Introduction
Myocardial infarction (MI), commonly known as a
heart attack, occurs when blood flow stops to a part of the heart,
causing damage to the heart muscle (1). Common symptoms are chest pain and
acute circulatory dysfunction (2).
According to clinical processes and electrocardiographic results,
myocardial infarction is classified into three phases; acute,
subacute and chronic. Its clinical symptoms predominantly appear
during the acute stage (3).
Acute MI (AMI) is a serious type of coronary heart
disease (4). AMI as a result of
coronary artery disease occurs when the blood supply of coronary
artery reduces sharply or suspends completely, resulting in lasting
and severe acute ischemia of the corresponding myocardium (5). Therefore, myocardial ischemic
necrosis of this myocardium occurs.
In 2008, as a non-pseudo-peptide thrombin inhibitor,
Pradaxa, which was developed by Boehringer Ingelheim, came into the
market in Germany and the UK as a novel oral anticoagulant
(6). In October 2010, it was
approved by Food and Drug Administration to be employed to prevent
cerebral apoplexy for patients with auricular fibrillation
(7). Pradaxa is effective to
reduce risks of patients with atrial fibrillation (8). In addition, it can avoid the
reinforcement of intracranial hemorrhage (9). It is preferable than Warfarin and
provides a new choice for anticoagulant therapy of atrial
fibrillation (10). In the present
study it was investigated whether effects of dabigatran regulate
the no-reflow phenomenon, inflammation and oxidative stress in AMI
rabbits.
Materials and methods
Surgical and experimental
procedures
Male New Zealand White rabbits (2.3–3.0 kg, 4–5
months age, n=24) were purchased from the Experimental Center of
Hebel Medical University (Hebel, China) and housed at 22–24°C with
a 12-h light/dark cycle in 55–60% humidity with access to food and
water. All rabbits were used as AMI rabbits and anesthetized with
ketamine (75 mg/kg) and xylazine (5 mg/kg). Rabbits were ventilated
mechanically following intubation and its catheters were inserted
into the jugular vein, carotid artery and left atrial appendage.
The heart rate and arterial blood pressure were monitored and
recorded. The coronary artery was occluded for 30 min following a
stabilization period, then were reperfused for 3 h. Blue pigment
was injected with the re-occlusion of the coronary artery, which
set the limits of the ischemic risk zone. Zones of necrosis stained
with triphenyltetrazolium chloride were distinguished. The anatomic
no-reflow zone was measured using thioflavin S (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Intact endothelium stained with
fluorescent yellow dye served as a marker of regional perfusion.
Areas receiving blood flow appear brightly fluorescent viewed under
ultraviolet light, and the no-reflow zone appears as a
nonfluorescent, dark area. Experiments were approved by the Medical
Ethics Committee of Cangzhou City Central Hospital (Cangzhou,
China).
Treatment groups
All rabbits were randomly distributed into the
control, AMI vehicle and dabigatran group. In the control or AMI
vehicle groups, normal rabbits and AMI model rabbits received an
equivalent volume of vehicle. In the dabigatran group, AMI model
rabbits were administered as an intravenous bolus (0.5 mg/kg) and
concomitant infusion (0.15 mg/kg/h). Prior to treatment initiation,
the coronary artery was occluded for 15 min and then the infusion
was continued for 2.5 h during reperfusion.
Inflammation and oxidative stress
measurements
Blood samples were obtained at baseline, then and
centrifuged at 2,000 × g for 10 min at 4°C. After centrifugation,
the plasma was removed and the samples stored at −70°C until
required for analysis. The plasma was used to analyze the P65 of
nuclear factor (NF)-κB (H202), tumor necrosis factor (TNF)-α
(H052), interleukin (IL)-1β (H002), IL-6 (H007), catalase (CAT;
A007-1-1) and superoxide dismutase (SOD; A001-1-1) activities using
ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) in accordance with the manufacturer's instructions.
Western blotting
Heart samples were obtained at baseline and were
homogenated using a radioimmunoprecipitation acid lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). Following
centrifugation at 12,000 × g for 10 min at 4°C, supernatant liquor
was collected to quantify protein content using a Bicinchoninic
Acid Assay kit (Beyotime Institute of Biotechnology). Proteins (50
µg) were separated on a 10–12% SDS polyacrylamide gel and
transferred to a polyvinylidene difluoride membrane (Beyotime
Institute of Biotechnology). The membranes were incubated overnight
at 4°C with anti-inducible nitric oxide synthase (iNOS; cat. no.
sc-649; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-collagen I (cat. no. 84336; 1:500; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-transforming growth factor β1
(TGF-β1; cat. no. sc-9043; Santa Cruz Biotechnology, Inc.),
anti-α-smooth muscle actin (α-SMA; cat. no. 19245; 1:500; Santa
Cruz Biotechnology, Inc.), anti-connective tissue growth factor
(CTGF; cat. no. sc-25440; 1:500; Santa Cruz Biotechnology, Inc.)
and anti-β-actin (cat. no. sc-7210; 1:500; Santa Cruz
Biotechnology, Inc.) antibodies after membrane incubation in 5%
skimmed milk powder. Membranes were then washed twice with TBS with
0.1% Tween-20 and incubated for 1 h with the goat anti-rabbit
peroxidase-conjugated secondary antibody (cat. no. sc-2004;
1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C.
Statistical analysis
Data are presented as the mean and standard
deviation using SPSS version 17.0 software (SPSS, Inc., Chicago,
IL, USA) Statistical analysis was conducted using a one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of dabigatran on infarct size
in AMI rabbits
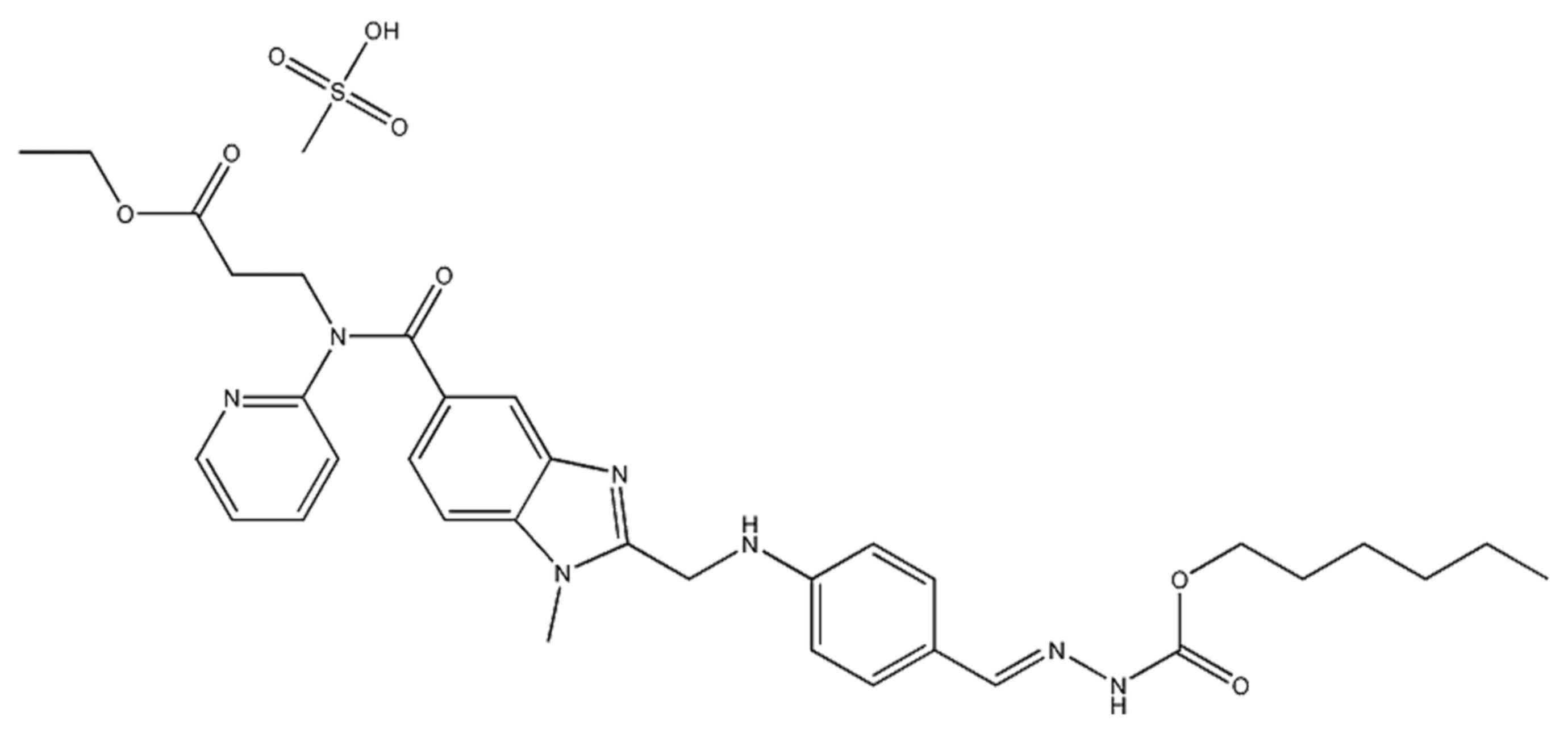
The chemical structure of dabigatran is presented in
Fig. 1. In order to examine the
effects of dabigatran on infarct size in AMI rabbits, the AMI model
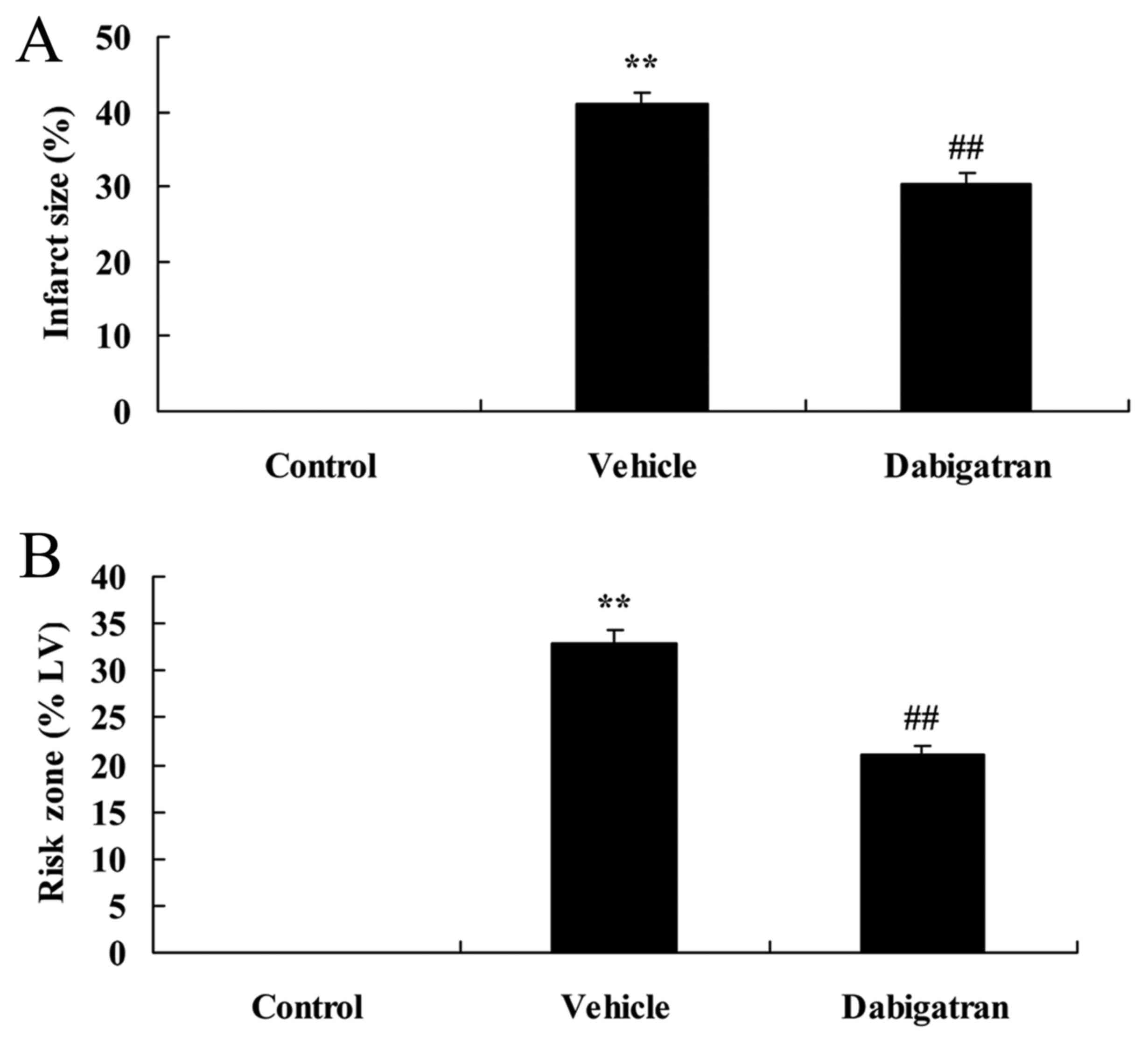
of rabbits was used. The results of Fig. 2 indicate that infarct size and risk
zone of infarct size in the AMI vehicle group were higher than that
of the control group. In addition, the effect of dabigatran
significantly inhibited the infarct size and risk zone of infarct
size in AMI rabbits, compared with AMI vehicle group (Fig. 2).
Effects of dabigatran on arterial
pressure in AMI rabbits
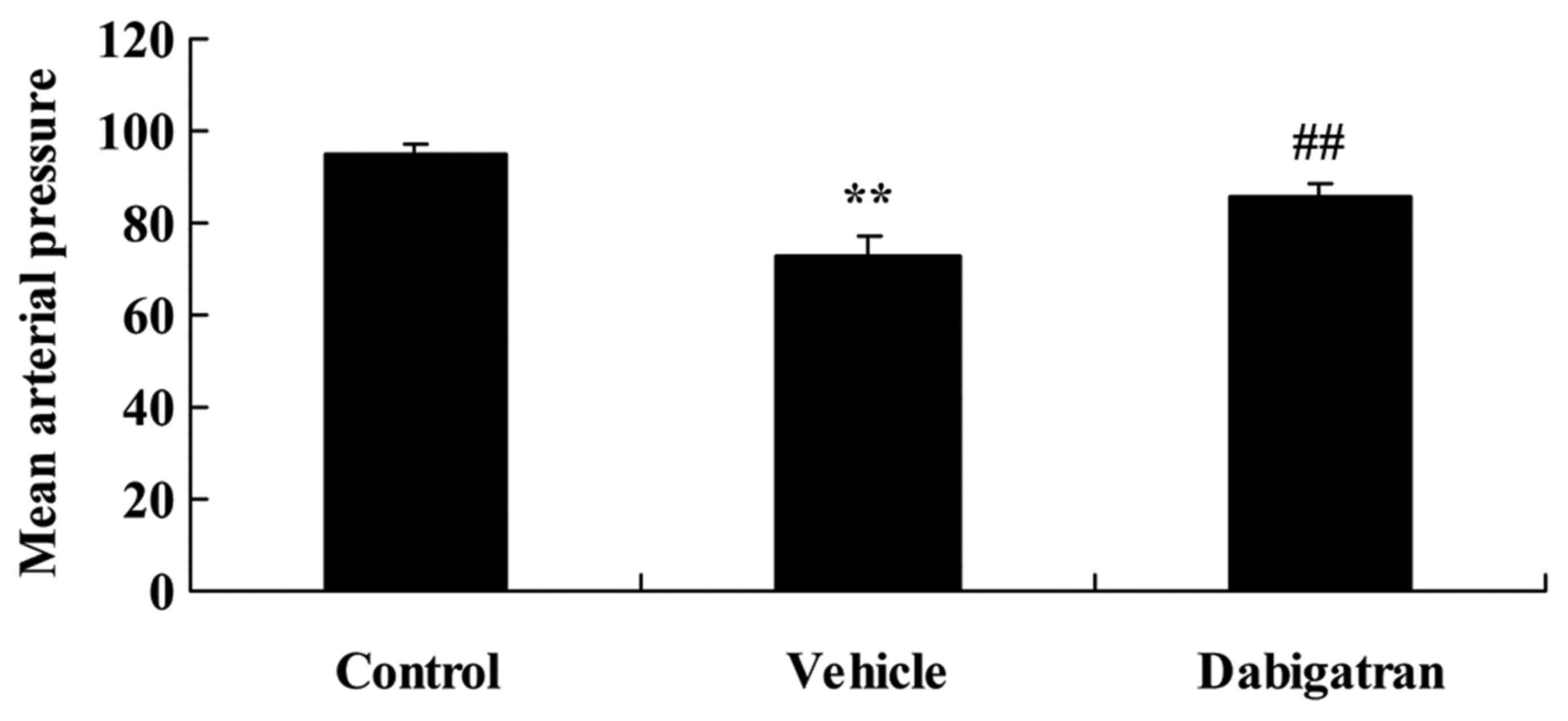
Subsequently, the effects of dabigatran on arterial
pressure in AMI rabbits were investigated. As presented in Fig. 3, AMI reduced arterial pressure in
AMI rabbits, compared with control rabbits. However, the effect of
dabigatran significantly increased arterial pressure in AMI
rabbits, compared with AMI vehicle rabbits (Fig. 3).
Effects of dabigatran on no-reflow
phenomenon in AMI rabbits
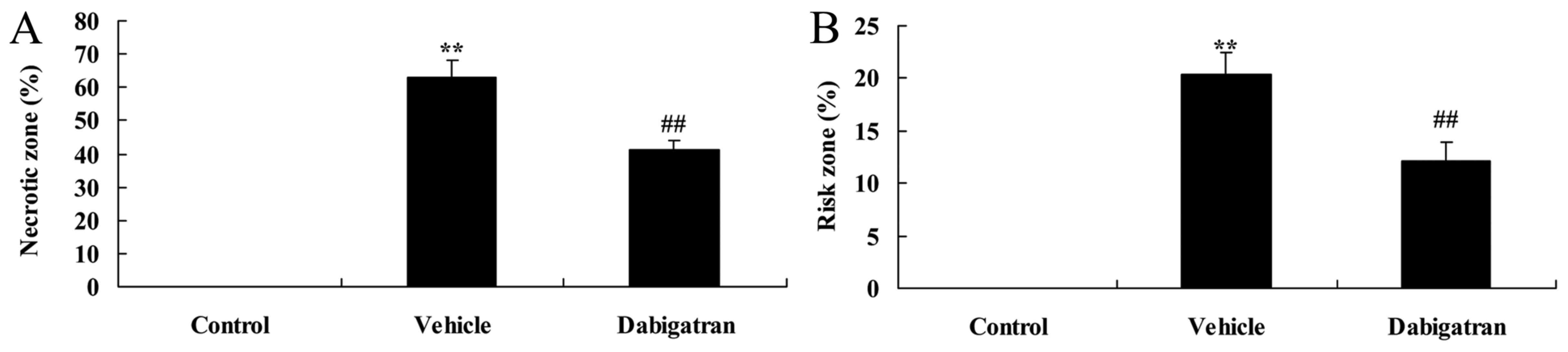
To examine the effects of dabigatran on arterial
pressure in AMI rabbits, no-reflow phenomenon was measured in the
current study. Fig. 4 indicates
that no-reflow phenomenon was higher in the vehicle group compared
with that of the control group. The effect of dabigatran
significantly inhibited the AMI-induced no-reflow phenomenon,
compared with AMI vehicle rabbits (Fig. 4).
Effects of dabigatran on inflammation
in AMI rabbits
The effects of dabigatran on arterial pressure in
AMI rabbits were established. There was a significant increase in
the activities of P65 of NF-κB, TNF-α, IL-1β and IL-6 in AMI
rabbits, compared with the normal control group (Fig. 5). Inhibition of the P65 of NF-κB,
TNF-α, IL-1β and IL-6 activities was observed in the dabigatran
group, compared with that of AMI rabbits (Fig. 5).
Effects of dabigatran on oxidative
stress in AMI rabbits
To investigate the effects of dabigatran on
oxidative stress in AMI rabbits, CAT and SOD activities were
detected using ELISA kits. Compared with normal control group, the
CAT and SOD activities were significantly inhibited in the AMI
rabbits group (Fig. 6). The effect
of dabigatran significantly enhanced the CAT and SOD activities in
the AMI rabbits, compared with AMI rabbits group (Fig. 6).
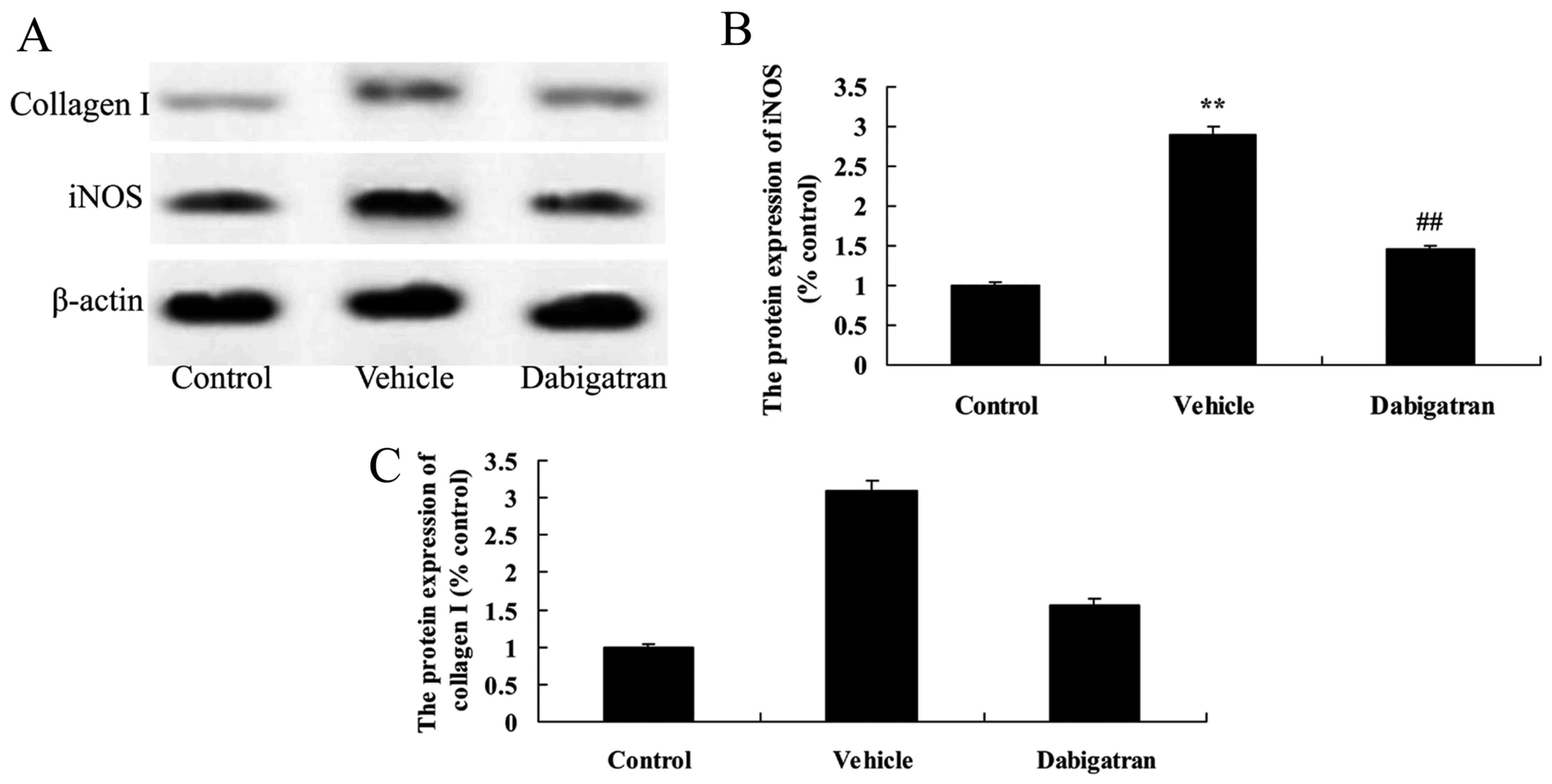
Effects of dabigatran on iNOS and
collagen I in AMI rabbits
To examine the effects of dabigatran on iNOS and
collagen I protein expression in AMI rabbits, we detected the
expression of iNOS and collagen I using western blotting. As
presented in Fig. 7, AMI
significantly activated the iNOS and collagen I protein expression
in rabbits, compared with the normal control group. However, the
effect of dabigatran significantly suppressed the iNOS and collagen
I protein expression in AMI rabbits, compared with AMI rabbits
group (Fig. 7).
Effects of dabigatran on TGF-β1, α-SMA
and CTGF in AMI rabbits
To further confirm the role of TGF-β1, α-SMA and
CTGF in the effects of dabigatran on AMI, TGF-β1 was measured using
western blotting. As presented in Fig.
8, there a significant increase in TGF-β1, α-SMA and CTGF
protein expression in AMI rabbits, compared with normal control
group. However, the effects of dabigatran significantly suppressed
the TGF-β1, α-SMA and CTGF protein expression in AMI rabbits,
compared with AMI rabbits group (Fig.
8).
Discussion
AMI is an acute event caused by atheromatous
thrombosis and is a major cause of angiocardiopathic mortality
(11). The most effective
treatment for myocardial ischemic injury is to recover the blood
supply as quickly as possible (11,12).
However, studies have identified that following a period of
ischemia, myocardial injuries worsen upon reperfusion; reversible
injuries of cardiomyocytes can become irreversible. Myocardial
ischemia/reperfusion injuries reduced cardiac function and
malignant arrhythmia (13). An AMI
rabbit model was used to verify that dabigatran significantly
inhibited the infarct size and increased arterial pressure in AMI
vehicle rabbits.
Lesion plaques of AMI patients easily rupture and
are unstable, resulting in acute and complete or incomplete
occlusive thrombus lesions of the coronary arteries (14). A previous study suggested that the
local inflammatory response could trigger atherosclerosis,
resulting in insatiability of plaques that are easy to rupture
(15). A literature review
indicated that following AMI, overexpression of IL may occur, which
suggested that inflammation additionally participates in the
occurrence of AMI (14). The
concentration of inflammatory cytokines in the serum is associated
with the severity of the disease. In the present study, dabigatran
significantly reduced the no-reflow phenomenon in AMI vehicle
rabbits.
Atherosclerosis is a pathogenesis basis of coronary
heart disease, while inflammation is an important feature of
atherosclerosis (16). With clear
biological effects, inflammatory factors are a type of endogenous
polypeptide produced by immunocytes (17). They can mediate various immune
responses and are closely associated with occurrence and
development of atherosclerosis (18). IL-6 is a type of inflammatory
cytokine with multiple functions, which is secreted by active
monocytes, macrophages, T lymphocytes, endothelial cells and
fibroblasts (19). IL-6 has been
reported to be the most powerful predictive factor of heart damage
and mortality for patients with AMI (20). TNF-α is a multi-functional protein
predominantly produced by active macrophage/monocyte system. Under
physiological states, myocardial cells do not generate TNF-α.
However, when myocardial infarction-induced pump failure occurs,
expression of TNF-α greatly increases, thus is a reliable indicator
for clinical prognosis of AMI (21). In the current study, it was
identified that dabigatran significantly inhibited P65 of NF-κB,
TNF-α, IL-1β and IL-6 activities and significantly enhanced CAT and
SOD activities in the AMI rabbits. De Boer et al (10) reported that the effect of
dabigatran inhibits allergic lung inflammation through suppression
of inflammation.
As a cellular messenger modulating functions of the
cardiovascular system, nervous system and immune system, NO is a
biological information transmitter (22). Numerous studies have observed that
NO possesses anti-platelet aggregation, inhibition of hyperplasia
of the vascular smooth muscle, interaction between endothelial
cells, regulation of angiostasis and mediation of immune and
cytotoxic effects (23). AMI may
serve an essential role in cardiovascular diseases, such as
hypertension (24). Previously, a
study identified that dabigatran significantly suppressed the iNOS,
collagen I, TGF-β1, α-SMA and CTGF protein expression in AMI
rabbits. Bogatkevich et al (25,26)
reported that dabigatran demonstrates antifibrotic effects on lung
fibroblasts through α-SMA and type I collagen expression and
dabigatran etexilate in a murine model of interstitial lung disease
through anti-inflammatory effects and TGF-β.
In conclusion, the effects of dabigatran on infarct
size, arterial pressure and no-reflow phenomenon, were observed to
include anti-inflammatory and anti-oxidative effects. The effects
of dabigatran may be mediated through downregulation of iNOS,
collagen I, TGF-β1, α-SMA and CTGF expression in AMI rabbits. There
is a requirement to conduct clinical trials to fully assess the
effects of dabigatran no-reflow phenomenon in AMI rabbits and
assess the risks involved in longterm therapy with this novel oral
anticoagulant agent.
References
|
1
|
Gili M, Ramirez G, Béjar L and López J: Is
cocaine-associated acute myocardial infarction the same as
myocardial infarction associated with recent cocaine consumption?
Response. Rev Esp Cardiol (Engl Ed). 67:965–966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kidawa M, Chizyński K, Zielińska M,
Kasprzak JD and Krzeminska-Pakula M: Real-time 3D echocardiography
and tissue Doppler echocardiography in the assessment of right
ventricle systolic function in patients with right ventricular
myocardial infarction. Eur Heart J Cardiovasc Imaging.
14:1002–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolte D, Khera S, Aronow WS, Mujib M,
Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH and
Fonarow GC: Trends in incidence, management, and outcomes of
cardiogenic shock complicating ST-elevation myocardial infarction
in the United States. J Am Heart Assoc. 3:e0005902014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sondergaard CS, Hess DA, Maxwell DJ,
Weinheimer C, Rosová I, Creer MH, Piwnica-Worms D, Kovacs A,
Pedersen L and Nolta JA: Human cord blood progenitors with high
aldehyde dehydrogenase activity improve vascular density in a model
of acute myocardial infarction. J Transl Med. 8:242010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malik MA, Khan Alam S, Safdar S and Taseer
IU: Chest Pain as a presenting complaint in patients with acute
myocardial infarction (AMI). Pak J Med Sci. 29:565–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dzeshka MS and Lip GY: Warfarin versus
dabigatran etexilate: An assessment of efficacy and safety in
patients with atrial fibrillation. Expert Opin Drug Saf. 14:45–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grottke O, van Ryn J, Spronk HM and
Rossaint R: Prothrombin complex concentrates and a specific
antidote to dabigatran are effective ex-vivo in reversing the
effects of dabigatran in an anticoagulation/liver trauma
experimental model. Crit Care. 18:R272014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanabria C, Cabrejos J, Olortegui A,
Guevara C and Lecca Garrido S: Costo-efectividad De apixaban con
otros noacs (Dabigatran Y Rivaroxaban) En El tratamiento De La
fibrilacion auricular no valvular (Fanv) En pacientes De La
seguridad social De Perú. Value Health. 18:A8292015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hajiri Mohd M, Shaharuddin S, Long CM,
Hashim R, Zulkifly HH, Kasim SS and Lim CW: Preliminary study of
safety and efficacy of warfarin versus dabigatran in atrial
fibrillation patients in a tertiary hospital in Malaysia. Value
Health. 18:A3782015. View Article : Google Scholar
|
|
10
|
de Boer JD, Berkhout LC, de Stoppelaar SF,
Yang J, Ottenhoff R, Meijers JC, Roelofs JJ, van't Veer C and van
der Poll T: Effect of the oral thrombin inhibitor dabigatran on
allergic lung inflammation induced by repeated house dust mite
administration in mice. Am J Physiol Lung Cell Mol Physiol.
309:L768–L775. 2015.PubMed/NCBI
|
|
11
|
Feistritzer HJ, Klug G, Reinstadler SJ,
Mair J, Seidner B, Mayr A, Franz WM and Metzler B: Aortic stiffness
is associated with elevated high-sensitivity cardiac troponin T
concentrations at a chronic stage after ST-segment elevation
myocardial infarction. J Hypertens. 33:1970–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lax A, Sanchez-Mas J, Asensio-Lopez MC,
Fernandez-Del Palacio MJ, Caballero L, Garrido IP, Pastor-Perez FJ,
Januzzi JL and Pascual-Figal DA: Mineralocorticoid receptor
antagonists modulate galectin-3 and interleukin-33/ST2 signaling in
left ventricular systolic dysfunction after acute myocardial
infarction. JACC Heart Fail. 3:50–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Studer M, Zuber M, Jamshidi P, Buser P and
Erne P: Thromboembolic acute myocardial infarction in a congenital
double chambered left ventricle. Indian Heart J. 63:289–290.
2011.PubMed/NCBI
|
|
14
|
Seropian IM, Cerliani JP, Toldo S, Van
Tassell BW, Ilarregui JM, González GE, Matoso M, Salloum FN,
Melchior R, Gelpi RJ, et al: Galectin-1 controls cardiac
inflammation and ventricular remodeling during acute myocardial
infarction. Am J Pathol. 182:29–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brener SJ, Cristea E, Kirtane AJ,
McEntegart MB, Xu K, Mehran R and Stone GW: Intra-procedural stent
thrombosis: A new risk factor for adverse outcomes in patients
undergoing percutaneous coronary intervention for acute coronary
syndromes. JACC Cardiovasc Interv. 6:36–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Exaire JE, Fathi RB, Brener SJ, Karha J,
Ellis SG and Bhatt DL: Impaired myocardial perfusion score and
inflammatory markers in patients undergoing primary angioplasty for
acute myocardial infarction. Arch Cardiol Mex. 76:376–382.
2006.PubMed/NCBI
|
|
17
|
Hiroi T, Wajima T, Negoro T, Ishii M,
Nakano Y, Kiuchi Y, Mori Y and Shimizu S: Neutrophil TRPM2 channels
are implicated in the exacerbation of myocardial
ischaemia/reperfusion injury. Cardiovasc Res. 97:271–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohseni M, Vafa M, Zarrati M, Shidfar F,
Hajimiresmail SJ and Forushani Rahimi A: Beneficial effects of
coenzyme Q10 supplementation on lipid profile and intereukin-6 and
intercellular adhesion molecule-1 reduction, preliminary results of
a double-blind trial in acute myocardial infarction. Int J Prev
Med. 6:732015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu W, Tang Y, Zhang Z, Zhang X, Yao Y, Fu
C, Wang X and Ma G: Inhibiting the mobilization of Ly6C(high)
monocytes after acute myocardial infarction enhances the efficiency
of mesenchymal stromal cell transplantation and curbs myocardial
remodeling. Am J Transl Res. 7:587–597. 2015.PubMed/NCBI
|
|
20
|
Kretzschmar D, Betge S, Windisch A,
Pistulli R, Rohm I, Fritzenwanger M, Jung C, Schubert K, Theis B,
Petersen I, et al: Recruitment of circulating dendritic cell
precursors into the infarcted myocardium and pro-inflammatory
response in acute myocardial infarction. Clin Sci (Lond).
123:387–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez AE, Fernandez M, Santaera O,
Larribau M, Bernardi V, Castaño H and Palacios LF: Coronary
stenting in patients undergoing percutaneous transluminal coronary
angioplasty during acute myocardial infarction. Am J Cardiol.
77:685–689. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aygül N, Aygül MU, Ozdemir K and
Altunkeser BB: Emergency revascularization procedures in patients
with acute ST-elevation myocardial infarction due to acute total
occlusion of unprotected left main coronary artery: A report of
five cases. Turk Kardiyol Dern Ars. 38:131–134. 2010.PubMed/NCBI
|
|
23
|
Ahmed RP, Haider KH, Shujia J, Afzal MR
and Ashraf M: Sonic Hedgehog gene delivery to the rodent heart
promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One.
5:e85762010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ribeiro DA, Buttros JB, Oshima CT,
Bergamaschi CT and Campos RR: Ascorbic acid prevents acute
myocardial infarction induced by isoproterenol in rats: Role of
inducible nitric oxide synthase production. J Mol Histol.
40:99–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bogatkevich GS, Ludwicka-Bradley A and
Silver RM: Dabigatran, a direct thrombin inhibitor, demonstrates
antifibrotic effects on lung fibroblasts. Arthritis Rheum.
60:3455–3464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bogatkevich GS, Ludwicka-Bradley A,
Nietert PJ, Akter T, van Ryn J and Silver RM: Antiinflammatory and
antifibrotic effects of the oral direct thrombin inhibitor
dabigatran etexilate in a murine model of interstitial lung
disease. Arthritis Rheum. 63:1416–1425. 2011. View Article : Google Scholar : PubMed/NCBI
|