Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is one of
the most severe types of hemorrhage in the brain, with mortality
rates ranging between 25 and 35% in developed countries, and up to
48% in developing countries (1).
SAH leads to poor outcomes, particularly attributed to progressive
brain injury following the initial hemorrhage. Early cerebral
injury during the first few days following SAH has been recognized
to be associated with increased mortality and morbidity among
survivors (2). Further damage from
SAH may occur following a 1–2 week delay, induced by
vasospasm-associated ischemic injury (3). Therefore, it is important to
administer effective treatment as early as possible following SAH
to prevent ischemic injury. Limb remote ischemic post-conditioning
(RIPostC) is a novel post-conditioning procedure which involves
repeated occlusion/release cycles on bilateral limb arteries
(4). Unlike classical pre- or
post-ischemic conditioning, limb RIPostC is easily adaptable in
clinical practice and particularly suitable for long term
rehabilitation (5–7). Numerous studies have demonstrated
that limb RIPostC improves neurological outcomes in ischemic animal
models (5,8). However, there are few studies
regarding the effect on hemorrhagic stroke and the mechanisms
behind RIPostC.
Autophagy is an important cellular pathway for the
degradation of intracellular macromolecules or organelles for
subsequent reuse, which helps to maintain intracellular homeostasis
of physiological conditions (9).
Previous studies have suggested that autophagy is an important
arbiter of cell death-survival decisions, by degrading harmful
aggregates and organelles in inflammation, malignancy and
neurodegeneration (10,11). Autophagy-lysosomal system
activation is reported to be involved in SAH-induced brain injury
(12). Certain studies have
indicated that autophagy activation may exhibit a neuroprotective
effect in SAH-associated injury (13,14).
Therefore, in the present study, the methodology of
limb RIPostC was optimized with the aim of investigating the short
and long term neuroprotective effects and possible role of
autophagy activation in limb RIPostC, using a puncture rat model of
SAH.
Materials and methods
Experimental animals and groups
All experiments were approved by the ethics
committee of the Animal Care and Experimental Committee of the
School of Medicine of Shanghai JiaoTong University (Shanghai,
China). The animals were maintained on a 12-h light/dark cycle
under controlled temperature conditions (22±2°C), and given
standard food and water ad libitum. A total of 77
Sprague-Dawley male rats (obtained from the School of Medicine of
Shanghai Jiao Tong University, Shanghai, China), weighing between
260 and 300 g, were divided randomly into five weight-matched
groups for the short-term experiments: Sham-operated (sham; n=12),
SAH (n=18), SAH treated with early RIPostC (eRIPostC; n=16), SAH
treated with delayed RIPostC (dRIPostC; n=16) and SAH treated with
repeated RIPostC (rRIPostC; n=15). For the long-term experiments, a
total of 48 rats were assigned to three groups: Sham-operated
(sham; n=12), SAH (n=20) and daily repeated RIPostC (RIPostC;
n=16). Prior to surgery, all rats fasted for 72 h with free access
to water. A total of 14 rats which succumbed to the surgery, and 12
rats which succumbed during the month following surgery, were
discarded from the present study.
Rat model of SAH and RIPostC
Rats were anesthetized by mechanical ventilation
with 2% isoflurane in oxygen (2:1) and received a subcutaneous
injection of Marcaine (2 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to provide topical analgesia prior to incision.
During the operation, a temperature-controlled heating pad was used
to maintain the rectal temperature at 37.5°C. SAH was induced using
endovascular perforation on the internal carotid artery (ICA)
bifurcation with a sharpened monofilament 4–0 nylon suture as
described by Bederson et al (15). Following exposure of the left
carotid artery and its branches, it was transected distally and
fashioned into a stump. The suture was advanced into the left ICA
through the common carotid bifurcation and further advanced into
the left intracranial ICA until resistance was felt, ~18–20 mm from
the common carotid artery bifurcation. The suture was further
advanced for ~3 mm to perforate the circle of Willis, and withdrawn
after ~15 sec. The ICA was subsequently re-perfused. Sham-operated
rats underwent the same procedure, except that the suture was
removed once resistance was felt without puncture. Cerebral blood
flow was measured prior and subsequent to SAH using a PR407-1
straight needle LDF-probe (Perimed, Järfälla, Sweden) connected to
a standard laser Doppler monitor (PF5010 LDPM Unit and PF5001 main
unit; Perimed). SAH was confirmed by cerebral blood flow detection
and autopsy in each rat. All animals were allowed to recover
following surgery and housed individually until euthanasia. In the
RIPostC groups, limb RIPostC was carried out by three cycles of 10
min occlusion/10 min release of the bilateral femoral artery using
an aneurysm clip at 0 min (early RIPostC) and 30 min (delayed
RIPostC) of SAH. For rRIPostC, all rats were subjected to 3 cycles
of 10 min occlusion/10 min release every day for 3 days. For the
long-term study, the limb RIPostC was performed every day for 1
month. The methodology of limb RIPostC in each group is presented
in Fig. 1A.
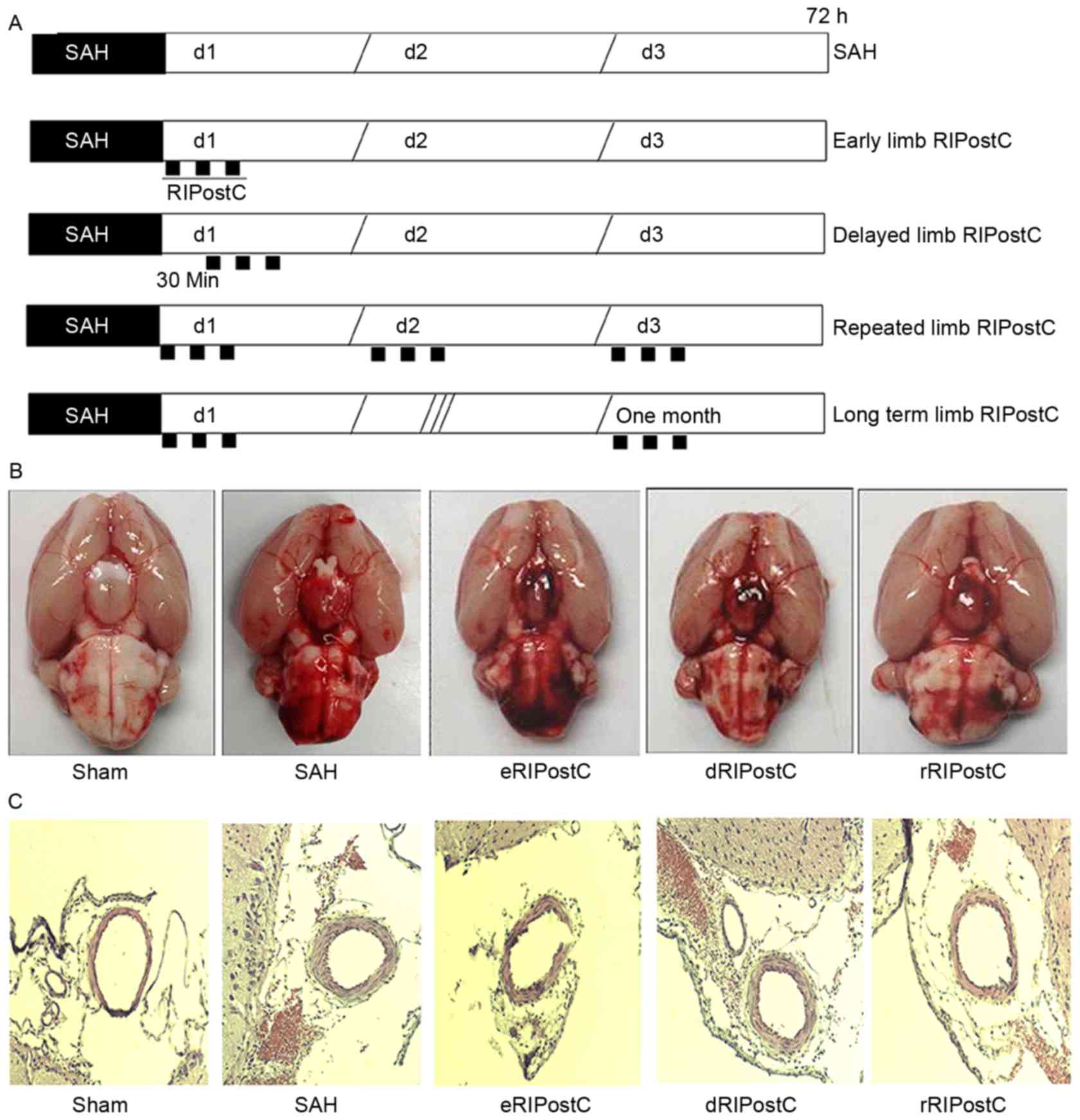 | Figure 1.The effect of rRIPostC on bleeding and
vasospasm. (A) The schematic protocol for limb RIPostC. Limb
RIPostC was performed with three cycles of 10 min occlusion (black
rectangles)/10 min release on the bilateral femoral artery using an
aneurysm clip. According to the methodology of post-conditioning,
RIPostC rats were divided into early RIPostC (start at 0 min),
delayed RIPostC (start at 30 min), repeated RIPostC (days 0, 1, and
2) and long term RIPostC (undergo three cycles every day for 1
month). (B) SAH score: Sham, 0; SAH, 12.57±2.50; eRIPostC,
13.01±1.56; dRIPostC, 13.25±1.71; and rRIPostC, 12.25±1.93. (C)
Hematoxylin-eosin histological analysis: Sham, 0.48±0.02; SAH,
0.37±0.05; eRIPostC, 0.31±0.06; dRIPostC 0.35±0.07; and rRIPostC,
0.35±0.03. eRIPostC, early remote ischemic post-conditioning;
dRIPostC, delayed RIPostC; rRIPostC, repeated RIPostC; SAH,
subarachnoid hemorrhage. |
SAH grade
The extent of SAH in all animals was evaluated as
described previously by Sugawara et al (16), 72 h following surgery. Following
removal of the brain, an image of the basal cistern was captured
and it was divided into 6 parts (left and right frontal, left and
right temporal, and upper and lower brain stem). Depending on the
prevalence of subarachnoid blood clots, each segment was given a
grade from 0 to 3 as follows: Grade 0, no subarachnoid blood
clotting; grade 1, minimal subarachnoid blood clotting; grade 2,
moderate blood clotting with recognizable arteries; and grade 3,
blood clotting obliterating all arteries within the segment. The
total score (maximum, 18) was calculated as the sum of all of the
sub-scores.
Brain water content
At 72 h post-SAH, rats were euthanized and brains
were harvested. Each left hemisphere, right hemisphere, cerebellum
and brain stem, were separated and weighed immediately (wet
weight), and subsequent to drying at 100°C for 72 h (dry weight).
The percentage water content was calculated as: [(Wet weight-dry
weight)/wet weight]x100.
Neurological evaluation and behavior
tests
Neurological scores were evaluated prior to
euthanasia in a blinded fashion at 72 h, with a
previously-described 18-point scoring system for assessing
sensorimotor deficits. There were six categories in the assessment,
including spontaneous activity, symmetrical movements of limbs,
forelimb outstretching, climbing a wall of a wire cage, axillary
touch response and vibrissae touch response. The worst score was 0
and the best was 3 for each subtest, and the total score was
calculated from all of the subtests. Beam walking and grid walking
were applied to evaluate motor function following SAH. A 0–6
grading beam walking test was used to assess ability to walk across
and maintain balance on a beam (2.5×2.5×80 cm). The response scores
were assigned as follows: Score 0, traversed the beam with no foot
slip; score 1, traversed with grasping of the lateral side of the
beam; score 2, exhibited difficulty crawling across the beam,
although able to traverse; score 3, required >10 sec to traverse
the beam due to difficulty in walking; score 4, unable to traverse
the beam; score 5, unable to move the body or any limb on the beam;
and score 6, unable to stay on the beam for >10 sec. Grid
walking ability was assessed by placing the animal on a stainless
steel grid floor (20×40 cm, with a mesh size of 2×2 cm). The total
number of steps was counted to a maximum of 50 steps. The number of
foot fault errors was defined as the frequency of misplacement of a
forelimb or hind limb through the grid floor.
Rotarod test
Long term neurological function, including motor
coordination and learning, was measured using an accelerating
rotarod (San Diego Instruments, Inc., San Diego, CA, USA). Between
days 1 and 9, each rat was placed on the 2.75 cm-diameter rod for
training. The 2nd, 3rd and 4th days were testing days. The time for
which each rat was able to remain on the rotating rod before
falling was measured. The maximum duration was 300 sec. All of the
tests were repeated three times in a blinded fashion.
Morris water maze (MWM) test
Rats were tested for spatial learning and memory in
the MWM test. The MWM consisted of a circular tank measuring 1.5 m
in diameter, with the water temperature maintained at ~21°C and
made opaque using non-toxic white paint powder (Reeves & Poole,
North York, ON, Canada) on the surface. Four points around the edge
of the pool were arbitrarily designated as north (N), south (S),
east (E) and west (W), allowing the apparatus to be divided into
four corresponding quadrants (NE, SE, NW and SW). A transparent
Plexiglas escape platform was submerged ~2 cm below the water
surface and placed in the NE quadrant of the maze. Extra-maze cues
consisted of laboratory furniture and lights (kept constant
throughout the experiment). A video camera was mounted above the
center of the pool and all performance was recorded for subsequent
analyses. Rats were given 3 trials/day for each of 5 test days (120
sec trial, 120 sec inter-trial interval during which time the rat
remained on the escape platform). If the rat did not find the
escape platform within the allotted time, it was guided to the
finish by the experimenter. Escape latencies and intertribal
behavior were recorded by observers who were blind to the
experimental treatment.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)-DAPI staining
In order to detect DNA fragmentation in degenerating
neurons, animals were sacrificed 3 days subsequent to reperfusion,
and coronal sections (5 µm) of freshly-frozen rat brain were cut
using a cryotome. The TUNEL assay was carried out, according to the
manufacturer's protocol (Roche Diagnostics GmbH, Mannheim,
Germany). The sections were fixed in 4% paraformaldehyde for 20 min
at room temperature and permeabilized using 0.1% Triton X-100
(Sigma-Aldrich; Merck KGaA) and 0.1% sodium citrate for 2 min at
4°C. Each slide was incubated with 50 µl TUNEL reaction mixture at
37°C for 1 h. Slides were mounted with DAPI (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Images were viewed
under an ECLIPSE Ti fluorescence microscope (Nikon Corporation,
Tokyo, Japan) and captured using a CoolSNAP camera (Photometrics,
Tucson, AZ, USA). TUNEL-positive cells were photographed at ×100
magnification, at 3 fields close to the infarct border. Apoptotic
cells were quantitatively evaluated by diagnostic software
NIS-Elements BR (version 3.2; Nikon Corporation).
Hematoxylin and eosin staining for
vessel lumen measurement
Brain sections were cut every 10 µm over the SAH
affected area, including the circle of Willis and basilar arteries,
and were stained with hematoxylin (10 min at room temperature) and
eosin (2 min at room temperature). Histological photographs were
captured using a microscope camera at ×400 magnification. In order
to determine the degree of vasospasm, the ratio of luminal diameter
to total arterial diameter was measured using Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA). Five measurements per rat were taken, and the mean score was
calculated; the mean values of all of the rats in each group were
used to calculate the group mean.
Western blotting analysis for the
detection of autophagy
The frozen brain samples were mechanically lysed in
20 mM Tris (pH 7.6), containing 0.2% SDS, 1% Triton X-100, 1%
deoxycholate, 1 mM phenylmethylsulfonyl fluoride, and 0.11 IU/ml
aprotinin (all Sigma-Aldrich; Merck KGaA). The lysates were
centrifuged at 12,000 × g for 20 min at 4°C. The protein
concentration was estimated using the Bradford method with a
Nanjing Jiancheng protein assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The samples (60 µg/lane)
were separated by SDS-PAGE on an 8% gel and electro-transferred
onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membrane was blocked with 5% skimmed
milk for 2 h at room temperature and incubated overnight with
primary antibodies against LC-3 (cat. no. 12741; 1:200) and
Beclin-1 (cat. no. 3495; 1:150) (both Cell Signaling Technology,
Inc., Danvers, MA, USA) at 4°C. GAPDH (diluted 1:6,000;
Sigma-Aldrich; Merck KGaA) was used as the loading control. The
membrane was washed six times in PBS with Tween-20 (PBST) for 10
min, and was subsequently incubated at room temperature for 2 h
with horseradish peroxidase-conjugated secondary antibody (1:400;
cat. no. 7074; Cell Signaling Technology, Inc.). The blotted
protein bands were visualized by enhanced chemiluminescence western
blot detection reagents (GE Healthcare Bio-Sciences, Pittsburgh,
PA, USA) and exposed to X-ray film. The developed films were
digitized using an Epson Perfection 2480 scanner (Epson, Nagano,
Japan). The results were quantified using Quantity One Software
(version 4.6.2; Bio-Rad Laboratories, Inc.). The band density
values were calculated as a ratio of light chain 3 (LC3)/actin or
Beclin-1/actin.
Transmission election microscopy
Rat brain samples for electron microscopy were fixed
in phosphate-buffered glutaraldehyde overnight at 4°C (2.5%;
Sigma-Aldrich; Merck KGaA) and osmium tetroxide at room temperature
for 2 h (1%; Sigma-Aldrich; Merck KGaA). Dehydration of the cortex
was accomplished in acetone solutions at increasing concentrations.
The tissue was embedded in an epoxy resin. Semi-thin (1 µm)
sections through the sample were stained with toluidine blue (30
min at room temperature). Sections of 0.06 µm were created from a
selected area of tissue defined by the semi-thin section, and these
were stained with lead citrate and uranyl acetate at room
temperature for 1 h. The ultrastructure of the brain was observed
under a transmission electron microscope (JEM-1200X; JEOL USA,
Inc., Peabody, MA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. The statistical significance was examined using analysis
of variance, followed by Student's t-test and post-hoc Fisher's
tests. P<0.05 was considered to indicate a statistically
significant difference. The statistical software SPSS (version
13.0; SPSS Inc., Chicago, IL, USA) was used for the statistical
analyses.
Results
Repeated limb RIPostC decreases brain
edema, and exhibits no effect on bleeding and vasospasm
On the 3rd day post-surgery, the rats were
sacrificed and images were taken of the base of the brain in order
to calculate the scale of SAH (Fig.
1B). The SAH score demonstrated that none of the limb RIPostC
groups exhibited a notable effect on bleeding. Hematoxylin-eosin
histological analysis was performed to detect the vasodilative
effect of RIPostC (Fig. 1C). The
ratio of luminal diameter to total arterial diameter did not
notably alter among all limb RIPostC groups.
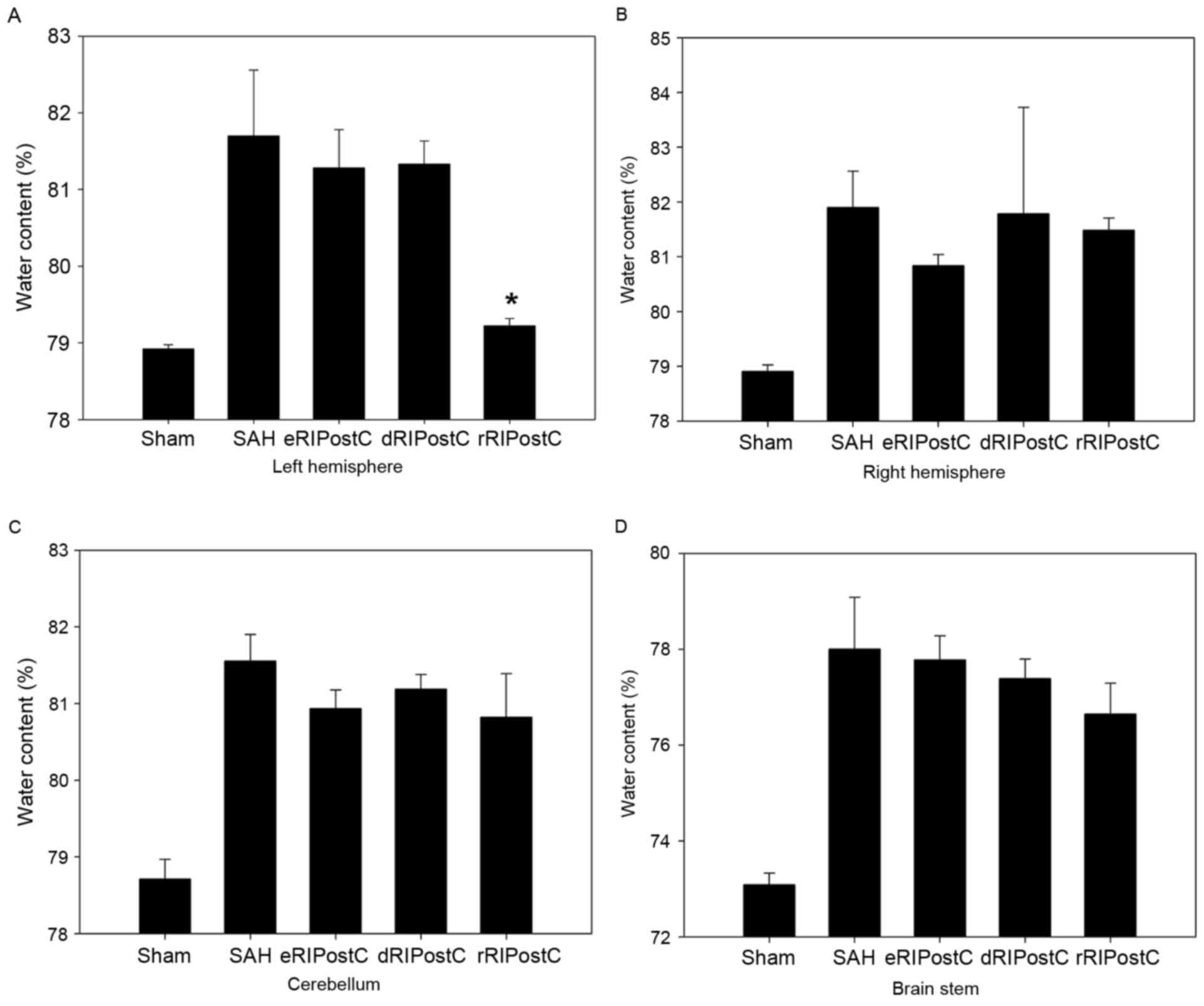
A total of four parts of the brain, including left
hemisphere, right hemisphere, cerebellum and brain stem, were
analyzed for brain edema using the drying procedure described above
(Fig. 2). It was observed that
only repeated limb RIPostC was able to decrease brain edema
compared with the other groups, and only in the left
hemisphere.
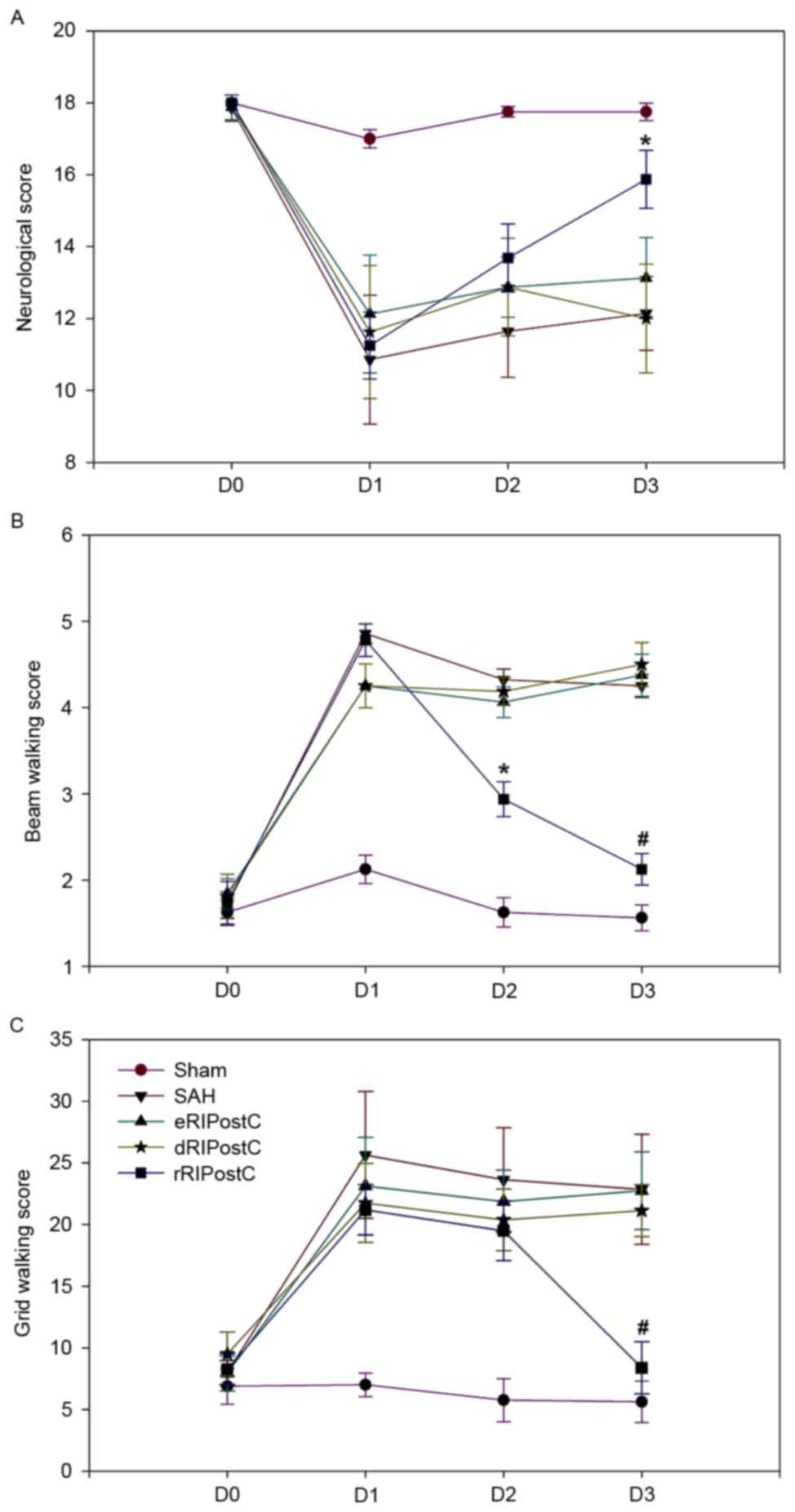
Repeated limb RIPostC improves the
short-term neurological evaluation scales and motor function of SAH
model rats
An 18-point score system was used to assess
short-term sensorimotor deficits on days 0, 1, 2 and 3 following
SAH. Compared with the other groups, the score of the rRIPostC
group was significantly increased on day 3, indicating that it was
able to alleviate short-term neurological deficits (Fig. 3A).
Short-term motor function was assessed using beam
walking and grid walking tests on days 0, 1, 2 and 3 following SAH
(Fig. 3B and C). The beam walking
test (sham group, 1.56±0.14; SAH group, 4.25±0.13; eRIPostC group,
4.37±0.24; dRIPostC group, 4.50±0.25; and rRIPostC group,
2.12±0.18) and grid walking scores (sham group, 5.62±1.68; SAH
group, 22.85±4.46; eRIPostC group, 22.75±3.15; dRIPostC group,
21.12±2.10; and rRIPostC group, 8.37±2.12) indicated that repeated
limb RIPostC was able to improve short-term motor function.
Repeated limb RIPostC protects neurons
from apoptotic cell death following SAH injury
The TUNEL assay was carried out to detect neuronal
apoptosis on day 3 following SAH. TUNEL-positive cells (green) were
observed at ×100 magnification, at 3 fields close to the infarct
border. All groups except sham exhibited neuronal apoptosis.
TUNEL-positive cells of all RIPostC groups were reduced compared
with the SAH group. Quantitative analysis demonstrated that
repeated limb RIPostC was able to prevent neuronal apoptosis
(Fig. 4).
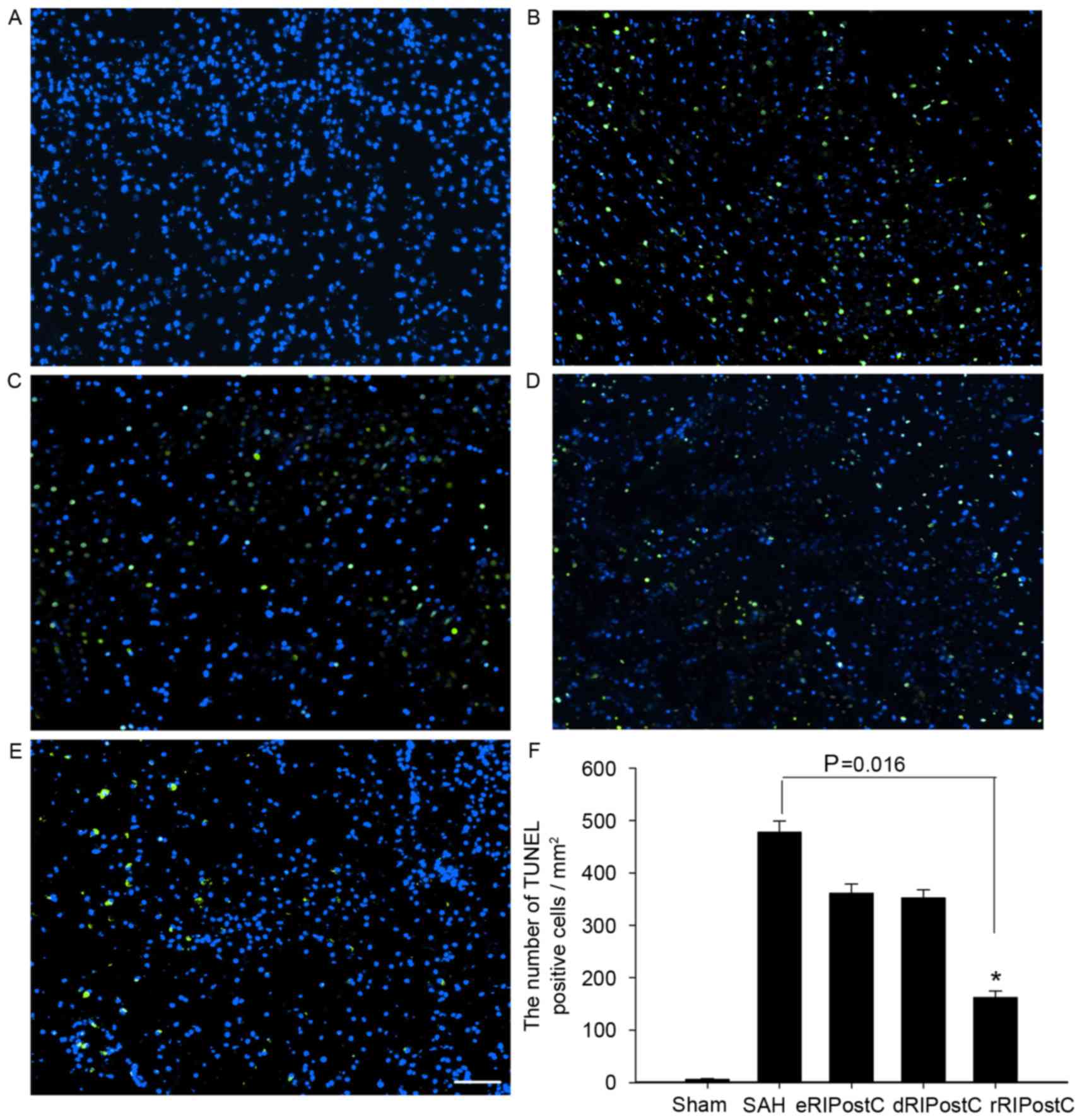 | Figure 4.TUNEL assay analysis of the cortex at
3 days following SAH. All groups except the sham exhibited neuronal
apoptosis. TUNEL-positive cells (green) of all RIPostC groups were
decreased compared with the SAH group. (A) Sham group, 6.22±1.28;
(B) SAH group, 477.77±21.23; (C) eRIPostC group, 361.11±17.73; (D)
dRIPostC group, 352.34±15.56; (E) rRIPostC group, 162.23±12.22. (F)
Quantitative analysis demonstrated that the number of
TUNEL-positive cells in the rRIPostC group decreased, suggesting
that rRIPostC was able to protect neurons from apoptosis.
*P<0.05. SAH, subarachnoid hemorrhage; eRIPostC, early remote
ischemic post-conditioning; dRIPostC, delayed RIPostC; rRIPostC,
repeated RIPostC; TUNEL, terminal deoxynucleotidyl transferase dUTP
nick end labeling. |
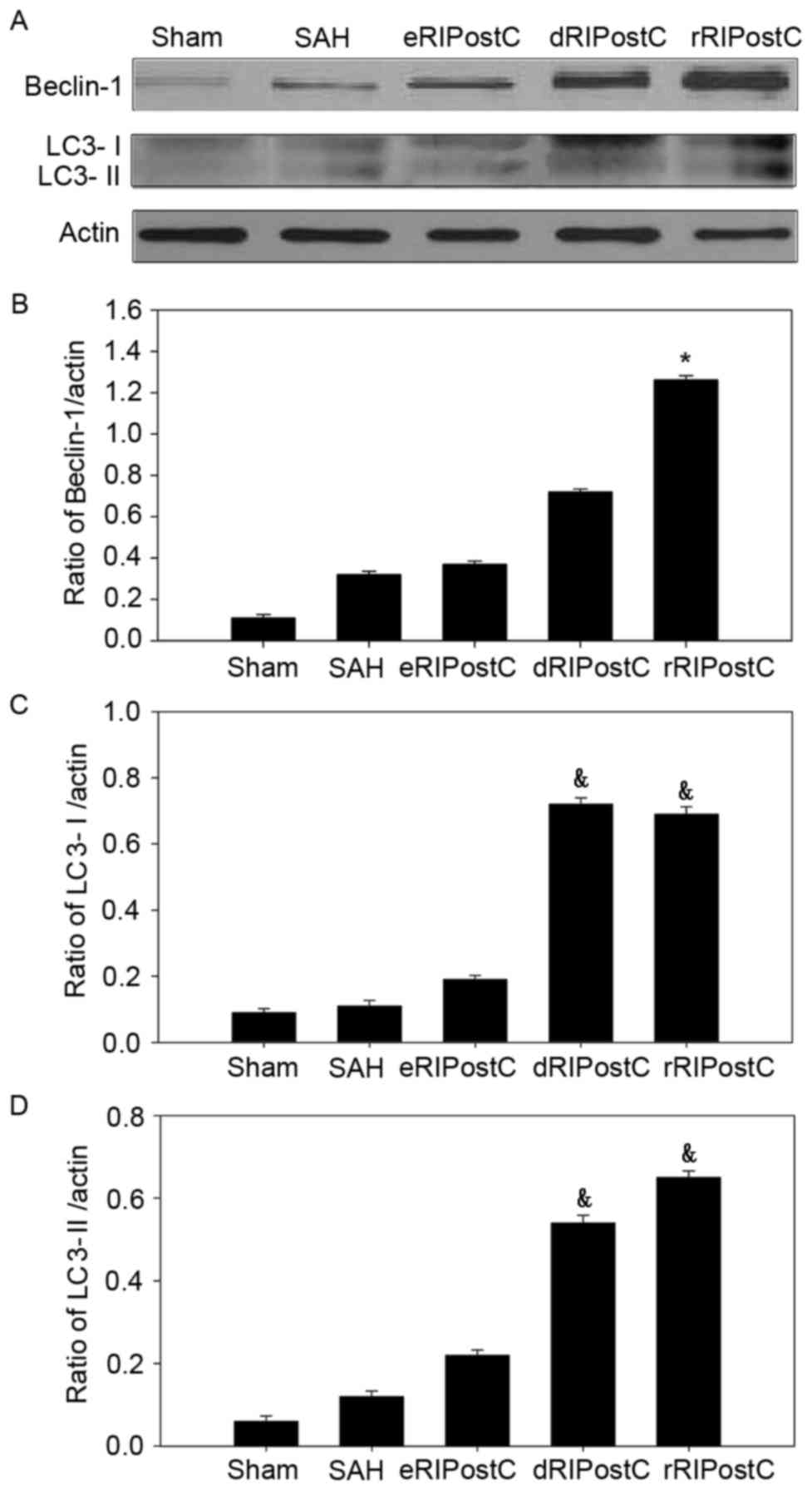
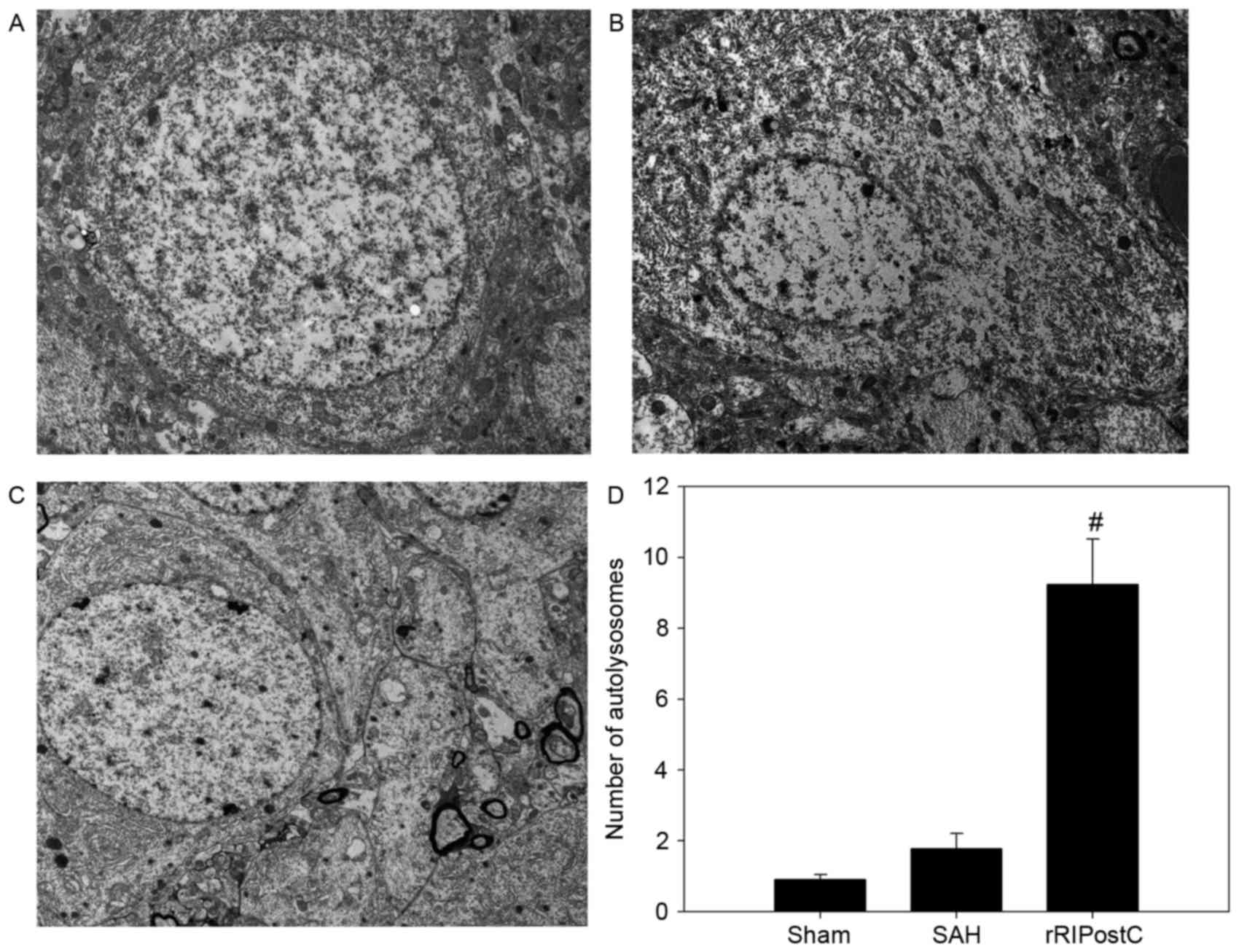
Repeated limb RIPostC promotes
autophagy in the brain cortex following SAH injury
In order to investigate the extent of autophagic
activation, the autophagy-related proteins Beclin-1 and LC3 I/II
were detected using western blot analysis (Fig. 5). In the sham group, little protein
was detected. SAH was able to increase the protein level of
Beclin-1 and LC3. Following RIPostC treatment, only repeated limb
RIPostC increased the Beclin-l level (Fig. 5A and B). Delayed and repeated limb
RIPostC increased the level of LC3 I/II (Fig. 5A, C and D).
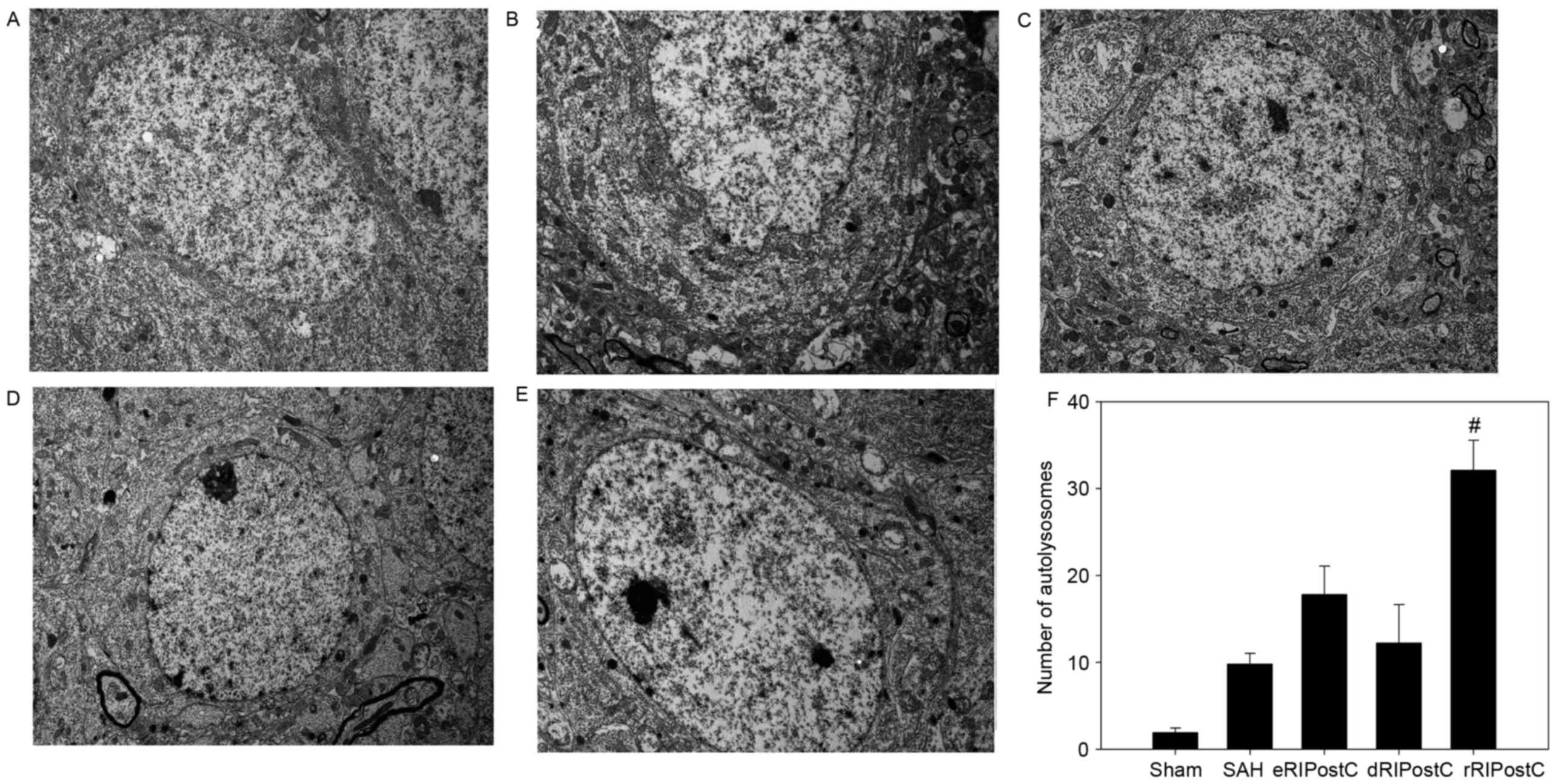
Ultrastructural alterations were observed using
transmission election microscopy (Fig.
6). Neurons and glial cells in the sham group appeared healthy
with normal endoplasmic reticulum, mitochondria, lysosomes and
nucleus (Fig. 6A). SAH injury
induced accumulation of autophagosomes and autolysosomes, and
mitochondrial swelling (Fig. 6B).
The number of autolysosomes in the SAH group was significantly
increased compared with rats in the sham group (P<0.05; Fig. 6B and F). The eRIPostC and dRIPostC
treatment group exhibited the same effect on the autolysosomes
(Fig. 6C and D); however, rRIPostC
further upregulated the number of autolysosomes compared with the
SAH group (P<0.01; Fig. 6E and
F).
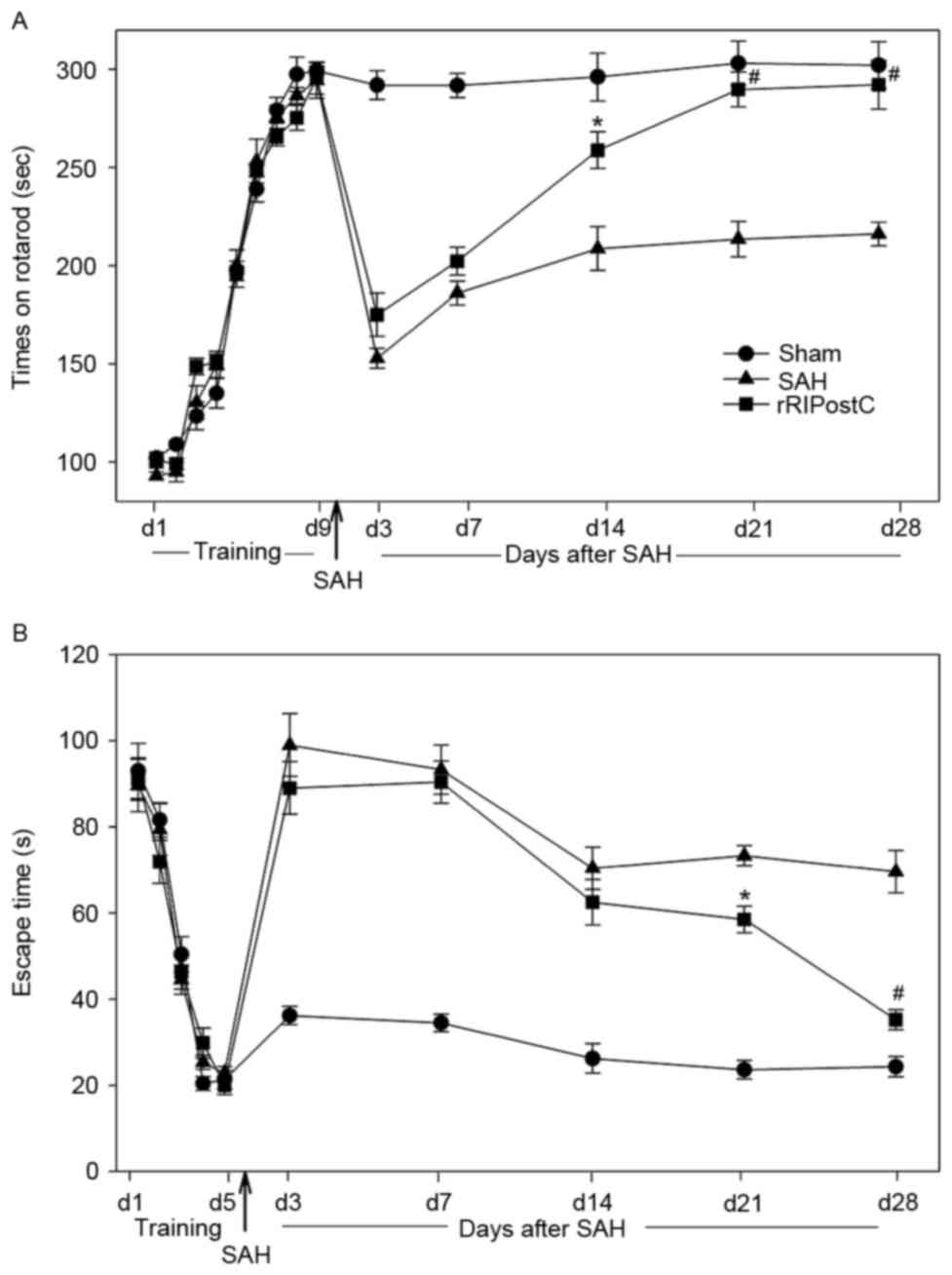
Repeated limb RIPostC improves
long-term behavior and memory recovery following SAH injury
All rats learned the rotarod test and plateaued at
day 9 (Fig. 7A). Sham rats
exhibited similar rotarod performance on day 28 post-testing,
indicating that the rats had achieved maximum performance prior to
surgery and that sham surgery did not affect their ability to
perform the rotarod task. However, a decrease in rotarod
performance was observed in the SAH and rRIPostC groups on day 3
following SAH. The rRIPostC treatment rats exhibited a better
recovery compared with the SAH group. On day 14 following SAH, the
rRIPostC group manifested a markedly improved performance in the
rotarod test compared with the SAH group (Fig. 7A).
For the Morris water maze test, all of the rats were
able to find the escape platform on day 5. The sham rats maintained
the ability to find the platform on day 28 post-surgery,
demonstrating that the rats had achieved good memory prior to
surgery and that sham surgery exerted no effect on the memory.
Following SAH injury, the rats in the SAH and RIPostC groups
exhibited impaired memory, and exhibited no amelioration during the
first two weeks post-surgery. However, following two weeks of
repeated limb RIPostC treatment, the rats exhibited a significant
recovery of memory compared with the SAH group (P<0.05 in week
3, P<0.01 in week 4 vs. SAH group; Fig. 7B).
Repeated limb RIPostC maintains active
autophagy for 1 month following SAH injury
The accumulation of autolysosomes in the SAH group
decreased to a level which was equal to that of the sham group on
day 28 following SAH. However, the number of autolysosomes in the
rRIPostC group remained significantly increased compared with the
SAH and sham groups (P<0.01; Fig.
8).
Discussion
The results of the present study demonstrated that
repeated RIPostC: i) Induces neuroprotective effects against
SAH-associated injury; ii) improves long-term neurological function
and memory recovery in rats; iii) markedly upregulates Beclin-1 and
LC3 in the cortex; iv) significantly increases the number of
autolysosomes; and v) activates autophagy, which may be maintained
for 1 month.
Aneurysmal SAH is among the most important and
common causes for disability and mortality. Survivors may suffer
from a variety of difficulties, including sensory disturbance,
dyskinesia, memory impairment and emotional problems, following the
early brain injury and subsequent ischemic injury (17). Although numerous treatments have
been performed, inducing neuroprotection remains a challenge. A
number of the pharmacological therapies are ineffective, or lead to
side effects. Consequently, the activation of endogenous
neuroprotection may be a promising strategy.
Brain ischemic conditioning has been recognized as
an effective therapeutic strategy to induce endogenous
neuroprotection. The protective stimulus may be applied prior to
(ischemic preconditioning) (18)
or following (ischemic post-conditioning) (19) the onset of disease. Compared with
preconditioning, post-conditioning, including delayed
post-conditioning, pharmacological post-conditioning and remote
post-conditioning, is more adaptable. However, previous research
has paid more attention to rapid or local ischemic
post-conditioning, and the underlying protective mechanisms of
remote post-conditioning remain unclear.
Remote post-conditioning is induced by cycles of
occlusion and release on a limb artery. Previous studies
demonstrated that limb RIPostC is able to improve neurological
function, reduce infarct size and attenuate brain edema in ischemic
stroke animal models; this indicates that limb RIPostC may be an
efficacious neuroprotective strategy, and more amenable to clinical
translation compared with RIPostC on the common carotid artery or
other remote organs (20–22). However, few studies have
demonstrated the effect of limb RIPostC on hemorrhagic stroke,
including SAH. Several neuroprotective strategies have been
observed to exhibit only transient effects (22,23).
Due to stroke-induced continued neurological injury, assessment of
long-term outcome is essential. Therefore, in the present study,
the methodology of limb RIPostC was optimized in order to examine
the short and long term effects, and investigate the underlying
mechanisms, in an SAH rat model. The present study demonstrated
that early limb RIPostC and delayed limb RIPostC exert no notable
effect on injury following SAH, which is contrary to certain
previous reports (5,24). It is hypothesized that this
discrepancy between the present study and previous studies may
result from the different RIPostC protocol and observation time
window used in the present study. Additionally, dRIPostC may serve
a protective role through other mechanisms (25). The results of the present study
demonstrated that dRIPostC increased LC3, although the specific
mechanisms require further investigation. Therefore, in the present
study, repeated RIPostC was investigated and the results indicated
that it improved short and long term neurological function, and
enhanced memory recovery in an SAH rat model.
The autophagic process may be involved in the
neuroprotective effect of repeated RIPostC. Autophagy is a cellular
self-clearance system, including the engulfment of cytoplasmic
material and intracellular organelles within double-membrane
vesicles. Lee et al (26)
reported that the autophagy pathway served a role in SAH. There are
two important biomarkers of autophagy: Beclin-1 and LC3. Beclin-1,
the mammalian ortholog of yeast vacuolar protein sorting-associated
protein 30, is involved in the regulation of autophagy, with
numerous other proteins. LC3, the mammalian ortholog of yeast
autophagy-related protein 8, is synthesized as pro-LC3 and cleaved
to LC3-I by cysteine protease ATG4. Conversion between LC3-I and
LC3-II is regarded as biochemical evidence of autophagosome
formation. In the present study, autophagic biomarkers, including
LC3 and Beclin-1, were observed to be upregulated simultaneously in
the brain cortex following treatment with RIPostC. The LC3 and
Beclin-1 were further upregulated in the rRIPostC group.
Ultrastructural observations demonstrated that autolysosomes
markedly increased in number 3 days subsequently, and were
maintained for 1 month. The specific mechanism of autophagy
activated by RIPostC remain unclear. Takagi et al (27) reported that adenosine
59-monophosphate-activated protein kinase (AMPK) was a positive
regulator of autophagy. AMPK is a sensor of ATP, and is activated
when the ratio of ATP/ADP is decreased during exercise, hypoxia,
oxidative stress and glucose deprivation. Han et al
(28) observed that limb RIPostC
was able to activate autophagy by enhancing the phosphorylation of
AMPK in myocardial ischemia-reperfusion injury, demonstrating that
RIPostC-induced AMPK served a role in the autophagic process.
Additionally, Qi et al (29) demonstrated that limb RIPostC was
able to increase the phosphorylation of protein kinase B (AKT) and
glycogen synthase kinase 3β (GSK3β), to activate autophagy in
cerebral ischemic rat models. The results of the present study also
demonstrated that rRIPostC induced neuroprotection via the
upregulation of autophagy; however, due to the specificity of the
animal model, whether this was through the same pathway requires
further investigation.
The most common pathological sequelae following
diffuse or focal neurodegenerative injury are apoptosis and
necrosis. Previous studies have emphasized a potential crosstalk
between autophagy and apoptosis, and examined the cytoprotective
function of autophagy via negative modulation of apoptosis
(30–32). The interaction between Beclin-1 and
apoptosis regulator Bcl-2 (Bcl-2)/Bcl-2-like protein 1 has been
recognized as the possible mechanism. Beclin-1 is a novel
Bcl-2-homology (BH)-3 domain-only protein, primarily located in
cytoplasmic structures, including the endoplasmic reticulum,
mitochondria and the perinuclear membrane. Previous studies have
investigated the possible mechanisms of the dissociation of Bcl-2
and Beclin-1, which is necessary for induction of autophagy.
Beclin-1 is additionally a target of death-associated protein
kinase (DAPK), a calcium/calmodulin-regulated Ser/Thr kinase.
DAPK-mediated phosphorylation of Beclin-1 on Thr119 at the BH-3
domain promotes the dissociation of Beclin-1 from its inhibitor
Bcl-2-family members, thereby activating Beclin-1 to induce
autophagy. Previous studies have demonstrated that limb RIPostC is
able to downregulate expression of caspase-3 via the autophagic
process through the AKT signaling pathway, involving the targeting
key molecules in autophagy, including mammalian target of rapamycin
and phosphatidylinositol-3 kinase (33,34).
The results of the present study demonstrated that rRIPostC
prevented neuronal apoptosis following SAH injury, and that
autophagy may serve an important role.
In the present study, the short and long term
neuroprotective effects of repeated limb RIPostC have been
demonstrated in an endovascular puncture SAH rat model. Further
investigations demonstrated that the neuroprotective and
anti-apoptosis effect induced by repeated limb RIPostC may involve
the autophagic process. However, the specific molecular mechanism
requires further research.
In conclusion, the ability to reduce brain injury in
the short and long term following SAH injury suggests that repeated
limb RIPostC may be a promising noninvasive therapy. It is proposed
that repeated limb RIPostC activates the autophagic pathway, which
may modulate apoptosis and reduce cell loss in an endovascular
puncture SAH rat model. Further investigation is required into the
precise autophagy-associated mechanism. The ability to reduce long
term injury and improve memory is promising for the potential
translation of this treatment to clinical rehabilitation for
hemorrhagic stroke.
Acknowledgements
The present study was supported by the National
Nature Science Foundation (grant no. 81471333).
References
|
1
|
Feigin VL, Lawes CM, Bennett DA,
Barker-Collo SL and Parag V: Worldwide stroke incidence and early
case fatality reported in 56 population-based studies: A systematic
review. Lancet Neurol. 8:355–369. 2009. View Article : Google Scholar
|
|
2
|
Ostrowski RP, Colohan AR and Zhang JH:
Molecular mechanisms of early brain injury after subarachnoid
hemorrhage. Neurol Res. 28:399–414. 2006. View Article : Google Scholar
|
|
3
|
Dorsch N: A clinical review of cerebral
vasospasm and delayed ischaemia following aneurysm rupture. Acta
Neurochir Suppl. 110:5–6. 2011.
|
|
4
|
Andreka G, Vertesaljai M, Szantho G, Font
G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS,
et al: Remote ischaemic postconditioning protects the heart during
acute myocardial infarction in pigs. Heart. 93:749–752. 2007.
View Article : Google Scholar :
|
|
5
|
Burda R, Danielisova V, Gottlieb M,
Nemethova M, Bonova P, Matiasova M, Morochovic R and Burda J:
Delayed remote ischemic postconditioning protects against transient
cerebral ischemia/reperfusion as well as kainate-induced injury in
rats. Acta Histochem. 116:1062–1067. 2014. View Article : Google Scholar
|
|
6
|
Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang
N and Zhou J: Remote ischemic postconditioning protects the brain
from global cerebral ischemia/reperfusion injury by up-regulating
endothelial nitric oxide synthase through the PI3K/Akt pathway.
Brain Res. 1445:92–102. 2012. View Article : Google Scholar
|
|
7
|
Wang Q, Zhang X, Ding Q, Hu B, Xie Y, Li
X, Yang Q and Xiong L: Limb remote postconditioning alleviates
cerebral reperfusion injury through reactive oxygen
species-mediated inhibition of delta protein kinase C in rats.
Anesth Analg. 113:1180–1187. 2011. View Article : Google Scholar
|
|
8
|
Liu X, Zhao S, Liu F, Kang J, Xiao A, Li
F, Zhang C, Yan F, Zhao H, Luo M, et al: Remote ischemic
postconditioning alleviates cerebral ischemic injury by attenuating
endoplasmic reticulum stress-mediated apoptosis. Transl Stroke Res.
5:692–700. 2014. View Article : Google Scholar
|
|
9
|
Rubinsztein DC, DiFiglia M, Heintz N,
Nixon RA, Qin ZH, Ravikumar B, Stefanis L and Tolkovsky A:
Autophagy and its possible roles in nervous system diseases, damage
and repair. Autophagy. 1:11–22. 2005. View Article : Google Scholar
|
|
10
|
Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX and
Liu C: Activating cannabinoid receptor 2 alleviates pathogenesis of
experimental autoimmune encephalomyelitis via activation of
autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther.
20:1021–1028. 2014. View Article : Google Scholar
|
|
11
|
Wang P and Miao CY: Autophagy in the
disorders of central nervous system: Vital and/or fatal? CNS
Neurosci Ther. 18:955–956. 2012. View Article : Google Scholar
|
|
12
|
Kubota C, Torii S, Hou N, Saito N,
Yoshimoto Y, Imai H and Takeuchi T: Constitutive reactive oxygen
species generation from autophagosome/lysosome in neuronal
oxidative toxicity. J Biol Chem. 285:667–674. 2010. View Article : Google Scholar
|
|
13
|
Liu J, Wang F, Liu Y, He G, Chen J and Zhu
F: Retrospective study of cerebral vasospasm-related risk factors
in elderly patients with subarachnoid hemorrhage. Neurosurg Quart.
20:258–262. 2010. View Article : Google Scholar
|
|
14
|
Luo S, Zhang X, Yu M, Yan H, Liu H, Wilson
JX and Huang G: Folic acid acts through DNA methyltransferases to
induce the differentiation of neural stem cells into neurons. Cell
Biochem Biophys. 66:559–566. 2013. View Article : Google Scholar
|
|
15
|
Bederson JB, Germano IM and Guarino L:
Cortical blood flow and cerebral perfusion pressure in a new
noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke.
26:1086–1092. 1995. View Article : Google Scholar
|
|
16
|
Sugawara T, Ayer R, Jadhav V and Zhang JH:
A new grading system evaluating bleeding scale in filament
perforation subarachnoid hemorrhage rat model. J Neurosci Methods.
167:327–334. 2008. View Article : Google Scholar
|
|
17
|
Frontera JA, Ahmed W, Zach V, Jovine M,
Tanenbaum L, Sehba F, Patel A, Bederson JB and Gordon E: Acute
ischaemia after subarachnoid haemorrhage, relationship with early
brain injury and impact on outcome: A prospective quantitative MRI
study. J Neurol Neurosurg Psychiatry. 86:71–78. 2015. View Article : Google Scholar
|
|
18
|
Selim M and Wang M: Ischemic
preconditioning: The long-awaited savior of Neuroprotection. Has It
Arrived? Neurotherapeutics. 12:655–656. 2015. View Article : Google Scholar :
|
|
19
|
Buchholz B, Donato M, D'Annunzio V and
Gelpi RJ: Ischemic postconditioning: Mmechanisms, comorbidities,
and clinical application. Mol Cell Biochem. 392:1–12. 2014.
View Article : Google Scholar
|
|
20
|
Zhao H: The protective effects of ischemic
postconditioning against stroke: From rapid to delayed and remote
postconditioning. Open Drug Discov J. 5:138–147. 2011.
|
|
21
|
Geng X, Ren C, Wang T, Fu P, Luo Y, Liu X,
Yan F, Ling F, Jia J, Du H, et al: Effect of remote ischemic
postconditioning on an intracerebral hemorrhage stroke model in
rats. Neurol Res. 34:143–148. 2012.
|
|
22
|
Sun J, Tong L, Luan Q, Deng J, Li Y, Li Z,
Dong H and Xiong L: Protective effect of delayed remote limb
ischemic postconditioning: Role of mitochondrial K(ATP) channels in
a rat model of focal cerebral ischemic reperfusion injury. J Cereb
Blood Flow Metab. 32:851–859. 2012. View Article : Google Scholar :
|
|
23
|
Xu J, Sun S, Lu X, Hu X, Yang M and Tang
W: Remote ischemic pre- and postconditioning improve
postresuscitation myocardial and cerebral function in a rat model
of cardiac arrest and resuscitation. Crit Care Med. 43:e12–e18.
2015. View Article : Google Scholar
|
|
24
|
Perera Drunalini PN, Hu Q and Tang J, Li
L, Barnhart M, Doycheva DM, Zhang JH and Tang J: Delayed remote
ischemic postconditioning improves long term sensory motor deficits
in a neonatal hypoxic ischemic rat model. PLoS One. 9:e902582014.
View Article : Google Scholar :
|
|
25
|
Sun J, Tong L, Luan Q, Deng J, Li Y, Li Z,
Dong H and Xiong L: Protective effect of delayed remote limb
ischemic postconditioning: Role of mitochondrial K(ATP) channels in
a rat model of focal cerebral ischemic reperfusion injury. J Cereb
Blood Flow Metab. 32:851–859. 2012. View Article : Google Scholar :
|
|
26
|
Lee Y, He Y, Sagher O, Keep R, Hua Y and
Xi G: Activated autophagy pathway in experimental subarachnoid
hemorrhage. Brain Res. 1287:126–135. 2009. View Article : Google Scholar
|
|
27
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar
|
|
28
|
Han Z, Cao J, Song D, Tian L, Chen K, Wang
Y, Gao L, Yin Z, Fan Y and Wang C: Remote limb ischemic
postconditioning on myocardial ischemia/reperfusion injury in
normal mice, but not diabetic mice. PLoS One. 9:e868382014.
View Article : Google Scholar :
|
|
29
|
Qi ZF, Luo YM, Liu XR, Wang RL, Zhao HP,
Yan F, Song ZJ, Luo M and Ji XM: AKT/GSK3β-dependent autophagy
contributes to the neuroprotection of limb remote ischemic
postconditioning in the transient cerebral ischemic rat model. CNS
Neurosci Ther. 18:965–973. 2012. View Article : Google Scholar
|
|
30
|
Zhao GX, Pan H, Ouyang DY and He XH: The
critical molecular interconnections in regulating apoptosis and
autophagy. Ann Med. 47:305–315. 2015. View Article : Google Scholar
|
|
31
|
Granato M, Chiozzi B, Filardi MR, Lotti
LV, Di Renzo L, Faggioni A and Cirone M: Tyrosine kinase inhibitor
tyrphostin AG490 triggers both apoptosis and autophagy by reducing
HSF1 and Mcl-1 in PEL cells. Cancer Lett. 366:191–197. 2015.
View Article : Google Scholar
|
|
32
|
Katsiougiannis S, Tenta R and Skopouli FN:
Endoplasmic reticulum stress causes autophagy and apoptosis leading
to cellular redistribution of the autoantigens Ro/Sjögren's
syndrome-related antigen A (SSA) and La/SSB in salivary gland
epithelial cells. Clin Exp Immunol. 181:244–252. 2015. View Article : Google Scholar :
|
|
33
|
Cui J, Hu YF, Feng XM, Tian T, Guo YH, Ma
JW, Nan KJ and Zhang HY: EGFR inhibitors and autophagy in cancer
treatment. Tumor Biol. 35:11701–11709. 2014. View Article : Google Scholar
|
|
34
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015:4175902015. View Article : Google Scholar :
|