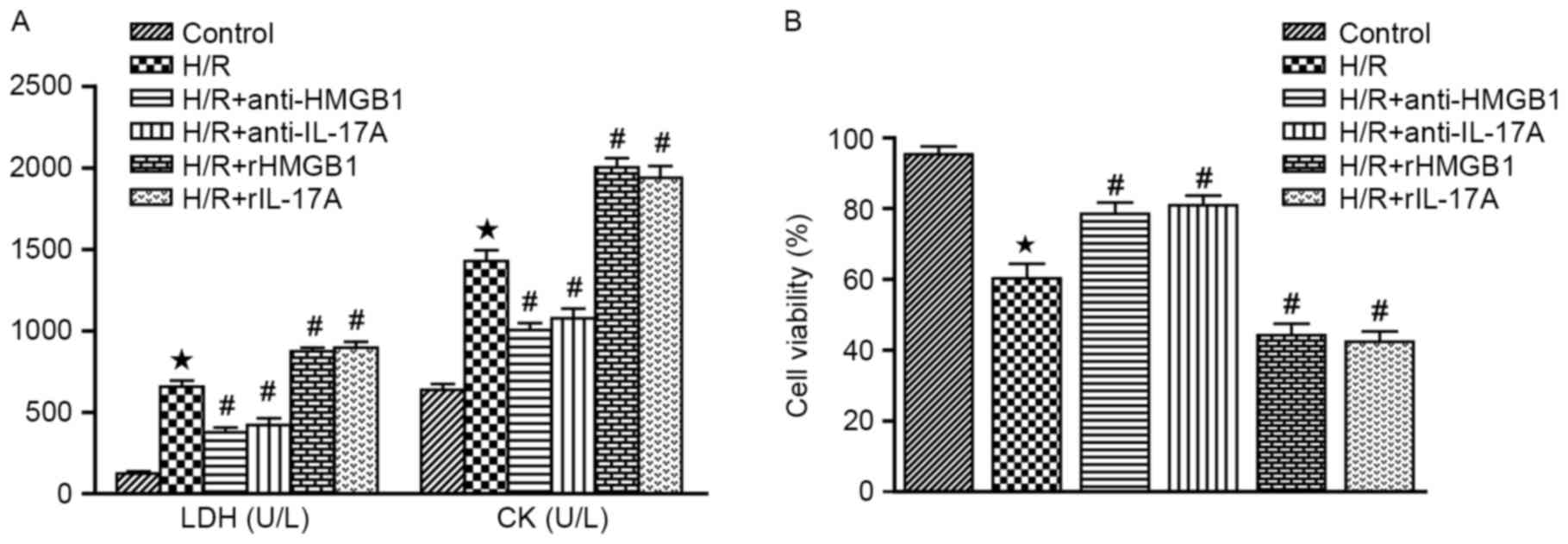
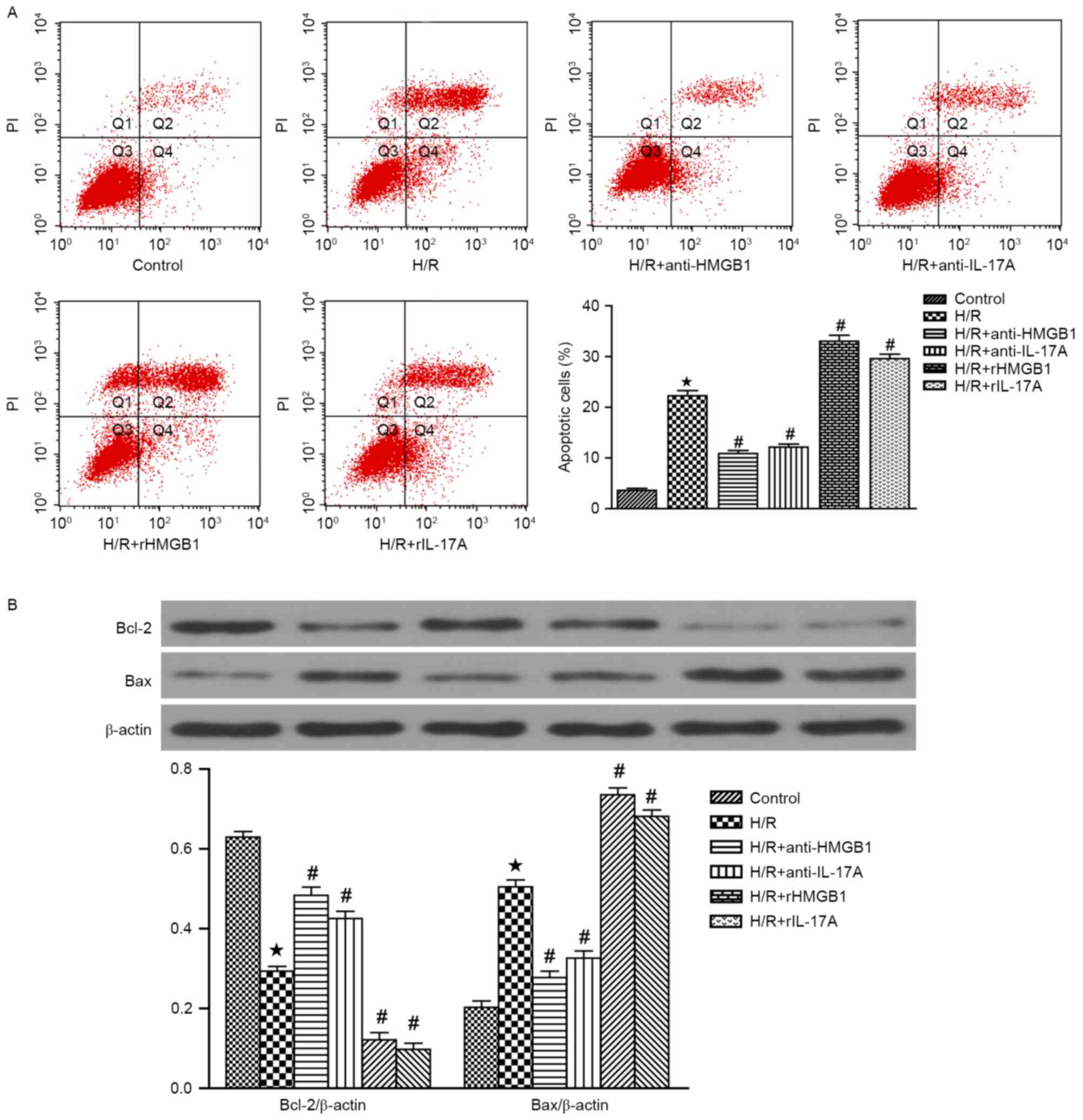
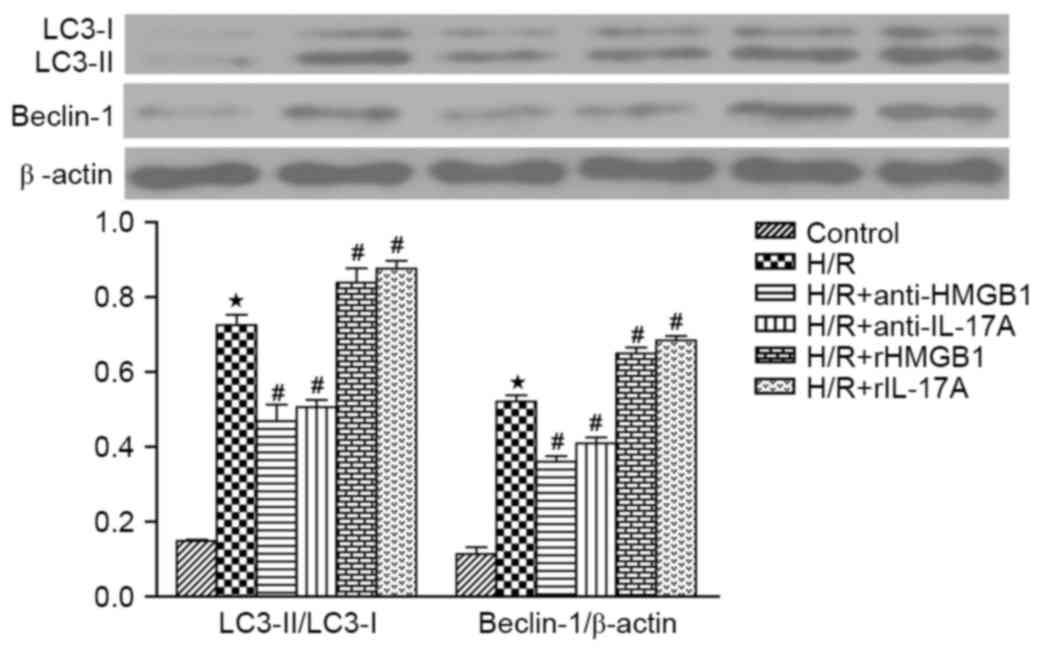
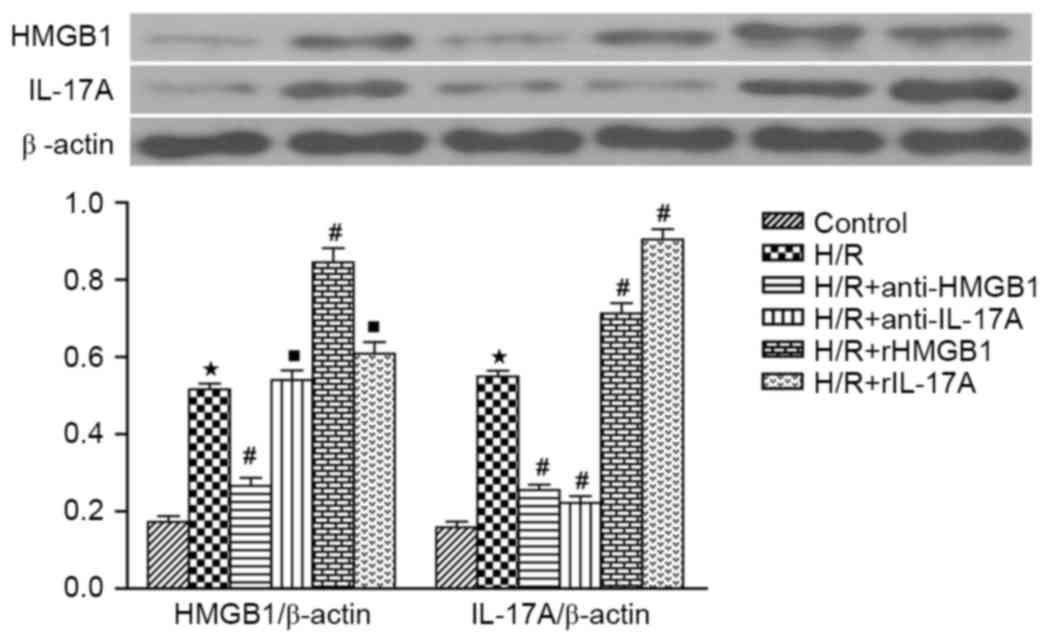
|
1
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottlieb RA and Engler RL: Apoptosis in
myocardial ischemia-reperfusion. Ann N Y Acad Sci. 874:412–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gurusamy N, Lekli I, Gorbunov NV,
Gherghiceanu M, Popescu LM and Das DK: Cardioprotection by
adaptation to ischaemia augments autophagy in association with
BAG-1 protein. J Cell Mol Med. 13:373–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX,
Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, et al: Interleukin-17A
contributes to myocardial ischemia/reperfusion injury by regulating
cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll
Cardiol. 59:420–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et
al: HMGB1 release and redox regulates autophagy and apoptosis in
cancer cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrassy M, Volz HC, Igwe JC, Funke B,
Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK,
et al: High-mobility group box-1 in ischemia- reperfusion injury of
the heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Wu Y, Weng Z, Zhou T, Feng T and
Lin Y: Glycyrrhizin protects brain against ischemia-reperfusion
injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain
Res. 1582:176–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Xu W and Jiang H: HMGB1/IL-17A axis:
An important mechanism for myocardial ischemia-reperfusion injury.
Int J Cardiol. 174:447–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou SF, Yuan J, Liao MY, Xia N, Tang TT,
Li JJ, Jiao J, Dong WY, Nie SF, Zhu ZF, et al: IL-17A promotes
ventricular remodeling after myocardial infarction. J Mol Med
(Berl). 92:1105–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Li J, Wang S, Liu K, Wang L and
Huang L: Hmgb1-TLR4-IL-23-IL17A axis promote ischemia-reperfusion
injury in a cardiac transplantation model. Transplantation.
95:1448–1454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen
H, Lu Z and Jiang H: Minocycline protects against myocardial
ischemia and reperfusion injury by inhibiting high mobility group
box 1 protein in rats. Eur J Pharmacol. 638:84–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu X, Cui B, Zhou X, Xu C, Lu Z and Jiang
H: Ethyl pyruvate reduces myocardial ischemia and reperfusion
injury by inhibiting high mobility group box 1 protein in rats. Mol
Biol Rep. 39:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Sun R, Wei H and Tian Z:
High-mobility group box 1 (HMGB1)-Toll-like receptor
(TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced
damage-associated lethal hepatitis: Interaction of γδ T cells with
macrophages. Hepatology. 57:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abbate A, Bussani R, Amin MS, Vetrovec GW
and Baldi A: Acute myocardial infarction and heart failure: Role of
apoptosis. Int J Biochem Cell Biol. 38:1834–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Neblina F, Toledo AH and
Toledo-Pereyra LH: Molecular biology of apoptosis in ischemia and
reperfusion. J Invest Surg. 18:335–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Chua CC, Ho YS, Hamdy RC and Chua
BH: Overexpression of Bcl-2 attenuates apoptosis and protects
against myocardial I/R injury in transgenic mice. Am J Physiol
Heart Circ Physiol. 280:H2313–H2320. 2001.PubMed/NCBI
|
|
20
|
Costa R, Morrison A, Wang J, Manithody C,
Li J and Rezaie AR: Activated protein C modulates cardiac
metabolism and augments autophagy in the ischemic heart. J Thromb
Haemost. 10:1736–1744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar
M, Massover WH, Yang G, Matsui Y, Sadoshima J and Vatner SF:
Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci
USA. 102:13807–13812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: Enhancing macroautophagy protects against ischemia/reperfusion
injury in cardiac myocytes. J Biol Chem. 281:29776–29787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma X, Liu H, Foyil SR, Godar RJ,
Weinheimer CJ, Hill JA and Diwan A: Impaired autophagosome
clearance contributes to cardiomyocyte death in
ischemia/reperfusion injury. Circulation. 125:3170–3181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong
H, Ding S, Liu J, Peng X, Gao E, et al: Vitamin D receptor
activation protects against myocardial reperfusion injury through
inhibition of apoptosis and modulation of autophagy. Antioxid Redox
Signal. 22:633–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu W, Jiang H, Hu X and Fu W: Effects of
high-mobility group box 1 on the expression of Beclin-1 and LC3
proteins following hypoxia and reoxygenation injury in rat
cardiomyocytes. Int J Clin Exp Med. 7:5353–5357. 2014.PubMed/NCBI
|