Introduction
Muscarinic acetylcholine receptors (mAChRs) are
primary receptors in cholinergic transmission, and mAChRs
immunoreactivities in the hippocampus are present in pyramidal
cells, granule cells, and GABAergic interneurons (1). It is well established that systemic
injection of scopolamine (Scop), a muscarinic cholinergic receptor
antagonist, produces learning and memory deficits through
dysregulation of cholinergic mechanisms in the hippocampus
(2).
Septo-hippocampal cholinergic pathway, which
originates in the medial septum/diagonal band, is one of crucial
circuits for cognitive function (3). Also, it is well known that
septo-hippocampal GABAergic pathway plays important roles in
learning and memory processes (4).
Important inputs to the hippocampus are provided by GABAergic
fibers as well as cholinergic fibers from the medial septum, and
most of GABAergic projections terminate on many hippocampal
interneurons including neurons containing calcium binding proteins
(CBPs), such as calbindin D-28k (CB), calretinin (CR), and
parvalbumin (PV) (5–7). GABAergic nonpyramidal cells transmit
hippocampal feedback to GABAergic neurons in the medial septum,
which forms septo-hippocampo-septal circuit that regulates the
activity of GABAergic septo-hippocampal projections (8).
The maintenance of intracellular calcium metabolism
in the hippocampus is an important factor in synaptic plasticity
and memory performance (9,10). Alterations in numbers or
expressions of CBPs in hippocampal neurons are observed in
post-mortem brain tissues in schizophrenia (11) and kainate-induced seizures
(12), and these changes
contribute to cognitive impairments in neurological disorders
(13,14). However, in Scop-induced amnesia
models, alterations in CBPs expressions have not been fully
studied.
In the present study, therefore, we examined
temporal and spatial alterations of CBPs expressions in hippocampal
subregions after chronic systemic treatment with Scop in mice, and
investigated the relationship between hippocampal CBPs expressions
and memory deficits after Scop treatment.
Materials and methods
Experimental animals
Male ICR mice (8-week-old) were purchased from
Orient Bio Inc. (Seongnam, South Korea) and used after 7 days of
acclimation. The procedures for animal handling and care adhered to
guidelines that are in compliance with the current international
laws and policies (Guide for the Care and Use of Laboratory
Animals, The National Academies Press, 8th edition, 2011), and the
experimental protocol of the present study was reviewed and
approved based on ethical procedures and scientific care by the
Kangwon National University-Institutional Animal Care and Use
Committee (approval no. KW-160802-3).
Experimental groups and drug
treatment
Experimental mice were divide into five groups (n=14
at each point in time per group): i) Control group; ii) 1 week
(wk)-Scop group, which received Scop treatment for 1 week; iii) 2
wk-Scop group; iv) 3 wk-Scop group; and v) 4 wk-Scop group. Normal
saline or 1 mg/kg Scop was dissolved in normal saline and
intraperitoneally injected once a day for 1 to 4 weeks (15–17).
The mice in each group were sacrificed 24 h after the final
administration.
Behavioral performance
Morris water maze test (MWMT)
The MWMT for spatial memory was performed according
to our published procedure (18).
The animals (n=7) in each group were tested 1 day before sacrifice
following training for 3 days to find the platform. Shortly, the
time to find the platform within 120 sec (latency time), and the
time in the target quadrant were recorded in each animal. The whole
process was monitored by a digital camera and a computer
system.
Passive avoidance test (PAT)
PAT for short-term memory was performed according to
our published method (19). The
animals (n=7) in each group were tested 1 day before sacrifice
following training for 1 day. Briefly, we used the Gemini Avoidance
system (GEM 392; San Diego Instruments, San Diego, CA, USA) that
consists of light and dark compartments with a grid floor. For
training, the animals in each group were allowed to explore
environments in both light and dark compartments for 1 min and
given an inescapable foot-shock (0.3 mA for 3 sec) after entering
the dark compartment. The animals were tested 15 min after the
training without applying the foot-shock. The interval between the
beginning of the test and the entry into the dark compartment was
defined as the latency time.
Immunohistochemistry
Immunohistochemistry for CB, CR, and PV was carried
out according to our published method (20). Briefly, mice (n=7 in each
group) were anesthetized with 30 mg/kg Zoletil 50 (Virbac, Carros,
France) and perfused transcardially with 4% paraformaldehyde in 0.1
M phosphate buffer (PB; pH 7.4). Their brains were serially
sectioned into 30-µm coronal sections in a cryostat (Leica
Microsystems GmbH, Wetzlar, Germany). The sections were incubated
with diluted goat anti-CB (1:200; Santa Cruz Biotechnology, Santa
Cruz, CA, USA), rabbit anti-CR antibody (1:1,000; Chemicon
International, Inc., Temecula, CA, USA) or goat anti-PV (1:200;
Santa Cruz Biotechnology) overnight and subsequently exposed to
biotinylated rabbit anti-goat or goat anti-rabbit IgG, and
streptavidin peroxidase complex (1:200; both from Vector
Laboratories, Inc., Burlingame, CA, USA). In order to establish the
specificity of the immunostaining, a negative control resulted in
the absence of immunoreactivity in the stained sections.
Data analysis
As previously described (19), digital images of CB-, CR-, and
PV-immunoreactive structures were captured with an AxioM1 light
microscope equipped with a digital camera (Axiocam; both from Carl
Zeiss AG, Oberkochen, Germany) connected to a PC monitor. The
density of each immunoreactive structures was evaluated on the
basis of optical density (OD), which was obtained after the
transformation of the mean gray level using the formula: OD=log
(256/mean gray level). After the background was subtracted, a ratio
of the OD of image file was calibrated as % [relative optical
density (ROD)] using Adobe Photoshop version 8.0 and NIH Image J
software (National Institutes of Health, Bethesda, MD, USA). The
mean value of the OD of the control group was designated as 100%,
and the ROD of each group was calibrated and expressed as % of the
control group.
Statistical analysis
The data shown here represent the means ± SEM.
Differences of the means among the groups were statistically
analyzed by analysis of variance with a post hoc
Bonferroni's multiple comparison test in order to elucidate effects
of long-term Scop treatment among groups. Statistical significance
was considered at P<0.05.
Results
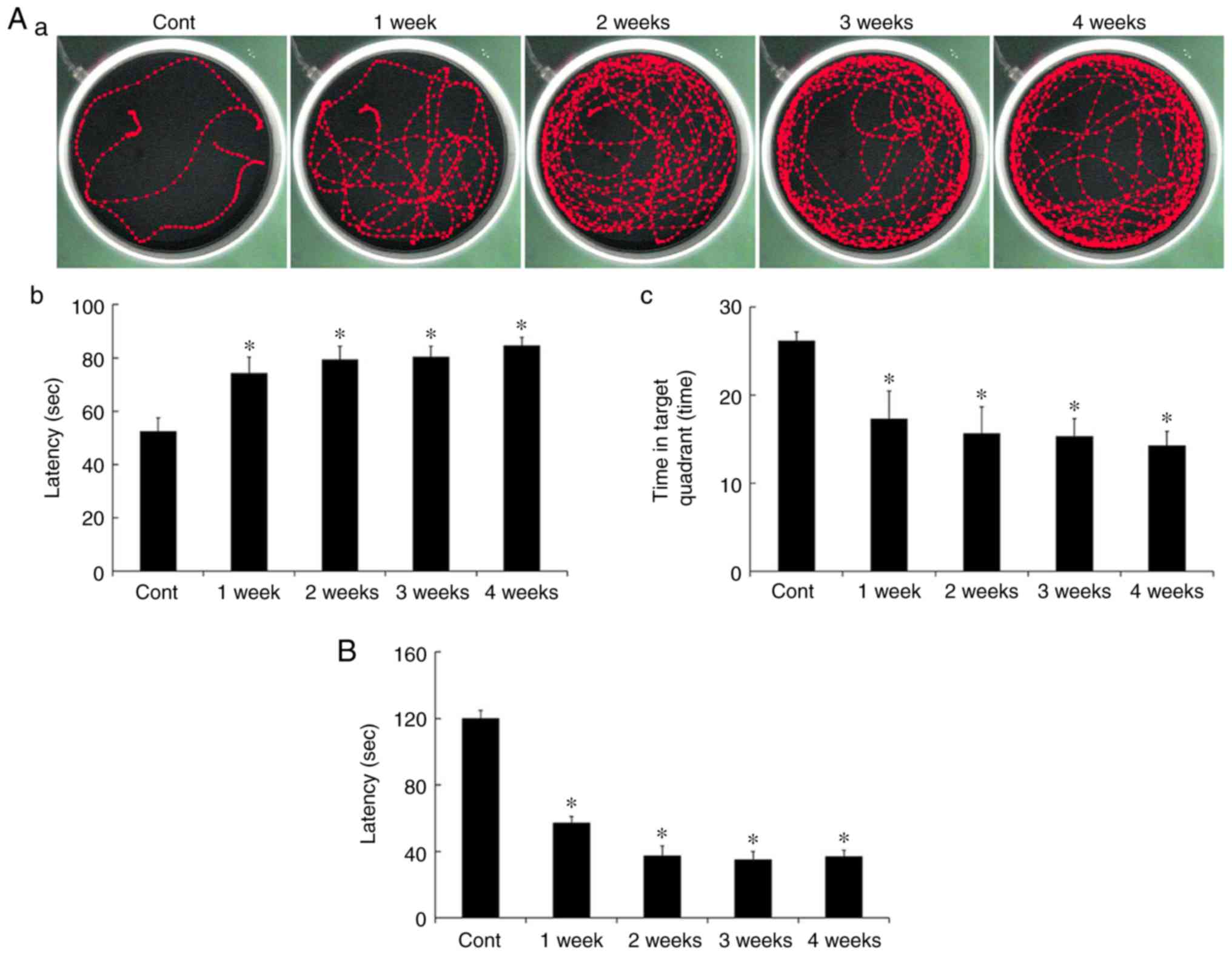
MWMT
Spatial memory was evaluated using the MWMT. Escape
latency in all the Scop treated groups was significantly increased
compared to that in the control group (Fig. 1Aa and Ab). In addition, duration of
the time in the target quadrant was significantly decreased in all
the Scop treated groups (Fig. 1Aa and
Ac). However, there were no significant differences among the
Scop treated groups (Fig. 1Ab and
Ac).
PAT
Short-term memory was evaluated using PAT. Similar
to results of the MWMT, latency in all the Scop treated groups was
significantly decreased compared to that in the control group
(Fig. 1B), however, the latency
was not significantly different among the Scop treated groups
(Fig. 1B).
CB immunoreactivity
CB immunohistochemistry results across weeks 1 to 4
are presented in Fig. 2. In the
control group, CB immunoreactivity was identified in neurons of the
stratum pyramidale (SP) in the CA1 region, in mossy fibers of the
stratum lucidum in the CA3 region, and in cells of the granule cell
layer (GCL) in the dentate gyrus (DG) (Fig. 2Aa-Ac). In the 1 wk-Scop group, the
distribution pattern of CB immunoreactivity in all the subregions
was similar to that in control group, however, the CB
immunoreactivity in all the subregions was significantly decreased
compared to that in the control group (Fig. 2Ba-Bc and F). In the 2 wk-Scop
group, CB immunoreactivity was more reduced compared with that in
the 1 wk-Scop group (Fig. 2Ca-Cc and
F). In the 3 wk- and 4 wk-Scop groups, CB immunoreactivity was
hardly shown in the CA1 and CA3 regions (Fig. 2Da, Db, Ea, Eb and F), and very weak
in the DG (Fig. 2Dc, Ec and
F).
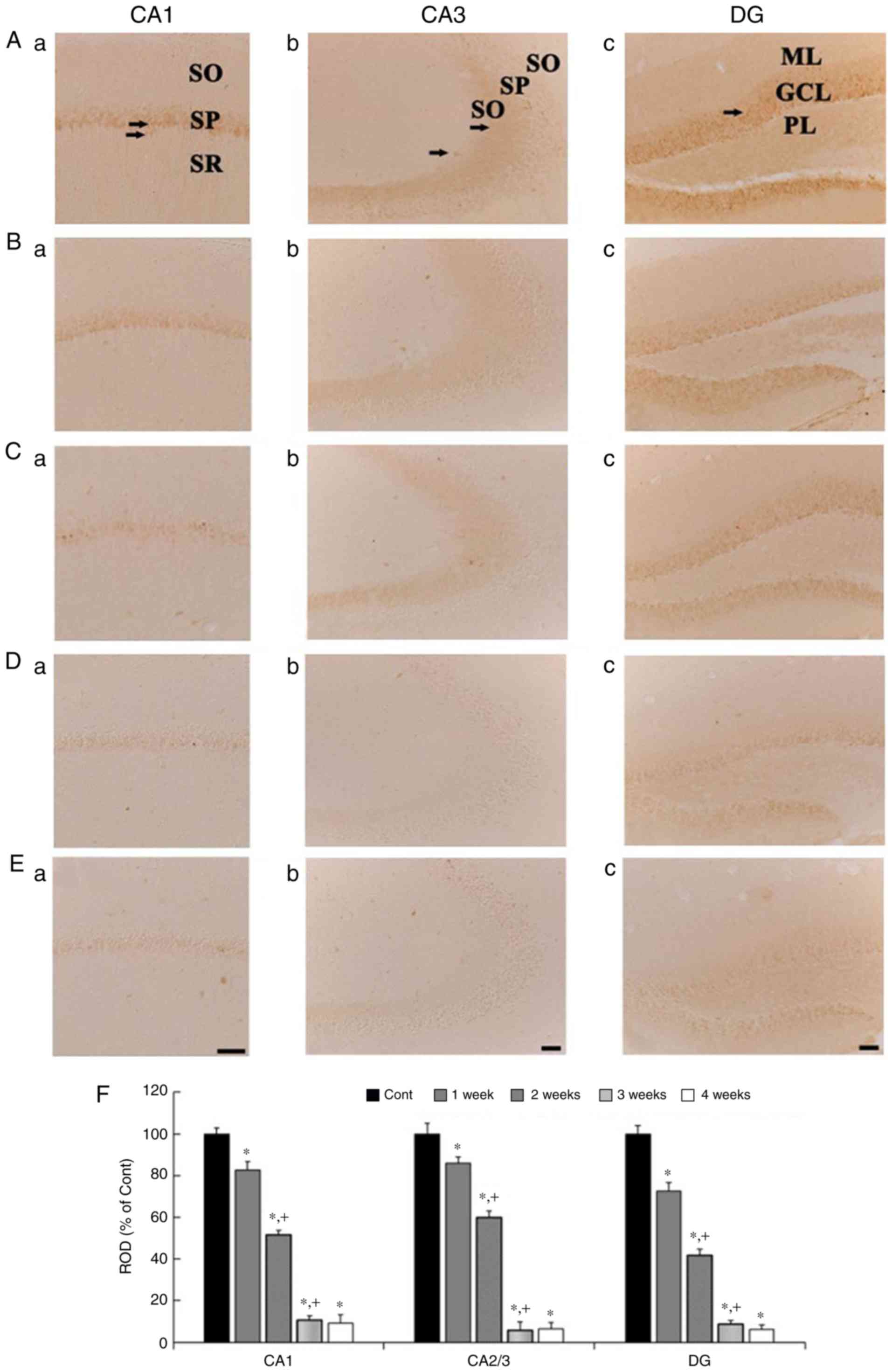 | Figure 2.CB immunohistochemistry in the
hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2
wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. CB
immunoreactivity in the control group is shown in cells the stratum
pyramidale (SP, arrows) in the CA1 region, in the mossy fibers
(arrows) in the CA3 region, and cells in the granule cell layer
(GCL, arrow) in the DG. CB immunoreactivity in the Scop groups is
gradually and significantly decreased with time in all the
subregions. Scar bar, 50 µm. (F) ROD of CB immunoreactive
structures in the control and Scop groups (n=7 per group;
*P<0.05 vs. the control group; †P<0.05 vs. the
pre-time point Scop group). The bars indicate the SEM. CB,
calbindin D-28k; wk, week; Scop, scopolamine; SP, stratum
pyramidale; GCL, granule cell layer; DG, dentate gyrus; ML,
molecular layer; PL, polymorphic layer; SO, stratum oriens; SR,
stratum radiatum; ROD, relative optical density. |
CR immunoreactivity
CR immunohistochemistry results across weeks 1 to 4
are presented in Fig. 3. In the
control group, CR immunoreactivity in the CA1 and CA3 regions was
shown in cells scattered in the strata oriens and radiatum
(Fig. 3Aa and Ab), and, in the DG,
CR immunoreactivity is found in the GCL and cells scatted in the
polymorphic layer (PL) (Fig. 3Ac).
In the Scop groups, CR immunoreactivity was gradually decreased
with time in the CA1 region (Fig. 3Ba,
Ca, Da, Ea and F), not significantly altered in the CA3 region
(Fig. 3Bb, Cb, Db, Eb and F). In
the DG, CR immunoreactivity was gradually decreased, in particular,
CR immunoreactivity in the 3- and 4-wk Scop groups was shown in a
few cells in the PL (Fig. 3Bc, Cc, Dc,
Ec and F).
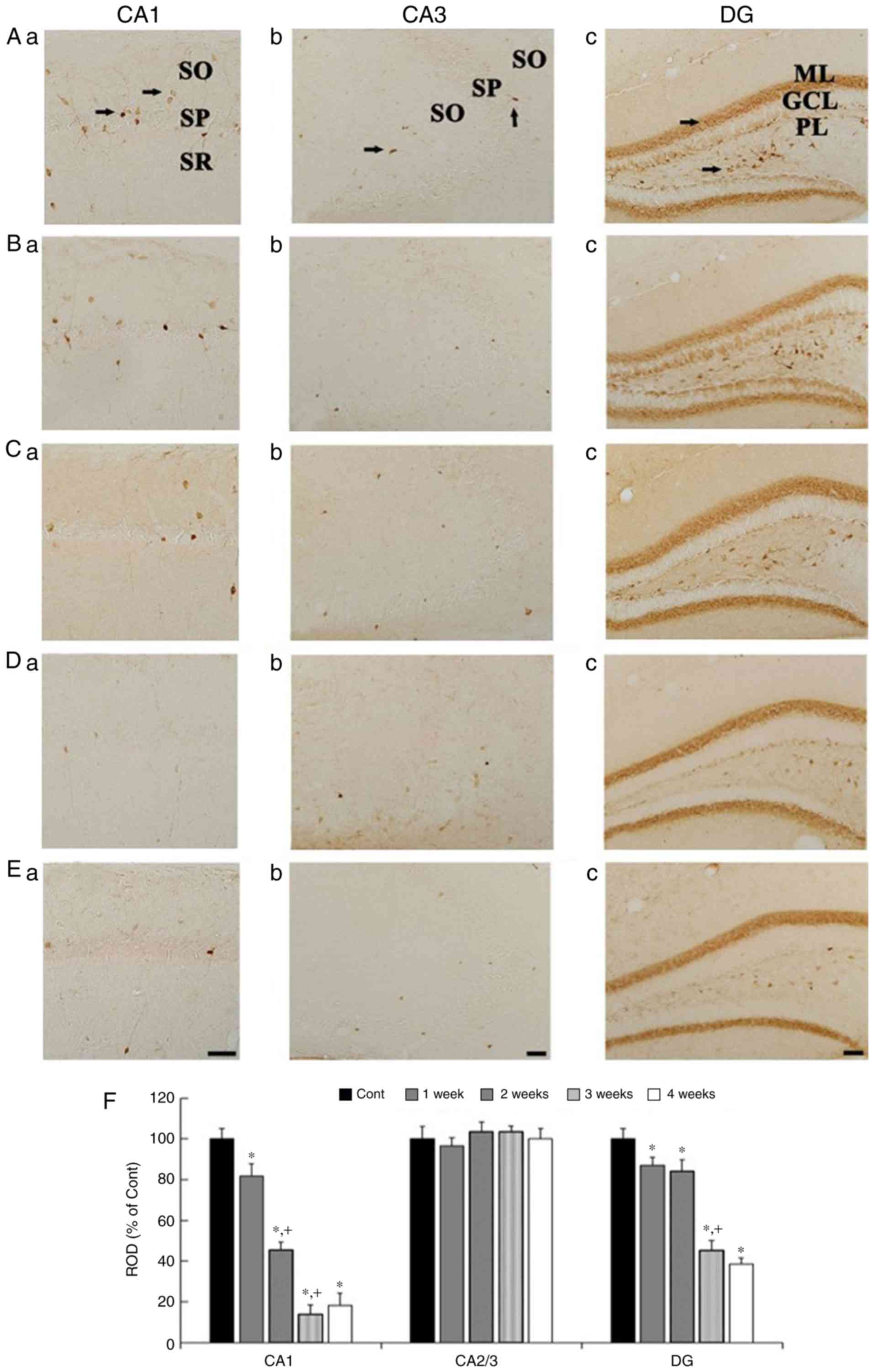 | Figure 3.CR immunohistochemistry in the
hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2
wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. CR
immunoreactivity in the control group is shown cells (arrows)
scattered in the CA1 and CA3 region, and cells in the granule cell
layer (GCL, arrow) and cells in the polymorphic layer (PL, arrow)
in the DG. CR immunoreactivity is significantly reduced in the CA1
region and the DG with time after Scop treatment, but not in the
CA3 region. Scar bar, 50 µm. (F) ROD of CR immunoreactive
structures in the control and Scop groups (n=7 per group;
*P<0.05 vs. the control group; †P<0.05 vs. the
pre-time point Scop group). The bars indicate the SEM. CR,
calretinin; wk, week; Scop, scopolamine; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; GCL, granule cell layer;
PL, polymorphic layer; ML, molecular layer; DG, dentate gyrus; ROD,
relative optical density. |
PV immunoreactivity
PV immunohistochemistry results across weeks 1 to 4
are presented in Fig. 4. In the
control group, strong PV immunoreactivity was shown in cells
scattered in the CA1 and 3 regions (Fig. 4Aa and Ab), and in the DG (4Ac), in
addition, weak PV immunoreactivity was detected in the SP in the
CA1 and 3 regions (Fig. 4Aa and
Ab), and in the GCL in the DG (Fig. 4Ac). In the Scop groups, PV
immunoreactivity in the CA1 region was similar to that in the
control group at 1 week (Fig. 4Ba and
F), thereafter, PV immunoreactivity in the CA1 region was
gradually and significantly reduced with time (Fig. 4Ca, Da, Ea and F). In the CA3
region, PV immunoreactivity was not significantly altered at any
time after Scop treatment (Fig. 4Bb,
Cb, Db, Eb and F). In the DG, PV immunoreactivity was not
changed at 1 week after Scop treatment (Fig. 4Bc and F), and significantly reduced
from 2 weeks after Scop treatment (Fig. 4Cc, Dc, Ec and F).
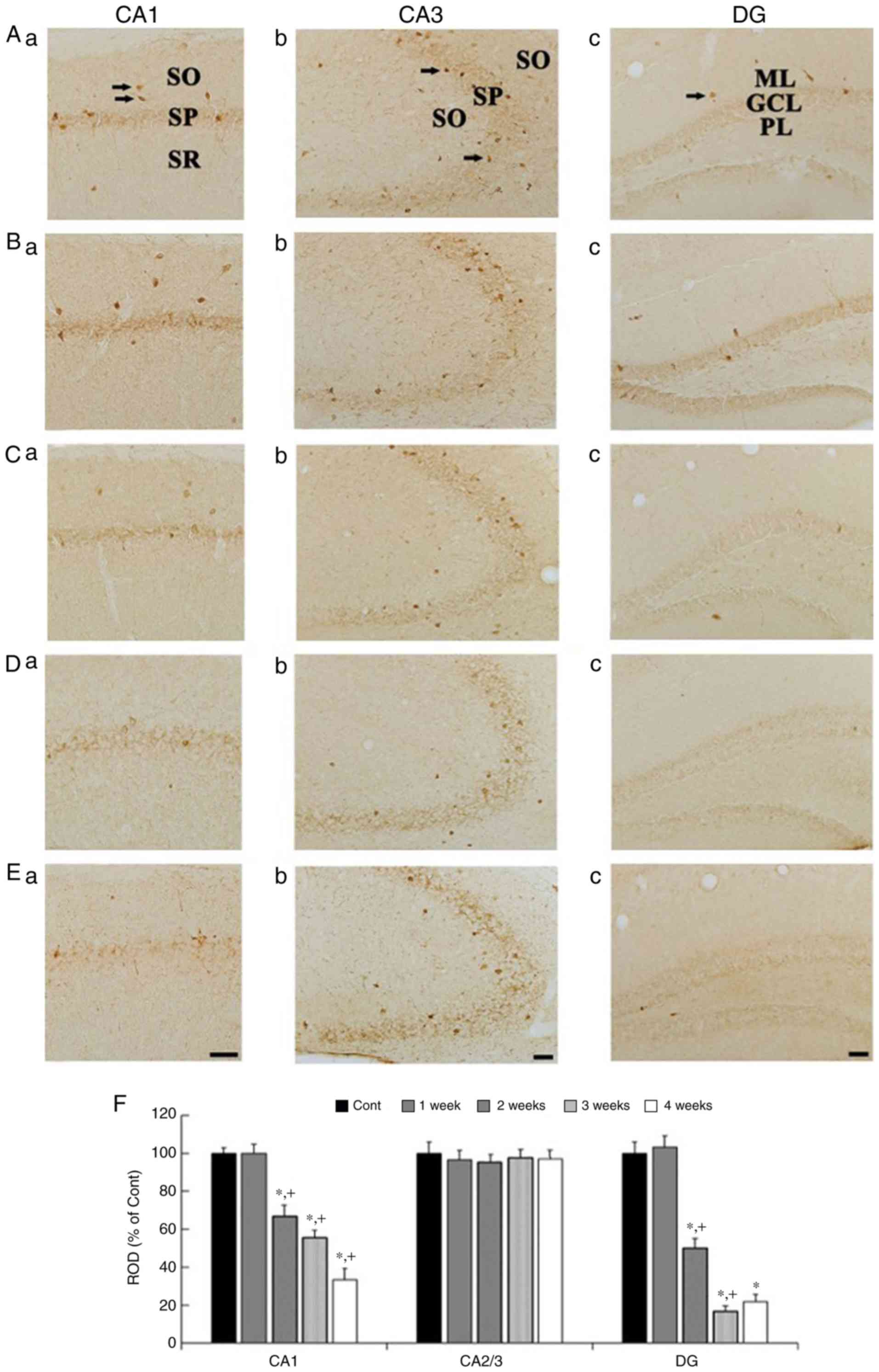 | Figure 4.PV immunohistochemistry in the
hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2
wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. In the
control group, strong PV immunoreactivity is shown in scattered
cells (arrows) in the CA1, CA3 region, and DG, and weak PV
immunoreactivity is detected in cells in the SP of the CA1 and CA3
regions, and in the GCL of the DG. PV immunoreactivity is gradually
and significantly decreased in the CA1 region and the DG, not in
the CA3 region, from 2 weeks after Scop treatment. Scar bar, 50 µm.
(F) ROD of PV immunoreactive structures in the control and Scop
groups (n=7 per group; *P<0.05 vs. the control group;
†P<0.05 vs. the pre-time point Scop group). The bars
indicate the SEM. PV, parvalbumin; wk, week; Scop, scopolamine; DG,
dentate gyrus; GCL, granule cell layer; ML, molecular layer; PL,
polymorphic layer; SO, stratum oriens; SP, stratum pyramidale; SR,
stratum radiatum; ROD, relative optical density. |
Discussion
In the present study, we investigated long-term
changes of spatial and short-term memory, and CBPs expressions in
the hippocampal subregions for 4 weeks after systemic Scop
treatment in mice and found that significant spatial and short-term
memory impairments were caused at 1 week post-treatment and
maintained until 4 weeks post-treatment using the MWMT and PAT.
These results were consistent with a previous study that showed
that long-term Scop treatment induced spatial memory deficits at
the beginning of 1 week after Scop injection (21). Acetylcholine has been implicated in
memory, for instance, hippocampal-dependent memory (2). It has been well established that
systemic Scop injection produces learning and memory impairments
via dysregulation of cholinergic mechanisms in the hippocampus
(2). For modulation of memory
impairments, on the other hand, it has been reported that
intraseptal muscarinic agonists alleviate systemic Scop
induced-memory impairments (22).
In addition, the septo-hippocampal GABAergic fibers are more
excited by exogenous muscarine (4), and excitation of septo-hippocampal
GABA neurons produces a powerful disinhibition of pyramidal neurons
and promotes long-term potentiation in the hippocampus via
hippocampal interneurons (4,22).
Based on these reports, it is likely that modulation of muscarinic
receptors could affect Scop-induced memory impairments through
hippocampal interneurons. We, therefore, examined alterations of
CB, CR, and PV immunoreactivities in the mouse hippocampus to
investigate the link between cognitive functions and hippocampal
interneurons containing CBPs after long-term systemic Scop
treatment.
In the present study, CB immunoreactivity was
significantly decreased in all hippocampal subregions from 1 week
after Scop treatment, and rarely observed from 3 weeks after Scop
treatment. We recently reported that muscarinic acetylcholine
receptor M1 (M1R) was expressed in neurons of the SP and granular
cell layer, the expression pattern was similar to that of CB in the
CA1 area and DG, and Scop treatment significantly reduced M1R
immunoreactivity in the hippocampus (23). On the other hand, a previous study
reported that decreased CB immunoreactivity in the hippocampal CA1
region and the DG was directly correlated with decreased
recognition memory performance in aged mice (24). Therefore, we suggest that CB
immunoreactivity is progressively reduced from 1 week and decreased
to the lowest immunoreactivity at least 3 weeks after systemic Scop
treatment, although impaired spatial and short-term memory
functions are induced from 1 week after systemic Scop
treatment.
We found, in this study, that CR and PV
immunoreactivities were also severely decreased in the CA1 region
and in the DG from at least 3 weeks after Scop treatment; in the
CA3 region, the immunoreactivities were not significantly altered
until 4 weeks after Scop treatment. Pascual et al reported
that CR- and PV-immunoreactive interneurons located in the CA1-3
region selectively received GABAergic axons originated from the
medial septum in mice (5). In
addition, it was reported that CBPs containing interneurons in the
hippocampus were involved in inhibition of GABAergic
septo-hippocampal neurons, which were the source of disinhibition
of pyramidal neurons in the hippocampus in the mouse, rat and
monkey (5–7). Furthermore, some researchers reported
that about 50% of all muscarinic cholinoceptive nonpyramidal
neurons contained PV, and about 90% of the PV containing neurons
expressed mAChR in rats (25,26).
Those previous findings as well as our present results suggest that
markedly decreased CR and PV immunoreactivities in the CA1 region
and in the DG of the mouse might be related to Scop-induced memory
impairments. However, it needs to study what maintained CR and PV
immunoreactivity in the CA3 region during chronic Scop treatment is
related to in memory after or during Scop treatment.
Finally, in the present study, we found that PV
immunoreactivity was not changed in all hippocampal subregions 1
week after Scop treatment, not like CB and CR immunoreactivities
that were decreased from 2 weeks after Scop treatment. To the best
of our knowledge, it is likely that PV containing neurons are
relatively resistant to short-term (1 week) Scop treatment than CB
and CR containing neurons in the hippocampus.
In conclusion, this study identified that CBPs
immunoreactivities in the mouse hippocampus were differently
decreased during period of systemic Scop treatment, and suggests
that Scop-induced CBPs changes or reductions might be differently
related according to types of CBPs to spatial and short-term memory
deficits.
Acknowledgements
This study was supported by the Bio-Synergy Research
Project (NRF-2015M3A9C4076322) of the Ministry of Science, ICT and
Future Planning through the National Research Foundation, and by a
Priority Research Centers Program grant (NRF-2009-0093812) through
the National Research Foundation of Korea funded by the Ministry of
Science, ICT and Future Planning.
References
|
1
|
Van der Zee EA and Luiten PG: Muscarinic
acetylcholine receptors in the hippocampus, neocortex and amygdala:
A review of immunocytochemical localization in relation to learning
and memory. Prog Neurobiol. 58:409–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Easton A, Douchamps V, Eacott M and Lever
C: A specific role for septohippocampal acetylcholine in memory?
Neuropsychologia. 50:3156–3168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alreja M, Wu M, Liu W, Atkins JB, Leranth
C and Shanabrough M: Muscarinic tone sustains impulse flow in the
septohippocampal GABA but not cholinergic pathway: Implications for
learning and memory. J Neurosci. 20:8103–8110. 2000.PubMed/NCBI
|
|
4
|
Wu M, Shanabrough M, Leranth C and Alreja
M: Cholinergic excitation of septohippocampal GABA but not
cholinergic neurons: Implications for learning and memory. J
Neurosci. 20:3900–3908. 2000.PubMed/NCBI
|
|
5
|
Pascual M, Pérez-Sust P and Soriano E: The
GABAergic septohippocampal pathway in control and reeler mice:
Target specificity and termination onto Reelin-expressing
interneurons. Mol Cell Neurosci. 25:679–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gulyás A, Seress L, Tóth K, Acsády L,
Antal M and Freund TF: Septal GABAergic neurons innervate
inhibitory interneurons in the hippocampus of the macaque monkey.
Neuroscience. 41:381–390. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acsády L, Halasy K and Freund TF:
Calretinin is present in non-pyramidal cells of the rat
hippocampus-III. Their inputs from the median raphe and medial
septal nuclei. Neuroscience. 52:829–841. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tóth K, Borhegyi Z and Freund TF:
Postsynaptic targets of GABAergic hippocampal neurons in the medial
septum-diagonal band of broca complex. J Neurosci. 13:3712–3724.
1993.PubMed/NCBI
|
|
9
|
Mattson MP: Calcium and neurodegeneration.
Aging Cell. 6:337–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foster TC: Calcium homeostasis and
modulation of synaptic plasticity in the aged brain. Aging Cell.
6:319–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang ZJ and Reynolds GP: A selective
decrease in the relative density of parvalbumin-immunoreactive
neurons in the hippocampus in schizophrenia. Schizophr Res.
55:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang IK, Nam YS, Chung DW, Lee HS, Yoon
YS, Yoo KY, Kang TC, Lee IS and Won MH: Changes in the expression
of calbindin D-28k in the gerbil hippocampus following seizure.
Neurochem Int. 44:145–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reynolds GP, Abdul-Monim Z, Neill JC and
Zhang ZJ: Calcium binding protein markers of GABA deficits in
schizophrenia-postmortem studies and animal models. Neurotox Res.
6:57–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holmes GL, Yang Y, Liu Z, Cermak JM,
Sarkisian MR, Stafstrom CE, Neill JC and Blusztajn JK:
Seizure-induced memory impairment is reduced by choline
supplementation before or after status epilepticus. Epilepsy Res.
48:3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan BC, Park JH, Chen BH, Cho JH, Kim IH,
Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, et al: Long-term
administration of scopolamine interferes with nerve cell
proliferation, differentiation and migration in adult mouse
hippocampal dentate gyrus, but it does not induce cell death.
Neural Regen Res. 9:1731–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Onaolapo OJ, Onaolapo AY, Abiola AA and
Lillian EA: Central depressant and nootropic effects of daytime
melatonin in mice. Ann Neurosci. 21:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doguc DK, Delibas N, Vural H, Altuntas I,
Sutcu R and Sonmez Y: Effects of chronic scopolamine administration
on spatial working memory and hippocampal receptors related to
learning. Behav Pharmacol. 23:762–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo DY, Kim W, Lee CH, Shin BN, Nam SM,
Choi JH, Won MH, Yoon YS and Hwang IK: Melatonin improves
D-galactose-induced aging effects on behavior, neurogenesis, and
lipid peroxidation in the mouse dentate gyrus via increasing pCREB
expression. J Pineal Res. 52:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JH, Choi JH, Park JH, Kim IH, Cho JH,
Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al: Long-term
exercise improves memory deficits via restoration of myelin and
microvessel damage, and enhancement of neurogenesis in the aged
gerbil hippocampus after ischemic stroke. Neurorehabil Neural
Repair. 30:894–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae EJ, Chen BH, Shin BN, Cho JH, Kim IH,
Park JH, Lee JC, Tae HJ, Choi SY, Kim JD, et al: Comparison of
immunoreactivities of calbindin-D28k, calretinin and parvalbumin in
the striatum between young, adult and aged mice, rats and gerbils.
Neurochem Res. 40:864–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JH, Choi HY, Cho JH, Kim IH, Lee TK,
Lee JC, Won MH, Chen BH, Shin BN, Ahn JH, et al: Effects of chronic
scopolamine treatment on cognitive impairments and myelin basic
protein expression in the mouse hippocampus. J Mol Neurosci.
59:579–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Givens B and Olton DS: Bidirectional
modulation of scopolamine-induced working memory impairments by
muscarinic activation of the medial septal area. Neurobiol Learn
Mem. 63:269–276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen BH, Park JH, Kim DW, Park J, Choi SY,
Kim IH, Cho JH, Lee TK, Lee JC, Lee CH, et al: Melatonin improves
cognitive deficits via restoration of cholinergic dysfunction in a
mouse model of scopolamine-induced amnesia. ACS Chem Neurosci. Sep
27–2017.(Epub ahead of print). View Article : Google Scholar
|
|
24
|
Soontornniyomkij V, Risbrough VB, Young
JW, Soontornniyomkij B, Jeste DV and Achim CL: Hippocampal
calbindin-1 immunoreactivity correlate of recognition memory
performance in aged mice. Neurosci Lett. 516:161–165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van der Zee EA and Luiten PG: GABAergic
neurons of the rat dorsal hippocampus express muscarinic
acetylcholine receptors. Brain Res Bull. 32:601–609. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van der Zee EA, de Jong GI, Strosberg AD
and Luiten PG: Parvalbumin-positive neurons in rat dorsal
hippocampus contain muscarinic acetylcholine receptors. Brain Res
Bull. 27:697–700. 1991. View Article : Google Scholar : PubMed/NCBI
|