Introduction
Colorectal cancer (CRC) is a common malignancy
around the world. CRC is the result of multistep processes in which
the sequential mutations of oncogenes or tumor suppressor genes and
chromosomal instability are considered as the main oncogenic
factors (1). According to the
difference of clinic pathological entities, CRC can be classified
as various histological subtypes, including sporadic, familial, and
hereditary forms. In the CRC cases, different CRC form exhibits
different proportion. In general, the sporadic form are most CRC
form. 10–30% of CRC cases correspond to the familial form, while
hereditary is the least (2). In
the treatment of CRC, early detection and surgical resection are
the primary approach. Although there are significant improvements
in the diagnostic method and surgical therapy, the cure rate of
patients with CRC is very poor (1,3).
Therefore, furthermore knowledge about the pathology of CRC
progression are urgently needed to improve the therapeutic
strategies for human CRC.
As endogenous regulators, microRNAs (miRNAs) is a
family of non-coding 19–22 nucleotides small single-stranded RNAs
molecules, and exert key roles in the expression of genes (4). It has been recognized that
dysregulation of miRNAs expression is found and proven to be
implicated in various human diseases, including glioma, metastatic
prostate carcinoma and other cancers (5,6). In
terms of CRC, aberrant regulation of miRNAs has been reported
widely in patients and is associated with the pathogenesis of human
CRC, miR-29a, miR-135b, miR-18b, miR-124, miR-222 and miR-31
(7–9). Members of miR-17-92 cluster has been
proven to be commonly dysregulated in various cancers and plays
role in the development and maintenance of tumor (10). In the present study, we determined
that miR-17-3P expression is aberrantly regulated in human CRC cell
model.
Prostate apoptosis responde-4 (Par4), also known as
PAWR, is a WT-1-interacting protein in variety of tissues, and is
required as a tumor suppressor (11). Par4 has a critical role in cell
apoptosis. Accumulating evidences suggest that lowly expressed Par4
is associated with the tumorigenesis of human cancers (12–14).
However, whether the dysfunction of PAR4 is involved in the
progression of CRC is still unexplored. Herein, we propose that
miR-17-3P contributes to the pathogenesis of human CRC by targeting
Par4. miR-17-3P expression was found to be abnormally expressed in
human CRC cells, and exerts regulatory roles in the survival of CRC
cells by directly targeting Par4. These results may provide a novel
finding about human CRC.
Materials and methods
Ethics statement
All patients gave the informed consent. This study
was approved by the Ethics Committee of Tianjin Medical
University.
CRC tissues and cells
Primary CRC biopsies and adjacent tissues were
collected from 40 patients undergoing surgery resection at Tianjin
Medical University. CRC cell lines SW480, LoVo and HCT-116 were
obtained from ATCC (Manassas, VA, USA). NCM460 normal colon
epithelial cells were obtained from Rongbai Biotechnology, Co.,
Ltd., (Shanghai, China). HCT-116 cells were maintained in McCoy's
5a medium, LoVo cells were maintained in F12-K medium and SW480
cells were cultured in DMEM medium containing 10% FBS at 37°C.
NCM460 cells were maintained in DMEM-H medium as the control.
Cell transfection
Using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA), 100 nM miR-17-3P mimic, inhibitor or negative control miRNA
(RiboBio Co., Ltd., Guangzhou, China) were transfected into
cultured cells in accord with manufacturer's instructions. The
plasmid of Par4 (kindly provided by Dr Sun) was also transfected
into cultured cells in absence or presence of miR-17-3P mimic.
After 48 h, the transfection efficacy was evaluated by RT-PCR and
western blotting.
Western blot analysis
All proteins were collected from cell lysates and
counted by bicinchoninic acid (BCA) assay. Then equal amount of
proteins were separated by SDS-PAGE, and transfered to PVDF
membrane. Primary antibodies were incubated with the blots (mouse
anti-Par4 1:2,000 and mouse anti-β-actin 1:2,000 dilution; Abcam,
Cambridge, MA, USA) overnight at 4°C temperature. The blots were
probed by specific secondary antibodies for 1 h at RT, then
performed by visualization with ECL.
Quantitative RT-PCR
Total RNA in tissues or cells were collected by
Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA). Expression of
miR-17-3P and Par4 were detected using microRNA First-Strand
Synthesis and miRNA Quantitation kits and CellAmp Direct RNA Prep
kit (Takara, Dalian, China), respectively. The reaction: 95°C for
10 min; 40 cycles of 95°C for 1 min, 63°C for 2 min, 72°C for 1
min; final 72°C for 10 min. Cq values of U6 and GAPDH expression in
tissues or cells were used as the internal control,
respectively.
Cell apoptosis
Cell apoptosis assay was evaluated by annexin
V-fluorescein isothiocyanate (FITC) assay. After 48 h of
transfection, transfected cells (5×106) were seeded in
the DMEM medium (serum-free) for another 12 h, followed by
harvesting using ice-cold PBS and re-suspending by binding buffer.
Cells were then treated with 0.6 µg/ml annexin V-FITC and 0.5 µg/ml
PI for 15 min in dark, and the apoptosis rate of cells were
analyzed by the FACS Calibur™ system (Becton-Dickinson, San Jose,
CA, USA).
Cell proliferation assay
After 48 h, transfected cell proliferation was
tested by CCK-8/WST-8 (Bioroot, Shanghai, China) in accordance with
manufacturer's instructions. Briefly, after 48 h, transfected cells
were planted in the 96-well plates (6×104 cells/ml). 10 µl CCK-8
solution was then added into the cells at 12, 24, 48 and 72 h.
Cells were maintained for 4 h and measured at 450 nm.
Luciferase reporter assay
The assay was performed as described previously
(15). In brief, 3′UTR of Par4
mRNA was cloned into pGL3 vector (Promega, Madison, WI, USA).
QuikChange Lightning Site-Directed Mutagenesis kit (Stratagene) was
applied to introduce the site-directed mutagenesis into predicted
miR-17-3P binding site on mRNA of Par4. The recombinant vectors
were then transfected into cultured CRC cells in the absence or
presence of miR-17-3P mimic for 36 h. And the luciferase activity
was then tested by Dual Luciferase Assay (Promega).
Statistical analysis
Data were expressed as mean ± SEM. SPSS15.0 was used
to analyze the differences between groups by one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-17-3P is up-regulated in human CRC
tissues and cells
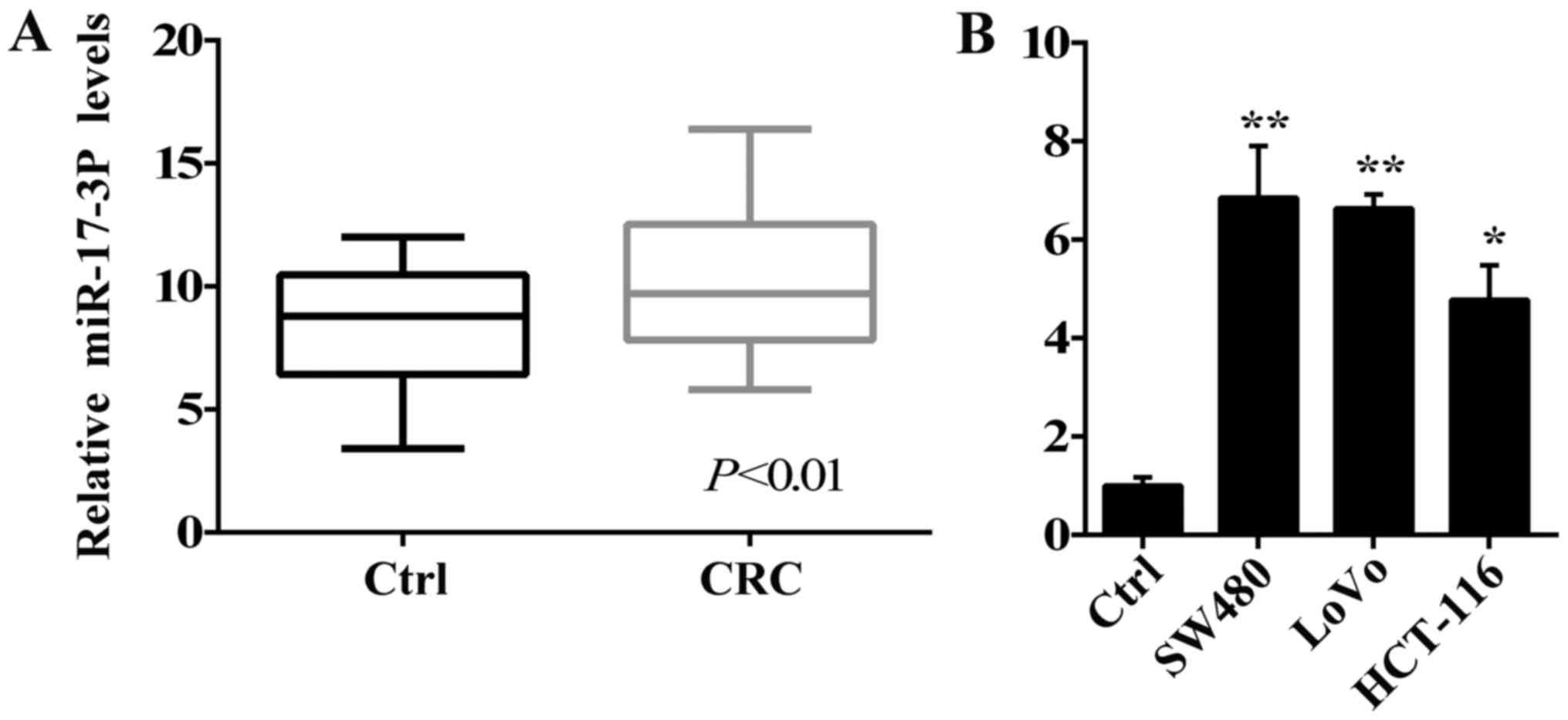
We firstly identified miR-17-3P expression in tumor
tissues of patients with CRC to determine the potential role of
miR-17-3P in human CRC. As seen in Fig. 1A, miR-17-3P was significantly
increased in tumor tissues of patients with CRC, while it was
maintained at relatively low expression levels in adjacent colonic
tissues. Further, we investigated the levels of miR-17-3P
expression in human CRC SW480, HCT-116 and LoVo cells. As seen in
Fig. 1B, miR-17-3P expression were
aberrantly up-regulated in the SW480, HCT-116 and LoVo cells
compared with the colon epithelial cells NCM460. Taken together,
miR-17-3P expression may have a positive effect on the
tumorigenesis of human CRC.
miR-17-3P promotes the survival of
human CRC cells
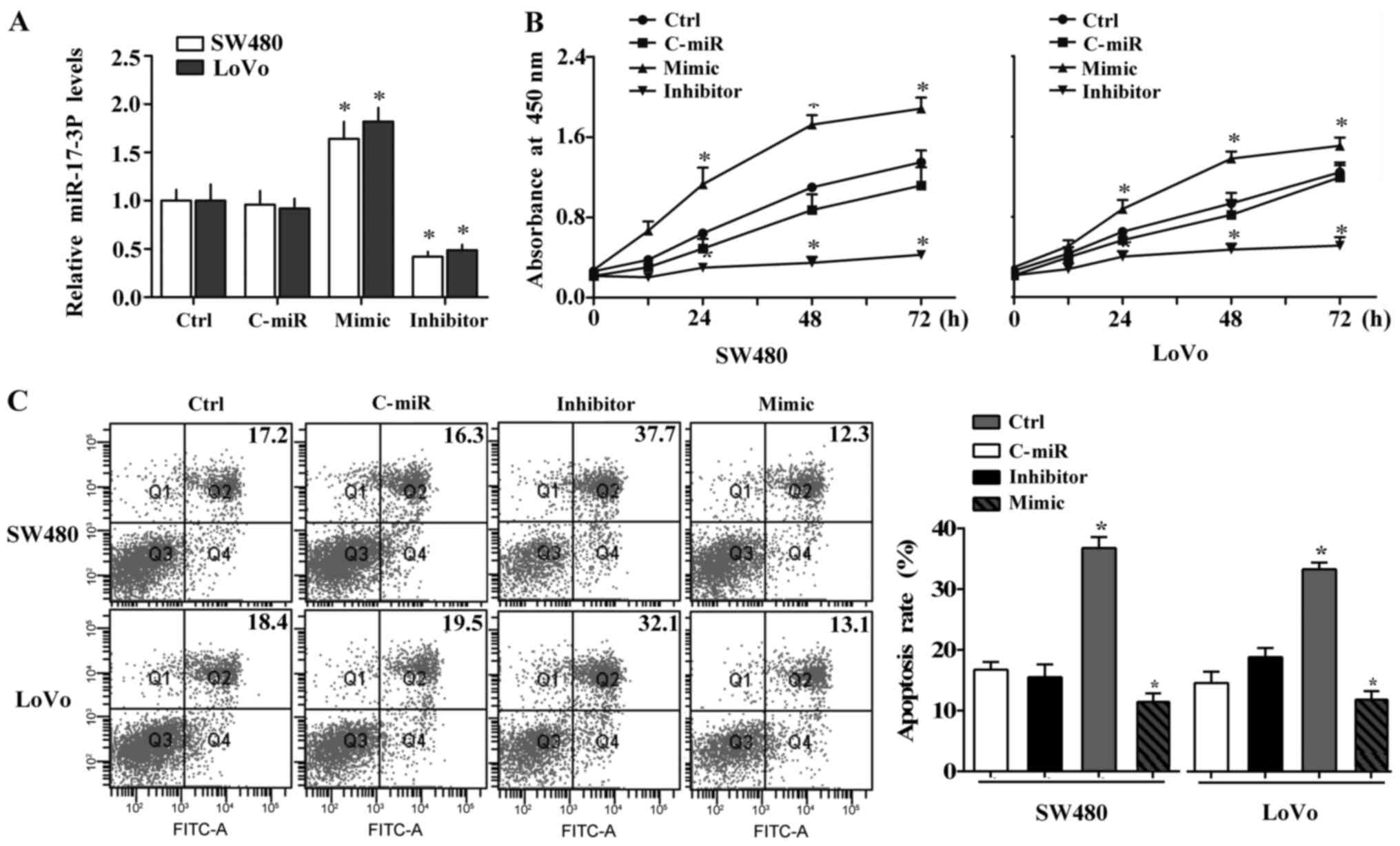
We then employed the specific mimic and inhibitor of
miR-17-3P to further identify the possible effect of high miR-17-3P
levels on human CRC cells. According to the difference in
expression of miR-17-3P, SW480 and LoVo cells were used as cell
models in the subsequent experiments. Efficiency of the mimic and
inhibitor were confirmed using RT-PCR assay. The specific mimic
significantly increased, while the inhibitor inhibited, miR-17-3P
expression in both SW480 and LoVo cells compared to the negative
control miRNA (Fig. 2A).
In the CCK-8 assay, the proliferation of CRC cells
were examined. As shown in Fig.
2B, the proliferation were remarkably increased by miR-17-3P
mimic in SW480 and LoVo cells compared to the negative control,
while miR-17-3P silencing significantly suppressed the
proliferation capacity of these two CRC cells. Furthermore, we
investigated the apoptosis levels of CRC cells. miR-17-3P
inhibition increased but miR-17-3P overexpression inhibited the
apoptosis of SW480 and LoVo cells compared to control (Fig. 2C). These data imply that miR-17-3P
may contribute to the human CRC progression by regulating the
survival of CRC cells.
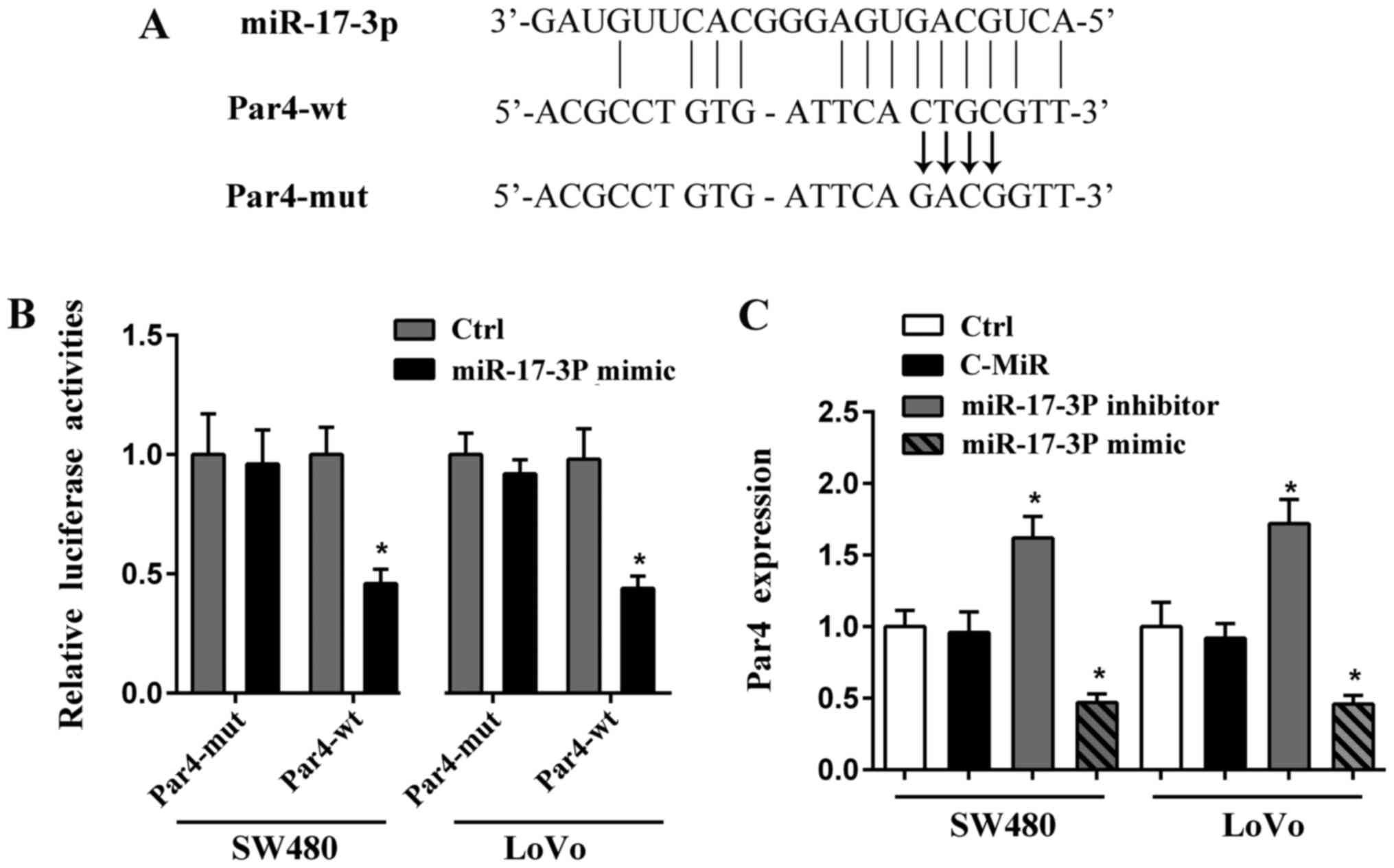
Par4 is the target of miR-17-3P
Par4 is a tumor suppressor in various cancers,
including human CRC (11). We
predicated the potential targets of miR-17-3P using miRDB and
TargetScan databases, and found that there is possible binding site
of miR-17-3P on 3′-UTR of Par4 (Fig.
3A). To identify the prediction and elucidate the underlying
mechanism by which miR-17-3P regulates CRC cells survival, we
conducted the luciferase reporter assay in SW480 and LoVo cells. As
seen in Fig. 3A, the wild or
mutant 3′-UTR of Par4 were cloned into luciferase vector,
respectively. Luciferase activities of wild Par4-3′UTR were
strongly reduced by miR-17-3P mimic, but there was no difference in
the luciferase activities of mutant Par4-3′UTR in CRC cells
(Fig. 3B).
Furthermore, we determined that the expression of
Par4 in CRC cells was up-regulated by miR-17-3P suppression and
reduced by miR-17-3P mimic (Fig.
3C). In summary, these data imply that Par4 is direct target of
miR-17-3P in CRC cells.
Par4 is involved in the
miR-17-3P-mediated regulation of CRC cells survival
Previous studies reported that Par4 is a
pro-apoptotic factor and is required for cell apoptosis (16). To identify whether Par4 is involved
in the miR-17-3P-mediated regulation of CRC cells survival, we
further employed a Par4 plasmid to specifically induce the
expression of Par4 in both SW480 and LoVo cells. As seen in
Fig. 4A, efficiency of Par4
plasmid was confirmed by RT-PCR assay. Par4 expression was strongly
enhanced by the specific recombinant plasmid in both CRC cells.
And Par4 expression reduced the proliferation of CRC
cells induced by miR-17-3P, as shown in Fig. 4B. On the contrary, miR-17-3P
inhibited apoptosis of SW480 and LoVo cells was reversed by Par4
compared to the control (Fig. 4C).
These findings indicate that miR-17-3P regulated CRC cells survival
by targeting Par4.
Discussion
Endogenous miRNAs have crucial roles as key
regulators of gene expression in the physiological or pathological
processes of human development and diseases (17). Differential expression of miRNAs
have been reported in previous studies about CRC, and the
dysregulation of miRNAs has been considered to play positive or
negative roles in colorectal carcinogenesis, including miR-29a,
miR-135b, miR-18b, miR-124, miR-222 and miR-31 (18–20).
miR-17-3P is derived from the miR-17 miRNA cluster,
and has different roles in the physiological processes of many
human diseases, such as development and remodeling of heart and
lung, spinal neural progenitor patterning and cardiac fibroblast
senescence (16,21). Dysfunction of miR-17-3P also exerts
a tumor oncogenic role in tumorigenesis. Shan et al
(22) reported that the passenger
strand miR-17-3p was largely expressed in transgenic mice and could
induce the development of hepatocellular carcinoma by targeting
PTEN and GalNT7 via various signal pathways. In another study, the
increase in miR-17-3p expression was found to be associated with
the stress response in glioblastoma cells. miR-17-3p could inhibit
cell proliferation and drug-resistance in glioblastoma cells by
repressing MDM2 levels (23). In
human CRC, miR-17-3p expression was also identified to be
associated with the tumorigenesis of CRC, and is involved in
diagnosis as well as prognosis of CRC (24,25).
Herein, we demonstrated a novel role of miR-17-3p in CRC. miR-17-3p
expression is significantly up-regulated in tumor tissues of
patients with CRC and cultured cells. The dysregulation of
miR-17-3p is associated with the regulation of survival of CRC
cells by promoting cell proliferation and inhibiting apoptosis,
contributing to the human CRC tumorigenesis.
Par4 is encoded by the Pawr gene and also
known as PAWR. In various tissues, Par4 could interact with WT-1
protein and has an important role in cell apoptosis (26). As a tumor suppressor, Par4 is has
been identified to be expressed at low levels in multiply cancers,
and is correlated with the tumorigenesis of cancers (27). By online bioinformatic prediction
and luciferase reporter assay, we confirmed that there is a binding
site of miR-17-3P in Par4 3′-UTR, and Par4 is a direct target of
miR-17-3p in human CRC cells. The expression level of Par4 was
significantly repressed by miR-17-3p in human CRC cells, suggesting
the involvement of miR-17-3p and Par4 in human CRC tumorigenesis.
Our result is consistent with a previous report, in which miR-17-3p
inhibits cardiac aging and cardiac fibroblast cellular senescence
as a negative modulator by targeting Par4 and regulating the
downstream proteins, including FAK, CEBPB, vimentin, N-cadherin,
Oct4 and Sca-1 (16).
In previous studies, Par4 has been reported to be a
crucial regulator of tumor cell survival. Induced Par4 expression
could enhance the death in some cancer cells, and the
down-regulation of Par4 expression might be a prognostic factor in
cancer (28). Herein, we also
found the involvement of miR-17-3p and Par4 in human CRC cells
survival. Par4 expression reduced the proliferation of CRC cells
induced by miR-17-3P. On the contrary, miR-17-3P inhibited
apoptosis of SW480 and LoVo cells was reversed by Par4 compared to
the control. These findings indicate that miR-17-3P regulated CRC
cells survival by targeting Par4.
In conclusion, we determined that increased
miR-17-3P level plays crucial role in CRC cells survival by
targeting Par4, contributing to colorectal carcinogenesis. This may
indicate a novel finding about human CRC progression.
References
|
1
|
Haigis KM: Molecular pathogenesis of
colorectal cancer. Anticancer Res. 11:609–633. 2014.
|
|
2
|
Fearon ER: Molecular genetics of
colorectal cancer. Ann Rev Pathol Mechanisms Dis. 6:479–507. 2011.
View Article : Google Scholar
|
|
3
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Tang H, Wang Z, Zhang B, Liu W,
Lu H, Xiao L, Liu X, Wang R, Li X, et al: MiR-185 targets the DNA
methyltransferases 1 and regulates global DNA methylation in human
glioma. Mol Cancer. 10:1242011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. MicroRNA Cancer Regulation Springer.
1–20. 2013. View Article : Google Scholar
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo X, Burwinkel B, Tao S and Brenner H:
MicroRNA signatures: Novel biomarker for colorectal cancer? Cancer
Epidemiol Biomarkers Prev. 20:1272–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koga Y, Yasunaga M, Takahashi A, Kuroda J,
Moriya Y, Akasu T, Fujita S, Yamamoto S, Baba H and Matsumura Y:
MicroRNA expression profiling of exfoliated colonocytes isolated
from feces for colorectal cancer screening. Cancer Prev Res
(Phila). 3:1435–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bandres E, Agirre X, Bitarte N, Ramirez N,
Zarate R, Roman-Gomez J, Prosper F and Garcia-Foncillas J:
Epigenetic regulation of microRNA expression in colorectal cancer.
Int J Cancer. 125:2737–2743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olive V, Jiang I and He L: mir-17-92, a
cluster of miRNAs in the midst of the cancer network. Int J Biochem
Cell Biol. 42:1348–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hebbar N, Shrestha-Bhattarai T and
Rangnekar VM: Cancer-selective apoptosis by tumor suppressor par-4.
Adv Exp Med Biol. 818:155–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pereira MC, de Bessa-Garcia SA, Burikhanov
R, Pavanelli AC, Antunes L, Rangnekar VM and Nagai MA: Prostate
apoptosis response-4 is involved in the apoptosis response to
docetaxel in MCF-7 breast cancer cells. Int J Oncol. 43:531–538.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Da Bessa-Garcia SA, Pereira M and Nagai
MA: Regulation of PAWR expression by estrogen and growth factors in
breast cancer cells. Int J Mol Med. 24:2009.
|
|
14
|
Yang K, Shen J, Chen SW, Qin J, Zheng XY
and Xie LP: Upregulation of PAWR by small activating RNAs induces
cell apoptosis in human prostate cancer cells. Oncol Rep.
35:2487–2493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zheng D, Xiong Y, Xue C, Chen G,
Yan B and Ye Q: miR-202 suppresses cell proliferation in human
hepatocellular carcinoma by downregulating LRP6
post-transcriptionally. FEBS Lett. 588:1913–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du WW, Li X, Li T, Li H, Khorshidi A, Liu
F and Yang BB: The microRNA miR-17-3p inhibits mouse cardiac
fibroblast senescence by targeting Par4. J Cell Sci. 128:293–304.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi Y, Formentini A, Chien M, Weir DB,
Russo JJ and Ju J, Kornmann M and Ju J: Prognostic values of
microRNAs in colorectal cancer. Biomarker Insights. 2:113–121.
2006.PubMed/NCBI
|
|
19
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
20
|
Lanza G, Ferracin M, Gafà R, Veronese A,
Spizzo R, Pichiorri F, Liu CG, Calin GA, Croce CM and Negrini M:
mRNA/microRNA gene expression profile in microsatellite unstable
colorectal cancer. Mol Cancer. 6:542007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen JA, Huang YP, Mazzoni E, Tan GC,
Zavadil J and Wichterle H: Mir-17-3p controls spinal neural
progenitor patterning by regulating Olig2/Irx3 cross-repressive
loop. Neuron. 69:721–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shan SW, Fang L, Shatseva T, Rutnam ZJ,
Yang X, Du W, Lu WY, Xuan JW, Deng Z and Yang BB: Mature miR-17-5p
and passenger miR-17-3p induce hepatocellular carcinoma by
targeting PTEN, GalNT7 and vimentin in different signal pathways. J
Cell Sci. 126:1517–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H and Yang BB: Stress response of
glioblastoma cells mediated by miR-17-5p targeting PTEN and the
passenger strand miR-17-3p targeting MDM2. Oncotarget. 3:1653–1668.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faltejskova P, Bocanek O, Sachlova M,
Svoboda M, Kiss I, Vyzula R and Slaby O: Circulating miR-17-3p,
miR-29a, miR-92a and miR-135b in serum: Evidence against their
usage as biomarkers in colorectal cancer. Cancer Biomark.
12:199–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Y and Emergency DO: Application of
miRNA-17-3p combined with CEA in the diagnosis and treatment of
colorectal cancer. J Clin Med Practice. 2014.
|
|
26
|
Chen X, Sahasrabuddhe AA, Szankasi P,
Chung F, Basrur V, Rangnekar VM, Pagano M, Lim MS and
Elenitoba-Johnson KS: Fbxo45-mediated degradation of the
tumor-suppressor Par-4 regulates cancer cell survival. Cell Death
Differ. 21:1535–1545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goswami A, Burikhanov R, de Thonel A,
Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T and Rangnekar
VM: Binding and phosphorylation of par-4 by Akt is essential for
cancer cell survival. Mol Cell. 20:33–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ranganathan P and Rangnekar VM: Regulation
of cancer cell survival by par-4. Ann N Y Acad Sci. 1059:76–85.
2005. View Article : Google Scholar : PubMed/NCBI
|