Introduction
Myocardial ischemia/reperfusion (I/R) injury is a
complex pathophysiological process, including an inflammatory
response, apoptotic cell death and autophagy, which may result in
myocardial cell damage (1–3). Therefore, prevention and treatment of
myocardial I/R injury has become one of the most important tasks
for patients with ischemic heart disease.
The interleukin (IL)-17 cytokine family consists of
six members (IL-17A-F). IL-17A and IL-17F share the greatest
similarity, with 55% homology at the amino acid level. IL-17A is a
member of the IL-17 cytokine family, secreted by CD4+αβ
T cells, rδT cells, natural killer cells and neutrophils (4). In myocardial I/R injury, it has been
indicated that IL-17A is produced primarily by rδT cells and plays
a pathogenic role by inducing cardiomyocyte apoptosis and
neutrophil infiltration (5). These
indicated that IL-17A serves a critical role in myocardial I/R
injury.
High-mobility group box 1 protein (HMGB1), a highly
conserved nuclear protein that is released from necrotic cells and
secreted by activated macrophages, natural killer cells and mature
dendritic cells, can function as an extracellular signaling
molecule during inflammation, cell differentiation, cell migration
and tumor metastasis (6). Andrassy
et al (7) demonstrated that
the expression of HMGB1 was significantly increased during the
reperfusion period and could exaggerate myocardial I/R injury,
while inhibiting HMGB1 could reduce myocardial I/R injury. A recent
study indicated that HMGB1 could aggravate the cerebral I/R injury
by upregulating the expression of IL-17A, as well as increasing
neuronal apoptosis (8). In
addition, a previous study of the authors reported that
HMGB1-IL-17A axis serves an important role in the pathogenesis of
myocardial I/R injury (9).
However, whether the HMGB1-IL-17A axis is involved in regulating
cardiomyocyte apoptosis and autophagy in myocardial I/R injury
remains unclear. The current study, therefore, aims to investigate
the HMGB1-IL-17A axis in a cardiomyocyte hypoxia/reoxygenation
(H/R) injury model to elucidate its possible role in regulating
cardiomyocyte apoptosis and autophagy in myocardial I/R injury.
Materials and methods
Cell culture and experimental
design
The cell extraction protocol for cardiomyocyte
primary culture was approved by the Institutional Animal Care and
Use Committee of Wuhan University (Wuhan, China). A total of 100
Sprague-Dawley rats (1-3-day-old) were purchased from the Centre of
Experimental Animals at Wuhan University (Wuhan, China). Primary
cultures of neonatal rat cardiomyocytes were prepared from the
ventricles of rats. Briefly, the hearts were harvested and finely
minced with scissors for about 1 min and then dissociated with
0.125% (w/v) trypsin and 0.08% collagenase I for five times at
37°C. The collected enzyme solution is next centrifuged (170 × g, 4
min, 4°C), the supernatant discarded, and cells re-suspended in
Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) containing 15% (v/v) fetal bovine serum
(FBS), 1% penicillin (100 U/ml) and 1% streptomycin (100 µg/ml). To
reduce fibroblast contamination, cells were incubated at 37°C and
5% CO2 for 1 h, then non-adherent cells (cardiomyocytes)
in DMEM were collected and seeded in six-well plates at a density
of 1×106 cells/ml. Cardiomyocytes were incubated for 4
days prior to conducting the experiment, then the cells were
randomly assigned into six groups. The group descriptions are as
follows: Control group (Control), cardiomyocytes were incubated in
DMEM F12 with 15% FBS; Hypoxia and reoxygenation (H/R) group,
cardiomyocytes were incubated in DMEM following 24 h of
synchronization, subjected to hypoxia for 1 h at 37°C and 95%
N2 at 5% (v/v) CO2 prior to incubation in
DMEM F12 for 4 h; H/R+anti-HMGB1 group, cardiomyocytes were
pretreated with HMGB1-neutralizing antibody (1:100 dilution;
#BM3965; Wuhan Boster Bioengineering Co, Ltd., Wuhan, China) at
37°C for 24 h before incubation in DMEM, and then were subjected to
H/R, as described above; H/R+anti-IL-17A group, cardiomyocytes were
pretreated with IL-17A-neutralizing antibody (1:100 dilution;
#A00421; Wuhan Boster Bioengineering Co, Ltd.) at 37°C for 24 h
before incubation in DMEM, and then were subjected to H/R, as
described above; H/R+rHMGB1 group, cardiomyocytes were pretreated
with recombinant HMGB1 (rHMGB1; 200 ng/ml) at 24 h before
incubation in DMEM, and then were subjected to H/R, as described
above; and the H/R+rIL-17A group, cardiomyocytes were pretreated
with recombinant IL-17A (rIL-17A; 100 ng/ml) at 24 h before
incubation in DMEM, and then were subjected to H/R, as described
above.
Assessment of cell injury
The extent of cell injury was assessed by the
concentrations of lactate dehydrogenase (LDH) and creatine kinase
(CK) in the culture medium. The protocols were followed according
to the manufacturer's instructions (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
Assay of cell viability
Cell viability was determined using the Cell
Counting Kit (CCK)-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), and the experimental procedure was based on the
manufacturer's recommendations. Cardiomyocytes were seeded in
96-well plates at 1×105 cells/well and incubated for 4 d
prior to treatments as described above. The absorbance of each well
at 490 nm was measured with a microplate reader (Bio-Rad
Laboratories, Hercules, CA). The percent cell viability was
calculated using the following formula: % cell viability=(mean
absorbance in test wells)/(mean absorbance in control wells)
×100.
Flow cytometry
Apoptosis rate was assessed by flow cytometric
analysis of propidium iodide (PI) and Annexin V double staining.
The cardiomyocytes were harvested after treatment, then rinsed in
PBS and suspended in 500 µl binding buffer, and finally incubated
with 5 µl Annexin V and 5 µl propidium iodide (Nanjing KeyGen
Biotech. Inc., Nanjing, China). The stained cells were analyzed by
using a BD flow cytometer (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA).
Western blot analysis
The cultured primary cardiomyocytes were extracted
to harbor protein solution in radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Nantong, China) after
indicated experimental protocols. A BCA Protein Assay Kit (Wuhan
Boster Biological Technology Co., Ltd.) was used to detect
concentration of the proteins. Extracts containing 20 µg of protein
were added into each well and subjected to 10% SDS-PAGE, which were
then transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Subsequently, the membranes were
blocked by 5% BSA for 60 min at 37°C, paralleling the incubation of
primary antibodies overnight at 4°C with anti-HMGB1 (1:1,000
dilution; #SAB2701809; Sigma-Aldrich; Merck KGaA), anti-IL-17A
(1:800 dilution; #13838; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti- microtubule-associated proteins 1A/1B light chain
3B (LC3) (1:600 dilution; #SAB1306269; Sigma-Aldrich; Merck KGaA),
anti-Beclin-1 (1:800 dilution; #3738; Cell Signaling Technology,
Inc.), anti-Bcl-2 (1:800 dilution; #15071; Cell Signaling
Technology, Inc.) and anti-Bcl-2-associated X protein (Bax) (1:600
dilution; #2774; Cell Signaling Technology, Inc.). After being
washed in Tris buffered saline with Tween 20 for three times (10
min), the membranes were exposed to horseradish
peroxidase-conjugated secondary antibody (mouse anti-rat IgG
antibody; 1:1,000 dilution; #bs-0293M; Beijing Bioss Biological
Technology Co., Ltd, Beijing, China) at 37°C for 2 h. The enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to visualize protein blots. The
expression of protein was normalized to β-actin (mouse anti-β-actin
antibody; 1:500 dilution; #bsm-33036M; Beijing Bioss Biological
Technology Co., Ltd) expression. Image Lab software 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was adopted to measure the
grey value of each band.
Statistical analysis
Statistical analysis was conducted with SPSS 18.0
software package (SPSS Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard deviation. One-way analysis of
variance or the Welch test was used for comparisons among groups,
and the Student-Newman-Keuls test or Dunnett's T3 test was used for
post-hoc multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
HMGB1 and IL-17A aggravate H/R-induced
cell injury
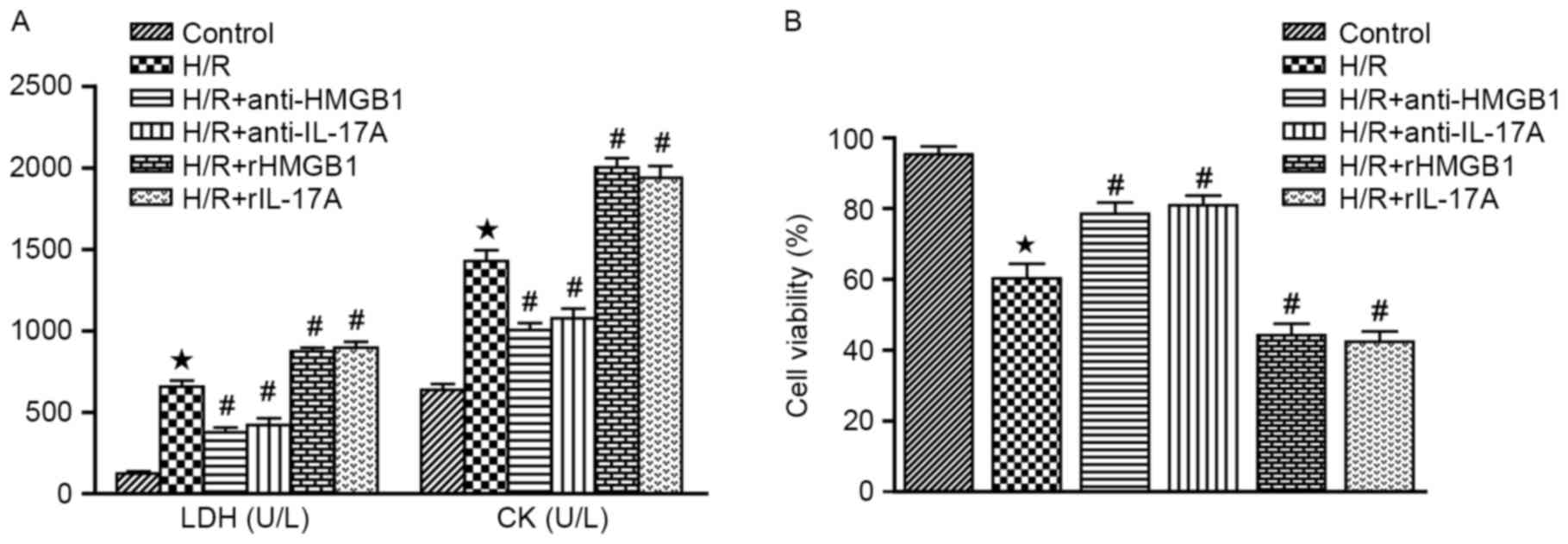
As demonstrated in Fig.
1A, cardiomyocytes subjected to hypoxia and reoxygenation were
able to cause the significant release of LDH and CK, when compared
with the control group (P<0.05). Furthermore, rHMGB1 or rIL-17A
increased the levels of LDH and CK, while HMGB1 or IL-17A antibody
were able to suppress their release (all P<0.05 vs. H/R
group).
HMGB1 and IL-17A inhibit the cell
viability
The authors assessed cell viability with CCK-8
assays in each groups. The cell viability was markedly inhibited
following H/R treatment, when compared with the control group
(P<0.05). Interestingly, pretreatment with HMGB1 or IL-17A
antibody significantly improved cell viability, while rHMGB1 or
rIL-17A could inhibit the cell viability (all P<0.05 vs. H/R
group). These findings indicated that HMGB1 or IL-17A could inhibit
the cell viability in cardiomyocyte subjected to H/R (Fig. 1B).
HMGB1 and IL-17A aggravate H/R-induced
cardiomyocyte apoptosis
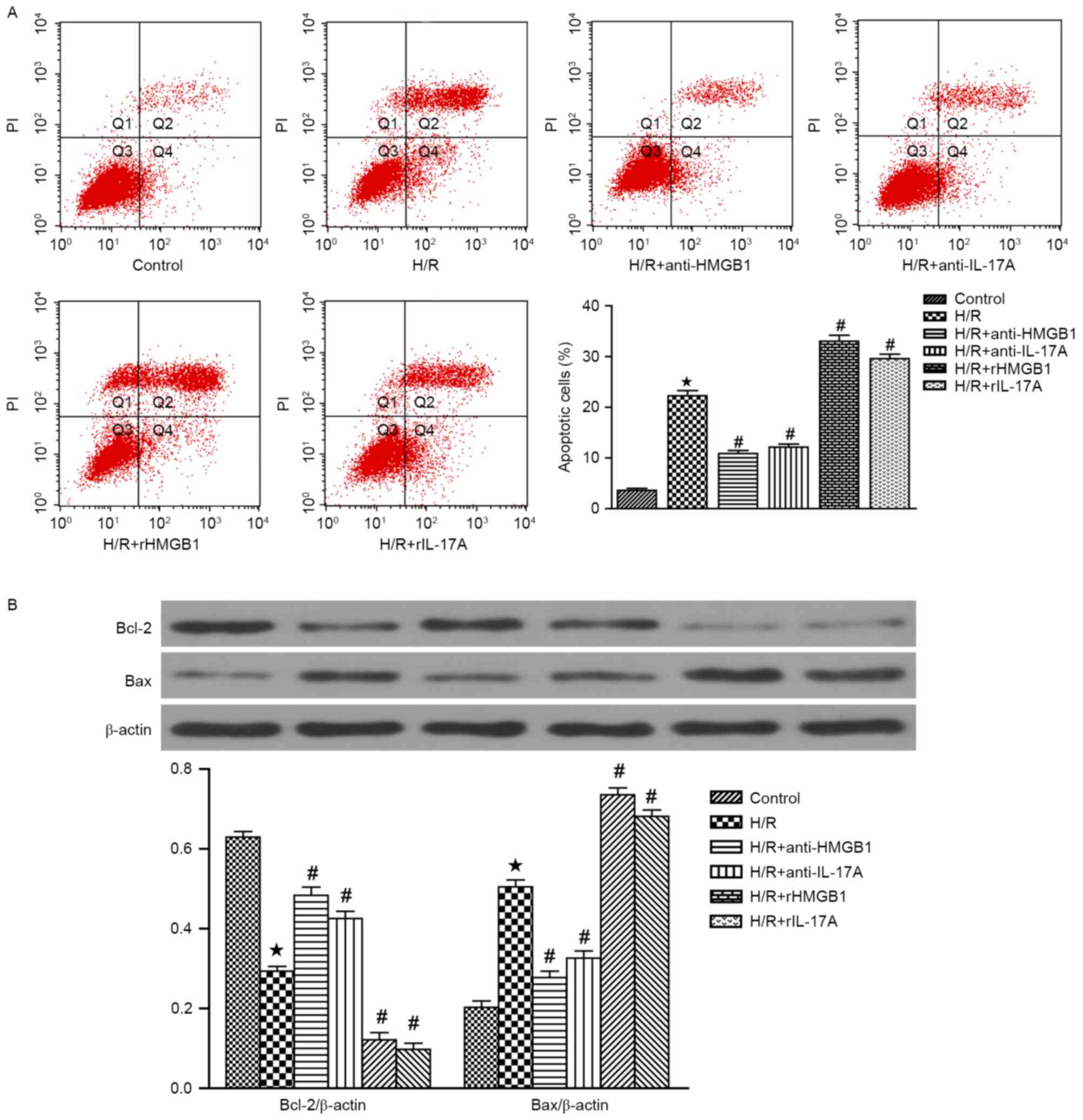
Annexin-V FITC/PI staining was used to quantify the
pro-apoptotic effects of HMGB1 and IL-17A. The apoptosis rate in
H/R group was significantly increased (P<0.05 vs. Control).
However, pretreatment with HMGB1 or IL-17A antibody could
significantly attenuate the elevated H/R-induced cardiomyocyte
apoptosis, while rHMGB1 or rIL-17A could aggravate the
cardiomyocyte apoptosis (all P<0.05 vs. H/R group) (Fig. 2A). In addition, Bcl-2
(anti-apoptotic protein) and Bax (pro-apoptotic protein) expression
were also measured by western blotting (Fig. 2B). A significant decrease in Bcl-2
expression and a increase in Bax expression were observed in H/R
group, when compared with the control group (P<0.05). Meanwhile,
HMGB1 or IL-17A antibody could increase the expression of Bcl-2 and
decrease the expression of Bax (both P<0.05 vs. H/R group). In
contrast, treatment with rHMGB1 or rIL-17A could attenuate the
increased expression of Bcl-2 and decreased the expression of Bax
(both P<0.05 vs. H/R group). These results strongly demonstrated
that HMGB1 or IL-17A could aggravate H/R-induced cardiomyocyte
apoptosis.
HMGB1 and IL-17A enhanced the
expression levels of autophagy markers in cardiomyocytes subjected
to H/R
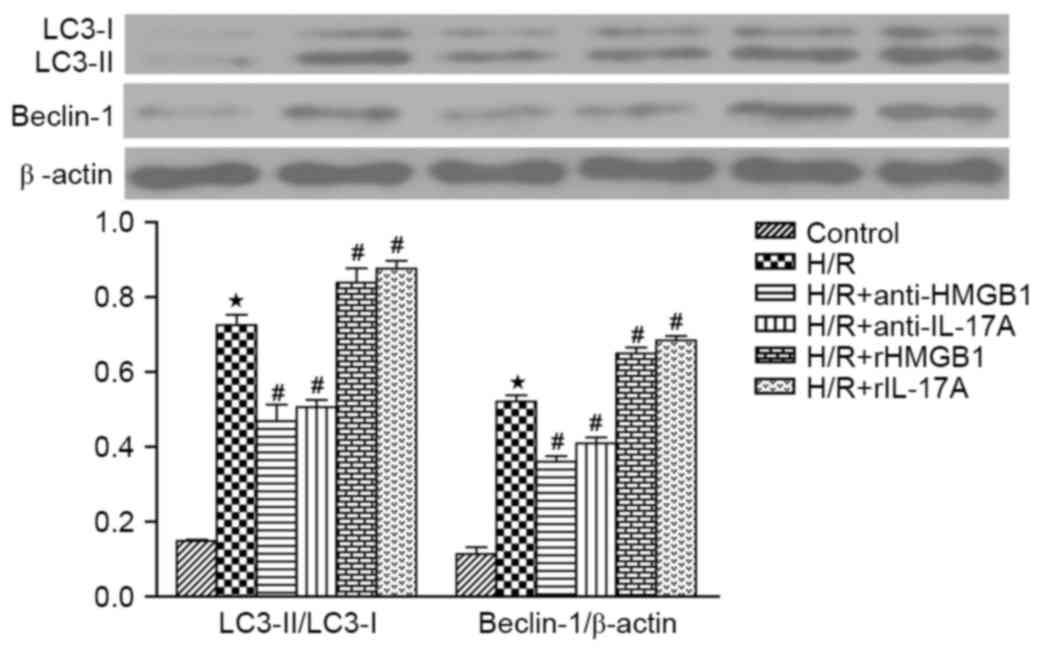
It is well-understood that autophagy is regulated by
autophagy-related proteins, such as, LC3-II/LC3-I and Beclin-1
(10). The ratio of LC3-II to
LC3-I and the Beclin-1 expression were significantly increased in
H/R group, when compared with the control group (P<0.05). The
HMGB1 or IL-17A antibody could markedly decreased the ratio of
LC3-II to LC3-I and the Beclin-1 expression when compared to H/R
group, while those were even higher in H/R+rHMGB1 group or
H/R+rIL-17A group (all P<0.05). It can be concluded that the
autophagy was upregulated in cardiomyocytes exposed to H/R, and
enhanced by HMGB1 or IL-17A prior to H/R (Fig. 3).
HMGB1 regulated the expression of
IL-17A
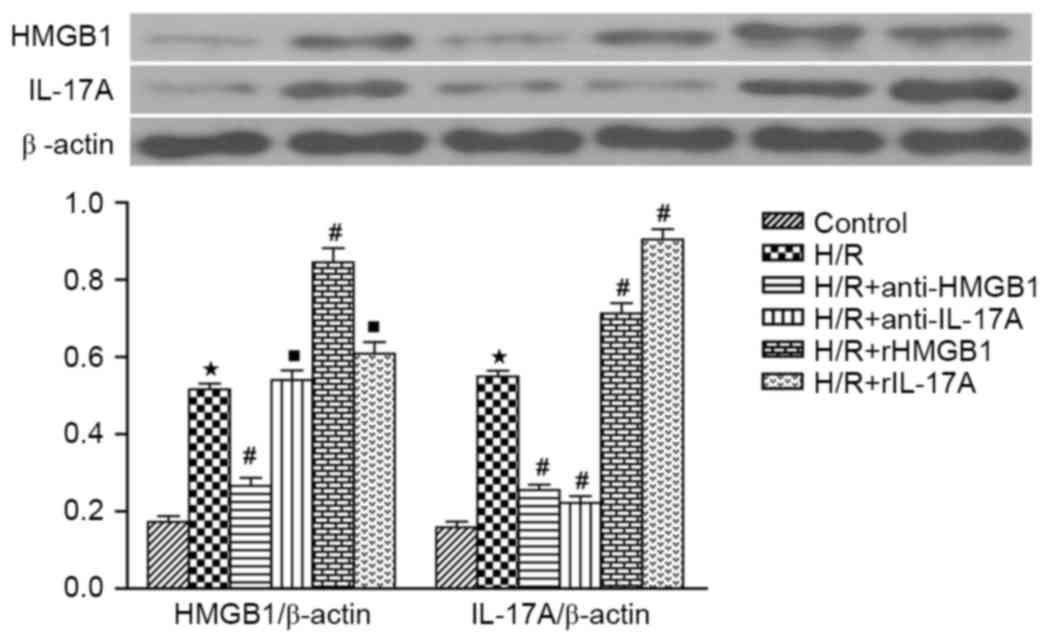
According to the western blotting, both HMGB1 and
IL-17A expression in H/R group were markedly increased compared to
those in control groups (P<0.05). Compared with the H/R group,
IL-17A expression was significantly decreased in H/R+anti-HMGB1
group, while it was increased in H/R+rHMGB1 group (both P<0.05).
Meanwhile, IL-17A antibody or rIL-17A had no significant effect on
HMGB1 expression when compared to H/R groups (both P>0.05)
(Fig. 4).
Discussion
The present study revealed a crucial role for the
HMGB1-IL-17A axis in cardiomyocyte H/R injury. HMGB1 promotes the
expression of IL-17A, then aggravates cardiomyocyte apoptosis and
autophagy, and finally augments cardiomyocyte H/R injury.
Recently, a number of studies have indicated that
local inflammation, cell apoptosis and autophagy all contribute to
myocardial I/R injury. IL-17A is an inflammatory cytokine with
robust effects on different cell types, resulting in inflammatory
cytokine production, leukocyte recruitment and creating a link
between innate and adaptive immunity (11). It has been reported that IL-17A
induces cardiomyocyte apoptosis and promotes ventricular remodeling
following myocardial infarction (12). In addition, IL-17A was demonstrated
to be significantly increased in a cardiac transplantation model,
and could induce cardiomyocyte apoptosis, promote the release of
proinflammatory mediators and serve an important role in myocardial
I/R injury (5,13). HMGB1, which is a novel
pro-inflammatory cytokine actively released by macrophages and
monocytes, has been proven to function as an early mediator of
inflammation and cell injury during myocardial I/R. In addition,
HMGB1 may promote the release of classical early pro-inflammatory
cytokines such as tumor necrosis factor-α (TNF-α) and IL-6.
Conversely, HMGB1 A box peptide (a specific HMGB1 antagonist) may
inhibit the release of TNF-α and IL-6, and reduce myocardial I/R
injury. This is due to the mRNA and protein expression of HMGB1
being increased as early as at 30 min following ischemia, and being
significantly increased at 6 h reperfusion (7). A previous study of the authors
demonstrated that the downregulation of HMGB1 by several drugs,
such as minocycline and ethyl pyruvate, can reduce myocardial
ischemia/reperfusion injury in rats (14,15).
A previous study showed that there is an association
between HMGB1 and IL-17A (16).
Wang, Sun and Tian (16) indicated
that both HMGB1 and IL-17A were significantly increased during
liver I/R injury, and that HMGB1 may stimulate the production of
IL-17A by γδ T cells in a TLR4-dependent manner. In the present
study, that HMGB1 antibody significantly reduced IL-17A expression
and ameliorated cardiomyocyte H/R injury. In contrast, rHMGB1
increased IL-17A expression and markedly increased H/R injury,
confirming the crucial role played by HMGB1 in the myocardial I/R.
However, the mechanisms underlying the regulation of IL-17A by
HMGB1 during myocardial H/R injury require further
investigation.
Cardiomyocyte apoptosis is a well-known key cellular
event in ischemic hearts (17). In
the current study, flow cytometry was performed to examine
cardiomyocyte apoptosis. Pretreatment with IL-17A antibody
significantly decreased cardiomyocyte apoptosis while rIL-17A have
an opposite effect. Apoptosis-related proteins, such as Bcl-2, Bax
and caspase-3, play critical roles in apoptosis (18). Overexpression of Bcl-2 in mice
attenuates apoptosis and alleviates myocardial IR injury (19). The present results indicated that
H/R markedly downregulated Bcl-2 and upregulated Bax. Meanwhile,
pretreatment with rIL-17A significantly decreased Bcl-2 expression
and increased Bax expression, yet the IL-17A antibody increased
Bcl-2 expression and decreased Bax expression. These indicated that
IL-17A may exert its pro-apoptotic effects through regulating Bcl-2
expression.
Autophagy, another major factor leading to cell
death, can be observed in both acute and chronic myocardial
ischemia and heart failure (20,21).
There is vast evidence that autophagy is upregulated during
myocardial ischemia and reperfusion (22). Autophagy may aggravate myocardial
I/R injury during the reperfusion phase due to the excessive
degradation of essential proteins and organelles (23). A previous study indicated that
impaired autophagosome clearance could trigger mitochondrial
permeabilization, and then lead to a necrotic mechanism of cell
death in cardiac IR injury (24),
while attenuating autophagy dysfunction could contribute to
cardioprotection (25). These
findings are consistent with the present study. The LC3 and Beclin1
proteins are two important markers of autophagosomes, which are
upregulated during the reperfusion period and signify ongoing
autophagy and cellular damage. The authors' previous study
presented that HMGB1 could regulate LC3 and Beclin-1 levels
following H/R injury in rat cardiomyocytes (26). In the present study, it was
observed that both rHMGB1 and rIL-17A dramatically induced
autophagosome formation (as evidenced by the increase of the
LC3-II/LC3-I ratio and the upregulation of Beclin 1 expression) in
cardiomyocytes subjected to H/R, while HMGB1 and IL-17A antibodies
have an opposite effect. All these results suggested that HMGB1 may
promote autophagy through upregulating the expression of
IL-17A.
In conclusion, the present current in vitro
study contributes to a better understanding of the role of the
HMGB1-IL-17A axis in inducing cardiomyocyte apoptosis and promoting
autophagy to cell damage following H/R. IL-17A is a key point in
controlling HMGB1-induced cardiomyocyte H/R injury. Considering its
important role in cardiomyocyte apoptosis and autophagy, a
therapeutic approach involving the HMGB1-IL-17A axis and its
related mechanisms may constitute a new strategy for myocardial I/R
injury.
Acknowledgements
This study was partially supported by grants from
the National Natural Science Foundation of China (grant no.
81370308) and grants from the Natural Science Foundation of Hubei
(grant nos. 2013CFB250 and 2015CFB701).
References
|
1
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottlieb RA and Engler RL: Apoptosis in
myocardial ischemia-reperfusion. Ann N Y Acad Sci. 874:412–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gurusamy N, Lekli I, Gorbunov NV,
Gherghiceanu M, Popescu LM and Das DK: Cardioprotection by
adaptation to ischaemia augments autophagy in association with
BAG-1 protein. J Cell Mol Med. 13:373–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX,
Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, et al: Interleukin-17A
contributes to myocardial ischemia/reperfusion injury by regulating
cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll
Cardiol. 59:420–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et
al: HMGB1 release and redox regulates autophagy and apoptosis in
cancer cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrassy M, Volz HC, Igwe JC, Funke B,
Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK,
et al: High-mobility group box-1 in ischemia- reperfusion injury of
the heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Wu Y, Weng Z, Zhou T, Feng T and
Lin Y: Glycyrrhizin protects brain against ischemia-reperfusion
injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain
Res. 1582:176–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Xu W and Jiang H: HMGB1/IL-17A axis:
An important mechanism for myocardial ischemia-reperfusion injury.
Int J Cardiol. 174:447–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou SF, Yuan J, Liao MY, Xia N, Tang TT,
Li JJ, Jiao J, Dong WY, Nie SF, Zhu ZF, et al: IL-17A promotes
ventricular remodeling after myocardial infarction. J Mol Med
(Berl). 92:1105–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Li J, Wang S, Liu K, Wang L and
Huang L: Hmgb1-TLR4-IL-23-IL17A axis promote ischemia-reperfusion
injury in a cardiac transplantation model. Transplantation.
95:1448–1454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen
H, Lu Z and Jiang H: Minocycline protects against myocardial
ischemia and reperfusion injury by inhibiting high mobility group
box 1 protein in rats. Eur J Pharmacol. 638:84–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu X, Cui B, Zhou X, Xu C, Lu Z and Jiang
H: Ethyl pyruvate reduces myocardial ischemia and reperfusion
injury by inhibiting high mobility group box 1 protein in rats. Mol
Biol Rep. 39:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Sun R, Wei H and Tian Z:
High-mobility group box 1 (HMGB1)-Toll-like receptor
(TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced
damage-associated lethal hepatitis: Interaction of γδ T cells with
macrophages. Hepatology. 57:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abbate A, Bussani R, Amin MS, Vetrovec GW
and Baldi A: Acute myocardial infarction and heart failure: Role of
apoptosis. Int J Biochem Cell Biol. 38:1834–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Neblina F, Toledo AH and
Toledo-Pereyra LH: Molecular biology of apoptosis in ischemia and
reperfusion. J Invest Surg. 18:335–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Chua CC, Ho YS, Hamdy RC and Chua
BH: Overexpression of Bcl-2 attenuates apoptosis and protects
against myocardial I/R injury in transgenic mice. Am J Physiol
Heart Circ Physiol. 280:H2313–H2320. 2001.PubMed/NCBI
|
|
20
|
Costa R, Morrison A, Wang J, Manithody C,
Li J and Rezaie AR: Activated protein C modulates cardiac
metabolism and augments autophagy in the ischemic heart. J Thromb
Haemost. 10:1736–1744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar
M, Massover WH, Yang G, Matsui Y, Sadoshima J and Vatner SF:
Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci
USA. 102:13807–13812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: Enhancing macroautophagy protects against ischemia/reperfusion
injury in cardiac myocytes. J Biol Chem. 281:29776–29787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma X, Liu H, Foyil SR, Godar RJ,
Weinheimer CJ, Hill JA and Diwan A: Impaired autophagosome
clearance contributes to cardiomyocyte death in
ischemia/reperfusion injury. Circulation. 125:3170–3181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong
H, Ding S, Liu J, Peng X, Gao E, et al: Vitamin D receptor
activation protects against myocardial reperfusion injury through
inhibition of apoptosis and modulation of autophagy. Antioxid Redox
Signal. 22:633–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu W, Jiang H, Hu X and Fu W: Effects of
high-mobility group box 1 on the expression of Beclin-1 and LC3
proteins following hypoxia and reoxygenation injury in rat
cardiomyocytes. Int J Clin Exp Med. 7:5353–5357. 2014.PubMed/NCBI
|