Introduction
Acute ischemic stroke is a type of focal brain
injury, which causes functional depression due to a disruption in
normal signal propagation between the ischemic area and regions
that are connected to it by nerve fiber bundles (1). Crossed cerebellar diaschisis (CCD),
which was first mentioned by Baron et al (2), is a condition in which blood flow and
metabolism on the side contralateral to a damaged cerebral area are
decreased (3). It has previously
been reported that an interruption in corticopontocerebellar
pathways is the most likely mechanism underlying CCD (3–5).
Following a supratentorial stroke, cortical excitability cannot be
transmitted to the contralateral cerebellum due to pathway
disruption, which leads to functional inhibition and a decrease in
metabolism in the contralateral cerebellar hemisphere (6). To date, numerous techniques,
including single photon emission computed tomography, positron
emission tomography, dynamic susceptibility contrast magnetic
resonance (MR) perfusion imaging, arterial spin-labeling MR imaging
and computed tomography perfusion, have been used to estimate CCD
within stroke patients (7–14). These techniques attempt to diagnose
CCD based on the rate of regional cerebral blood flow and oxygen
metabolism in the brain; however, the mechanisms underlying CCD
remain unclear.
Regional metabolic differences in the mammalian
brain, including glucose and glycogen stores, have been detected in
ex vivo analyses (15), and
have also been determined from non-invasive measurements in humans
and mice (16–18). Håberg et al (1) reported that glucose metabolism and
the metabolic activity of intermediates from the astrocytic
tricarboxylic acid (TCA) cycle were markedly decreased in the whole
rat cerebellum in the superacute stage of middle cerebral artery
occlusion (MCAO), as determined using 13C MR spectra. In
addition, it was demonstrated that the cerebellum could control
hemispheric activity. Therefore, it may be suggested that, to
enhance the recovery of cerebral hemispheric function, it would be
beneficial to maintain the cerebellum in a low-activity state.
However, this previous study did not explore metabolism in the
bilateral cerebellum or observe variations between left and right
sides. Previous studies have reported that CCD may not be just a
concomitant phenomenon of stroke, but may be regarded as a crucial
prognostic indicator, which may benefit the treatment and
rehabilitation of brain ischemia (10,19).
The present study aimed to identify the effects of
MCAO on alterations in cerebral metabolism in the ischemic brain
regions and in the contralateral cerebellum in rats 1, 3, 9 and 24
h following ischemia using proton nuclear MR (1H NMR)
spectroscopy. In addition, the study aimed to: i) Evaluate the
regional metabolic differences induced by CCD between the ischemic
cerebral hemisphere and the contralateral cerebellum; and ii) to
identify the mechanisms underlying CCD.
Materials and methods
Animal preparation and treatment
A total of 38 male Sprague-Dawley rats (8–10 weeks
old; 250–320 g; Shanghai SLAC Laboratory Animal Co., Ltd.,
Shanghai, China) were maintained in the Specific-Pathogen-Free
Animal Experimental Center of Wenzhou Medical University (Wenzhou,
China). All rats were kept under a temperature of 23±20°C and a
relative humidity of 55±10%, and were maintained under a 12-h
light/dark cycle with free access to food and drink. The present
study was approved by the Animal Ethics Committee of Wenzhou
Medical University and was strictly conducted according to the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (20).
Development of the MCAO model
An MCAO model was developed using the intraluminal
filament technique, as previously described (21). After 12 h of fasting, the rats were
anesthetized with 10% chloral hydrate (300 mg/kg; intraperitoneal).
Initially, an incision was made into the middle cervical fascia and
the left common carotid artery (CCA) was exposed. The external
carotid artery (ECA) and internal carotid artery (ICA) were then
separated. Subsequently, the bifurcation close to the ECA was
ligated with the filament. A ready-made suture (Beijing Sunbio
Biotech Co., Ltd., Beijing, China) was inserted via the left CCA
into the ICA, in order to occlude the MCA. The depth of the suture
within the vessel was 16–18 mm and the redundant part was cut off
with a ligature. In this procedure, the suture was maintained
around the vessel. Finally, the incision was stitched and the rats
were fed separately to improve survival rate. The temperature was
maintained at 25–26°C during the surgery. In the sham operation
group (n=10), the neck was incised to expose the left CCA; however,
the MCA was not occluded. MCAO rats (n=28) were randomly sacrificed
by prompt decapitation at the following time points: 1, 3, 9 and 24
h after MCAO (n=7/group). The rats in the sham operation group were
decapitated 24 h after surgery and were compared with the MCAO rats
at all other time points. Tissue specimens were obtained from the
left cerebral hemisphere, and the left and right cerebellum, within
15 sec; tissue specimens were frozen at −80°C.
Preparation of cerebral samples
The frozen brain tissues were weighed and
homogenized in centrifuge tubes using an electric homogenizer. The
samples were then vortexed with 4 ml/g ice-cold methanol and 0.85
ml/g distilled water. Subsequently, 2 ml/g chloroform and 2 ml/g
distilled water were added to the tubes and mixed again. The
specimen tubes were placed on ice for 15 min and were then
centrifuged at 12,000 × g for 15 min at 4°C. The supernatant was
separated from the tubes and placed into a freeze-dryer to
lyophilize for 24 h. Finally, the obtained extracts were dissolved
in 500 µl 99.5% D2O for NMR spectroscopy.
Acquisition of 1H NMR
spectra
All 1H NMR spectra of the extracts were
obtained at 25°C with a 90° flip angle on a spectrometer (Bruker
Avance III 600-MHz; Bruker Corporation, Billerica, MA, USA). The
spectral width was set at 12,000 Hz and 32 K data points. The
collection time was 2.66 sec per scan and the number of scans was
128. In order to assure full relaxation, an extra 8 sec relaxation
delay was set. Exponential line-broadening of 0.3 Hz was used in
the free induction decay ahead of Fourier transformation and the
spectra were zero-filled to 64 K. All spectra were carefully
corrected by hand for phase as well as baseline. In addition, the
methyl peak of lactate (Lac) (CH3; 1.33 ppm) was used as
a reference point for the spectra. Peak area integration was
conducted using the Bruker Topspin software package (version 2.1;
Bruker Corporation) with standard routines.
Data and statistical analysis
NMR spectra (δ0.5–10.0) were segmented into integral
intervals with each width of 0.01 ppm (2.4 Hz) through AMIX package
(Bruker Topspin 2.1; Bruker Corporation), so that all metabolic
information embedded in the spectra could be exploited; the sum of
each spectrum was then standardized. The normalized integral values
were mean-centered for multivariate data analysis using software
(Umetrics SIMCA-P+12.0; Sartorius Stedim Data Analytics AB, Umeå,
Sweden). Partial least squares discriminate analysis (PLS-DA) was
conducted to identify metabolites according to the separation of
different groups (22). Data were
visualized using a principal component scores plot of the first two
principal components to provide the most efficient 2D
representation of the information (20). Data were confirmed in 2D
1H−1H correlation spectroscopy and total
correlation spectroscopy spectra. The position of each point
represents an individual spectrum of a sample. Differences in the
sample compositions between the different groups were determined by
PLS-DA, and differences in the metabolites between the groups were
revealed as coefficient of variation plots (23). Model quality was assessed with the
fitness of model (R2) and the predictive ability of
model (Q2).
All values are presented as the mean ± standard
deviation. SPSS software (version 13.0; SPSS, Inc., Chicago, IL,
USA) was used to determine the statistical differences between
groups using one-way analysis of variance followed by a least
significant difference post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
1H NMR spectral analysis of
samples
1H NMR was used to investigate metabolism
within the contralateral side to the damaged area; the typical
1H NMR spectra of the right rat cerebellum samples in
the sham operation, and 1, 3, 9 and 24 h MCAO model groups are
presented in Fig. 1. The
allocation of metabolites on the spectrograms was based on our
previous work (24). 2D
1H−1H correlation spectroscopy and total
correlation spectroscopy of the representative samples were
performed to confirm the allocations on the 1H NMR
spectra. Numerous endogenous metabolites were simultaneously
observed on the 1H NMR spectra of cerebral samples.
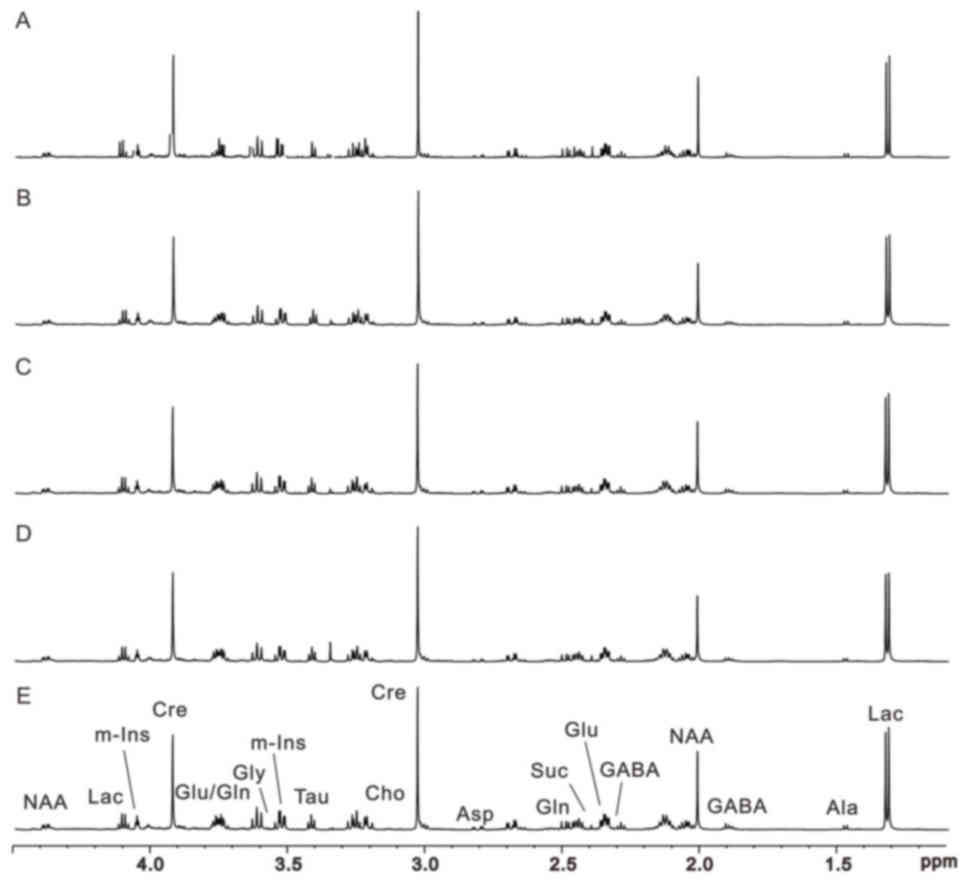 | Figure 1.Representative 600-MHz proton nuclear
magnetic resonance spectra of right cerebellum extracts obtained
from rats in (A-D) the model groups (1, 3, 9 and 24 h after middle
cerebral artery occlusion, respectively) and (E) the sham operation
group. Ala, alanine; Asp, aspartate; Cre, creatine; Cho, choline;
GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Lac,
lactate; m-Ins, myo-inositol; NAA, N-acetyl aspartate; Suc,
succinate. |
Pattern recognition of cerebral
extracts
The 1H NMR data of the left cerebral
hemisphere and the right cerebellum were used to determine
differences between metabolic profiling of rats in the MCAO and
sham groups after 1, 3, 9 and 24 h by multivariate data analysis.
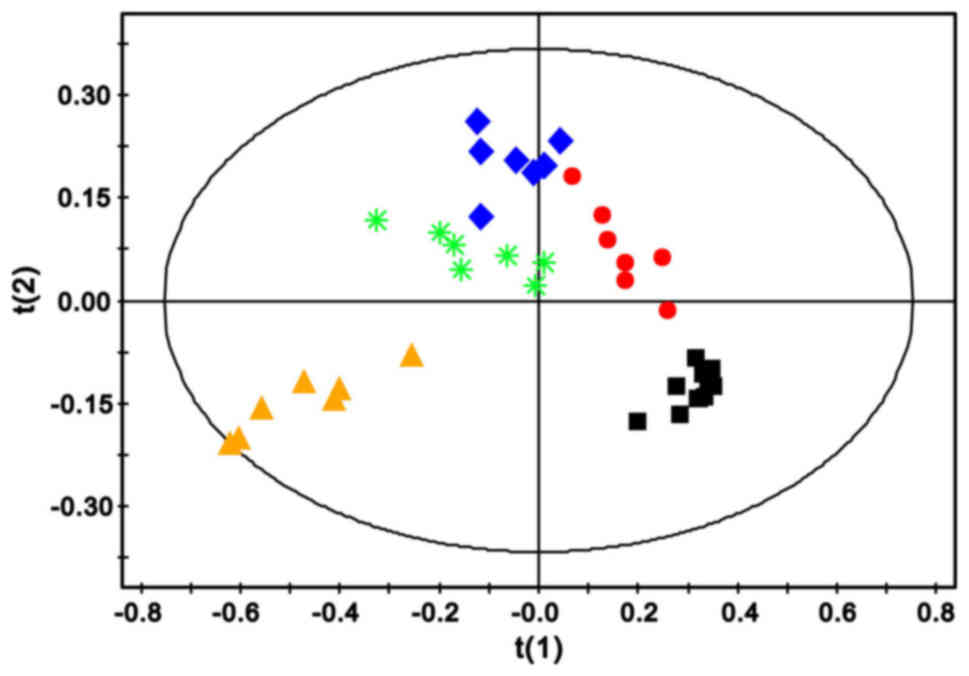
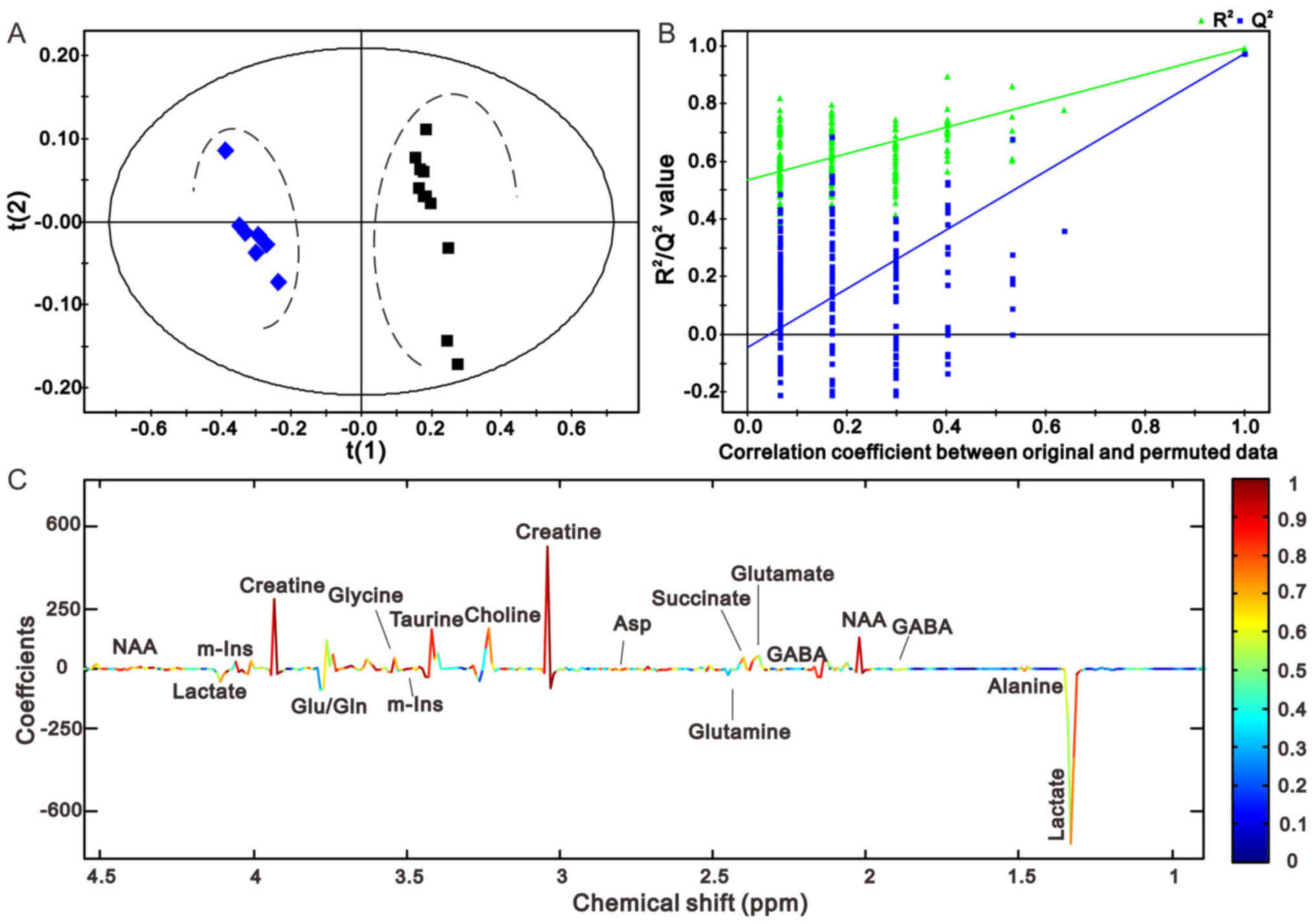
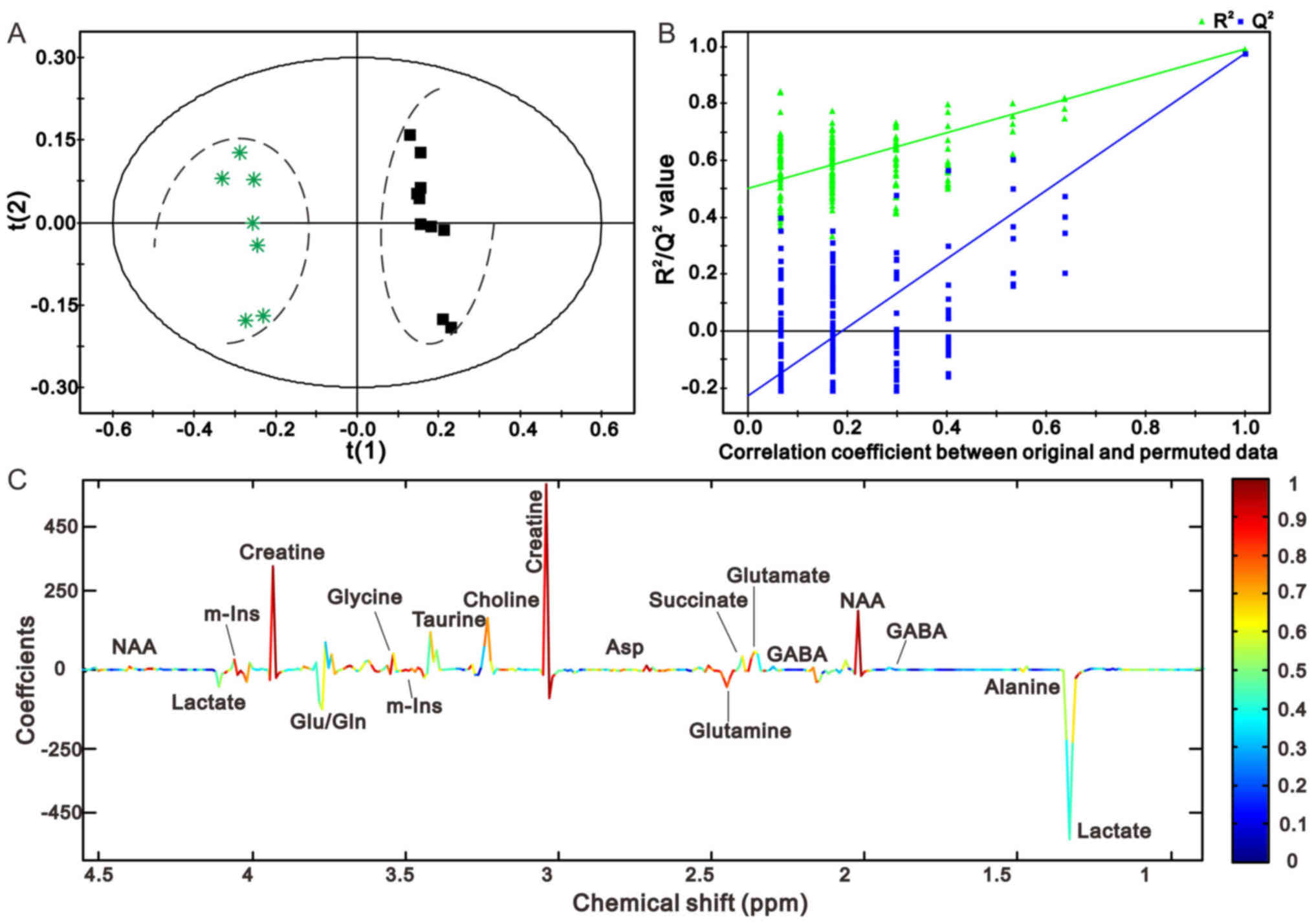
PLS-DA score plots displayed a prominent separation between the
sham and MCAO groups along the direction of t(1) in the left hemisphere (Fig. 2) and the right cerebellum (Fig. 3A), revealing a significant
metabolic disturbance. However, with the increase in ischemic
duration, the alteration in metabolic patterns moved gradually away
from the t(1) direction in the
left cerebral hemisphere (Fig. 2),
which was not detected in the right cerebellum at 9 h (Fig. 3B). Such alterations indicated that
the two brain regions have differences in metabolic pattern.
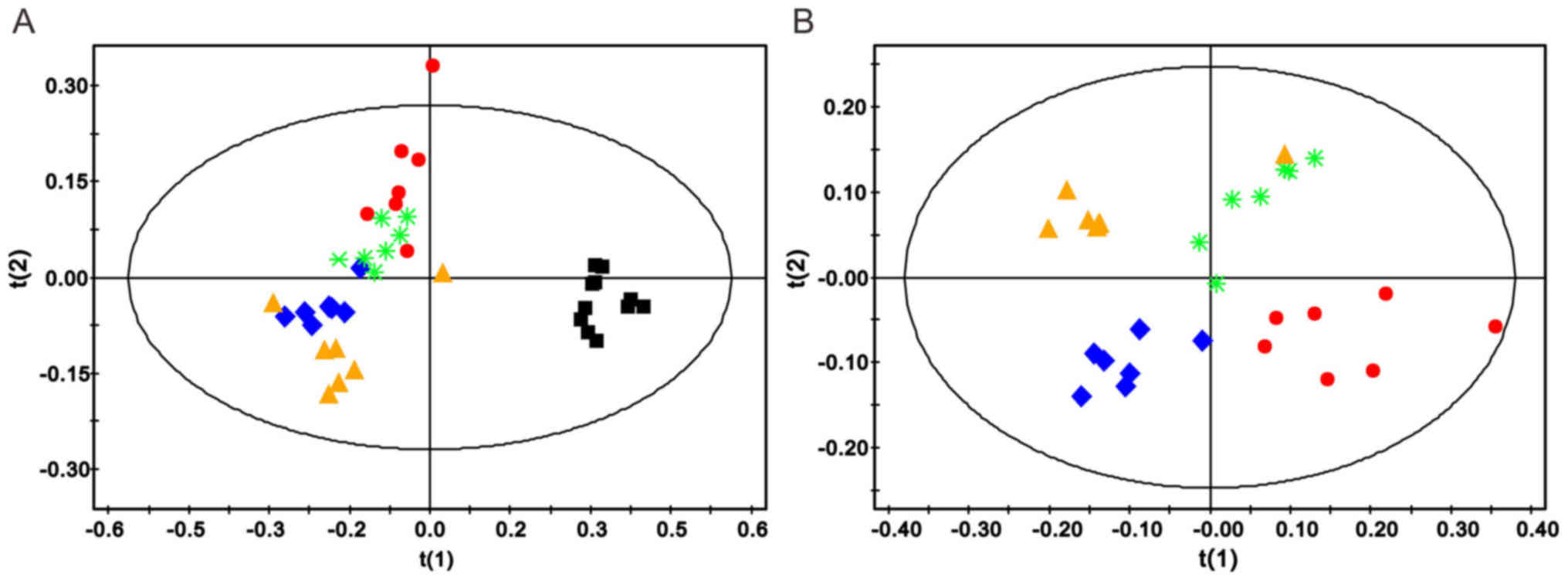
The comparisons between rats in the sham and 1 h
model groups, and the sham and 24 h model groups are presented in
Figs. 4 and 5, respectively. The PLS-DA score plots of
the sham operation group and the 1 h model group, and the sham
operation group and the 24 h model group are presented in Figs. 4A and 5A, respectively. The model groups may be
separated from the sham operation group along the first principal
components horizontal direction. The results demonstrated that
there was an obvious difference between the two groups with regards
to the spectral features in the right cerebellum. In addition, the
validation graph of permutation tests indicated that the PLS-DA
models were robust and credible (Figs.
4B and 5B).
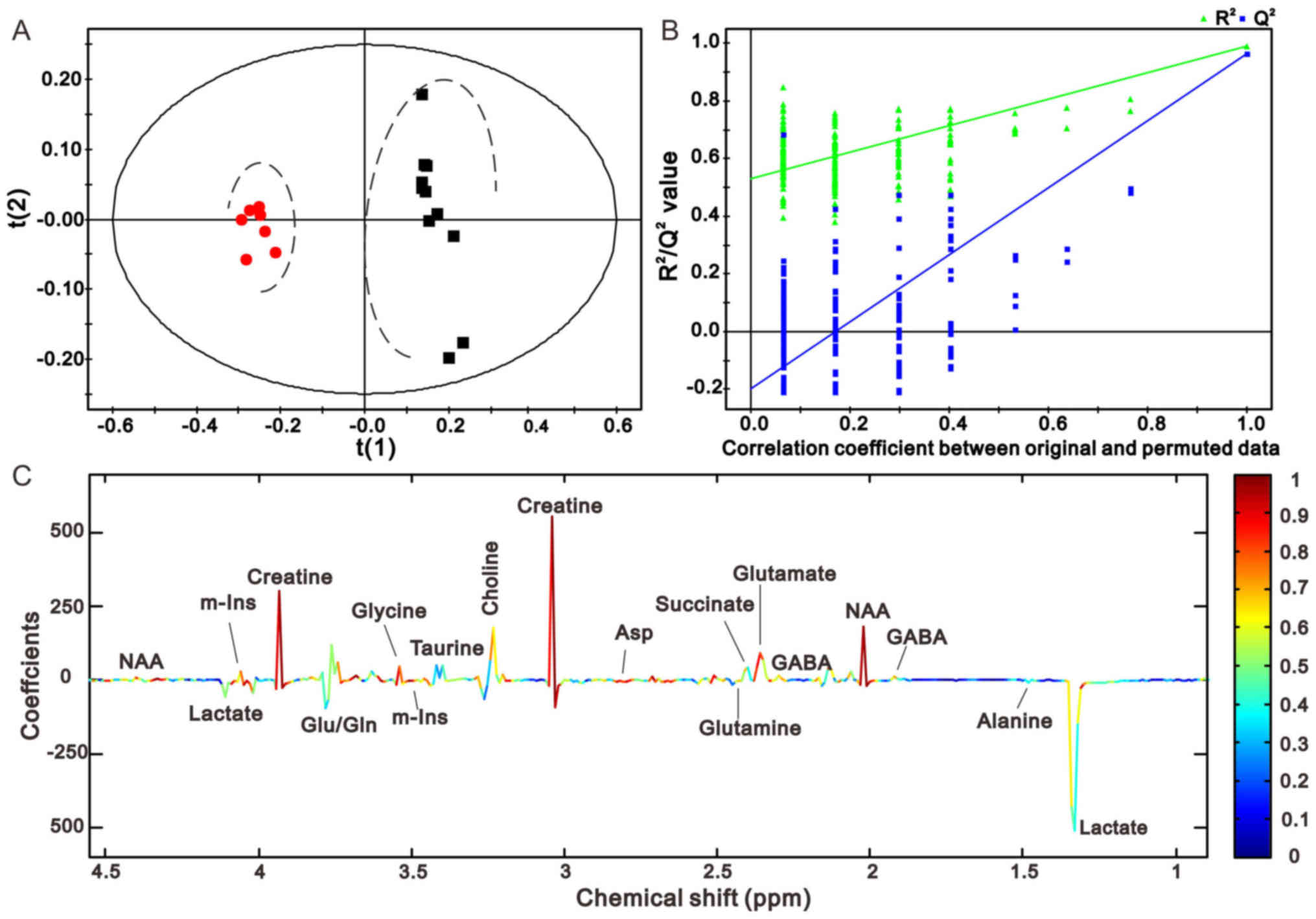
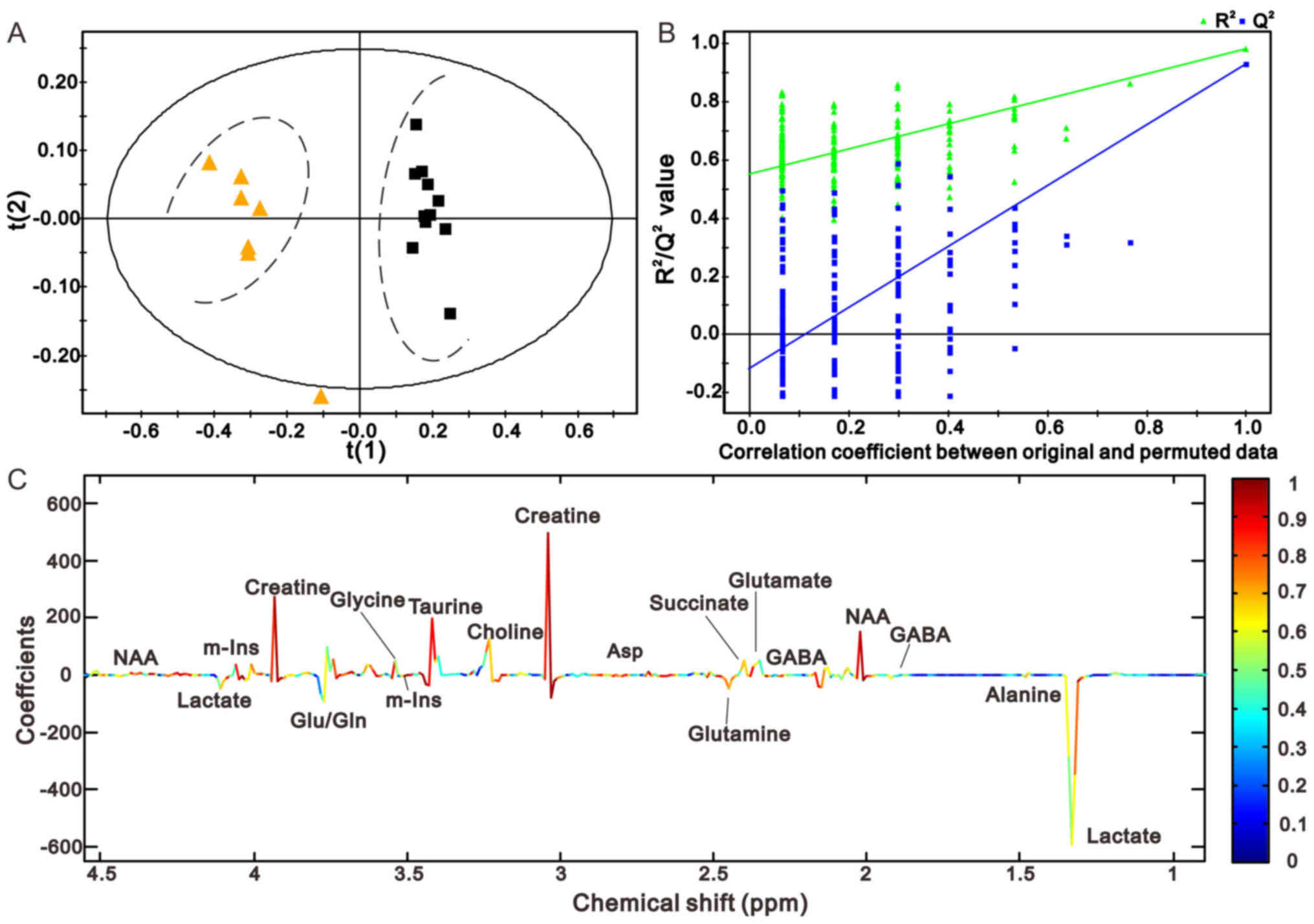
Figs. 4C and
5C illustrate the corresponding
loading plots of metabolites between the MCAO and sham groups using
color-coded correlation coefficients at 1 and 24 h in the right
cerebellum. The findings indicated that the separation of the
different groups may be due to variation in metabolite levels. The
square of the correlation coefficient was used as the weight of a
variable, and color-coding was used to indicate low and high values
(low, blue; high, red). An increase in the corresponding
metabolites in MCAO rats was displayed in the negative area,
whereas a decrease in the corresponding metabolites was displayed
in the positive area. The results demonstrated that MCAO rats had
lower levbels of N-acetyl aspartate (NAA), creatine (Cre),
glutamate (Glu) and succinate (Suc), and higher levels of lactate
(Lac), γ-aminobutyric acid (GABA) and glutamine (Gln) compared with
the control groups. The results at the other time points are
presented in Figs. 6 and 7, and were in accordance with those
presented in Figs. 4 and 5.
Alterations in the levels of cerebral
metabolites
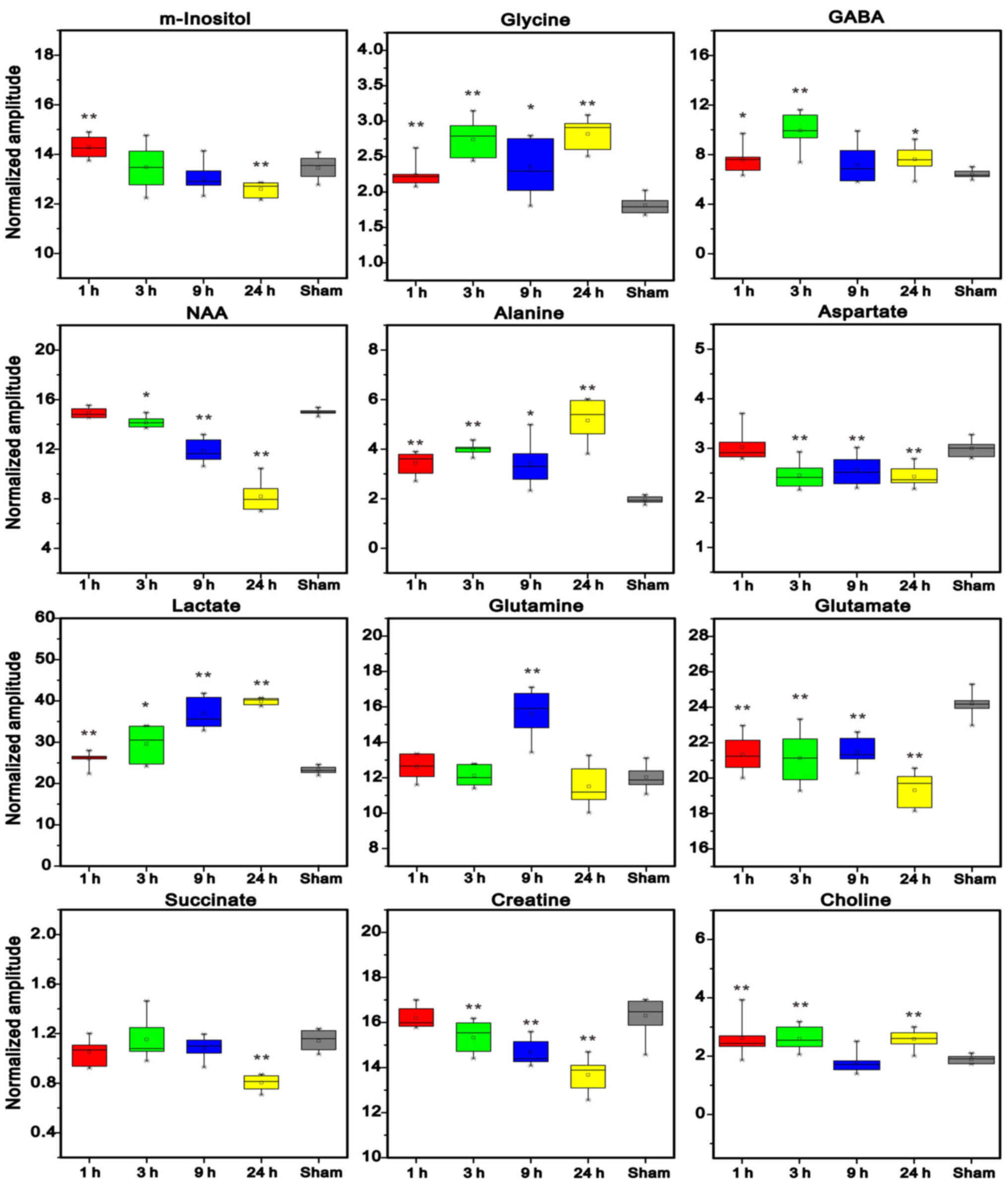
Metabolite levels were quantified, in order to
investigate the metabolic alterations in left cerebral tissue
(Fig. 8). The results demonstrated
that the levels of GABA, glycine (Gly), choline (Cho), Lac and
alanine (Ala) were markedly increased in the ischemic cerebral
hemisphere of rats compared with in the control group. The levels
of Gln were not markedly increased until 9 h post-MCAO. The levels
of Glu, aspartate (Asp), NAA and Cre were markedly decreased in the
left cerebral hemisphere 3, 9 and 24 h post-MCAO. In addition, the
levels of Suc were decreased 24 h post-MCAO, and an obvious
decrease in myo-inositol (m-Ins) levels were also detected 24 h
after ischemic insult in the left cerebral hemisphere.
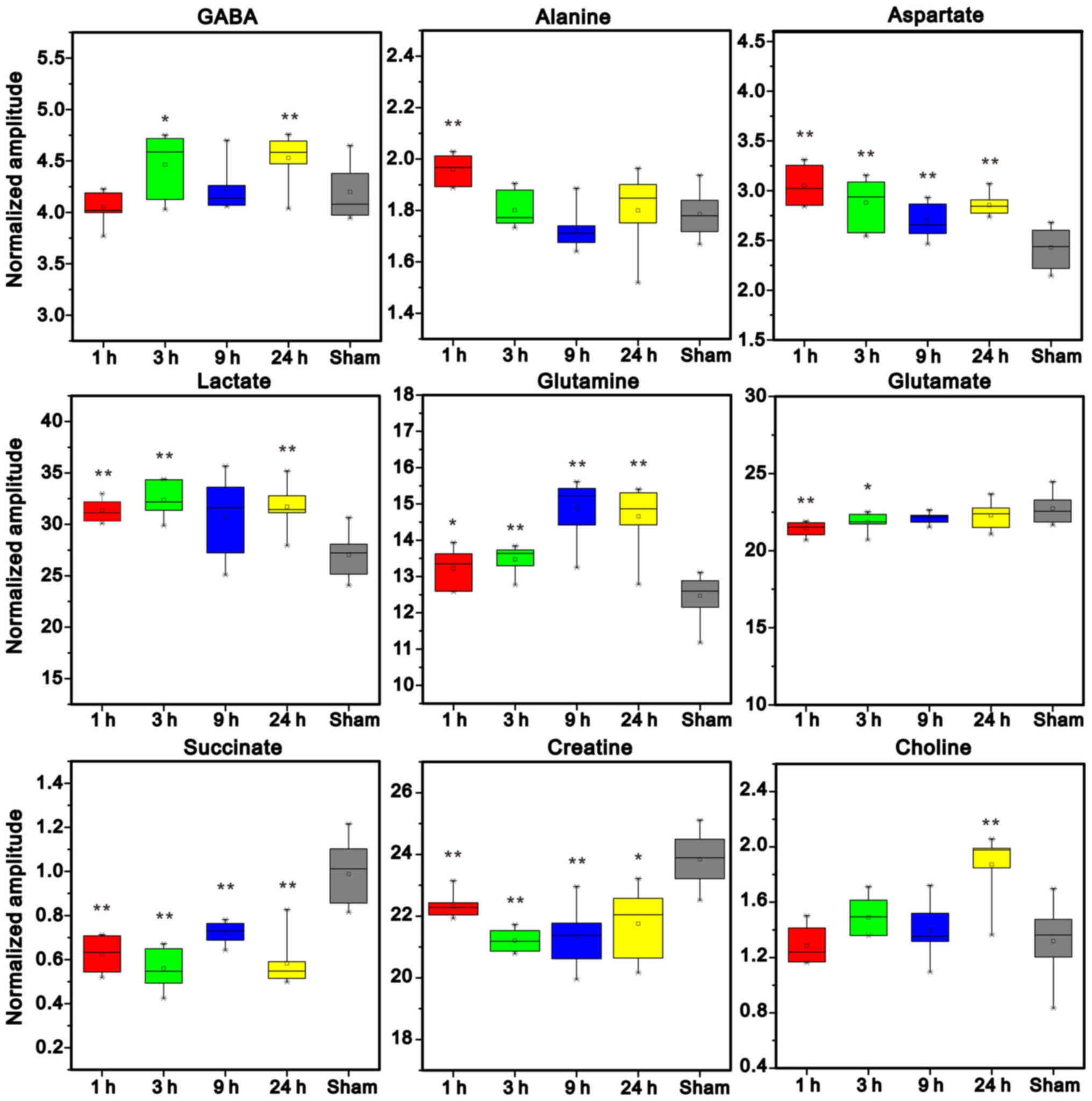
Conversely, ischemia induced marked increases in the
levels of Gln, Asp and Lac, and concomitant decreases in the levels
of Suc and Cre in the right cerebellum at all studied time points
post-MCAO (Fig. 9); however, there
were no significant differences in NAA levels between the groups
(data not shown). Cho levels were not markedly increased until 24 h
post-MCAO. In addition, Ala levels were increased at 1 h post-MCAO,
and GABA levels were elevated at 3 and 24 h following ischemic
insult. Conversely, Glu levels were significantly decreased at 1
and 3 h post-MCAO.
As presented in Fig.
10, there were variations in Glu, Gln, GABA and total levels
(Gln + Glu + GABA), and the Glu/GABA and Glu/Gln ratios in the
non-ischemic left cerebellum and right cerebellum 1, 3, 9, and 24 h
post-MCAO and sham operation. The alterations in Glu/Gln ratio and
Gln + Glu + GABA had just the same trend between the right and left
cerebellum; however, for Glu and Glu/GABA ratio, a similar trend
was observed between the right and left cerebellums. A marked
increase in Gln levels and a concomitant decrease in the Glu/Gln
ratio was detected in both regions at all studied time points
post-MCAO. In addition, an obvious increase in Gln was observed in
the right and left cerebellum at 3, 9 and 24 h post-MCAO.
Furthermore, the present study demonstrated that GABA levels were
decreased at 1 h in the left and right cerebellum and were
evidently increased at 24 h in the right cerebellum post-MCAO.
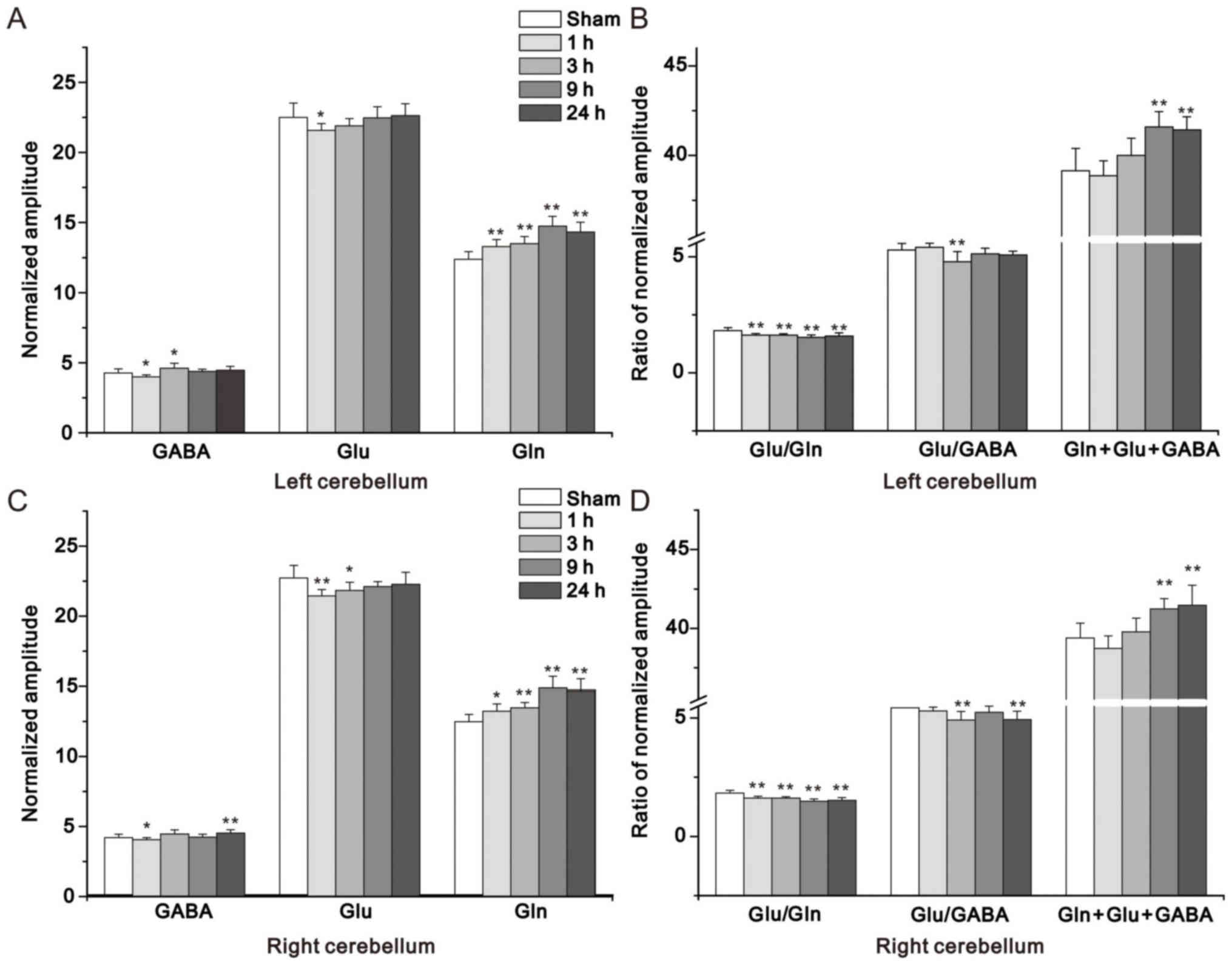 | Figure 10.Summed concentration of Glu, Gln,
GABA, Glu/Gln ratio, Glu/GABA ratio and Gln + Glu + GABA in (A and
B) the non-ischemic left cerebellum and (C and D) right cerebellum
in the 1, 3, 9 and 24 h middle cerebral artery occlusion, compared
with sham groups. *P<0.05; **P<0.01 vs. the sham group. GABA,
γ-aminobutyric acid; m-Ins, myo-inositol; Gln, glutamine; Glu,
glutamate. |
Discussion
Since the brain has high sensitivity to ischemic
hypoxia, focal ischemia can result in a reduced supply of glucose
and oxygen to the corresponding brain areas, resulting in
disruption to the TCA cycle (25).
Consequently, the levels of Lac, as the main product of anaerobic
glycolysis, were markedly increased in the present study in
response to ischemia, which is in agreement with the results of
previous studies (26–29). 1H MR spectroscopy (MRS)
has been widely used to research the pathological mechanism
underlying neuronal and cerebral metabolic alterations in response
to cerebral ischemia in humans and animals (26,30,31).
Alterations in the spectral peaks of patients with acute ischemic
stoke may have prognostic value in clinical practice (32,33).
The present study aimed to use 1H NMR
spectroscopy to analyze the metabolic alterations of CCD between
the left cerebral hemisphere and the contralateral cerebellum in
rats after permanent MCAO. The main finding of the present study
was that metabolic alterations were detected in the contralateral
cerebellum, which is a region involved in CCD, as determined using
PLS-DA. The results indicated that: i) Focal ischemia induced
marked increases in the levels of Lac, Ala, Gln and GABA, and
decreases in the levels of Cre, Suc and Glu in the left cerebral
hemisphere and the right cerebellum; ii) supratentorial ischemia
induced metabolic alterations between left and right cerebellum,
particularly in the contralateral cerebellum, iii) alterations in
Glu metabolism may be associated with CCD; however, further studies
are required.
Glu and Asp are the major excitatory amino acids,
which have significant roles in the central nervous system. In
previous studies, dynamic equilibrium of excitatory and inhibitory
amino acids (Glu and GABA) was elevated to the highest level 1–2 h
after ischemia; however, it was decreased 3 h after ischemic injury
in rats (34,35). The present study detected
alterations in the levels of Glu, Asp, NAA and Cre at 3, 9 and 24 h
in the brain of ischemic rats. In addition, GABA and Gly levels
were significantly increased at 1, 3 and 24 h post-MCAO, which
indicated that GABA and Gly had protective effects on ischemic
insult in the rat brain against excitatory amino acid toxicity.
Glu is a neurotransmitter that is released by
neurons and can be absorbed by astrocytes, where it is converted
into Gln. Subsequently, Gln can be transferred to neurons and once
again converted to Glu. Furthermore, Gln is a major precursor of
neuronal Glu and GABA. This important circulatory pathway between
astrocytes and neurons is known as the Gln-Glu-GABA cycle (36). Elevated levels of GABA and Gln, and
decreased levels of Glu and Asp, were observed in the ischemic
cerebral hemisphere of MCAO rats in the present study, which was
consistent with the results of a previous study (37). A recent study indicated that the
potential mechanisms underlying a decrease in Glu levels may be
associated with increased utilization and decreased synthesis
(1). Furthermore, Glu may undergo
retrograde transport in axons (38). Therefore, it may be hypothesized
that excitotoxic action in the ischemic region is increased if Glu
is transported from the non-ischemic cerebellum to the ischemic
cerebral hemisphere. In the present study, Glu concentrations were
significantly decreased in the left cerebral hemisphere 1, 3, 9 and
24 h post-MCAO; however, in the right cerebellum Glu was markedly
decreased at 1 and 3 h post-MCAO, but was increased at 9 h, without
reaching statistical significance. In addition, according to the
PLS-DA score plots, as the duration of ischemia increased, the
metabolic pattern in the left cerebral hemisphere moved gradually
away from the sham group along the t(1) direction; however, this was not the
case for the metabolic pattern in the right cerebellum at 9 h. It
may be hypothesized that Glu was not transferred from the
non-ischemic right cerebellum to the left ischemic brain at 9 h
post-MCAO. As a result, the excitotoxic burden in the ischemic
region is decreased. This finding also suggested that CCD is
reversible 9 h after ischemic injury. The metabolic alterations of
Glu may be associated with CCD. It has previously been reported
that following permanent ischemia, metabolic alterations can be
longitudinally followed using in vivo localized
1H MRS (39,40). Berthet et al (40) detected marked metabolic alterations
in the ischemic core following permanent focal ischemia, including
increases in GABA, Gly and Cre, and decreases in Gln, Glu and NAA.
However, the results of the present study, including the increases
in Gln levels in the ischemic cerebral hemisphere, were not
consistent with the previous in vivo results. Further
studies are required to assess these inconsistencies.
NAA is regarded as a sensitive marker of neuronal
function (41). The levels of NAA
were significantly decreased in the ischemic cerebral hemisphere of
rats. This indicated that the ischemic brain damage may lead to
obvious neuronal dysfunction. Cre is a biological marker for the
energy metabolism of neurons (42,43).
In the present study, the levels of Cre were markedly decreased in
the left cerebral hemisphere and the right cerebellum, which
revealed that energy metabolism of the brain was disordered in the
ischemic rats. Cho is involved in lipid metabolism and membrane
function (44). An increase in the
levels of Cho in the brain suggests a significant alteration in
membrane metabolism caused by ischemia. In addition, the reduction
in the levels of m-Ins detected in the present study was consistent
with previous findings (45) and
may be a suggestive of alterations in the local osmotic pressure of
cells induced by the ischemia.
The effects of ischemia on metabolism in the
ischemic cerebral hemisphere and in other brain regions have been
reported in a previous ex vivo analysis (1) and in an in vivo study
(46). However, some of the
findings from the ischemic cerebral hemisphere in the present study
were inconsistent with the findings of other studies regarding
permanent MCAO (1,39,40,47),
which may be due to numerous factors, as listed below.
Firstly, there are differences between the methods
used to generate successful models of ischemia, including the
control of cerebral blood circulation for filament
insertion-induced focal ischemia (5). This may result in differences in the
metabolic response to permanent ischemia in the cerebral cortex,
consequently resulting in increased variability in metabolic
results (21,39,40).
Secondly, fixation of cerebral tissue following
decapitation has potential postmortem effects on Lac, Cre and GABA,
which are key components used for PLS-DA in the present study. It
is well known that fixation and extraction procedures intrinsically
affect metabolic results. For example, decapitation is known to
induce postmortem effects on metabolism (2,48,49).
Typically, highly elevated Lac is expected following decapitation,
due to the degradation of glucose and glycogen in the brain, and
may also occur during extraction procedures. In addition, GABA is
known to increase and the levels of phosphocreatine are known to
immediately diminish after decapitation (48). The effects of fixation following
decapitation have also been observed in vivo in the ischemic
core following permanent ischemia using localized 1H MRS
in a horizontal 600MHz magnet (40). Therefore, the majority of the
significant metabolic alterations induced by permanent ischemia in
the present study may have been affected by the fixation method
used; for example, highly elevated Lac levels were detected in MCAO
rats compared with in sham-operated rats and therefore the
difference in Lac between sham-operated and ischemic rats was
significantly reduced. In addition, a three-fold elevation of GABA
(40) in vivo following
permanent ischemia was reduced; however, the difference was not so
marked ex vivo in the present study. Alternatively,
microwave fixation (50) and
funnel-freeze fixation (51), with
careful extraction procedures, have been reported to reserve all
carbohydrates, including glucose and glycogen, as illustrated by
the lower Lac levels detected in the control animals.
Finally, the selection of specimens may have effects
on the results. For example, the effects of focal ischemia were
limited to only part of one side of the cerebral hemisphere in
Igarashi et al (26),
Berthet et al (40) and
Håberg et al (1).
Therefore, the results from the selected specimen (part of the left
cerebral hemisphere) would intrinsically provide information
regarding the metabolic alterations in the ischemic cerebral
hemisphere and in some non-ischemic regions. This may explain the
discrepancies between the metabolic alterations detected in the
present study compared with other studies (1,39,40,47).
In conclusion, the present study used 1H
NMR-based metabonomics to evaluate cerebral metabolism in the
ischemic cerebral hemisphere and the non-ischemic cerebellum
post-MCAO in rats. The results indicated that focal ischemia
affected non-ischemic cerebellum activities, neurotransmitter
synthesis and metabolic balance, particularly in the contralateral
cerebellum. In addition, metabolic activity in the cerebellum,
particularly with regards to Glu, may serve an important role in
brain function reconstruction, which may help improve understanding
regarding cerebral infarction on a molecular level. Alterations in
Glu metabolism may be associated with CCD; however, this requires
further experimentation.
Acknowledgements
The present study was supported by the Fund of
Zhejiang Provincial Key Laboratory of Aging and Neurological
Disorder Research (grant nos. 2012E10008 and LH001); the National
Natural Science Foundation of China (grant nos. 81571626 and
21575105); the Natural Science Foundation of Zhejiang Province
(grant no. LY15H220001); the Medical Science and Technology Project
of Zhejiang Province (grant no. 2014KYA134); and the Science and
Technology Planning Project of Wenzhou City (grant no.
Y20140731).
Glossary
Abbreviations
Abbreviations:
|
MCAO
|
middle cerebral artery occlusion
|
|
1H NMR
|
proton nuclear magnetic resonance
|
|
CCD
|
crossed cerebellar diaschisis
|
|
TCA
|
tricarboxylic acid
|
|
CCA
|
common carotid artery
|
|
ECA
|
external carotid artery
|
|
ICA
|
internal carotid artery
|
|
PLS-DA
|
partial least squares discriminate
analysis
|
|
NAA
|
N-acetyl aspartate
|
|
Lac
|
lactate
|
|
Ala
|
alanine
|
|
GABA
|
γ-aminobutyric acid
|
|
Glu
|
glutamate
|
|
Gln
|
glutamine
|
|
Suc
|
succinate
|
|
Asp
|
aspartate
|
|
Cre
|
creatine
|
|
m-Ins
|
myo-inositol
|
|
Cho
|
choline
|
|
Gly
|
glycine
|
|
1H MRS
|
1H magnetic resonance
spectroscopy
|
References
|
1
|
Håberg AK, Qu H and Sonnewald U: Acute
changes in intermediary metabolism in cerebellum and contralateral
hemisphere following middle cerebral artery occlusion in rat. J
Neurochem. 109 Suppl 1:S174–S181. 2009. View Article : Google Scholar
|
|
2
|
Baron JC, Bousser MG, Comar D and
Castaigne P: ‘Crossed cerebellar diaschisis’ in human
supratentorial brain infarction. Trans Am Neurol Assoc.
105:459–461. 1981.PubMed/NCBI
|
|
3
|
Pantano P, Baron JC, Samson Y, Bousser MG,
Derouesne C and Comar D: Crossed cerebellar diaschisis. Further
studies. Brain. 109:677–694. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer JS, Obara K and Muramatsu K:
Diaschisis. Neurol Res. 15:362–366. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gold L and Lauritzen M: Neuronal
deactivation explains decreased cerebellar blood flow in response
to focal cerebral ischemia or suppressed neocortical function. Proc
Natl Acad Sci USA. 99:7699–7704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin G, Levy EI, Scarrow AM, Firlik AD,
Karakus A, Wechsler L, Jungreis CA and Yonas H: Remote effects of
acute ischemic stroke: A xenon CT cerebral blood flow study.
Cerebrovasc Dis. 10:221–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meneghetti G, Vorstrup S, Mickey B,
Lindewald H and Lassen NA: Crossed cerebellar diaschisis in
ischemic stroke: A study of regional cerebral blood flow by 133Xe
inhalation and single photon emission computerized tomography. J
Cereb Blood Flow Metab. 4:235–240. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito H, Kanno I, Shimosegawa E, Tamura H,
Okane K and Hatazawa J: Hemodynamic changes during neural
deactivation in human brain: A positron emission tomography study
of crossed cerebellar diaschisis. Ann Nucl Med. 16:249–254. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Karonen JO, Nuutinen J, Vanninen E,
Kuikka JT and Vanninen RL: Crossed cerebellar diaschisis in acute
ischemic stroke: A study with serial SPECT and MRI. J Cereb Blood
Flow Metab. 27:1724–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin DD, Kleinman JT, Wityk RJ, Gottesman
RF, Hillis AE, Lee AW and Barker PB: Crossed cerebellar diaschisis
in acute stroke detected by dynamic susceptibility contrast MR
perfusion imaging. AJNR Am J Neuroradiol. 30:710–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madai VI, Altaner A, Stengl KL, Zaro-Weber
O, Heiss WD, von Samson-Himmelstjerna FC and Sobesky J: Crossed
cerebellar diaschisis after stroke: Can perfusion-weighted MRI show
functional inactivation? J Cereb Blood Flow Metab. 31:1493–1500.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeon YW, Kim SH, Lee JY, Whang K, Kim MS,
Kim YJ and Lee MS: Brain Research Group: Dynamic CT perfusion
imaging for the detection of crossed cerebellar diaschisis in acute
ischemic stroke. Korean J Radiol. 13:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Guan M, Lian HJ, Ma LJ, Shang JK,
He S, Ma MM, Zhang ML, Li ZY, Wang MY, et al: Crossed cerebellar
diaschisis detected by arterial spin-labeled perfusion magnetic
resonance imaging in subacute ischemic stroke. J Stroke Cerebrovasc
Dis. 23:2378–2383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang KM, Sohn CH, Kim BS, Kim YI, Choi SH,
Yun TJ, Kim JH, Park SW, Cheon GJ and Han MH: Correlation of
asymmetry indices measured by arterial spin-labeling MR imaging and
SPECT in patients with crossed cerebellar diaschisis. AJNR Am J
Neuroradiol. 36:1662–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swanson RA, Sagar SM and Sharp FR:
Regional brain glycogen stores and metabolism during complete
global ischaemia. Neurol Res. 11:24–28. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pouwels PJ and Frahm J: Regional
metabolite concentrations in human brain as determined by
quantitative localized proton MRS. Magn Reson Med. 39:53–60. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duarte JM, Lei H, Mlynárik V and Gruetter
R: The neurochemical profile quantified by in vivo 1H NMR
spectroscopy. Neuroimage. 61:342–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Emir UE, Auerbach EJ, Van De Moortele PF,
Marjańska M, Uğurbil K, Terpstra M, Tkáč I and Oz G: Regional
neurochemical profiles in the human brain measured by (1)H MRS at 7
T using local B(1) shimming. NMR Biomed. 25:152–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szilágyi G, Vas A, Kerényi L, Nagy Z,
Csiba L and Gulyás B: Correlation between crossed cerebellar
diaschisis and clinical neurological scales. Acta Neurol Scand.
125:373–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao H, Dong B, Liu X, Xuan H, Huang Y and
Lin D: Metabonomic profiling of renal cell carcinoma:
High-resolution proton nuclear magnetic resonance spectroscopy of
human serum with multivariate data analysis. Anal Chim Acta.
624:269–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westerhuis JA, van Velzen EJ, Hoefsloot HC
and Smilde AK: Multivariate paired data analysis: Multilevel PLSDA
versus OPLSDA. Metabolomics. 6:119–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cloarec O, Dumas ME, Trygg J, Craig A,
Barton RH, Lindon JC, Nicholson JK and Holmes E: Evaluation of the
orthogonal projection on latent structure model limitations caused
by chemical shift variability and improved visualization of
biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal
Chem. 77:517–526. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao H, Xiang Y, Sun N, Zhu H, Wang Y, Liu
M, Ma Y and Lei H: Metabolic changes in rat prefrontal cortex and
hippocampus induced by chronic morphine treatment studied ex vivo
by high resolution 1H NMR spectroscopy. Neurochem Int. 50:386–394.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katsura K, de Turco Rodriguez EB,
Folbergrová J, Bazan NG and Siesjö BK: Coupling among energy
failure, loss of ion homeostasis, and phospholipase A2 and C
activation during ischemia. J Neurochem. 61:1677–1684. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Igarashi H, Kwee IL, Nakada T, Katayama Y
and Terashi A: 1H magnetic resonance spectroscopic imaging of
permanent focal cerebral ischemia in rat: Longitudinal metabolic
changes in ischemic core and rim. Brain Res. 907:208–221. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frykholm P, Hillered L, Långström B,
Persson L, Valtysson J and Enblad P: Relationship between cerebral
blood flow and oxygen metabolism, and extracellular glucose and
lactate concentrations during middle cerebral artery occlusion and
reperfusion: A microdialysis and positron emission tomography study
in nonhuman primates. J Neurosurg. 102:1076–1084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brouns R, Sheorajpanday R, Wauters A, De
Surgeloose D, Mariën P and De Deyn PP: Evaluation of lactate as a
marker of metabolic stress and cause of secondary damage in acute
ischemic stroke or TIA. Clin Chim Acta. 397:27–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cvoro V, Wardlaw JM, Marshall I, Armitage
PA, Rivers CS, Bastin ME, Carpenter TK, Wartolowska K, Farrall AJ
and Dennis MS: Associations between diffusion and perfusion
parameters, N-acetyl aspartate, and lactate in acute ischemic
stroke. Stroke. 40:767–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bruhn H, Frahm J, Gyngell ML, Merboldt KD,
Hänicke W and Sauter R: Cerebral metabolism in man after acute
stroke: New observations using localized proton NMR spectroscopy.
Magn Reson Med. 9:126–131. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wardlaw JM, Marshall I, Wild J, Dennis MS,
Cannon J and Lewis SC: Studies of acute ischemic stroke with proton
magnetic resonance spectroscopy: Relation between time from onset,
neurological deficit, metabolite abnormalities in the infarct,
blood flow, and clinical outcome. Stroke. 29:1618–1624. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Federico F, Simone IL, Lucivero V,
Giannini P, Laddomada G, Mezzapesa DM and Tortorella C: Prognostic
value of proton magnetic resonance spectroscopy in ischemic stroke.
Arch Neurol. 55:489–494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pereira AC, Saunders DE, Doyle VL, Bland
JM, Howe FA, Griffiths JR and Brown MM: Measurement of initial
N-acetyl aspartate concentration by magnetic resonance spectroscopy
and initial infarct volume by MRI predicts outcome in patients with
middle cerebral artery territory infarction. Stroke. 30:1577–1582.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graham SH, Chen J, Sharp FR and Simon RP:
Limiting ischemic injury by inhibition of excitatory amino acid
release. J Cereb Blood Flow Metab. 13:88–97. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Melani A, Pantoni L, Corsi C, Bianchi L,
Monopoli A, Bertorelli R, Pepeu G and Pedata F: Striatal outflow of
adenosine, excitatory amino acids, gamma-aminobutyric acid, and
taurine in awake freely moving rats after middle cerebral artery
occlusion: Correlations with neurological deficit and
histopathological damage. Stroke. 30:2448–2455. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iltis I, Koski DM, Eberly LE, Nelson CD,
Deelchand DK, Valette J, Ugurbil K, Lim KO and Henry PG:
Neurochemical changes in the rat prefrontal cortex following acute
phencyclidine treatment: An in vivo localized (1)H MRS study. NMR
Biomed. 22:737–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Håberg A, Qu H, Haraldseth O, Unsgård G
and Sonnewald U: In vivo injection of [1-13C]glucose and
[1,2-13C]acetate combined with ex vivo 13C nuclear magnetic
resonance spectroscopy: A novel approach to the study of middle
cerebral artery occlusion in the rat. J Cereb Blood Flow Metab.
18:1223–1232. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barbaresi P, Fabri M, Conti F and Manzoni
T: D-[3H]aspartate retrograde labelling of callosal and association
neurones of somatosensory areas I and II of cats. J Comp Neurol.
263:159–178. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gyngell ML, Busch E, Schmitz B, Kohno K,
Back T, Hoehn-Berlage M and Hossmann KA: Evolution of acute focal
cerebral ischaemia in rats observed by localized 1H MRS,
diffusion-weighted MRI, and electrophysiological monitoring. NMR
Biomed. 8:206–214. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berthet C, Xin L, Buscemi L, Benakis C,
Gruetter R, Hirt L and Lei H: Non-invasive diagnostic biomarkers
for estimating the onset time of permanent cerebral ischemia. J
Cereb Blood Flow Metab. 34:1848–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Demougeot C, Marie C, Giroud M and Beley
A: N-acetylaspartate: A literature review of animal research on
brain ischaemia. J Neurochem. 90:776–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miller BL: A review of chemical issues in
1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline.
NMR Biomed. 4:47–52. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li S, Huang M, Wang X, Wang X, Chen F, Lei
H and Jiang F: Retinal metabolic changes in an experimental model
of optic nerve transection by ex vivo 1H magnetic resonance
spectroscopy. Neurochem Res. 36:2427–2433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cecil KM and Jones BV: Magnetic resonance
spectroscopy of the pediatric brain. Top Magn Reson Imaging.
12:435–452. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang M, Wang S, Hao F, Li Y, Tang H and
Shi X: NMR analysis of the rat neurochemical changes induced by
middle cerebral artery occlusion. Talanta. 88:136–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alf MF, Lei H, Berthet C, Hirt L, Gruetter
R and Mlynarik V: High-resolution spatial mapping of changes in the
neurochemical profile after focal ischemia in mice. NMR Biomed.
25:247–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nonaka M, Yoshimine T, Kohmura E, Wakayama
A and Yamashita TTH: Changes in brain organic osmolytes in
experimental cerebral ischemia. J Neurol Sci. 157:25–30. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lowry OH, Passonneau JV, Hasselberger FX
and Schulz DW: Effect of ischemia on known substrates and cofactors
of the glycolytic pathway in brain. J Biol Chem. 239:18–30.
1964.PubMed/NCBI
|
|
49
|
Petroff OA, Ogino T and Alger JR:
High-resolution proton magnetic resonance spectroscopy of rabbit
brain: Regional metabolite levels and postmortem changes. J
Neurochem. 51:163–171. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kong J, Shepel PN, Holden CP, Mackiewicz
M, Pack AI and Geiger JD: Brain glycogen decreases with increased
periods of wakefulness: Implications for homeostatic drive to
sleep. J Neurosci. 22:5581–5587. 2002.PubMed/NCBI
|
|
51
|
Cruz NF and Dienel GA: High glycogen
levels in brains of rats with minimal environmental stimuli:
Implications for metabolic contributions of working astrocytes. J
Cereb Blood Flow Metab. 22:1476–1489. 2002. View Article : Google Scholar : PubMed/NCBI
|