Introduction
Aristolochic acid (AA) is a component present in
Chinese herbs (for example Asarum and Aristolochia)
from remedies for the treatment of arthritis pain, coughs and
gastrointestinal symptoms (1–4).
Previous studies have indicated that AA can lead to renal injury
(5,6) and this finding has led to further
studies (7,8). Previous studies have indicated that
renal damage from renal cell death and renal fibrosis is associated
with AA treatment (9,10).
AA-induced oxidative stress may serve an important
role in the development of renal injury (11–13).
Previous studies have demonstrated that oxidative stress causes
lipid peroxidation, DNA damage and protein peroxidation, and
results in cell damage (14–16).
O2− and H2O2 are key
reactive oxygen species (ROS) identified in cells (17,18).
Normally, O2− and H2O2
are produced in the mitochondria via electron transport chain
(19,20) and these ROS are removed by cellular
superoxide dismutase (SOD), glutathione peroxidase (Gpx) and
catalase (CAT) (21–23). However, various toxins also induce
O2− and H2O2 production
(24–26). The excessive
O2− and H2O2 lead to
cell injury (27,28) and it has additionally been reported
that AA-induced H2O2 leads to renal damage
(29).
Various studies have demonstrated that oxidative
stress can induce cell apoptosis or cell necrosis (30–32),
and consequently AA-induced oxidative stress can cause apoptosis or
necrosis of renal cells (29,33–35).
Concerning apoptosis, caspase-dependent and caspase-independent
pathways have been reported previously (36,37).
Although certain mechanisms of AA-induced cell death remain
unclear, the caspase activation may be associated with AA-induced
apoptosis (38,39). Previous studies indicated that AA
can activate caspase-9 and caspase-3 leading to cell apoptosis
(40–42).
The isoforms of vitamin E consist of α-tocopherol,
β-tocopherol, δ-tocotrienol and γ-tocotrienol (43). Among them, α-tocopherol possesses
anti-oxidative activities and has been used in a clinical setting
(44,45). In addition, previous studies have
suggested that α-tocopherol can inhibit renal fibrosis (46,47).
Due to the fact that AA-induced renal injury was associated with
oxidative damage and fibrotic renal injury (9,11–13),
the effects of α-tocopherol on AA-induced renal cell cytotoxicity
were studied. The results of the present study demonstrated that
α-tocopherol can inhibit the H2O2 level and
caspase-3 activities to attenuate renal tubular epithelial cell
death under AA treatment.
Materials and methods
Materials
The MTT assay kit was obtained from Bio Basic
Canada, Inc. (Markham, OT, Canada). Vitamin E (α-tocopherol),
luminol, lucigenin, tubulin polyclonal antibody and Hoechst 33342
were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Caspase-3 and cleaved caspase-3 polyclonal antibodies were obtained
from Cell Signaling Technology, Inc. (9662; 1:1,000; Danvers, MA,
USA). Fetal bovine serum, DMEM, non-essential amino acid,
L-glutamine, and penicillin/streptomycin were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
Rat renal tubular epithelial cells (NRK-52E) were
obtained from the Bioresource Collection and Research Center (Shin
Chu, Taiwan). NRK-52E cells were cultured with DMEM medium
containing 10% fetal bovine serum, 2 mM L-glutamine, 100 IU/ml
penicillin/streptomycin and 0.1 mM non-essential amino acids. Cells
were maintained in a humidified atmosphere containing 5%
CO2 at 37°C.
ROS detection
H2O2 and
O2− levels were measured by using the
lucigenin-amplified chemiluminescence method (48,49).
The culture supernatant (200 µl) were added with 0.2 mmol/l of
luminol solution (100 µl) and measured subsequently by using a
chemiluminescence analyzing system (CLA-FSI; Tohoko Electronic
Industrial Co., Ltd., Sendai, Japan) for the determination of
H2O2 levels. The samples (200 µl) were
treated with 0.1 mmol/l lucigenin solution (200 µl) and then
O2− levels were measured using the CLA-FSI
chemiluminescence analyzing system.
Cell survival rates determination
The cell survival rates were determined using the
MTT assay kit according to the manufacturer's instructions. In
brief, NRK-52E cells were cultured into 96-well plates at a density
of 8×103 cells/well and incubated for 24 h in 100 µl
DMEM medium. The suitable concentration and optimum exposure time
of AAI were determined as 5, 10, 20 and 100 µM at 6 h time
intervals. Cells were treated with MTT assay kit for 3 h at 37°C
and were measured at 570 nm absorbance using a Multiskan™ FC
microplate photometer (Molecular Devices, Inc., Sunnyvale, CA,
USA). The cell survival rate was calculated as the following
formula: Optical density (OD) 570 experimental group/OD 570 control
group ×100%.
Observation of apoptotic features
Apoptotic features containing DNA fragmentation and
nuclear condensation were observed by using Hoechst 33342
(23491-52-3; Sigma-Aldrich; Merck KGaA) nuclear staining (49,50).
Control and experimental cells were treated with Hoechst 33342 (10
µg/ml) at 37°C for 10 min. DNA fragmentation and nuclear
condensation were observed under an Olympus DP71 fluorescence
microscope (excitation, 352 nm; emission, 450 nm; Olympus
Corporation, Tokyo, Japan).
Western blotting
Cells were treated with radioimmunoprecipitation
assay buffer (20–188; EMD Millipore, Billerica, MA, USA). Following
10 min centrifugation (16,000 × g) at 4°C, proteins were obtained
from the supernatant layer and the concentration was determined by
using the protein assay kit (23200; Thermo Fischer Scientific,
Inc.). Equal quantities of samples were separated on a 13.3%
SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and then
transferred onto polyvinylidene difluoride membranes (EMD
Millipore). The membranes were blocked with 5% milk for 2 h at room
temperature. Next, the membranes were washed with
phosphate-buffered saline (PBS) then incubated with the primary
antibodies for 4 h. Following that, membranes were washed with PBS
and treated with anti-rabbit-horseradish peroxidase secondary
antibodies (NA934; 1:1,000 Amersham; GE Healthcare Life Sciences,
Chalfont, UK) for 1 h at room temperature. Finally, proteins were
observed by using Western Lightning Chemiluminescence Reagent Plus
(PerkinElmer, Inc., Waltham, MA, USA).
Statistical analysis
Student's t-test and two-way analysis of variance
were utilized for the analysis of the data using SPSS version 18.0
(SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard error. P<0.05 was considered statistically significant
different between values.
Results
Increases of
H2O2 and O2- levels by different
concentrations of AA treatment
Previous studies have demonstrated that AA induced
ROS generation in renal tubular cells (13,51).
H2O2 and O2−, two major
types of ROS, were measured in AA-treated renal tubular cells.
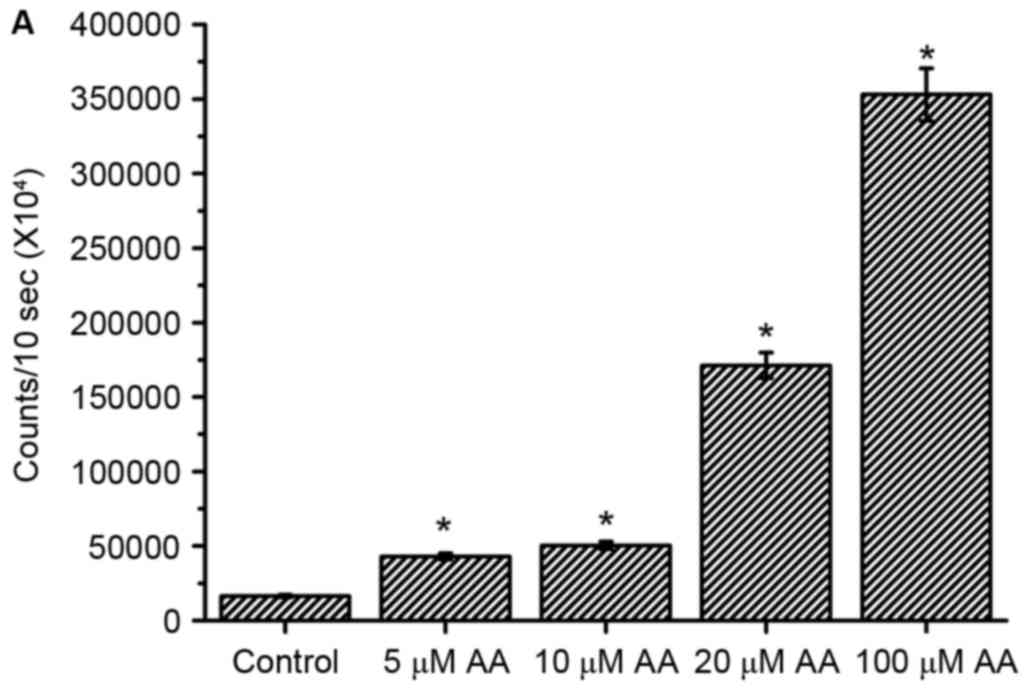
Experimental results indicated that H2O2
levels were increased dose-dependently in the AA-treated cells
(Fig. 1A). Compared with
H2O2 levels, O2− levels
were increased only at the 100 µM AA treatment however not at 20–50
µM AA concentrations (Fig. 1B).
The data suggested low-dose AA (5–20 µM) can induce increases in
H2O2 levels, but not
O2− levels. Additionally, high-dose AA (100
µM) can induce increases of H2O2 and
O2− levels.
AA decreased cell survival rates in
dose- and time- dependent manners
In order to determine the cytotoxic effects on
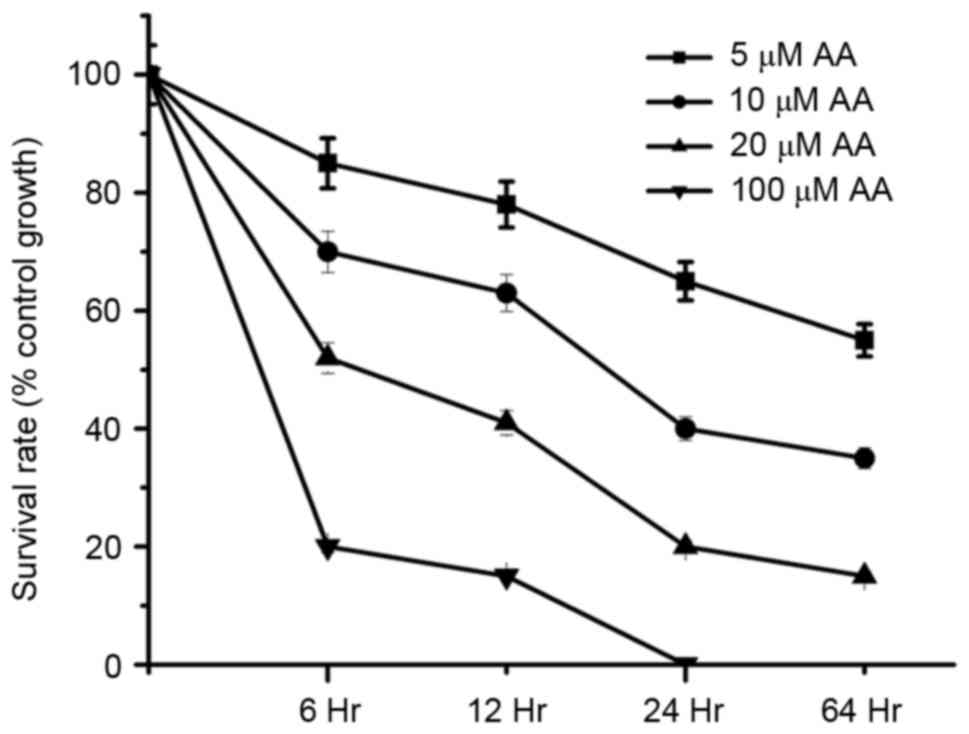
AA-treated renal tubular cells, various concentrations (5–100 µM)
of AA were studied. As presented in Fig. 2, the cell survival rates were below
50% at 100 µM AA (6 h), 20 µM AA (12 h), 10 µM AA (24 h) and 5 µM
AA (48 h) treatment. These results demonstrated AA-induced cell
cytotoxicity was dose- and time-dependent.
Apoptotic characteristics in
AA-treated renal tubular cells
Cell death can be described as apoptosis or necrosis
(52,53). Apoptotic cells can be removed by
macrophages in order to prevent serious inflammatory responses
(54,55), and previous studies have indicated
that nuclear condensation and DNA fragmentation are key apoptotic
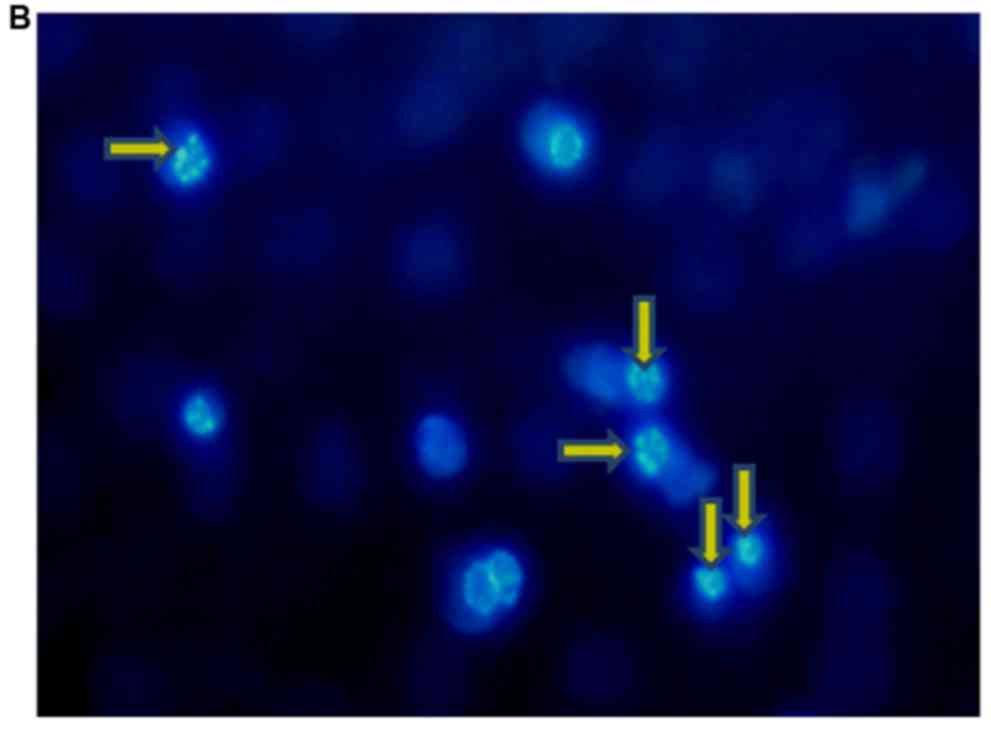
characteristics (49,56). In the present study, the cell
nuclei were observed by Hoechst 33342 staining (49,50).
As presented in Fig. 3, compared
with control cell, the nuclear condensation and DNA fragmentation
were identified in AA-treated renal tubular cells. The results
indicated that AA-induced cell death was associated with the
apoptotic death pathway.
α-tocopherol attenuated
H2O2 levels and increased cell survival in
AA-treated cells
The antioxidant stress activities of vitamin E
(α-tocopherol) has been demonstrated in clinical cases (44,45).
Due to the fact that AA elevated H2O2 levels
(Fig. 1A), it was investigated
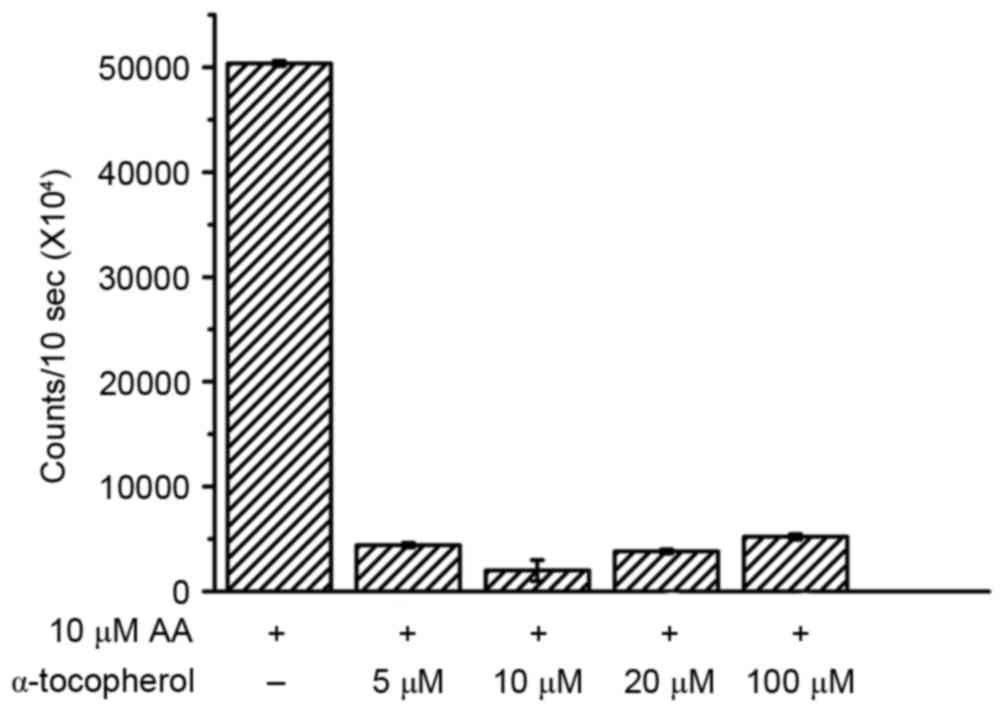
whether α-tocopherol could inhibit H2O2 in
AA-treated cells with various concentrations of α-tocopherol (5,
10, 20 and 100 µM). The data indicated that α-tocopherol attenuated
AA-induced H2O2 levels (Fig. 4). Notably, it was observed that 10
µM α-tocopherol appeared to have a more marked effect on AA-induced
H2O2 compared with other concentrations.
Subsequently, the effects of α-tocopherol on AA-induced renal cell
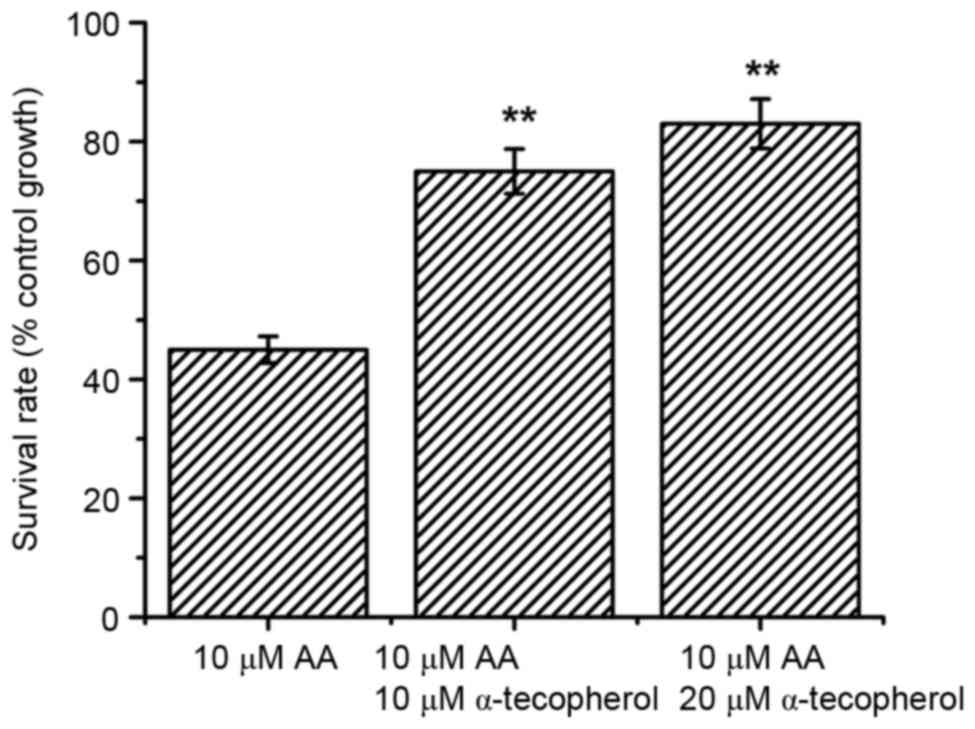
death were investigated. As presented in Fig. 5, the cell survival rates were
increased in AA-treated renal tubular cells undergoing 10 or 20 µM
α-tocopherol treatment. These results first demonstrated that
α-tocopherol attenuated AA-induced H2O2
levels and increased cell survival of AA-treated cells.
α-tocopherol reduced AA-activated
caspase-3
Caspase-3 activation is associated with the
apoptotic death pathway (40–42).
Due to the fact that apoptotic characteristics were predominantly
identified in AA-treated renal tubular cells (Fig. 3B), whether AA could activate
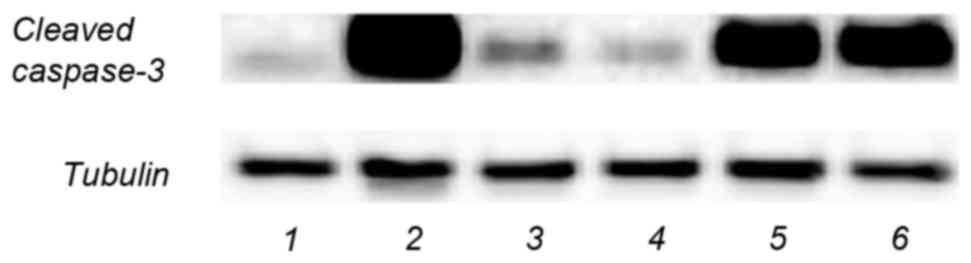
caspase-3 was investigated. As presented in Fig. 6, compared with the control group
(lane 1), the level of cleaved caspase-3 was markedly increased in
the AA-treated group (lane 2). AA was identified to induce
caspase-3 activity and the effect of α-tocopherol on AA-induced
caspase-3 was further determined. AA was reported to decrease
mitochondrial membrane depolarization and to lead to an increase of
caspase-3 (42). The results
demonstrated that cleaved caspase-3 levels were reduced in the AA
plus α-tocopherol group (lane 5 and 6) compared with the AA
treatment group (lane 2). Due to the fact that AA-induced
H2O2 levels are also reduced in the AA +
α-tocopherol group (Fig. 4), this
suggested that α-tocopherol attenuation of AA-induced cell
cytotoxicity may be associated with caspase-3 activity from the
reduce of H2O2 levels.
Discussion
The results of the current study indicated that AA
causes increases in H2O2 levels and a
reduction in cell survival rates in renal tubular cells and
α-tocopherol (10 and 20 µM) attenuated AA-induced
H2O2 levels and inhibited AA-induced
cytotoxicity in these cells. These data suggested that AA-induced
cell cytotoxicity may be associated with H2O2
levels. By contrast, α-tocopherol could not inhibit AA-induced
cytotoxicity under high-dose (100 µM) AA treatment (data not
shown), however it effectively ameliorated AA-induced cytotoxicity
under low-dose (5–20 µM) AA treatment. This suggested that
α-tocopherol ameliorated AA-induced renal cell damage was dependent
on AA dosage. Furthermore, high-dose AA alone elevated both
H2O2 and O2− levels.
Therefore, this may partially explain why α-tocopherol could not
inhibit high-dose AA-induced cell cytotoxicity.
CAT, Gpx and SOD are major cellular antioxidant
enzyme systems (57,58). CAT is a tetrameric iron-porphyrin
protein in peroxisomes that converts H2O2 to
H2O and O2. CAT and Cu/Zn-SOD are expressed
constitutively, whereas Mn-SOD expression within the mitochondria
is induced by oxidative stress. GSH is a sulfhydryl peptide that
may directly react with O2− or
N2− containing free radicals, or is able to
donate electrons in the enzymatic dismutation of
H2O2 to H2O and O2 by
GPx (59,60). Cellular CAT and Gpx can remove
H2O2, whereas SOD removes
O2− (61).
In the present study, the data indicated that AA induced increases
in H2O2 in a dose-dependent manner, however
the 100 µM AA alone was capable of increasing
O2− levels. This result suggested that
low-dose AA may influence the activity of CAT and Gpx while
high-dose AA may influence CAT, Gpx and SOD activities. However,
further studies are required to confirm this hypothesis.
Studies have indicated that antioxidant can
attenuate AA-induced renal damage (11,29,59)
and it has been additionally demonstrated that vitamin C attenuated
AA-induced renal damage (29).
However, the effect of antioxidant α-tocopherol on AA-induced renal
damage remains to be reported. The current study demonstrated that
α-tocopherol ameliorated AA-induced renal cell cytotoxicity.
Vitamin C and α-tocopherol are common antioxidants
and have been used in clinical cases (44,45,62,63).
Vitamin C and α-tocopherol both have antioxidant activities,
however their antioxidant mechanisms differ (64–66).
These studies indicated that α-tocopherol (lipid-soluble material)
is able to pass through the cell membrane and catch the free
radicals to protect the cell from oxidative damage. However,
vitamin C (water-soluble material) cannot pass through cell
membrane to remove free radicals directly. Based on the results of
a previous study (29) and the
current study, it is suggested that both vitamin C and α-tocopherol
scavenge H2O2 produced by AA-treated renal
cells, leading to an increase of survival rate. It was suggested
that AA-induced H2O2 existed not only in the
cytosol however additionally in the cell membrane. In addition,
another key antioxidant function of vitamin C is converting the
oxidized α-tocopherol radical back to α-tocopherol (62). It was suggested that the
combination of vitamin C and α-tocopherol may be more powerful for
protection of AA-induced renal damage. In patients with coronary
artery bypass surgery, vitamin cocktail (ascorbic acid and
α-tocopherol) effectively attenuated oxidative stress than control
(44). In summary, the results
demonstrated that α-tocopherol attenuates AA-induced
H2O2 and caspase-3 and ameliorated AA-induced
renal cell cytotoxicity.
Acknowledgements
The present study was supported by grants from the
Ministry of Science and Technology, Taiwan (grant nos. MOST103
2320-B-039-052-MY3 and MOST104-2321-B-039-005) and Ministry of
Health and Welfare (MOHW104-TDU-B-212-124-002).
References
|
1
|
Schaneberg BT and Khan IA: Analysis of
products suspected of containing Aristolochia or
Asarum species. J Ethnopharmacol. 94:245–249. 2004.
View Article : Google Scholar
|
|
2
|
Li XW, Morinaga O, Tian M, Uto T, Yu J,
Shang MY, Wang X, Cai SQ and Shoyama Y: Development of an Eastern
blotting technique for the visual detection of aristolochic acids
in Aristolochia and Asarum species by using a
monoclonal antibody against aristolochic acids I and II. Phytochem
Anal. 24:645–653. 2013. View
Article : Google Scholar
|
|
3
|
Tsai DM, Kang JJ, Lee SS, Wang SY, Tsai
IL, Chen GY, Liao HW, Wei-Chu L, Kuo CH and Tseng YJ: Metabolomic
analysis of complex chinese remedies: Examples of induced
nephrotoxicity in the mouse from a series of remedies containing
aristolochic acid. Evid Based Complement Alternat Med.
2013:2637572013. View Article : Google Scholar :
|
|
4
|
Heinrich M, Chan J, Wanke S, Neinhuis C
and Simmonds MS: Local uses of Aristolochia species and
content of nephrotoxic aristolochic acid 1 and 2-a global
assessment based on bibliographic sources. J Ethnopharmacol.
125:108–144. 2009. View Article : Google Scholar
|
|
5
|
Feng C, Xie X, Wu M, Li C, Gao M, Liu M,
Qi X and Ren J: Tanshinone I protects mice from aristolochic acid
I-induced kidney injury by induction of CYP1A. Environ Toxicol
Pharmacol. 36:850–857. 2013. View Article : Google Scholar
|
|
6
|
Luciano RL and Perazella MA: Aristolochic
acid nephropathy: Epidemiology, clinical presentation, and
treatment. Drug Saf. 38:55–64. 2015. View Article : Google Scholar
|
|
7
|
Zhang J, Zhang L, Wang W and Wang H: China
National Survey of Chronic Kidney Disease Working Group:
Association between aristolochic acid and CKD: A cross-sectional
survey in China. Am J Kidney Dis. 61:918–922. 2013. View Article : Google Scholar
|
|
8
|
De Broe ME: Chinese herbs nephropathy and
Balkan endemic nephropathy: Toward a single entity, aristolochic
acid nephropathy. Kidney Int. 81:513–515. 2012. View Article : Google Scholar
|
|
9
|
Pozdzik AA, Salmon IJ, Debelle FD,
Decaestecker C, Van den Branden C, Verbeelen D, Deschodt-Lanckman
MM, Vanherweghem JL and Nortier JL: Aristolochic acid induces
proximal tubule apoptosis and epithelial to mesenchymal
transformation. Kidney Int. 73:595–607. 2008. View Article : Google Scholar
|
|
10
|
Lin TC, Lee TC, Hsu SL and Yang CS: The
molecular mechanism of leptin secretion and expression induced by
aristolochic acid in kidney fibroblast. PLoS One. 6:e166542011.
View Article : Google Scholar :
|
|
11
|
Matsui K, Kamijo-Ikemorif A, Sugaya T,
Yasuda T and Kimura K: Renal liver-type fatty acid binding protein
(L-FABP) attenuates acute kidney injury in aristolochic acid
nephrotoxicity. Am J Pathol. 178:1021–1032. 2011. View Article : Google Scholar :
|
|
12
|
Bunel V, Antoine MH, Nortier J, Duez P and
Stévigny C: In vitro effects of Panax ginseng in
aristolochic acid-mediated renal tubulotoxicity: Apoptosis versus
regeneration. Planta Med. 81:363–372. 2015. View Article : Google Scholar
|
|
13
|
Yu FY, Wu TS, Chen TW and Liu BH:
Aristolochic acid I induced oxidative DNA damage associated with
glutathione depletion and ERK1/2 activation in human cells. Toxicol
In Vitro. 25:810–816. 2011. View Article : Google Scholar
|
|
14
|
Singh M, Kapoor A and Bhatnagar A:
Oxidative and reductive metabolism of lipid-peroxidation derived
carbonyls. Chem Biol Interact. 234:261–273. 2015. View Article : Google Scholar :
|
|
15
|
Dur A, Kocaman O, Koçyiğit A, Türkdoğan
KA, Sönmez E, Keskin S, Yiğit M, Gülen B, Kılıç E and Uysal Ö:
Oxidative status and lymphocyte DNA damage in patients with acute
pancreatitis and its relationship with severity of acute
pancreatitis. Turk J Gastroenterol. 27:68–72. 2016. View Article : Google Scholar
|
|
16
|
Kruk J, Kubasik-Kladna K and Aboul-Enein
HY: The role oxidative stress in the pathogenesis of eye diseases:
Current status and a dual role of physical activity. Mini Rev Med
Chem. 16:241–257. 2015. View Article : Google Scholar
|
|
17
|
Yao CW, Piao MJ, Kim KC, Zheng J, Cha JW
and Hyun JW: 6′-o-galloylpaeoniflorin protects human keratinocytes
against oxidative stress-induced cell damage. Biomol Ther (Seoul).
21:349–357. 2013. View Article : Google Scholar :
|
|
18
|
Sen S, Kawahara B, Fry NL, Farias-Eisner
R, Zhang D, Mascharak PK and Chaudhuri G: A light-activated NO
donor attenuates anchorage independent growth of cancer cells:
Important role of a cross talk between NO and other reactive oxygen
species. Arch Biochem Biophys. 540:33–40. 2013. View Article : Google Scholar
|
|
19
|
Goncalves RL, Quinlan CL, Perevoshchikova
IV, Hey-Mogensen M and Brand MD: Sites of superoxide and hydrogen
peroxide production by muscle mitochondria assessed ex vivo under
conditions mimicking rest and exercise. J Biol Chem. 290:209–227.
2015. View Article : Google Scholar
|
|
20
|
Goncalves RL, Rothschild DE, Quinlan CL,
Scott GK, Benz CC and Brand MD: Sources of
superoxide/H2O2 during mitochondrial proline
oxidation. Redox Biol. 2:901–909. 2014. View Article : Google Scholar :
|
|
21
|
Prasad AK and Mishra PC: Mechanism of
action of sulforaphane as a superoxide radical anion and hydrogen
peroxide scavenger by double hydrogen transfer: A model for iron
superoxide dismutase. J Phys Chem B. 119:7825–7836. 2015.
View Article : Google Scholar
|
|
22
|
Ludwig E and Eyer P: Reactivity of
glutathione adducts of 4-(dimethylamino)phenol. Involvement of
reactive oxygen species during the interaction with oxyhemoglobin.
Chem Res Toxicol. 8:363–368. 1995. View Article : Google Scholar
|
|
23
|
Tulunoglu O, Alacam A, Bastug M and
Yavuzer S: Superoxide dismutase activity in healthy and inflamed
pulp tissues of permanent teeth in children. J Clin Pediatr Dent.
22:341–345. 1998.
|
|
24
|
Gölz L, Memmert S, Rath-Deschner B, Jäger
A, Appel T, Baumgarten G, Götz W and Frede S: LPS from P.
gingivalis and hypoxia increases oxidative stress in
periodontal ligament fibroblasts and contributes to periodontitis.
Mediators Inflamm. 2014:9862642014. View Article : Google Scholar :
|
|
25
|
Magdalan J, Piotrowska A, Gomulkiewicz A,
Sozański T, Szelag A and Dziegiel P: Influence of commonly used
clinical antidotes on antioxidant systems in human hepatocyte
culture intoxicated with alpha-amanitin. Hum Exp Toxicol. 30:38–43.
2011. View Article : Google Scholar
|
|
26
|
Peng XL, Xu WT, Wang Y, Huang KL, Liang
ZH, Zhao WW and Luo YB: Mycotoxin Ochratoxin A-induced cell death
and changes in oxidative metabolism of Arabidopsis thaliana.
Plant Cell Rep. 29:153–161. 2010. View Article : Google Scholar
|
|
27
|
Maruf AA and O'Brien P:
Inflammation-enhanced drug-induced liver injury. Free Radic Biol
Med. 75 Suppl 1:S402014. View Article : Google Scholar
|
|
28
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar
|
|
29
|
Wu TK, Wei CW, Pan YR, Cherng SH, Chang
WJ, Wang HF and Yu YL: Vitamin C attenuates the toxic effect of
aristolochic acid on renal tubular cells via decreasing oxidative
stress-mediated cell death pathways. Mol Med Rep. 12:6086–6092.
2015. View Article : Google Scholar
|
|
30
|
Gomez C, Martinez L, Mesa A, Duque JC,
Escobar LA, Pham SM and Vazquez-Padron RI: Oxidative stress induces
early-onset apoptosis of vascular smooth muscle cells and neointima
formation in response to injury. Biosci Rep. 35:pii: e00227. 2015.
View Article : Google Scholar :
|
|
31
|
Hu XL, Niu YX, Zhang Q, Tian X, Gao LY,
Guo LP, Meng WH and Zhao QC: Neuroprotective effects of Kukoamine B
against hydrogen peroxide-induced apoptosis and potential
mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol. 40:230–240.
2015. View Article : Google Scholar
|
|
32
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar
|
|
33
|
Yang H, Dou Y, Zheng X, Tan Y, Cheng J, Li
L, Du Y, Zhu D and Lou Y: Cysteinyl leukotrienes synthesis is
involved in aristolochic acid I-induced apoptosis in renal proximal
tubular epithelial cells. Toxicology. 287:38–45. 2011. View Article : Google Scholar
|
|
34
|
Baudoux TE, Pozdzik AA, Arlt VM, De Prez
EG, Antoine MH, Quellard N, Goujon JM and Nortier JL: Probenecid
prevents acute tubular necrosis in a mouse model of aristolochic
acid nephropathy. Kidney Int. 82:1105–1113. 2012. View Article : Google Scholar
|
|
35
|
Yang L, Li X and Wang H: Possible
mechanisms explaining the tendency towards interstitial fibrosis in
aristolochic acid-induced acute tubular necrosis. Nephrol Dial
Transplant. 22:445–456. 2007. View Article : Google Scholar
|
|
36
|
Seo HS, Ku JM, Choi HS, Woo JK, Jang BH,
Go H, Shin YC and Ko SG: Apigenin induces caspase-dependent
apoptosis by inhibiting signal transducer and activator of
transcription 3 signaling in HER2-overexpressing SKBR3 breast
cancer cells. Mol Med Rep. 12:2977–2984. 2015. View Article : Google Scholar
|
|
37
|
Göke A, Göke R, Ofner A, Herbst A and
Lankat-Buttgereit B: The FGFR inhibitor NVP-BGJ398 induces NSCLC
cell death by activating caspase-dependent pathways as well as
caspase-independent apoptosis. Anticancer Res. 35:5873–5879.
2015.
|
|
38
|
Wang Y, Fu W, Wang H, Liang Y, Wang Y, Yao
W, Chen W, Li Q, Ying PH, Shi X and Peng W: Renal microvascular
injury in chronic aristolochic acid nephropathy and protective
effects of Cozaar. Ren Fail. 34:60–67. 2012. View Article : Google Scholar
|
|
39
|
Zhang L, Li J, Jiang Z, Sun L, Mei X, Yong
B and Zhang L: Inhibition of aquaporin-1 expression by RNAi
protects against aristolochic acid I-induced apoptosis in human
proximal tubular epithelial (HK-2) cells. Biochem Biophys Res
Commun. 405:68–73. 2011. View Article : Google Scholar
|
|
40
|
Yuan SY, Yang CR, Cheng CL, Hsu SL, Liao
JW, Lin CC, Chou YY and Cheng YW: Comparative nephrotoxicity of
aristolochic acid and tetrandrine in vitro and in vivo. Int J
Toxicol. 30:35–46. 2011. View Article : Google Scholar
|
|
41
|
Kwak DH, Park JH, Lee HS, Moon JS and Lee
S: Aristolochic acid I induces ovarian toxicity by inhibition of
akt phosphorylation. Chem Res Toxicol. 27:2128–35. 2014. View Article : Google Scholar
|
|
42
|
Qi X, Cai Y, Gong L, Liu L, Chen F, Xiao
Y, Wu X, Li Y, Xue X and Ren J: Role of mitochondrial permeability
transition in human renal tubular epithelial cell death induced by
aristolochic acid. Toxicol Appl Pharmacol. 222:105–110. 2007.
View Article : Google Scholar
|
|
43
|
Cook-Mills JM: Isoforms of vitamin E
differentially regulate PKC α and inflammation: A review. J Clin
Cell Immunol. 4:pii: 1000137. 2013. View Article : Google Scholar :
|
|
44
|
Stanger O, Aigner I, Schimetta W and
Wonisch W: Antioxidant supplementation attenuates oxidative stress
in patients undergoing coronary artery bypass graft surgery. Tohoku
J Exp Med. 232:145–154. 2014. View Article : Google Scholar
|
|
45
|
Park OJ, Kim HY, Kim WK, Kim YJ and Kim
SH: Effect of vitamin E supplementation on antioxidant defense
systems and humoral immune responses in young, middle-aged and
elderly Korean women. J Nutr Sci Vitaminol (Tokyo). 49:94–99. 2003.
View Article : Google Scholar
|
|
46
|
Kutlubay R, Oğuz EO, Güven C, Can B, Sinik
Z and Tuncay OL: Histological and ultrastructural evidence for
protective effects on aluminium-induced kidney damage by
intraperitoneal administration of alpha-tocopherol. Int J Toxicol.
26:95–101. 2007. View Article : Google Scholar
|
|
47
|
Tasanarong A, Kongkham S, Duangchana S,
Thitiarchakul S and Eiam-Ong S: Vitamin E ameliorates renal
fibrosis by inhibition of TGF-beta/Smad2/3 signaling pathway in UUO
mice. J Med Assoc Thai 94 Suppl. 7:S1–S9. 2011.
|
|
48
|
Lin BR, Yu CJ, Chen WC, Lee HS, Chang HM,
Lee YC, Chien CT and Chen CF: Green tea extract supplement reduces
D-galactosamine-induced acute liver injury by inhibition of
apoptotic and proinflammatory signaling. J Biomed Sci. 16:352009.
View Article : Google Scholar :
|
|
49
|
Yiang GT, Yu YL, Lin KT, Chen JN, Chang WJ
and Wei CW: Acetaminophen induces JNK/p38 signaling and activates
the caspase-9-3-dependent cell death pathway in human mesenchymal
stem cells. Int J Mol Med. 36:485–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu YL, Yiang GT, Chou PL, Tseng HH, Wu TK,
Hung YT, Lin PS, Lin SY, Liu HC, Chang WJ and Wei CW: Dual role of
acetaminophen in promoting hepatoma cell apoptosis and kidney
fibroblast proliferation. Mol Med Rep. 9:2077–2084. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen YY, Chung JG, Wu HC, Bau DT, Wu KY,
Kao ST, Hsiang CY, Ho TY and Chiang SY: Aristolochic acid
suppresses DNA repair and triggers oxidative DNA damage in human
kidney proximal tubular cells. Oncol Rep. 24:141–153.
2010.PubMed/NCBI
|
|
52
|
Kataki A, Skandami V, Memos N,
Nikolopoulou M, Oikonomou V, Androulis A, Konstadoulakis MM and
Zografos CG: Similar immunity profiles in patients with meningioma
and glioma tumors despite differences in the apoptosis and necrosis
of circulating lymphocyte and monocyte populations. J Neurosurg
Sci. 58:9–15. 2014.PubMed/NCBI
|
|
53
|
Song AS, Najjar AM and Diller KR:
Thermally induced apoptosis, necrosis and heat shock protein
expression in 3D culture. J Biomech Eng. 136:2014. View Article : Google Scholar :
|
|
54
|
Wang Q, Zeng P, Liu Y, Wen G, Fu X and Sun
X: Inhibition of autophagy ameliorates atherogenic inflammation by
augmenting apigenin-induced macrophage apoptosis. Int
Immunopharmacol. 27:24–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Martin KR, Ohayon D and Witko-Sarsat V:
Promoting apoptosis of neutrophils and phagocytosis by macrophages:
Novel strategies in the resolution of inflammation. Swiss Med Wkly.
145:w140562015.PubMed/NCBI
|
|
56
|
Yiang GT, Chou PL, Hung YT, Chen JN, Chang
WJ, Yu YL and Wei CW: Vitamin C enhances anticancer activity in
methotrexate-treated Hep3B hepatocellular carcinoma cells. Oncol
Rep. 32:1057–1063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Valle A, Oliver J and Roca P: Role of
uncoupling proteins in cancer. Cancers (Basel). 2:567–591. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cai H: Hydrogen peroxide regulation of
endothelial function: Origins, mechanisms, and consequences.
Cardiovasc Res. 68:26–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expressio. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Flora SJ: Structural, chemical and
biological aspects of antioxidants for strategies against metal and
metalloid exposure. Oxid Med Cell Longev. 2:191–206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Costa A, Scholer-Dahirel A and
Mechta-Grigoriou F: The role of reactive oxygen species and
metabolism on cancer cells and their microenvironment. Semin Cancer
Biol. 25:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anichini C, Lotti F, Pietrini A, Lo Rizzo
C, Longini M, Proietti F, Felici C and Buonocore G: Antioxidant
effects of potassium ascorbate with ribose in costello syndrome.
Anticancer Res. 33:691–695. 2013.PubMed/NCBI
|
|
63
|
Moore DF, Ye F, Brennan ML, Gupta S,
Barshop BA, Steiner RD, Rhead WJ, Brady RO, Hazen SL and Schiffmann
R: Ascorbate decreases Fabry cerebral hyperperfusion suggesting a
reactive oxygen species abnormality: An arterial spin tagging
study. J Magn Reson Imaging. 20:674–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Packer L, Weber SU and Rimbach G:
Molecular aspects of alpha-tocotrienol antioxidant action and cell
signalling. J Nutr. 131:369S–373S. 2001.PubMed/NCBI
|
|
65
|
Chaudière J and Ferrari-Iliou R:
Intracellular antioxidants: From chemical to biochemical
mechanisms. Food Chem Toxicol. 37:949–962. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh M, Singh S and Kale RK:
Chemomodulatory potential of Asparagus adscendens against
murine skin and forestomach papillomagenesis. Eur J Cancer Prev.
20:240–247. 2011. View Article : Google Scholar : PubMed/NCBI
|