany). The protein concentration was determined
by the bicinchoninic acid assay (Pierce; Thermo Fisher Scientific,
Inc.). Total proteins (25 µg) was separated by a 7% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The antibodies and dilutions used included
anti-β-catenin (cat. no. C19220; 1:2,000; BD Biosciences, San Jose,
CA, USA) and anti-β-actin (cat. no. 81178, 1:100; Santa Cruz
Biotechnology Inc., Dallas, TX, USA), both incubated for 5 h at
room temperature and following extensive washing the membranes were
incubated with anti-mouse immunoglobulin G-horseradish
peroxidase-conjugated antibody (cat. no. Nr7074, 1:5,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h at room
temperature and revealed with Western Lightning Chemiluminescence
Reagent Plus (PerkinElmer, Inc., Waltham, MA, USA). Membranes were
probed for β-actin to normalize for loading.
Statistical analysis
Data were analysed using the GraphPad Prism 5.0
statistical program (GraphPad Software, Inc., La Jolla, CA, USA)
and statistical differences were evaluated using one-way analysis
of variance followed by Tukey's multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
β-catenin expression and
transcriptional activity of the β-catenin/TCF complex in cervical
cancer cell lines
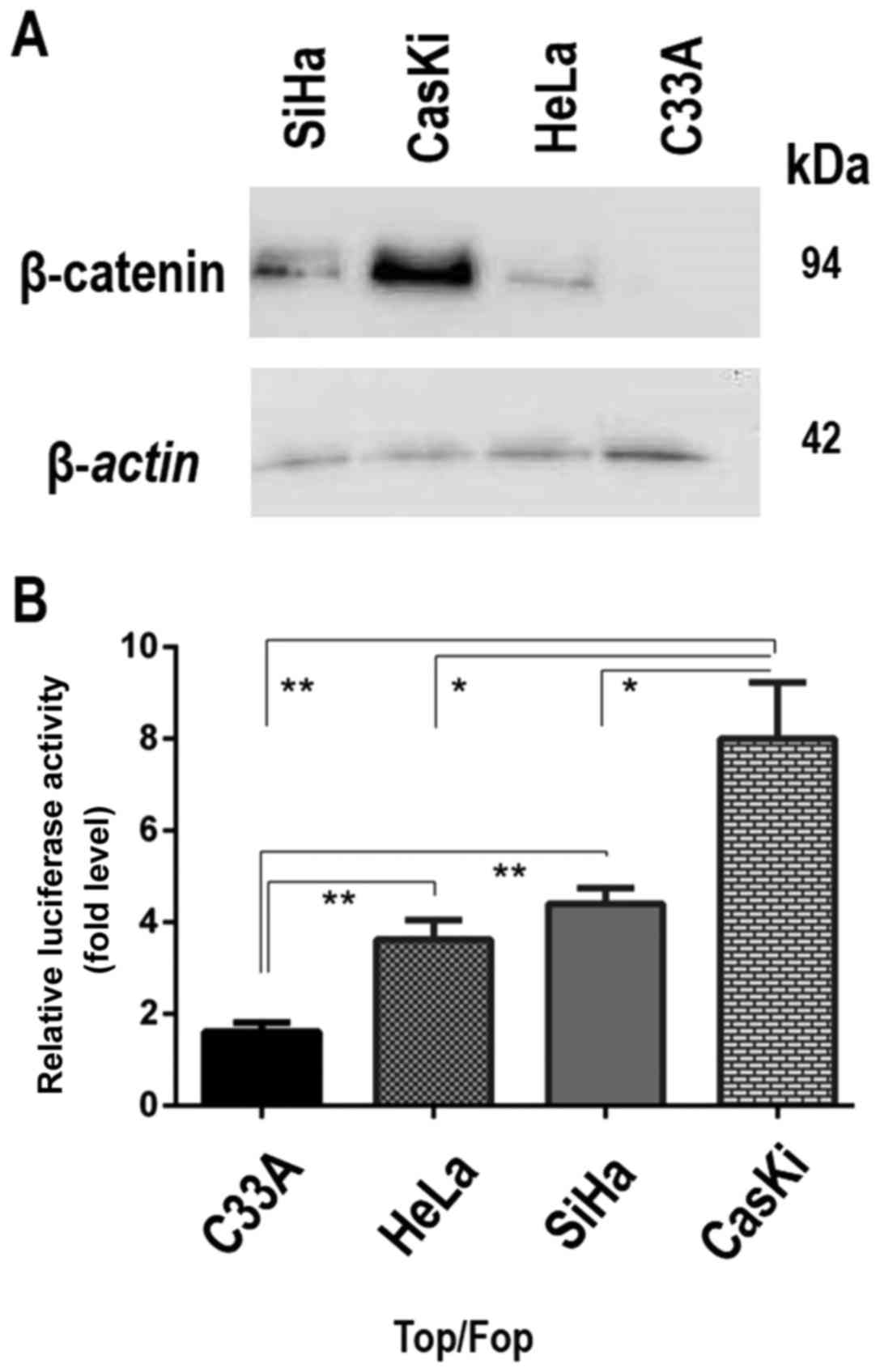
β-catenin levels in different cell lines were
analyzed using western blotting and there was abundant β-catenin in
CaSki cells, low levels in HeLa cells, intermediate levels in SiHa
and none detected in C33A cancer-derived cells (Fig. 1A). The transcriptional activity of
the β-catenin/TCF complex in these four cell lines (CaSki, SiHa,
HeLa and C33A) was subsequently analyzed using a luciferase
reporter assay with TCF reporter constructs (TOPflash and
FOPflash). The results demonstrated that the activity of the TCF
reporter TOPflash was increased compared with the FOPflash reporter
in all cell lines except C33A cells. The transcriptional activity
of the β-catenin/TCF complex was higher in CaSki cells, with higher
β-catenin levels, compared with the transcriptional activity
observed in SiHa and Hela cells (Fig.
1B). The CaSki cells, infected with HPV16, thus exhibited the
highest levels of β-catenin expression and transcriptional
activity. These results demonstrated that the activation of the
Wnt/β-catenin signaling pathway responded in a
concentration-dependent manner to the levels of β-catenin in these
cell lines.
APC gene mutations in cervical cancer
cell lines
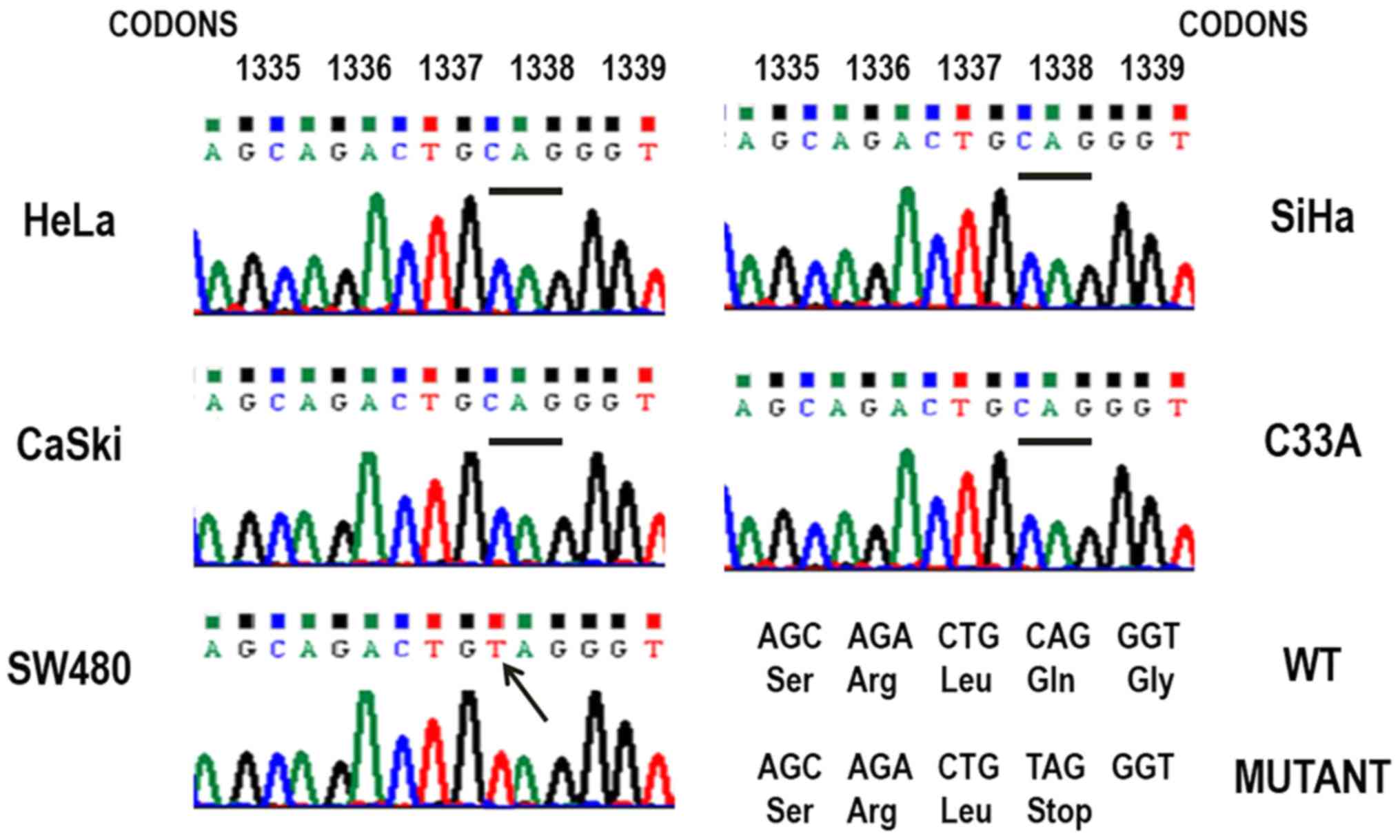
To investigate the existence of sequence variation
in APC, one of the most important members of the β-catenin
degradation complex, the MCR region, was evaluated via PCR and
direct sequencing. As a positive control, DNA extracted from the
colon cancer cell line SW480 was used, homozygous for a mutation at
codon 1338 that generates a truncated APC protein (17), leading to abnormal
accumulation/delocalization of β-catenin. The sequence analysis of
the MCR did not reveal any mutations that were associated with the
activation of APC in the cervical cancer cell lines CaSki, SiHa,
HeLa and C33A (Fig. 2). None of
the known ‘hot-spots’ in codons 1061, 1338 and 1450 were mutated,
the most common sites of mutation in colorectal cancers (18). The mutation at codon 1338 (CAG/TAG)
in SW480 cell line was detected (Fig.
2), confirming the single base specificity of the sequence
analysis detection level. These results suggested that APC
mutations are not responsible for the β-catenin accumulation
observed in the cervical cancer cell lines analyzed.
Methylation of the APC gene promoter
1A in cervical cancer cell lines and biopsies
It has been demonstrated that APC downregulation may
be associated with epigenetic rather than genetic alterations in
other tumor cell types (19,20).
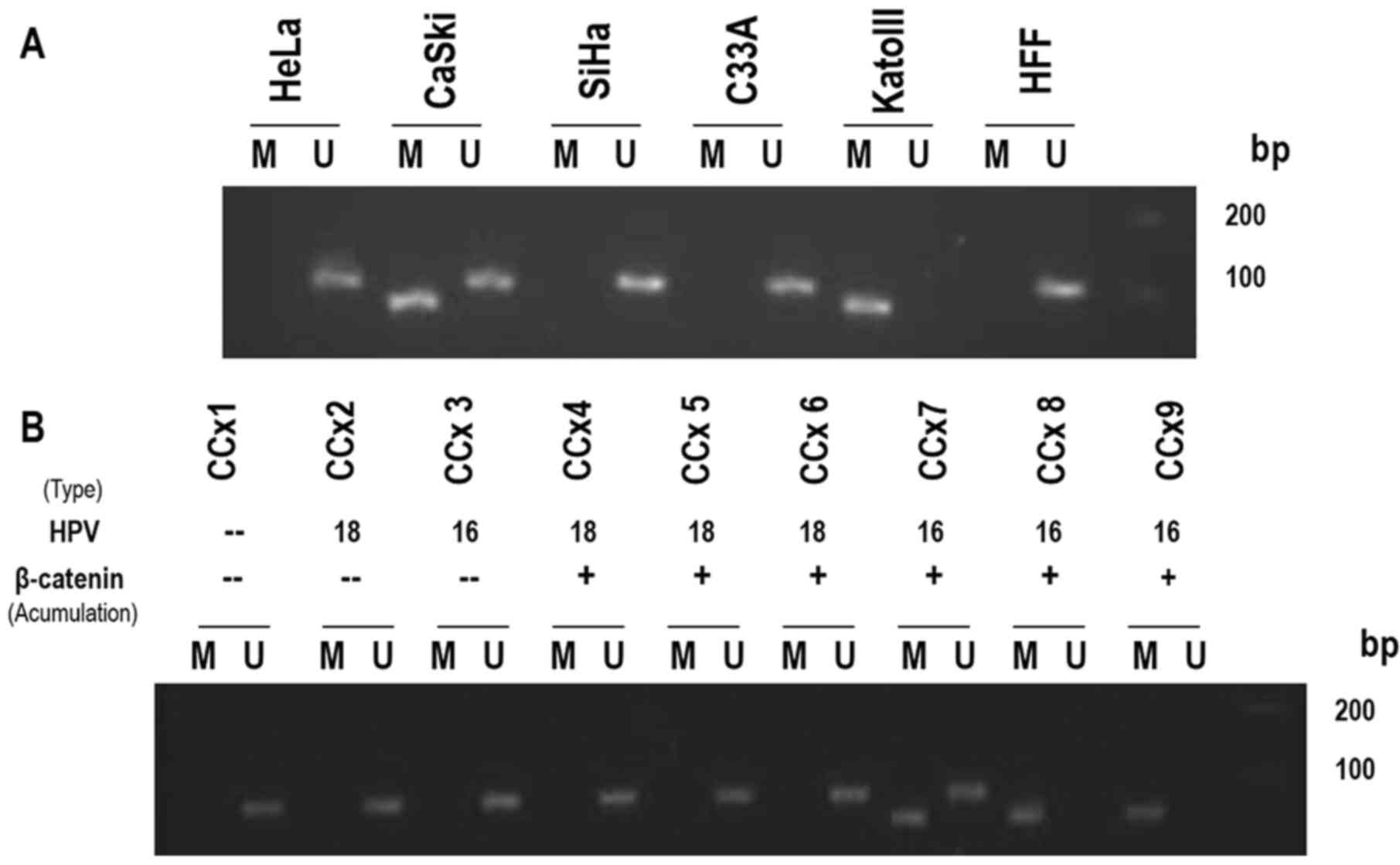
To investigate that possibility, the methylation status of the APC
gene promoter 1A in cervical cancer cell lines was analyzed by MSP.
The gastric cancer cell line KATOIII, which exhibits bi-allelic
methylation (12) and HFF, without
methylation, were used as positive and negative controls,
respectively. The APC gene promoter 1A did not exhibit DNA
methylation at CpG dinucleotides in the C33A, HeLa and SiHa cell
lines. However, CaSki cells demonstrated heterozygous signals,
indicating methylated and unmethylated alleles of the APC gene
promoter 1A. (Fig. 3A).
To further investigate a possible association
between the methylated status of the APC promoter, the levels of
expression of β-catenin or its aberrant localization and HPV
infection, the same MSP analysis was applied to a small group of
biopsies from tumors of the uterine cervix. Different samples, with
variations in the delocalization/accumulation of β-catenin and the
types of HPV (HPV-16 or −18), were analyzed (7). Notably, the MSP results demonstrated
that methylated alleles (homozygous) or unmethylated/methylated
alleles (heterozygous) of the APC gene promoter 1A were only
amplified in cervical tumor samples that exhibited abnormal
subcellular localization of β-catenin and were infected by the
HPV16 type (Fig. 3B). These
observations indicated a clear association between the methylation
status of the APC gene promoter 1A and the abnormal localization of
β-catenin observed in cervical carcinoma specimens infected by
HR-HPV16.
APC expression and Aza/TSA
treatment
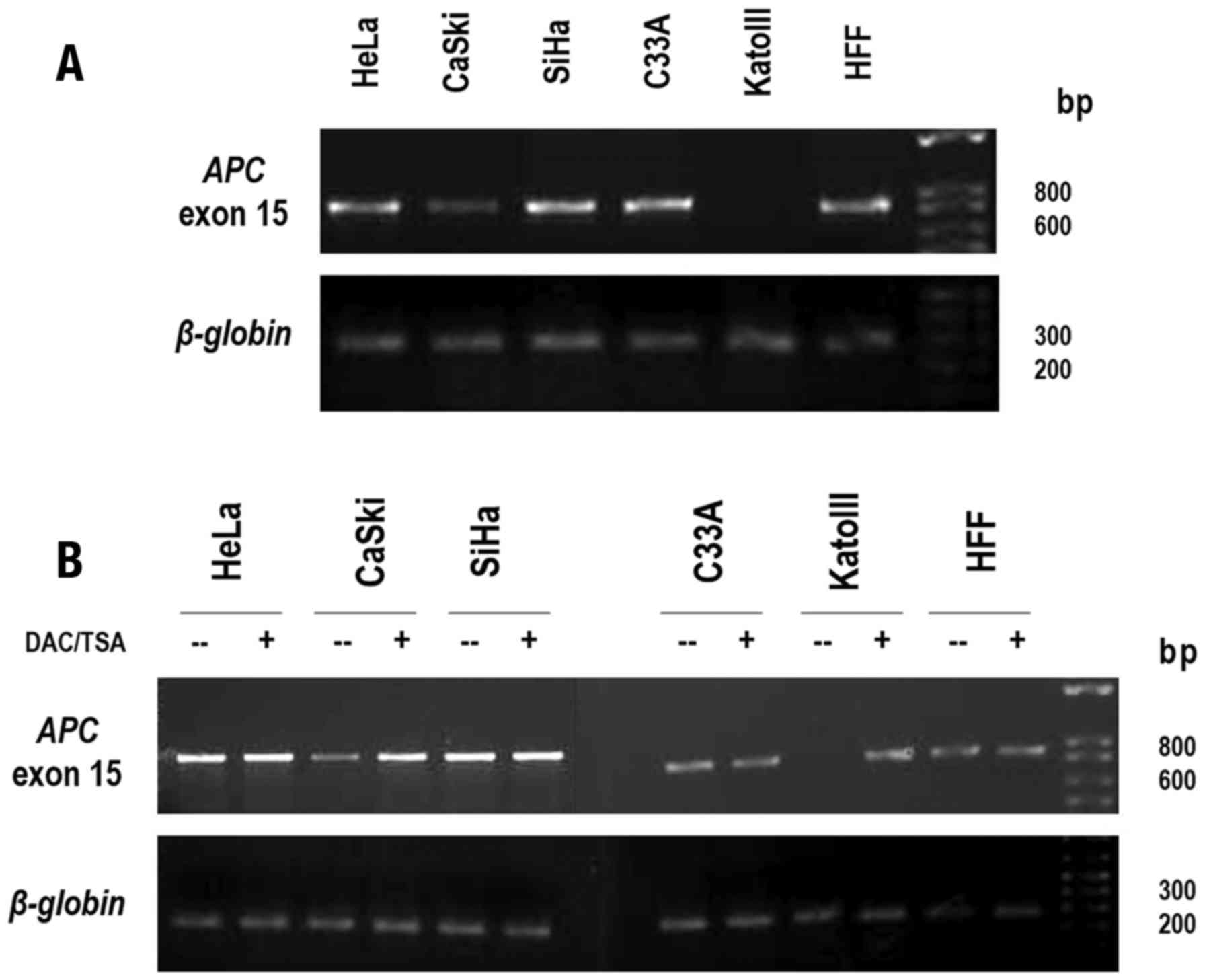
To determine how the methylation patterns of APC
gene promoter 1A identified in certain cervical cancer cell lines
may affect APC transcript levels, their abundance was examined by
quantifying exon 15 of APC by RT-PCR analysis. Abundant APC
transcript expression in C33A, SiHa and Hela cell lines and HFF was
identified where the APC promoter 1A was not methylated, whereas
the KATOIII cell line, which demonstrated bi-allelic methylation,
exhibited undetectable levels of APC mRNA. As expected, a marked
reduction of APC transcripts in CaSki cells, which presented a
heterozygous methylation pattern, was identified (Fig. 4A). These observations indicated
that methylation of the APC gene promoter 1A led to the repression
of APC expression. To further confirm this role of the methylation
of APC gene promoter 1A in the transcriptional repression of the
APC gene, KATOIII cells carrying fully methylated APC alleles and
all cervical cancer cell lines were treated with the DNA
methyltransferase inhibitor, Aza, together with TSA. Following
treatment, the KATOIII cells demonstrated high levels of APC
transcripts, a result associated with the demethylation of APC gene
promoter 1A and the chromatin remodeling by histone acetylation
(13). Of note, CaSki cells also
demonstrated a marked increase in APC transcript expression
following treatment (Fig. 4B),
whereas the HFF, C33A, SiHa and HeLa cells did not demonstrate
changes in APC expression levels. Taken together, these results
highlighted that the levels of APC gene expression were negatively
regulated by the methylation status of the promoter 1A in CaSki and
KATOIII cells.
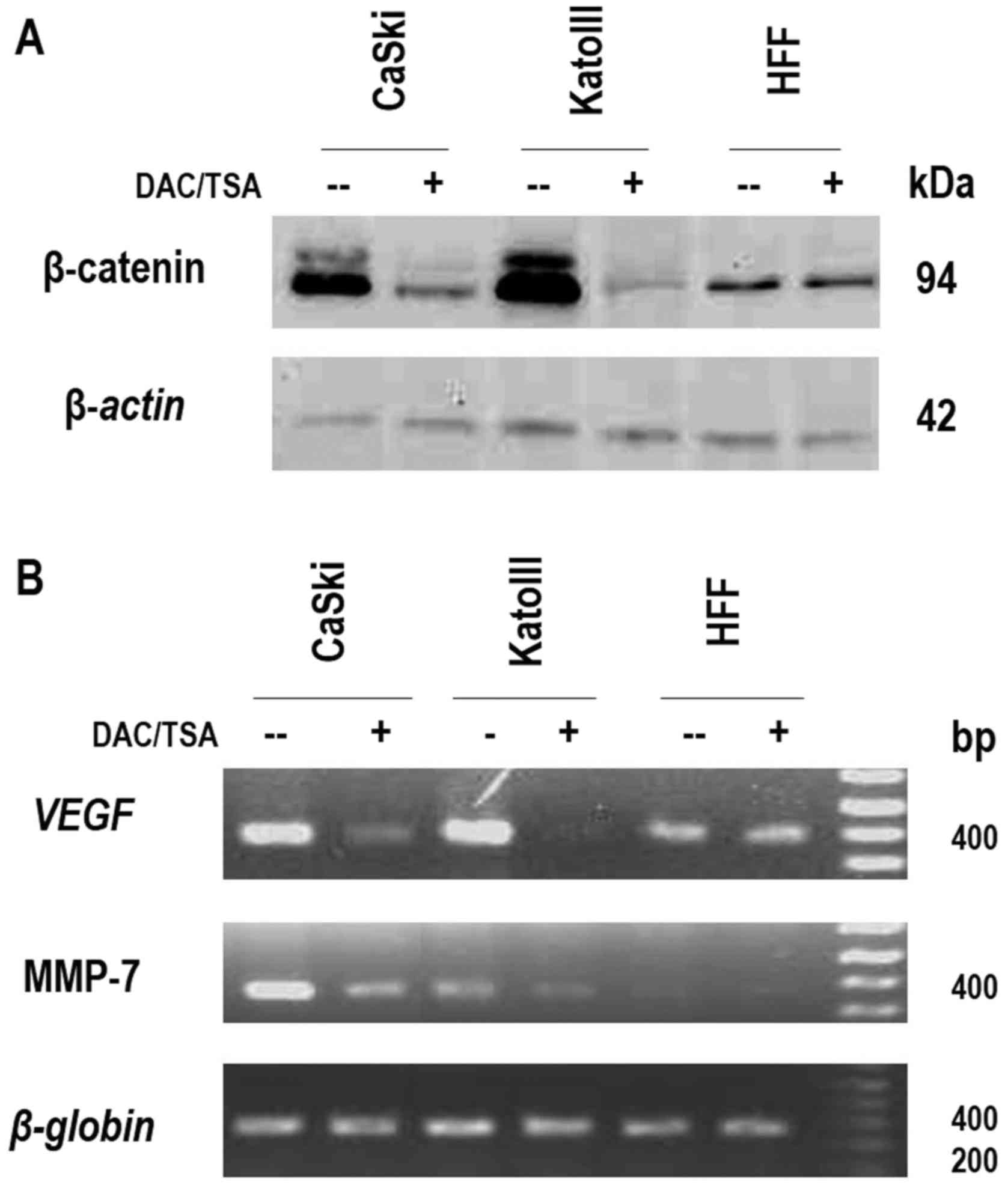
β-catenin expression and Wnt signaling
pathway activity
It was observed that low levels of APC were linked
to the methylation status of its promoter 1A. Since APC is a direct
regulator of β-catenin, it was hypothesized that the reactivation
of APC gene expression may lead to a reduction in β-catenin
accumulation and a decrease in Wnt signaling pathway activity. To
test this hypothesis, the expression of endogenous β-catenin and
the expression of two β-catenin/TCF complex target genes in CaSki
cells were evaluated prior to and following treatment with Aza and
TSA. Prior to treatment, CaSki cells demonstrated abundant
β-catenin protein levels, while following treatment; the β-catenin
levels were significantly reduced. Consistently, alterations in
β-catenin protein expression levels were observed in KATOIII cells,
prior to and following treatment with Aza and TSA. In HFF, the
negative control, the β-catenin protein levels did not alter
following treatment (Fig. 5A). To
further confirm the link between the presence of high β-catenin
levels and high β-catenin/TCF complex activity, RT-PCR analysis was
used to evaluate the expression of VEGF and MMP-7, two well-known
target genes of the Wnt canonical pathway. The VEGF and MMP-7 mRNA
levels were notably reduced following treatment in CaSki and
KATOIII cells, while no alteration was observed in HFF (Fig. 5B). Taken together, these results
confirmed that the methylation of the APC gene promoter 1A in CaSki
cells had a direct effect on the expression of β-catenin and on the
activity of the β-catenin/TCF complex, leading to increased levels
of at least two Wnt target genes: VEGF and MMP-7. This suggested
that this epigenetic modification of the APC gene promoter 1A may
directly contribute to the expression of Wnt signaling pathway
target genes involved in tumor growth and invasion.
Discussion
It has been widely accepted that abnormal activation
of the Wnt signaling pathway leads to the stabilization and
accumulation of β-catenin in the cytoplasm, then to its
translocation to the nucleus, where it promotes the transcription
of multiple genes involved in tumor growth and invasion (2,21,22).
In certain types of cancer, including colorectal and hepatocellular
carcinomas, deregulation of this pathway occurs almost invariably
through mutation of the two key regulators: APC and β-catenin.
These mutations lead to the reduced regulatory activity of APC and
enhanced protein stability of β-catenin, respectively (23,24).
No mutations in CTNNB1 (β-catenin gene) have yet been detected in
cervical neoplasia or cancer-derived cell lines (8,9).
Therefore, the present study aimed to identify mutations in the APC
gene. However, no significant mutations were observed at the
‘hot-spot’ codons (1061, 1338 and 1450) within the MCR, the most
common site of alterations that usually lead to a truncated protein
lacking β-catenin binding and regulation sites (25).
A number of studies have suggested aberrant
methylation in promoter regions as an epigenetic mechanism leading
to the deregulation of tumor suppressor genes. Esteller et
al (16) first demonstrated
the mono- or bi-allelic methylation of APC gene promoter 1A in
tumors of the gastrointestinal tract, including colon, gastric,
pancreatic, esophageal and hepatic carcinomas. Subsequent studies
have detected a similar status of promoter 1A methylation in
several cancers, although in gastric cancer it has been identified
with particularly high frequencies, in >50% of the cases
(12,13,26).
Fu et al (27) suggested
that the hypermethylation of APC gene promoter 1A led to moderate
activation of Wnt signaling pathway in colorectal cancer, instead
of the usual mutations involving the APC and β-catenin genes. In
apparent contradiction with these results, studies in cervical
cancer samples have reported a great diversity in the APC gene
promoter 1A methylation status (28,29),
although none evaluated the association between APC promoter
methylation, APC gene expression and the aberrant activation of the
Wnt signaling pathway. To reconcile these data, the methylation
patterns of the APC gene promoter 1A were evaluated in cell lines
and biopsies from cervical cancer, in order to determine the
frequency of this epigenetic regulation. These samples exhibited
different levels of accumulation/delocalization of β-catenin and
were either infected by HR-HPV type 16 or 18. The results
demonstrated that the methylation frequency ranged from 1/4 (25%)
to 3/9 (~33%) in cell lines and biopsies, respectively. These
results are in conflict with a previous report, which demonstrated
a 90% frequency of APC promoter 1A methylation in biopsies from
cervical cancer (30). However,
the results of the present study are comparable to other studies,
which have proposed that the methylation of the APC promoter 1A,
analyzed by MSP or by quantitative MSP, is not as common in
cervical cancer cells, ranging between 12 and 35% (31–33).
Notably, the results of the present study indicated a strong
correlation between the methylation status of the APC gene promoter
1A and the delocalization/accumulation of β-catenin observed in
biopsies that were infected by HPV16. Furthermore, alleles of APC
promoter 1A which were completely unmethylated (homozygous) were
observed in C33A (HPV negative), and HeLa cells (HPV18). The
mechanism through which HPV16 promotes Wnt signaling requires
further investigation. However, knocking down HPV16 E6/E7 reduced
the Wnt signal in CaSki (HPV16) cells, while overexpressing HPV16
E6 and/or E7 increased the Wnt signal in C33A cells (34).
To confirm the role of the methylation of promoter
1A in the transcriptional regulation of the APC gene, APC gene
expression was examined over the same panel of cervical cancer cell
lines. The results demonstrated no significant difference in APC
transcript levels in C33A, SiHa and Hela cell lines when compared
with HFF, where the APC gene promoter 1A was not methylated. The
absence of APC mRNA in the KATOIII cell line, which presented
bi-allelic hypermethylation, was confirmed (12), and it was identified that CaSki
cells, which demonstrated a heterogeneous methylation pattern,
exhibited a marked reduction of APC mRNA levels. The RT-PCR and MSP
analyses thus demonstrated a correlation between APC expression
levels and the APC gene promoter 1A methylation status in the cell
lines. Additionally, since some studies have demonstrated that the
lack of expression of tumor suppressor genes may be reversed by
treatment with epigenetic silencing inhibitors (inhibitor of DNMT
and HDAC) in cancer cells (35,36),
this strategy was employed to evaluate the recovery of APC gene
expression. The results demonstrated a significant increase in APC
mRNA levels following treatment with Aza and TSA in CaSki cells,
whereas no significant alteration was observed in C33A, SiHa and
HeLa cells. Similar results were observed in melanoma and gastric
cancer cell lines carrying a mono- or bi-allelic methylation of APC
gene promoter 1A, in which the low or absent APC expression levels
were modified following Aza and TSA treatment, leading to its
active transcription and recovery of APC expression (13,37).
These results indicated that the presence of promoter methylation
suppressed APC gene transcription and contributed to inactivating
the APC tumor suppressor function in CaSki cells.
Conversely, previous studies with human cervical
carcinoma samples observed an abnormal accumulation/delocalization
of β-catenin, which constitutes a hallmark of the activated Wnt
pathway (7,8). The present study confirmed through
western blot analyses and luciferase reporter assays that the
abnormal expression of β-catenin is associated, in a
concentration-dependent manner, with the transcriptional activity
of the β-catenin/TCF complex in CaSki, SiHa and HeLa cervical
cancer cells infected with HR-HPV16 or 18. In CaSki cells (HPV16),
with the greatest β-catenin expression, the strongest
transcriptional activity was observed, whereas neither expression
of β-catenin, nor β-catenin/TCF transcriptional activity was
observed in non-HPV infected C33A cervical cancer cells (38,39).
To determine the role of the APC promoter methylation in the
regulation of Wnt/β-catenin signaling, the expression of endogenous
β-catenin and the expression of β-catenin/TCF complex target genes
were evaluated following treatment with Aza and TSA. In CaSki
cells, a reduction of β-catenin levels was observed, in addition to
a decrease in the transcripts of two β-catenin/TCF target genes,
VEGF and MMP-7. These results suggested that stimulating the
expression of the APC gene via treatment with epigenetic silencing
inhibitors reduced the abnormal activation of the Wnt pathway in
CaSki cells. This conclusion is further supported by a study from
Svedlund et al (40), which
demonstrated that the treatment of a primary PC cell culture with
the DNA hypomethylating agent Aza induced APC expression and
reduced β-catenin levels. Taken together, these data suggested that
the re-expression of the APC gene in CaSki cells appears to be
sufficient to assemble a functional complex of
Axin/PP2A/GSK-3β/CK1/APC, capable of phosphorylating the β-catenin
protein, eventually leading to its degradation and switching off
the Wnt/β-catenin signaling pathway (41).
In conclusion, the present study proposed that
methylation-dependent silencing of the APC gene promoter 1A is a
mechanism that contributes to the activation of Wnt signaling
pathway in cervical cancer cells infected by high risk HPV16. The
reduction of APC levels induced by hypermethylation of the APC gene
promoter 1A, rather than direct mutations in the APC or β-catenin
genes, leads to accumulation of β-catenin, which in turn increases
the Wnt/β-catenin transcriptional activity. This mechanism may lead
to an increase in the transcription of genes involved in cancer
development. Therefore, the development of an epigenetic therapy to
reactivate the expression of APC could be advantageous for
the treatment of cervical cancer cells-infected with HPV16.
However, further studies need to be performed to confirm the above
results.
References
|
1
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:a0080522012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munemitsu S, Albert I, Souza B, Rubinfeld
B and Polakis P: Regulation of intracellular betacatenin levels by
the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc
Natl Acad Sci USA. 92:3046–3050. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moody CA and Laimins LA: Human
papillomavirus oncoproteins: Pathways to transformation. Nat Rev
Cancer. 10:550–560. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uren A, Fallen S, Yuan H, Usubütün A,
Kücükali T, Schlegel R and Toretsky JA: Activation of the canonical
Wnt pathway during genital keratinocyte transformation: A model for
cervical cancer progression. Cancer Res. 65:6199–6206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bulut G, Fallen S, Beauchamp EM, Drebing
LE, Sun J, Berry DL, Kallakury B, Crum CP, Toretsky JA, Schlegel R
and Üren A: Beta-catenin accelerates human papilloma virus type-16
mediated cervical carcinogenesis in transgenic mice. PLoS One.
6:e272432011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodríguez-Sastre M, González-Maya L,
Delgado R, Mohar A and García-Carrancá A: Abnormal distribution of
E-cadherin and β-catenin in different histologic types of cancer of
the uterine cervix. Gynecol Oncol. 97:330–336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shinohara A, Yokoyama Y, Wan X, Takahashi
Y, Mori Y, Takami T, Shimokawa K and Tamaya T: Cytoplasmic/nuclear
expression without mutation of exon 3 of the beta-catenin gene is
frequent in the development of the neoplasm of the uterine cervix.
Gynecol Oncol. 82:450–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pereira-Suárez A, Meraz MA, Lizano M,
Estrada-Chávez C, Hernández F, Olivera P, Pérez E, Padilla P, Yaniv
M, Thierry F and García-Carrancá A: Frequent alterations of the
beta-catenin protein in in cancer of the uterine cervix. Tumor
Biol. 23:45–53. 2002. View Article : Google Scholar
|
|
10
|
Nakamura Y, Nishisho I, Kinzler KW,
Vogelstein B, Miyoshi Y, Miki Y, Ando H, Horii A and Nagase H:
Mutations of the adenomatous polyposis coli gene in familial
polyposis coli patients and sporadic colorectal tumors. Princess
Takamatsu Symp. 22:285–292. 1991.PubMed/NCBI
|
|
11
|
Horii A, Nakatsuru S, Miyoshi Y, Ichii S,
Nagase H, Kato Y, Yanagisawa A and Nakamura Y: The APC gene,
responsible for familial adenomatous polyposis, is mutated in human
gastric cancer. Cancer Res. 152:3231–3233. 1992.
|
|
12
|
Tsuchiya T, Tamura G, Sato K, Endoh Y,
Sakata K, Jin Z, Motoyama T, Usuba O, Kimura W, Nishizuka S, et al:
Distinct methylation patterns of two APC gene promoters in normal
and cancerous gastric epithelia. Oncogene. 19:3642–3646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosoya K, Yamashita S, Ando T, Nakajima T,
Itoh F and Ushijima T: Adenomatous polyposis coli 1A is likely to
be methylated as a passenger in human gastric carcinogenesis.
Cancer Lett. 285:182–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Javadi A, Shamaei M, Ziazi Mohammadi L,
Pourabdollah M, Dorudinia A, Seyedmehdi SM and Karimi S:
Qualification study of two genomic DNA extraction methods in
different clinical samples. Tanaffos. 13:41–47. 2014.PubMed/NCBI
|
|
15
|
Miyoshi Y, Ando H, Nagase H, Nishisho I,
Horii A, Miki Y, Mori T, Utsunomiya J, Baba S, Petersen G, et al:
Germ-line mutations of the APC gene in 53 familial adenomatous
polyposis patients. Proc Natl Acad Sci USA. 89:4452–4456. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esteller M, Sparks A, Toyota M,
Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G,
Sidransky D, Meltzer SJ, et al: Analysis of adenomatous polyposis
coli promoter hypermethylation in human cancer. Cancer Res.
60:4366–4371. 2000.PubMed/NCBI
|
|
17
|
Rowan AJ, Lamlum H, Ilyas M, Wheeler J,
Straub J, Papadopoulou A, Bicknell D, Bodmer WF and Tomlinson IP:
APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’
and interdependence of the ‘two hits’. Proc Natl Acad Sci USA.
97:3352–3357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minde DP, Anvarian Z, Rüdiger SG and
Maurice MM: Messing up disorder: How do missense mutations in the
tumor suppressor protein APC lead to cancer? Mol Cancer.
10:1012011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiltunen M, Alhonen L, Koistinaho J,
Myöhänen S, Pääkkönen M, Marin S, Kosma VM and Jänne J:
Hypermethylation of the APC (adenomatous polyposis coli) gene
promoter region in human colorectal carcinoma. Int J Cancer.
70:644–648. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Virmani A, Rathi A, Sathyanarayana U,
Padar A, Huang C, Cunnigham H, Farinas A, Milchgrub S, Euhus D,
Gilcrease M, et al: Aberrant methylation of the adenomatous
polyposis coli (APC) gene promoter 1A in breast and lung
carcinomas. Clin Cancer Res. 7:1998–2004. 2001.PubMed/NCBI
|
|
21
|
Aguiler O, Muñoz A, Esteller M and Fraga
M: Epigenetic alterations of the Wnt/beta-catenin pathway in human
disease. Endocr Metab Immune Disord Drug Targets. 7:13–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kikuchi A: Tumor formation by genetic
mutations in the components of the Wnt signaling pathway. Cancer
Sci. 94:225–229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cieply B, Zeng G, Proverbs-Singh T, Geller
DA and Monga SP: Unique phenotype of hepatocellular cancers with
exon-3 mutations in beta-catenin gene. Hepatology. 49:821–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts DM, Pronobis MI, Poulton JS,
Waldmann JD, Stephenson EM, Hanna S and Peifer M: Deconstructing
the ßcatenin destruction complex: Mechanistic roles for the tumor
suppressor APC in regulating Wnt signaling. Mol Biol Cell.
22:1845–1863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai A, Tong J, To K, Chan M, Man E, Lo K,
Lee J, Sung J and Leung W: Promoter hypermethylation of
tumor-related genes in the progression of colorectal neoplasia. Int
J Cancer. 112:846–853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu X, Li J, Li K, Tian X and Zhang Y:
Hypermethylation of APC promoter 1A is associated with moderate
activation of Wnt signalling pathway in a subset of colorectal
serrated adenomas. Histopathology. 55:554–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang S, Kim J, Kang G, Lee S, Park N, Song
Y, Park S, Kang S and Lee H: Comparison of DNA hypermethylation
patterns in different types of uterine cancer: Cervical squamous
cell carcinoma, cervical adenocarcinoma and endometrial
adenocarcinoma. Int J Cancer. 118:2168–2171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang N, Nijhuis E, Volders H, Eijsink J,
Lendvai A, Zhang B, Hollema H, Schuuring E, Wisman G and van der
Zeea A: Gene promoter methylation patterns throughout the process
of cervical carcinogenesis. Cell Oncol. 32:131–143. 2010.PubMed/NCBI
|
|
30
|
Zambrano P, Segura-Pacheco B,
Perez-Cardenas E, Cetina L, Revilla-Vazquez A, Taja-Chayeb L,
Chavez-Blanco A, Angeles E, Cabrera G, Sandoval K, et al: A phase I
study of hydralazine to demethylate and reactivate the expression
of tumor suppressor genes. BMC Cancer. 5:442005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong S, Kim H, Rha S and Sidransky D:
Promoter hypermethylation of multiple genes in carcinoma of the
uterine cervix. Clin Cancer Res. 7:982–1986. 2001.
|
|
32
|
Wisman GB, Nijhuis ER, Hoque MQ,
Reesink-Peters N, Koning AJ, Volders HH, Buikema HJ, Boezen HM,
Hollema H, Schuuring E, et al: Assessment of gene promoter
hypermethylation for detection of cervical neoplasia. Int J Cancer.
119:1908–1914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van der Meide WF, Snellenberg S, Meijer
CJ, Baalbergen A, Helmerhorst TJ, van der Sluis WB, Snijders PJ and
Steenbergen RD: Promoter methylation analysis of WNT/β-catenin
signaling pathway regulators to detect adenocarcinoma or its
precursor lesion of the cervix. Gynecol Oncol. 123:116–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma C, Zeng C, Jin L, Yang Y, Li P, Chen L
and Wang J: GSK3β mediates the carcinogenic effect of HPV16 in
cervical cancer. Sci Rep. 5:165552015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cameron EE, Bachman KE, Myöhänen S, Herman
JG and Baylin SB: Synergy of demethylation and histone deacetylase
inhibition in the re-expression of genes silenced in cancer. Nat
Genet. 21:103–107. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Phillips DL, Ferguson AT, Nelson
WG, Herman JG and Davidson NE: Synergistic activation of functional
estrogen receptor (ER)-alpha by DNA methyltransferase and histone
deacetylase inhibition in human ER-alpha-negative breast cancer
cells. Cancer Res. 61:7025–7029. 2001.PubMed/NCBI
|
|
37
|
Worm J, Christensen C, Grønbaek K,
Tulchinsky E and Guldberg P: Genetic and epigenetic alterations of
the APC gene in malignant melanoma. Oncogene. 23:5215–5226. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2
suppress the transformation and invasion abilities of cervical
cancer cells through Wnt signal pathway. Gynecol Oncol.
112:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a beta-catenin-Tcf complex in
APC−/−colon carcinoma. Science. 275:1784–1787. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Svedlund J, Aurén M, Sundström M, Dralle
H, Akerström G, Björklund P and Westin G: Aberrant WNT/β-catenin
signaling in parathyroid carcinoma. Mol Cancer. 9:2942010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang M, Wang Y, Sun D, Zhu H, Yin Y,
Zhang W, Yang S, Quan L, Bai J, Wang S, et al: Identification of
genes regulated by Wnt/beta-catenin pathway and involved in
apoptosis via microarray analysis. BMC Cancer. 6:2212006.
View Article : Google Scholar : PubMed/NCBI
|