Introduction
Glioma originates from glial cells in the brain and
the most common intracranial tumor. In the last 30 years, the
incidence of primary malignant brain tumors has increased annually
with an annual growth rate of ~1.2%, particularly in the elderly
population (1). According to the
statistics of the Central Brain Tumor Registry of the United
States, malignant gliomas account for ~70 percent of primary
malignant brain tumors. The annual occurrence rate is ~5 in 10,000
individuals, with >14,000 newly diagnosed each year, and
incidence is significantly higher in people over the age of 65
(2). The annual mortality rate
from glioma has risen in China to 30,000. The characteristics of
glioblastoma include invasive growth, infiltration in to normal
brain tissue without clear boundaries, fast progression and short
survival time. Typically, gliomas are not limited to one lobe of
the brain; the tumor infiltrates and damages the surrounding brain
tissue (3). Currently, there is no
effective treatment to combat glioma. For the conventional
treatment of gliomas in China and other countries, surgery,
radiotherapy, chemotherapy, stereotactic photon therapy system and
stereotactic gamma ray radiation therapy are used (4). According to follow-up and
investigation, the recurrence time of World Health Organization
(WHO) grade III and/or IV glioma was between 1–6 months, and for
grade I–II level recurrence typically occur during 1–2 years after
surgery. Following comprehensive treatment of patient with
low-grade gliomas (WHO grade I–II grade), the median survival time
is between 8–10 years; for patients with aplastic glioma (WHO grade
III), the median survival time is 3–4 years; for patients with
glioblastoma (WHO grade IV), the median survival is only 14.6–17
months (2).
MicroRNAs (miRNAs) have various forms at different
stages of biogenesis. The initial form is pri-miRNA, which is
300–1,000 bases in length. Following primary processing, the
pri-miRNA becomes a pre-miRNA, also termed microRNA precursor, of
70–90 bases in length. Pre-miRNA is processed further through
digestion by Dicer enzyme, creating a small single-stranded miRNA
molecule of 21–23 bases in length. miRNAs are different from, but
closely associated with small interfering RNA (siRNA) duplexes
(5). The majority of miRNA genes
are present in the genome asa single copy, multiple copies or gene
clusters (5,6). For use in research, pre-miRNAs are
the most commonly used miRNA form. Numerous commercial miRNA
libraries contain RNAs in the pre-miRNA form. A previous study
demonstrated that miRNA arms serve an important role in the
formation of mature miRNA, thus, pri-miRNAs that retain the miRNA
arms are increasingly being adopted for use by researchers
(3).
Presumably, these non-coding small molecule RNAs are
involved in the regulation of gene expression, however its
mechanism is different from siRNA-mediated mRNA degradation. The
first characterized miRNAs were lin-4 and let-7, which were
discovered in nematodes (7).
Subsequently, hundreds of miRNAs were identified in various
species, including in humans, fruit flies and plants by numerous
research teams. miRNAs are involved in transcriptional regulation
of gene expression in plants and animals and have a variety of
important roles in the cell. Scientists previously identified
28,645 miRNA molecules in viruses, plants and animals (8). The majority of identified miRNAs have
a hairpin structure. Single stranded RNA hairpin precursorsof ~70
bases are generated through Dicer enzyme processing, and a 5′ end
phosphate group and a 3′ hydroxyl group are located at the 3′ or 5′
end of the RNA precursor, respectively (9). Each miRNA may have multiple target
genes, and the expression of one gene may be regulated by a
combination of several miRNAs resulting in a complex regulatory
network (10). Potentially, miRNAs
may regulate the expression of a third of human genes. The genes
that encode miRNAs are always located in transcription units
(11), the majority of which are
located in intronic regions (12).
The position of miRNA genes in introns is highly conserved among
different species. miRNA genes are not only conserved in position,
but also have high sequence homology (13–15).
The high conservation of miRNAs is closely associated with their
functions. miRNAs are also closely associated with the evolution of
their target genes, therefore, examining the evolutionary history
of miRNAs may help to understand the mechanism of action and
functions of miRNAs (9).
miRNAs have many biological functions in glioma,
including modulating glioma cell apoptosis and tumor angiogenesis,
and therefore, modulating glioma cell proliferation, invasion,
radio-resistance and migration (1). miRNAs are also involved in glioma
stem cell development and maintenance, and contribute to glioma
resistance to therapies. Briefly, miRNAs act as ‘gap fillers’,
‘amplifiers’, ‘fine-tuners’ and ‘crosstalk mediators’ in different
glioma-associated cellular signaling networks, demonstrating the
biological importance of miRNAs in glioma (1).
miRNA (miR)181a, miR181b and miR181c were originally
identified as downregulated miRNAs in glioblastoma cells and tumors
by miRNA microarrays (16).
miR181a, and to a greater extent miR181b, were subsequently
described as tumor suppressors that inhibit growth and induce
apoptosis of glioma cells (17).
miR181a overexpression sensitizes glioma cells to radiation
treatment concurrent with the downregulation of Bcl-2 expression
(18). Also, miR181b and miR181c
were reported to be significantly downregulated in patients that
responded to radiation therapy and temozolomide compared with
patients with progressive disease. Therefore, it was proposed that
expression levels of miR181b and miR181c may serve as a predictive
marker for response to radiation therapy and temozolomide in
patients with glioblastoma (19).
Materials and methods
Samples
Samples from 20 patients were used in the current
study. Samples were collected between March 2015 and February 2016
from the Center for Clinical Laboratory Medicine of PLA, Xijing
Hospital (Xi'An, China). There were five samples per group as
follows: Grade I, primary Grade I pilocyticastrocytomas,
infiltrated growth in the cerebral white matter (WHO I); Grade II
astrocytoma, neoplastic cells with almost unity nucleus and perfect
differentiation, widespread growth, thus, evident pathological
changes in the microcapsule (WHO II); Grade III anaplastic
astrocytomas, neoplastic cells with pleomorphism, cacoethic
differentiation, karyokinesis, capillary vessel generation (WHO
III); Grade IV glioblastoma multiforme, neoplastic cells have
pleomorphism, high density, undifferentiation, capillary vessel
generation, coagulation necrosis (WHO IV); and matched normal brain
tissues derived from the temporal lobes and saddle area 2 cm away
from tumor tissue (Table I). The
average of the ages of the WHO I to IV group were 56.4±2.5,
54.2±3.3, 54.6±3.1 and 55.4±3.1 respectively. There were 5 patients
in each group (M: F ratio: WHO I, 2:3; WHO II, 3:2; WHO III, 2:3;
WHO IV, 3:2). Each sample was divided into two parts, one was used
for RNA extraction (TRIzol method) and the other was used for
primary cell culture (tissues were immersed in DMEM, and maintained
on ice for a maximum of 4 h). Informed consent was acquired from
every patient prior to participation, and the study was approved by
the Ethics committee of First Affiliated Hospital of Fourth
Military Medical University (Certificate no. KY20153226-1).
 | Table I.Clinical information of the 20
patients. |
Table I.
Clinical information of the 20
patients.
| Patient (group) | Age | Sex | Lesion location |
|---|
| 1 (WHO I) | 63 | Male | Right frontal
lobe |
| 2 (WHO I) | 55 | Female | Right frontal
lobe |
| 3 (WHO I) | 61 | Female | Left frontal
lobe |
| 4 (WHO I) | 54 | Male | Left frontal
lobe |
| 5 (WHO I) | 49 | Female | Right frontal
lobe |
| 6 (WHO II) | 58 | Male | Left frontal
lobe |
| 7 (WHO II) | 57 | Male | Right frontal
lobe |
| 8 (WHO II) | 48 | Female | Right frontal
lobe |
| 9 (WHO II) | 63 | Female | Left frontal
lobe |
| 10 (WHO II) | 45 | Male | Left frontal
lobe |
| 11 (WHO III) | 47 | Male | Right frontal
lobe |
| 12 (WHO III) | 56 | Female | Right frontal
lobe |
| 13 (WHO III) | 48 | Female | Left frontal
lobe |
| 14 (WHO III) | 62 | Female | Left frontal
lobe |
| 15 (WHO III) | 60 | Male | Right frontal
lobe |
| 16 (WHO IV) | 51 | Male | Left frontal
lobe |
| 17 (WHO IV) | 59 | Female | Right frontal
lobe |
| 18 (WHO IV) | 46 | Male | Right frontal
lobe |
| 19 (WHO IV) | 57 | Female | Left frontal
lobe |
| 20 (WHO IV) | 64 | Male | Left frontal
lobe |
Primary glioma cell isolation and
culture
Primary glioma cells were isolated from patients
glioma tissue without selectively removing cells (containing all
tissue cells). Connective tissue and blood vessels were removed
from the tumor. The remaining tissue were dissected into small
pieces and digested for 10 min in 0.25% trypsin (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The dissociated cells
were cultured in high glucose Dulbecco's modified Eagle's medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc.), supplemented
with 10% fetal bovine serum (FBS; Biochrom Ltd., Cambourne, UK),
0.1 mM non-essential amino acids and 2 mM Glutamax at 37°C and 5%
CO2. Cells became confluent within 2–3 days and were
passaged in a 1:4 split. Primary glioma cells were used between
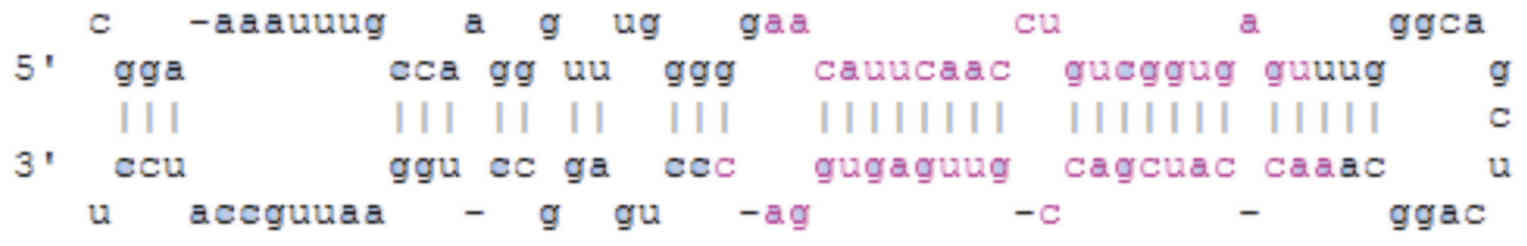
passages 2 and 4. The method of miR181c lentivirus
(pCDH-miR181c-GFP) packaging followed the protocol of a previous
report (20). Subsequently, the
lentivirus was used to infect glioma cells at a multiplicity of
infection (MOI; the number of viral particles per cell) of 10.
Control cells were transfected with pCDH-GFP. Following infection
for 8–12 h, cells were cultured in DMEM with 10% FBS. The miR181c
sequence is presented in Fig.
1.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol extraction of total RNA from glioma tissue
was performed according to the manufacturer's instructions
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Total RNA (500 ng)
was reversed transcribed using a miRcute miRNA First Strand cDNA
kit (KR201; Tiangen Biotech Co., Ltd., Beijing, China) and then
quantified using SYBR Green (Tiangen Biotech, Co., Ltd., FP401) on
an ABI 7500 machine (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 94°C 2 min, followed by
40 cycles of 94°C for 40 sec, 60°C for 40 sec and 72°C for 1 min.
Final extension was performed at 72°C for 5 min. PCR miRNA levels
were quantified using the 2−ΔΔCq method with 3 repeats
(21), and β-actin was used as the
reference gene. The following primers (5′-3′) were used for qPCR:
miR181a1, AGGTTGCTAGGACATCAACGG (F), AGA GTT AGT TTG GTA GCT GGC
(R); miR181a2, ATC AGG CCA GCC TTC AAT CT (F), ACT GGC AAC TAC AGG
AAC CC (R); miR181b1, TCA ACT GGA AGG GTC ACA CAT T (F), ATG TTG
TTG GGC GAA CCC TC (R); miR181b2, CTG ACT CAA CTT GTT GGC TGC (F),
GTG ACT AGT TAC TTA CAC GCC G (R); miR181c, TTT GAG TGG AAC TAG GCA
GGA C (F), GTA GCT GCA ACT CAC GGG TC (R); miR181d, GGT GAA TGT CCC
CTC CCC TA (F), TAC TTA CAA CAC CGA CCG CC (R); and β-actin, GGC
TGT ATT CCC CTC CAT CG (F), CCA GTT GGT AAC AAT GCC ATG T (R).
Immunofluorescence
Following overnight culture, Glioma cells were
plated at 5,000 cells/cm2, infected with lentivirues as
aforementioned, and then allowed to proliferate for further 5 days.
The cells were fixed with 4% paraformaldehyde (PFA) in 0.1 mol/l
PBS for 20 min at room temperature and blocked in 5% BSA with 0.1%
Triton X-100 in PBS for 30 min at room temperature. Primary
antibodies were diluted in antibody dilution solution (PBS with 1%
BSA and 0.1% Triton X-100) in the following ratios, and secondary
antibodies were diluted 1:1,000 in antibody dilute solution. Glioma
cells were incubated in primary antibodies overnight at 4°C and
with secondary antibodies were incubated for 60 min at room
temperature. The following antibodies were used: Rabbit anti-Ki67
(1:500; AB 9260, EMD Millipore, Billerica, MA, USA) and Alexa
Cy3-conjugated secondary antibody (1:1,000; 111-165-003, Jackson
ImmunoResearch Laboratories, Inc.-West Grove, PA, USA). Cells were
also stained with DAPI 5 min at room temperature (1:1,000; D9542,
Sigma-Aldrich). Proliferation was indicated by
Ki67+−staining and infected cells were detected by
observing GFP expression, using an Olympus IX71 microscope (Olympus
Corporation, Tokyo, Japan). The number of proliferating infected
cells was calculated as the percentage of Ki67+ +
GFP+ cells/total GFP+ cells. The value was
calculated as the average of three parallel wells (24 well) and 10
microscope fields per well.
Ball formation
Glioma cells were suspended at 100 cells/ml, plated
at 1 ml/well into 24-well plates, and cultured for 5 days. Cells
forming ball-like structures were considered to be cancer stem
cells. Cells which grew into a colony were selected, digested and
separated into single cells and then infected with the miR181c and
control lentivirus, and expanded in vitro at 100 cells/ml
for a further three days. The balling proportion was calculated as
the number of balls after 3 days/original cell number. The diameter
was calculated using the ruler tool on an Olympus IX71 microscope.
The ball diameter and balling proportion was calculated from the
average of 3 parallel wells (24 well).
Statistical analysis
Statistical analysis was evaluated using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA) and assessed with normality
and variance. Data were compared by t test, one-way analysis of
variance (ANOVA) for multiple comparisons, or two-way ANOVA for
repeated-measures, followed by the Games-Howell post hoc test. Data
were presented as the mean ± standard deviation in each experiment.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA181 family member expression
levels are decreased in glioma
Given the significance of miR181 in glioma, it is
important to investigate the role of miR181 family members in the
development and treatment of glioma. The current study examined the
expression of miR181a1 in the normal brain tissue from patient with
WHO Grade I–IV gliomas. Normal tissue was collected 2 cm away from
the tumor. The results demonstrated that, among all the WHO grades,
there was no significant difference in the relative expression of
miR181a1 in the adjacent normal tissue (Fig. 2A). This indicated that the
expression of the miRNA181 family is unaffected in the normal
tissue outside of tumor. Therefore, 1–2 patients in each Grade
(I–IV) were selected; a total of five people, and the expression of
each miRNA181 subtype was compared with the expression in normal
brain tissue. The results demonstrated that the expression of all
miR181 subtypes (miR181a1, Fig.
2B; miR181a2, Fig. 2C;
miR181b1, Fig. 2D; miR181b2,
Fig. 2E; miR181c, Fig. 2F; and miR181d, Fig. 2G) was significantly reduced in
glioma compared with the normal tissue (P<0.05), and the
expression level decline was greater with increasing WHO grade.
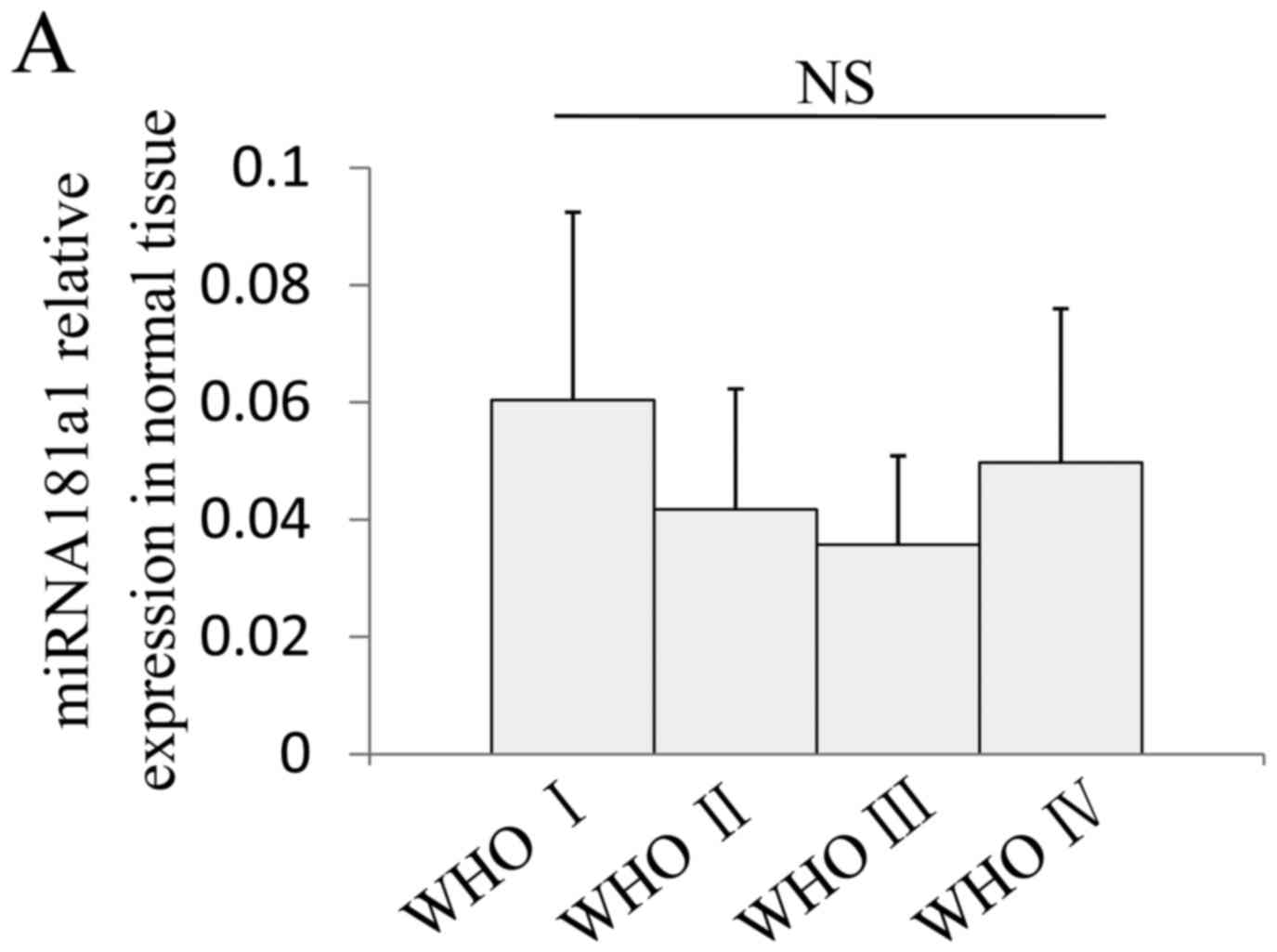 | Figure 2.miRNA181 family member expression
levels are decreased in glioma. (A) There is no difference between
the miRNA181a1 relative expression in the normal tissue adjacent to
I–IV grade gliomas. Normal tissue was used as the control and the
relative expression of each member of the miRNA181 family, (B)
miRNA181a1, (C) miRNA181a2, (D) miRNA181b1, (E) miRNA181b2, (F)
miRNA181c and (G) miRNA181d, was reduced significantly in glioma
compared with normal tissue, and further decreased with the gradual
progress of glioma. *P<0.05, **P<0.01, vs. normal; NS,
P>0.1. miRNA, microRNA; WHO, World Health Organization
grade. |
At the initial period of glioma development (WHO
Grade I), the six members of miRNA181 family were already
significantly reduced, with all decreased to <30% of the levels
in the normal tissue. In particular, the miR181c level (Fig. 2F) was markedly decreased in all
five of the patients with WHO I grade glioma, with the greatest
decrease to 4.2% of the normal tissue, and an average reduction to
3.3±0.6% of normal tissue levels. Expression levels of all members
of the miRNA181 family were decreased in the early glioma stage;
the expression of miRNA181c declined the most, which has important
implications for the early diagnosis and prognosis of glioma.
However, during the process of glioma development, the expression
of miR181 family members decreased further. As the WHO grade level
increased, the expression levels were decreased to 10–30% of the
preceding level. miR181b1 had the fastest decline rate of the
family, with the WHO Grade IV expression level even <0.1% of WHO
Grade I level, and only 0.024% of the level in normal tissues
(Fig. 2D). This result indicates
that miRNA181 is critical in glioma and is associated with the
level of malignancy; gradually decreasing expression of miRNA181
was accompanied by increasing stage and may be associated with
increased degeneration of glial cells.
miR181c inhibits glioma WHO Grade I
tumor cell proliferation
Subsequently, it was determined whether miR181
overexpression suppresses glioma cell growth and whether it may be
useful for treatment of glioma. Since miR181c exhibited a sharp
declined at the glioma initiation stage, miR181c may be an
important factor that inhibits glial cell malignant (Fig. 2F). Therefore, a plasmid was
constructed to overexpress miR181c, packaged as a lentivirus and
infected into primary glioma cells, to determine the role of
miR181c in the different stages of glioma. Initial experiments were
performed using WHO Grade I patients cultured in vitro.
RT-qPCR was performed to detect expression level of miR181c in
normal cells, uninfected glioma cells, control virus glioma cells
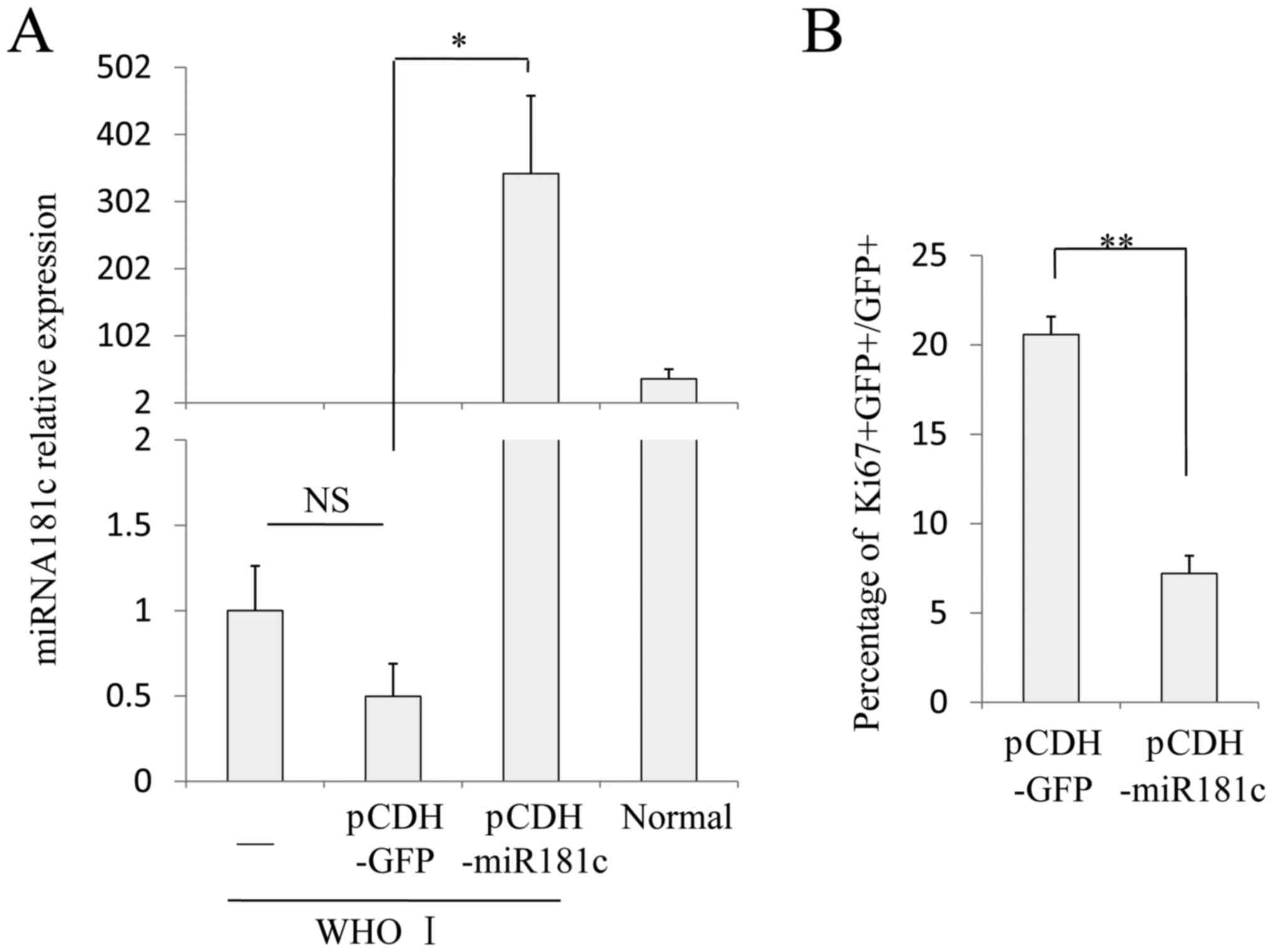
(pCDH-GFP) and glioma cells overexpressing miR181c. In cells
overexpressing miR181c (pCDH-miR181c), the miR181c levels were
significantly increased compared with control virus cells
(P=0.028), and also markedly increased compared with the levels in
normal cells (Fig. 3A). However,
there was no significant difference between the miR181c levels in
cells infected with the control virus compared with the uninfected
group. Subsequently, the proliferation ability of these cells was
analyzed (22). Cells infected
with the control virus proliferated rapidly during the logarithmic
growth phase, >20% of GFP+ cells were proliferative
(indicated by Ki67+ staining), and the GFP−
cells also had similar proliferation rates. Overexpression of
miR181c in WHO Grade I glioma cells significantly decreased cell
proliferation compared with cells infected with the control virus
(P=0.0097; Fig. 3B and C).
Following culture for the equivalent time (48 h), the cells number
(Dapi quantity) was lower in the miR181c overexpressing cells than
the control virus-infected group cells, and the proportion of
GFP+−Ki67+ cells was 7.21±0.56%,
significantly lower than the proportion in the infection control
virus group (Fig. 3B and C).
miRNA181c inhibits glioma WHO Grade IV
cancer stem cell proliferation
Subsequently, the current study investigated whether
miR181c also has an inhibitory effect on gliomablastoma cells. As
gliomablastoma cells have stem cell properties, colonies can form
as balls in suspension culture; cells that form balls within a
low-density suspension culture are regarded as gliomablastoma cells
(23). Therefore, the cells from
patients with WHO Grade IV glioma were cultured in suspension.
Cells that grew into a colony were selected, digested and separated
into single cells, and then infected with the miR181c and control
lentivirus. The virus titer was adjusted to MOI=10. Initially,
whether the infection of the gliomablastoma cells with the miR181c
lentivirus restored the expression of miR181c was determined by
RT-qPCR. The results demonstrated that miR181c expression was lower
in gliomablastoma cells than in Grade IV glioma cells, and after
infection of blastoma cells with pCDH-miR181c, the levels of
miR181c were significantly increased compared with pCDH-GFP cells
(P=0.027), and comparable to normal cell levels (Fig. 4A). Subsequently, the secondary ball
formation ability the infected cells was evaluated. Cells were
cultured at a starting density of 100 cells/ml in 24-well plates
for 72 h. The number of balls per well as the proportional to form
ball, and the ball diameter was measured. The results demonstrated
that the balling proportion of cells infected with the control
virus was 74.9±7.35% and the mean diameter of the ball was 158±13
µm, which indicated that ~75% of cells had cancer stem cell
properties and a high proliferation rate (Fig. 4B and C). However, the cell
proliferation was decreased by infection with the miR181c virus
compared with the control virus, and the balling proportion and
ball diameter were 1.53±0.8% and 28.2±6.65 µm, respectively
(P=0.0030 and P=0.00063 vs. pCDH-GFP, respectively). The results
demonstrated that restoration of miRNA181c expression appeared to
reverse the tumor cell (and also tumor stem cell) phenotype, by
reducing excessive proliferation, and restoring regular properties
observed in normal glial cells (Fig.
4B and C).
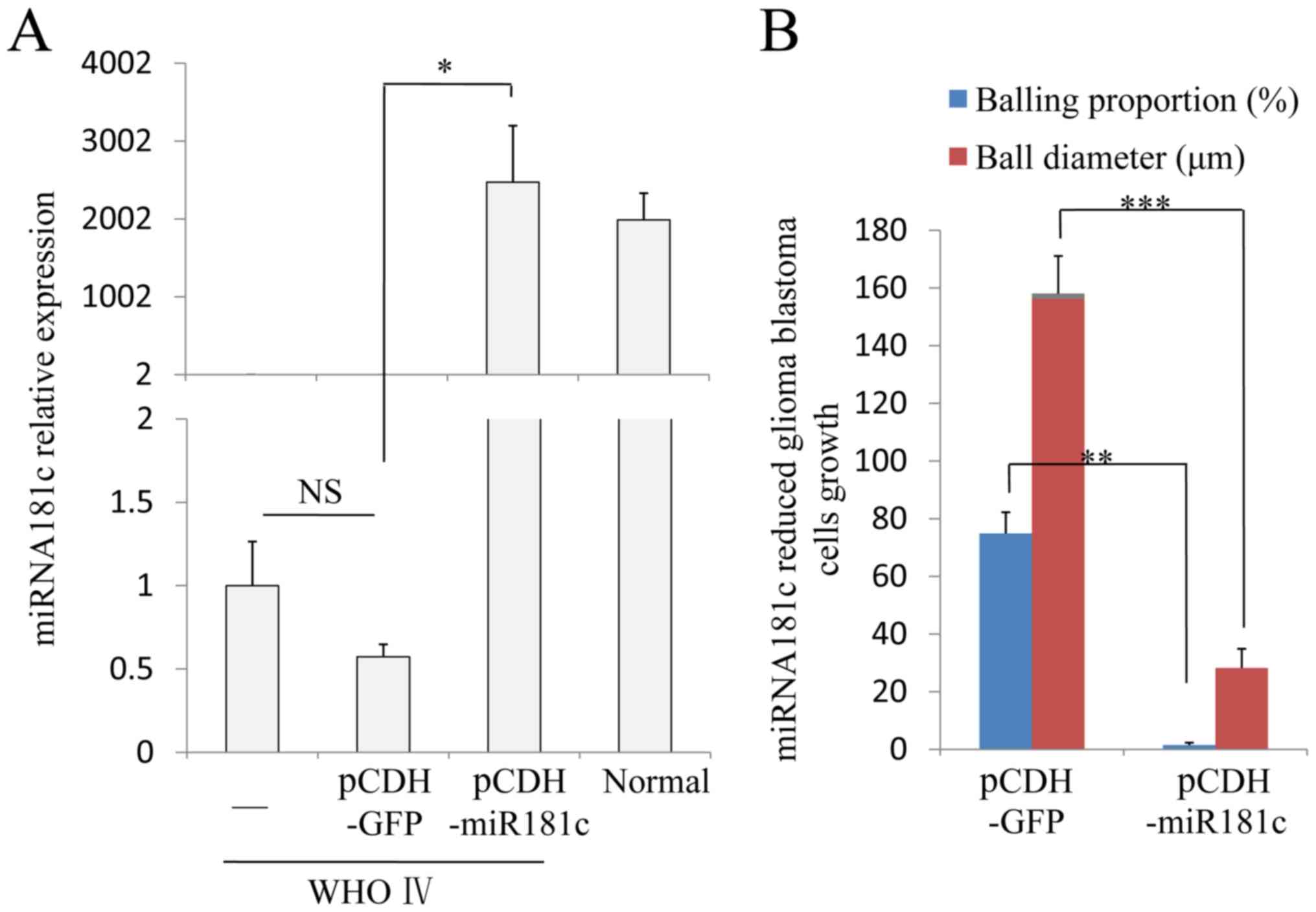 | Figure 4.miRNA181c inhibits glioma WHO Grade IV
cancer stem cell proliferation. (A) miRNA181c was over expressed in
the WHO Grade IV blastoma cells using a lentivirus
(pCDH-miRNA181c). The relative expression level of miRNA181c
reached normal tissues levels following infection with the
lentivirus. (B) In glioma cells cultured in suspension, cells
forming ball-like structures were considered to be cancer stem
cells. The ability to form balls was significantly decreased in
cells overexpressing miRNA181c compared with pCDH-GFP, in the
proportion of cells and the ball diameter. (C) In the control group
(pCDH-miRNA181c) ~75% of the cells formed balls, with the ball
diameter ~160 µm. Overexpressing miRNA181c reduced the balling
proportion to 1–2%, and the ball diameter down to~30 µm.
*P<0.05, **P<0.01, ***P<0.001, comparison indicated by
brackets; NS, P>0.1. Scale bars, 100 µm. miR, microRNA; NS, no
significance; WHO, World Health Organization grade. |
Discussion
miRNA181 is an ancient miRNA gene family that
originated in urochordata. miRNAs are endogenously expressed small
non-coding RNAs (22 nucleotides) that regulate the expression of
protein-coding genes post-transcriptionally. They predominantly
function by base-pairing to the 3-untranslated regions of mRNAs to
induce translational inactivation or RNA degradation. As a class of
important gene regulators throughout the evolutionary process,
miRNA-meditated gene regulation is widespread in animals, plants
and viruses. Previous studies have demonstrated the involvement of
the hsa-miR-181 family in gliomaoncogenesis, and miR181 expression
was typically downregulated in glioma tissue (17–19).
Hsa-miR-181a and hsa-miR-181b functioned as tumor suppressors that
inhibited growth, induced apoptosis and reduced invasion in glioma
cells. Furthermore, the tumor-suppressive effect of hsa-miR-181b in
glioma cells was more apparent than the effect of hsa-miR-181a,
which suggested that aberrantly downregulated hsa-miR-181a and
hsa-miR-181b may be critical factors that contribute to the
malignancy of human gliomas. Previous studies reported that miR-21
and 221 were overexpressed in glioma samples, whereas miRNA 181
family members were downregulated compared with normal brain tissue
(1,24). These findings suggest that aberrant
downregulation of miR-181a/miR-181b and miR-181c could serve as a
predictive marker that may contribute to malignant appearance in
human gliomas (17,19). Slaby et al (19) and Zhang et al (25) demonstrated that the expression
signature of miR-181 family members may be useful in predictive
glioblastoma biomarkers.
However, to the best of our knowledge, no detailed
analysis of specific trends within different glioma stages has been
performed. Thus, the current study determined the expression trends
of the six subtypes of the miR181 family in detail and evaluated
the changes in their expression in the four WHO glioma stages.
Additionally, the effects of miR181 on glioma cell proliferation
and malignancy were investigated. The current study demonstrated
that restoring the expression of miR181c reduced the proliferation
of glioma cells in the early or late stage (Grade I and Grade IV)
and in gliomablastoma cells, restoring normal glia cells
characteristics. Therefore, miR181 has important implications for
the diagnosis of glioma, and also may be useful as a therapeutic
method.
The results of the current study demonstrated that
the expression level of all six subtypes of miR181 were decreased
in glioma compared with normal tissue, particularly miR181c, which
exhibited the greatest decrease. With the progression of gliomas
from Grade I to IV, the expression levels of the miRNAs also
decreased, and miR181b1 decreased the fastest. Thus, the expression
level changes of the different miR181 subtypes are important for
the early diagnosis of glioma and for predicting prognosis.
As the miR181c expression level was greatly
decreased at the early stage of glioma, and continued to decline by
>1,000-fold with the development of glioma (to Grade IV),
miR181c maybe a crucial factor involved in the glioma cell
transformation and glioma progression. Thus, the effects of miR181c
on glioma cell proliferation and malignancy were also examined. The
present study demonstrated that, in the early and late stage,
miR181c expression recovery reduced the proliferation of glioma
cells and gliomablastoma cells, restoring normal glia cells
characteristics to these cells. Therefore, miR181 has important
implications for the diagnosis of glioma, and may also be useful as
a therapeutic for glioma treatment.
References
|
1
|
Li M, Li J, Liu L, Li W, Yang Y and Yuan
J: MicroRNA in human glioma. Cancers (Basel). 5:1306–1331. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kohler BA, Ward E, McCarthy BJ, Schymura
MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA and Edwards
BK: Annual report to the nation on the status of cancer, 1975–2007,
featuring tumors of the brain and other nervous system. J Natl
Cancer Inst. 103:714–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Floyd D and Purow B: Micro-masters of
glioblastoma biology and therapy: Increasingly recognized roles for
microRNAs. Neuro Oncol. 16:622–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A ‘state of the
science’ review. Neuro Oncol. 16:896–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Urquijo Aguirregomoscorta JI, Gastan
Menéndez I, Aparicio Abendaño S, López Quintana J, Saiz
Capelastegui A and Landa Urrutia I: Nap polysomnography: Sufficient
grounds for initiating CPAP treatment? Arch Bronconeumol.
37:302–306. 2001.PubMed/NCBI
|
|
6
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagga S, Bracht J, Hunter S, Massirer K,
Holtz J, Eachus R and Pasquinelli AE: Regulation by let-7 and lin-4
miRNAs results in target mRNA degradation. Cell. 122:553–563. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha M, Pang M, Agarwal V and Chen ZJ:
Interspecies regulation of microRNAs and their targets. Biochim
Biophys Acta. 1779:735–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurtan AM, Lu V, Bhutkar A and Sharp PA:
In vivo structure-function analysis of human Dicer reveals
directional processing of precursor miRNAs. RNA. 18:1116–1122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao W, Greenwold MJ and Sawyer RH:
Expressed miRNAs target feather related mRNAs involved in cell
signaling, cell adhesion and structure during chicken epidermal
development. Gene. 591:393–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim Y, Nam YJ and Lee C: Haplotype
analysis of single nucleotide polymorphisms in VEGF gene for
vascular dementia. Am J Med Genet B Neuropsychiatr Genet.
141B:332–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruvkun G: Molecular biology. Glimpses of a
tiny RNA world. Science. 294:797–799. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciafrè SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu
Z and You Y: hsa-mir-181a and hsa-mir-181b function as tumor
suppressors in human glioma cells. Brain Res. 1236:185–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
19
|
Slaby O, Lakomy R, Fadrus P, Hrstka R,
Kren L, Lzicarova E, Smrcka M, Svoboda M, Dolezalova H, Novakova J,
et al: MicroRNA-181 family predicts response to concomitant
chemoradiotherapy with temozolomide in glioblastoma patients.
Neoplasma. 57:264–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen LJ, He JL, Yang DH, Ding YB, Chen XM,
Geng YQ, Liu SJ, Liu XQ and Wang YX: Mmu-microRNA-200a
overexpression leads to implantation defect by targeting
phosphatase and tensin homolog in mouse uterus. Reprod Sci.
20:1518–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paulus W: GFAP, Ki67 and IDH1: Perhaps the
golden triad of glioma immunohistochemistry. Acta Neuropathol.
118:603–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirth T: A short perspective on gene
therapy: Clinical experience on gene therapy of gliomablastoma
multiforme. World J Exp Med. 1:10–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conti A, Aguennouz M, La Torre D,
Tomasello C, Cardali S, Angileri FF, Maio F, Cama A, Germanò A,
Vita G and Tomasello F: miR-21 and 221 upregulation and miR-181b
downregulation in human Grade II–IV astrocytic tumors. J
Neurooncol. 93:325–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Zhang J, Hoadley K, Kushwaha D,
Ramakrishnan V, Li S, Kang C, You Y, Jiang C, Song SW, et al:
miR-181d: A predictive glioblastoma biomarker that downregulates
MGMT expression. Neuro Oncol. 14:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|