Introduction
Marine sponge (Porifera) is the oldest animal phylum
in existence, and is also the simplest of animals with the most
primitive multicellular structures and partially differentiated
tissues. Sponge is a remarkable component of the marine benthos
throughout the tropical and polar habitats, and lives in areas with
strong currents or wave action (1). Sponges account for one fifteenth of
all marine species on the Earth, and the species found in China
accounts for approximately half of these.
The marine sponge surface forms a number of
streamline textures and may avoid destruction by waves and currents
and are able to defend against environmental factors such as
hunting, overgrowth by fouling the shells of abalone and oyster or
conquering spaces (2). Due to the
splinter-like spicules and toxic chemicals produced by the sponge,
the majority of carnivorous animals elude them (3). The disparate structural framework
makes the marine sponge source rich and display a battery of cogent
biological activities that are of potential interest to humans
(4).
Novel constituents have been identified from
Dysidea fragilis, such as azacyclopropene derivatives
(5), diketopiperazines (6,7),
polyhydroxylated sterols (8),
sesquiterpenoid (9) and brominated
diphenyl ethers (10). The
diversity of the chemical structures implies the potential of
various bioactivities, such as antibacterial, anti-inflammatory,
antiviral and anti-cardiovascular activity. Previous studies on
marine sponges of Dysidea genus from the Xisha Islands in
the South China Sea identified seven novel sesquiterpene quinones
(dysidavarones A-D and dysideanones A-C) (11,12)
from Dysidea avara, and 13 novel sesquiterpene aminoquinones
(dysidaminones A-M) (13) and
three other novel sesquiterpene aminoquinones based on a rearranged
avarone skeleton (dysifragilones A-C) (14) from Dysidea fragilis. These
newly isolated agents have been assessed for their inhibitory
activities on lipopolysaccharide (LPS)-stimulated production of
nitric oxide (NO) in RAW264.7 cells, and the results have
demonstrated that dysifragilone A exhibits potent inhibitory
activity. In the present research, the anti-inflammatory activities
in vitro and the regulatory effect on the inflammatory
signal transduction pathway of dysifragilone A were further
investigated.
Materials and methods
Animal material
Samples of Dysidea fragilis were collected
along the coast of Yongxing Island in the South China Sea on 11th
April 2011, and the extraction, isolation and identification of
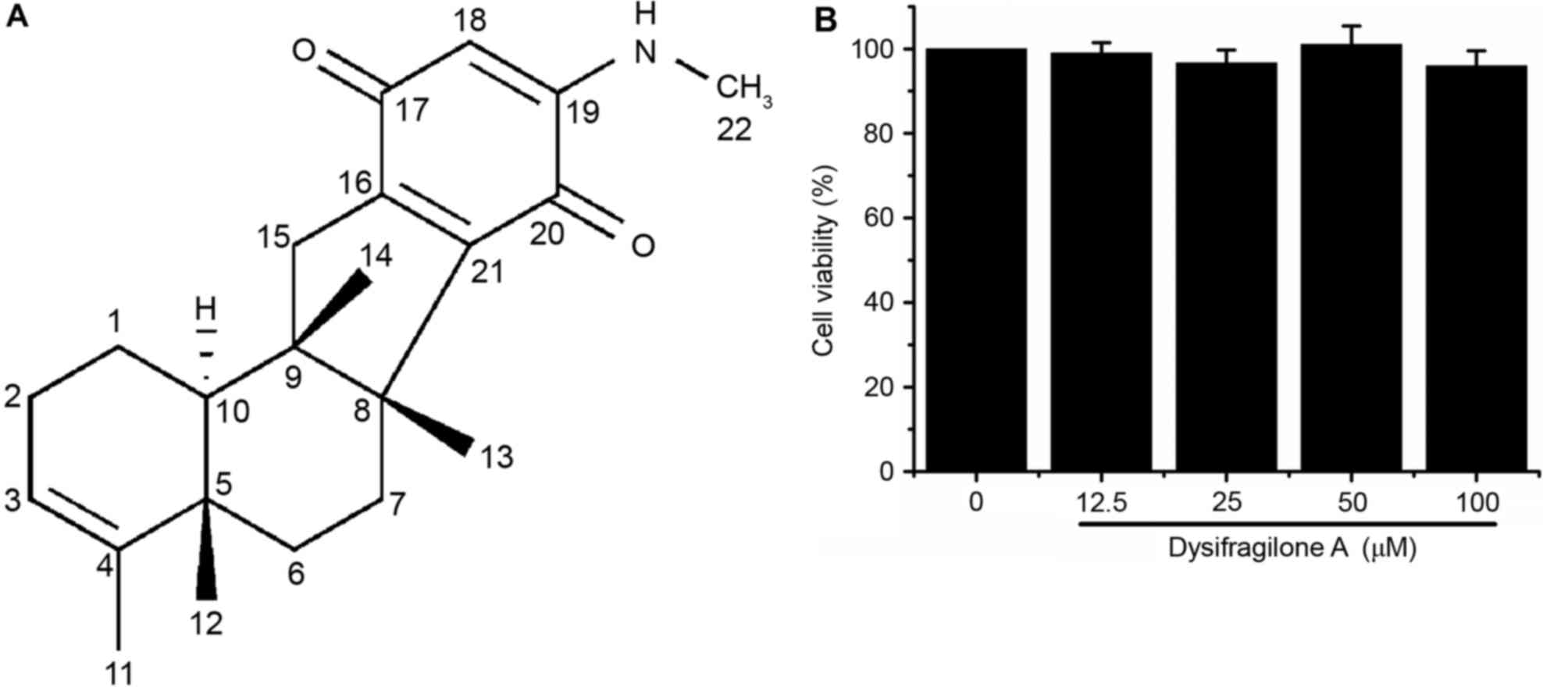
dysifragilone A (15.9 mg; Fig. 1A)
were performed by Shanghai Jiao Tong University (Shanghai, China)
(14). Subsequently, the purity
was determined by standardization of the peak area by high
performance liquid chromatography, which was found to be 98.7% (via
a UV detector). The data of 1H-nuclear magnetic
resonance (NMR) and 13C-NMR of dysifragilone A are
displayed in Table I.
 | Table I.1H-NMR and
13C-NMR spectroscopic data of dysifragilone A
(CDCl3, δ). |
Table I.
1H-NMR and
13C-NMR spectroscopic data of dysifragilone A
(CDCl3, δ).
| Position | δH | δC | Position | δH | δC |
|---|
| 1 | 1.55 (m) |
21.1 | 11 | 1.52 (s, br) |
17.9 |
| 1 | 1.56 (m) |
| 12 | 1.01 (s) |
18.9 |
| 2 | 1.92 (m) |
27.2 | 13 | 1.04 (s) |
23.4 |
| 2 | 2.05 (m) |
| 14 | 1.03 (s) |
16.3 |
| 3 | 5.15 (s, br) | 120.9 | 15 | 2.82 (d,
J=18.6 Hz) |
42.2 |
| 4 |
| 143.5 | 15 | 2.25 (d,
J=18.6 Hz) |
|
| 5 |
|
37.6 | 16 |
| 153.7 |
| 6 | 0.96 (td,
J=13.8 3.0 Hz) |
33.7 | 17 |
| 184.9 |
| 6 | 1.64 (dt,
J=12.6 3.0 Hz) |
| 18 | 5.29 (s) |
96.9 |
| 7 | 1.50 (m) |
26.6 | 19 |
| 149.0 |
| 7 | 2.56 (dt,
J=15.0 2.4 Hz) |
| 20 |
| 181.8 |
| 8 |
|
52.6 | 21 |
| 147.0 |
| 9 |
|
47.5 | 22 | 2.83 (d,
J=5.4 Hz) |
29.3 |
| 10 | 1.28 (m) |
46.2 | NH | 5.68 (s, br) |
|
Chemicals and reagents
E. coli LPS and MTT were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RPMI-1640 medium
and fetal bovine serum (FBS) were obtained from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Hydrocortisone
succinate (catalog no. 080-05581; lot CTE6574) was purchased from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The NO
concentration determination kit, mouse tumor necrosis factor-α
(TNF-α) ELISA kit (catalog no. SEM024), mouse interleukin-6 (IL-6)
ELISA kit (catalog no. SEM008) and the bicinchoninic acid protein
assay kit were obtained from Yantai Science and Biotechnology Co.,
Ltd. (Shandong, China). The mouse prostaglandin E2
(PGE2) ELISA kit (catalog no. KGE004B) was obtained from
R&D Systems, Inc. (USA). The NO synthase assay kit
(fluorimetric method) was purchased from Beyotime Biotechnology
(Haimen, China). The cyclooxygenase (COX) colorimetric inhibitor
screening assay kit (catalog no. 701050) was purchased from Cayman
Chemical Company (Ann Arbor, MI, USA). The mouse anti-rabbit
inducible nitric oxide synthase (iNOS) polyclonal antibody (catalog
no. 160862) and mouse anti-rabbit COX-2 polyclonal antibody
(catalog no. 160106) were purchased from Cayman Chemical Company.
Goat anti-rabbit phosphorylated (p)-extracellular signal-regulated
kinase (ERK) 1/2 polyclonal antibody (catalog no. AF1015), goat
anti-rabbit p-c-Jun N-terminal kinase (JNK) polyclonal antibody
(catalog no. AF3318), goat anti-rabbit p-p38 polyclonal antibody
(catalog no. AF3455), and horse radish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (H+L; catalog no. S0001) were products of
Affinity Biosciences (Cincinnati, OH, USA). Goat anti-rabbit
β-actin polyclonal antibody (catalog no. sc-1616) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Dysifragilone A was dissolved in cell culture grade dimethyl
sulfoxide (DMSO) (purity >99.9%) at 50 mM and stored at −20°C,
and then diluted to the concentration required.
Cell culture of RAW264.7 cells
RAW264.7 mouse monocyte-macrophage cells (TIB-71;
American Type Culture Collection, Manassas, VA, USA), were cultured
in RPMI-1640 medium containing 10% heat-inactivated FBS at 37°C in
a cell incubator with 5% CO2. The media was routinely
replaced every 2 days. RAW264.7 cells were passaged until they
achieved 80% of the petri dish area.
MTT assay for cytotoxicity
MTT assay was used to detect cell viability and
cytotoxicity. Succinate dehydrogenase of mitochondria in living
cells reduced the exogenous MTT reagent to formazan, which is
deposited in the cells, and the number of living cells is
proportional to the formazan crystals (15). RAW264.7 cells were seeded in a
96-well plate at a density of 1×106 cells/ml. After 1 h
incubation, the cells were treated with dysifragilone A at final
concentrations of 12.5, 25, 50, 100 µM and the control group
received an equal amount of 0.2% DMSO in the culture medium. After
24 h incubation, MTT solution (5 mg/ml) was then added to the
96-well plate, and the cells were incubated for another 4 h at 37°C
in cell incubator. After removal of the cell supernatant, 150 µl
DMSO was added to dissolve the formazan. The absorbance was
measured at 570 nm (reference, 630 nm) by using a microplate
reader. The untreated cells were regarded as having 100% viability.
Results are expressed as a percentage of viable cells compared with
the control group.
Determination of nitrite
concentration
RAW264.7 cells were seeded in a 96-well plate at the
density of 1×106 cells/ml. After 1 h incubation, the
cells were treated with LPS (1 µg/ml), various concentrations
(12.5, 25, 50 and 100 µM) of dysifragilone A with LPS (1 µg/ml),
hydrocortisone succinate (100 µM) with LPS (1 µg/ml) for 24 h. A
total of 100 µl cell culture supernatant was removed to detect the
NO concentration. The cell culture supernatant was added to 100 µl
Griess reagent (an equal mixture of reagent A and reagent B) and
then incubated for 10 min at room temperature. The absorbance at
540 nm was measured by using a microplate reader (16), and the nitrite concentrations were
calculated by interpolation of a standard curve.
Determination of PGE2
concentration
PGE2, a pro-inflammatory mediator, is
produced by COX-2. RAW264.7 cells were seeded in a 96-well plate at
a density of 5×105 cells/ml. After 1 h incubation, the
cells were treated with LPS (1 µg/ml), treated with various
concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A with
LPS (1 µg/ml) and treated with hydrocortisone succinate (100 µM)
with LPS (1 µg/ml) for 24 h at 37°C in a humidified atmosphere
containing 5% CO2. A total of 100 µl of the cell culture
supernatant was removed to detect the levels of PGE2 by
using a commercial mouse PGE2 ELISA kit in accordance
with the manufacturer's protocol. The ELISA data represent the mean
± standard deviation. Samples were tested in duplicate in more than
three independent experiments (17).
Determination of TNF-α and IL-6
RAW264.7 cells were seeded in a 96-well plate at a
density of 5×105 cells/ml. After 1 h incubation, the
cells were treated with LPS (1 µg/ml), treated with various
concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A with
LPS (1 µg/ml) and treated with hydrocortisone succinate (100 µM)
with LPS (1 µg/ml) for 6 h at 37°C in a humidified atmosphere
containing 5% CO2. A total of 100 µl of the cell culture
supernatant was taken out to detect the levels of TNF-α or IL-6 by
using the respective ELISA kit in accordance with the
manufacturer's protocol (18). The
ELISA data represent the mean ± standard deviation. Samples were
tested in duplicate in more than three independent experiments.
iNOS enzymatic activity
determination
The determination of iNOS enzymatic activity has
been previously reported (19).
Briefly, RAW264.7 cells at a density of 5×105 cells/ml
were treated with LPS (1 µg/ml), treated with various concentration
(12.5, 25, 50 and 100 µM) of dysifragilone A with LPS (1 µg/ml) and
treated with hydrocortisone succinate (100 µM) with LPS (1 µg/ml)
for 24 h at 37°C in a humidified atmosphere containing 5%
CO2, and the cell supernatant was removed from the
96-well plate. After adding 100 µl iNOS assay buffer (2X NOS assay
buffer was mixed with an equal volume of Milli-Q water), 100 µl of
NOS assay reaction solution (50% NOS assay buffer, 39.8% Milli-Q
water, 5% L-arginine solution, 5% 0.1 mM nicotinamide adenine
dinucleotide phosphate, 0.2%
4′-amino-5-methylamino-2′,7′-difluorofluorescein diacetate) was
added and incubated for 2 h at 37°C in an incubator. The
fluorescence was measured at excitation wavelength of 495 nm and
emission wavelength of 515 nm by using a fluorescence microplate
reader.
COX-2 enzymatic activity
determination
The enzymatic activity of COX-2 was assayed by using
a COX colorimetric inhibitor screening assay kit in a cell-free
system in accordance with the manufacturer's protocol (19). Briefly, 160 µl assay buffer, 10 µl
heme and 10 µl DMSO were added to three background wells. 150 µl
assay buffer, 10 µl heme, 10 µl COX-2 enzyme and 10 µl DMSO were
added to three 100% initial activity wells. 150 µl assay buffer, 10
µl heme, 10 µl COX-2 enzyme and 10 µl dysifragilone A (final
concentration 1 mM) were added to three inhibitor wells. After
carefully shaking the plate for a few sec, the plate was incubated
for 5 min at 25°C. After adding 20 µl colorimetric substrate
solution to all wells, 20 µl arachidonic acid was quickly added to
all wells. The plate was carefully shaken for a few sec and
incubated for 2 min at 25°C. The absorbance was measured at 590 nm
using a microplate reader, and the suppression ratio of COX-2
enzymatic activity was calculated in accordance with the
manufacturer's protocol.
Western blot analysis
RAW264.7 cells were treated with various
concentration (12.5, 25, 50 and 100 µM) of dysifragilone A with or
without LPS (1 µg/ml), and then washed with cold PBS (1X) and lysed
in ultrasonic cell disruptor with cold PBS buffer after 24 h at
37°C in a humidified atmosphere containing 5% CO2. The
cell debris was removed from the samples by centrifugation (1,1749
× g, 4°C, 6 min). After determination of the protein concentration
for each sample by the bicinchoninic acid method, 60 µl sample was
boiled in SDS-PAGE loading buffer (15 µl) for 5 min. A total of 30
µg protein was added to gels (8% gels for proteins of iNOS and
COX-2, 10% gels for proteins of p-ERK, p-JNK and p-p38), which were
subjected to electrophoresis prior to transfer onto nitrocellulose
membranes. Membranes were then incubated with blocking buffer [5%
nonfat-dried milk in Tris-buffered saline-Tween (TBS-T)] for 2 h at
room temperature. After being washed three times in TBS-T, the
membranes were incubated with anti-rabbit polyclonal primary
antibodies diluted at 1:1,000 (anti-iNOS, anti-COX-2,
anti-phospho-ERK 1/2, anti-phospho-JNK and anti-phospho-p38)
overnight at 4°C and anti-β-actin antibody. The membranes were
washed three times in TBS-T and incubated with HRP-conjugated goat
anti-rabbit IgG (H+L) (1:1,000) for 1 h at room temperature. The
membranes were then washed three times in TBS-T prior to
visualization of proteins by using an Enhanced Chemiluminescence
reagent (BeyoECL plus A and the same volume of BeyoECL plus B;
Beyotime Biotechnology) and exposed to photographic films (Kodak,
Rochester, NY, USA). Images of iNOS, COX-2, p-ERK 1/2, p-JNK,
p-p38, and β-actin proteins were collected and quantified by
densitometric analysis using the DigDoc100 program (Alpha Ease FC
2008 software; Alpha Innotech Corporation, San Leandro, CA, USA).
Expression of iNOS, COX-2, p-ERK 1/2, p-JNK, and p-p38 were
normalized to the expression levels of β-actin.
Statistical analysis
All experimental results are presented as mean ±
standard deviation. Statistical significance of differences between
groups was determined by a one-way analysis of variance followed by
the Least Significant Difference test using SPSS software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Dysifragilone A is not cytotoxic to
RAW264.7 cells
RAW264.7 cells were treated with the indicated
concentration of dysifragilone A for 24 h, and then the cell
viability was determined by MTT assay. Dysifragilone A (up to 100
µM) did not cause cytotoxicity against RAW264.7 cells (Fig. 1B). The highest dose for treatment
of cells in future experiments was 100 µM dysifragilone A, and was
used to determine the effect of dysifragilone A on
anti-inflammatory activities and the production of pro-inflammatory
factors, inflammatory mediators or protein expression levels. As
Dysifragilone A did not cause any significant cytotoxicity, this
implied that the observed inhibition activities were not a result
of cell death.
Effect of dysifragilone A on NO and
PGE2 production
RAW264.7 cells were exposed to LPS (1 µg/ml),
various concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A
with LPS (1 µg/ml) and hydrocortisone succinate (100 µM) with LPS
(1 µg/ml) for 24 h. The concentration of nitrite
(NO2−) was detected by Griess assay as an
indicator of NO production, and the levels of PGE2 were
determined by ELISA. Hydrocortisone succinate was used as a
positive control. As shown in Fig.
2A, dysifragilone A caused a significant reduction in the
production of NO stimulated by LPS, which was nearly equivalent to
treatment with hydrocortisone succinate when 100 µM dysifragilone A
was used. The production of PGE2 was also strongly
suppressed by dysifragilone A in a dose-dependent manner, compared
with LPS treatment (Fig. 2B).
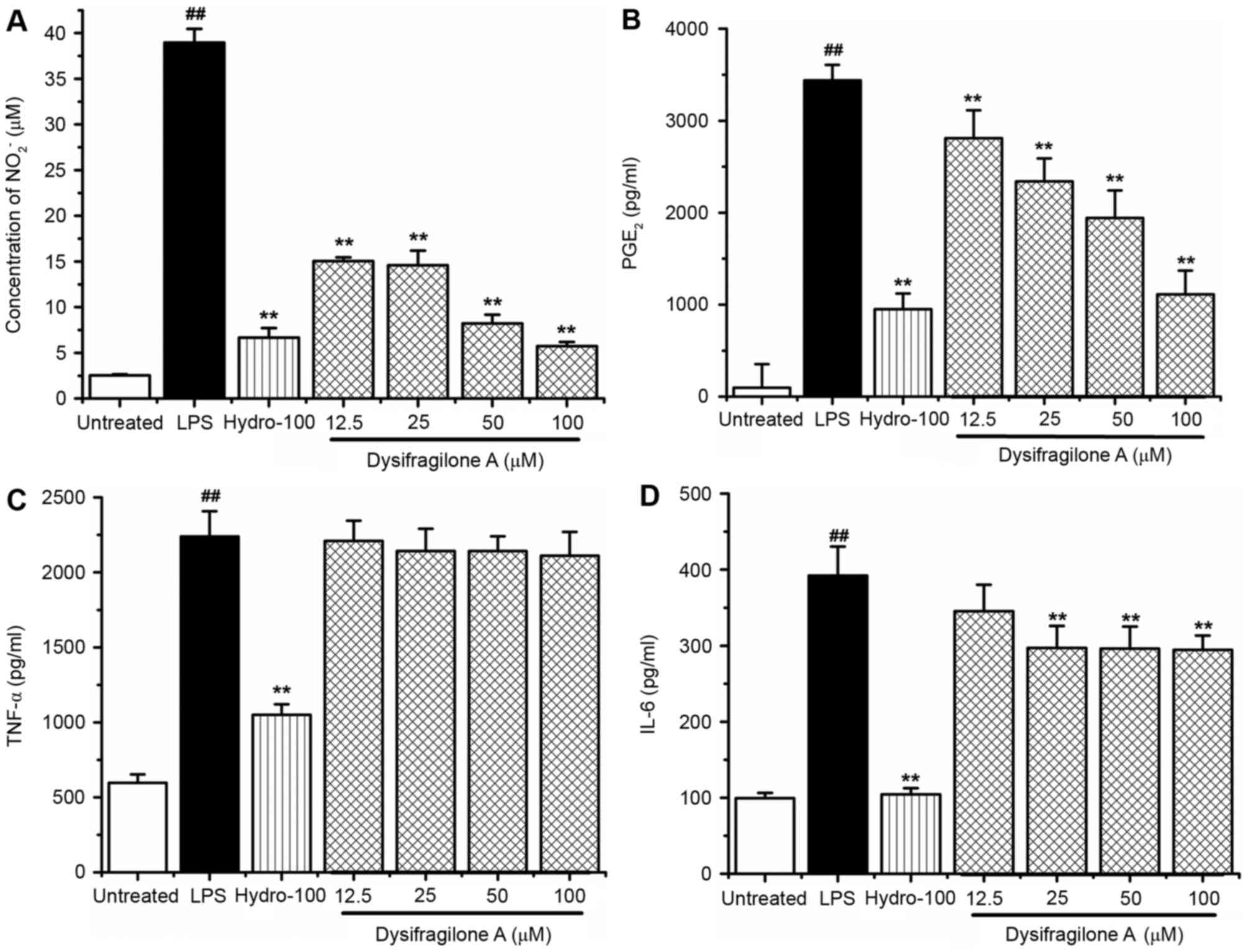 | Figure 2.Effect of dysifragilone A on the
production of NO, PGE2, TNF-α and IL-6 induced by LPS.
(A) RAW264.7 cells were treated with LPS (1 µg/ml), treated with
various concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A
with LPS (1 µg/ml) and hydrocortisone succinate (100 µM) with LPS
(1 µg/ml) for 24 h. The concentration of nitrite in the cells
culture supernatant was measured in triplicate, and the data
represent the mean ± standard deviation from three independent
experiments. (B) The levels of PGE2 in the cells culture
supernatant was measured in triplicate, and the data represent the
mean ± standard deviation from three independent experiments.
RAW264.7 cells were treated with LPS (1 µg/ml), treated with
various concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A
with LPS (1 µg/ml) and hydrocortisone succinate (100 µM) with LPS
(1 µg/ml) for 6 h. A total of 100 µl cell culture supernatant was
removed to detect the levels of TNF-α or IL-6 by using respective
ELISA kits. The experiment was performed in triplicate, and the
results represent the mean ± standard deviation of (C) TNF-α or (D)
IL-6 levels. ##P<0.01 vs. untreated group;
**P<0.01 vs. LPS treatment group. NO, nitric oxide; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; LPS, lipopolysaccharide;
Hydro-100, 100 µM hydrocortisone succinate; PGE2,
prostaglandin E2. |
Effect of dysifragilone A on TNF-α and
IL-6 release
RAW264.7 cells were exposed to LPS (1 µg/ml),
various concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A
with LPS (1 µg/ml) and hydrocortisone succinate (100 µM) with LPS
(1 µg/ml) for 6 h. The levels of pro-inflammatory cytokines TNF-α
and IL-6 were measured by using corresponding ELISA kits. As shown
in Fig. 2C, hydrocortisone
succinate significantly inhibited the release of TNF-α stimulated
by LPS. However, dysifragilone A did not cause inhibition of the
release of TNF-α induced by LPS. As displayed in Fig. 2D, hydrocortisone succinate
significantly inhibited the release of IL-6 stimulated by LPS,
while dysifragilone A only showed slight inhibitory activity on the
release of IL-6 at concentrations >12.5 µM.
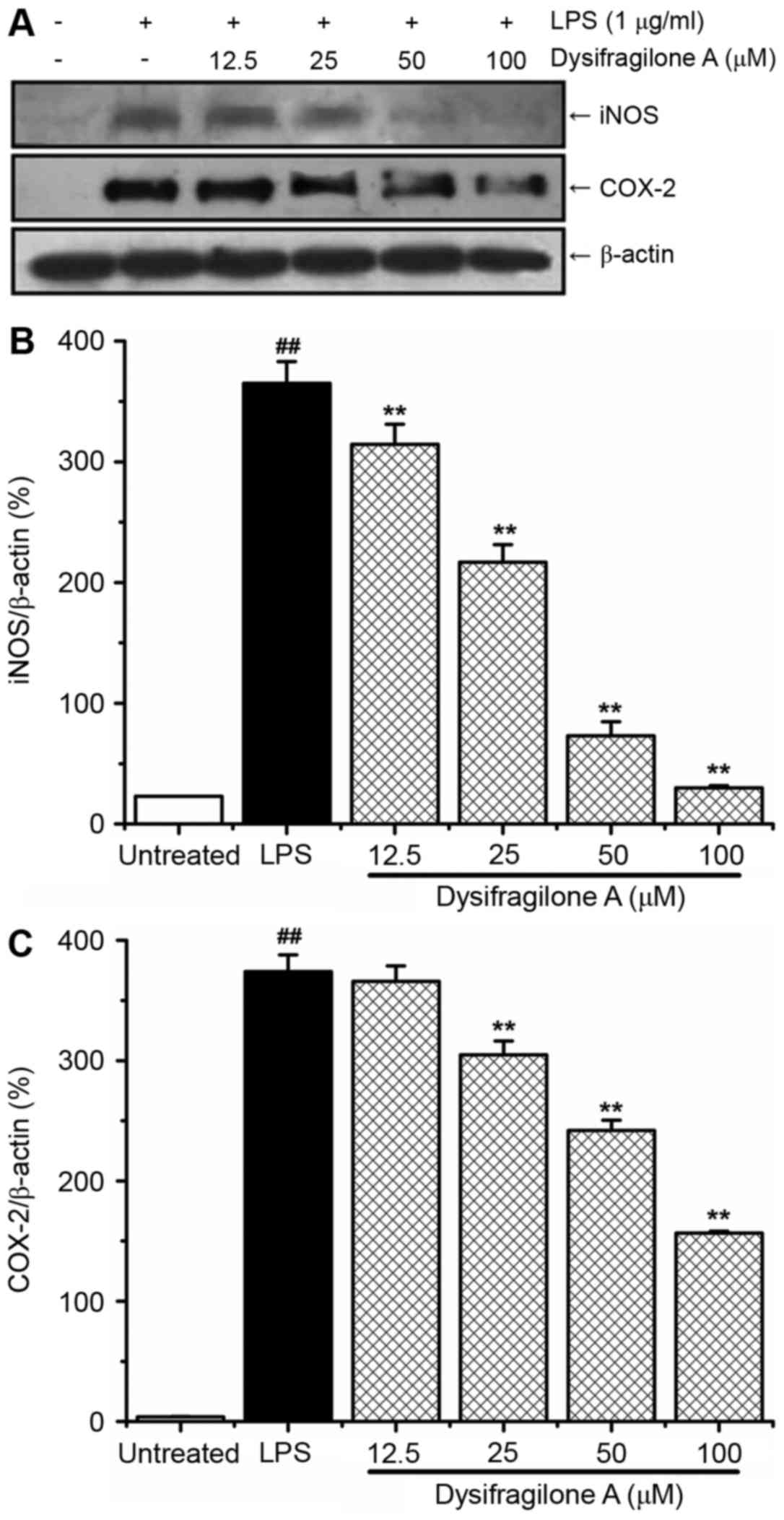
Effect of dysifragilone A on the
expression levels of iNOS and COX-2 proteins
NO and PGE2 are synthesized by iNOS and
COX-2 in the inflammatory reaction. High NO and PGE2
levels are associated with high expression levels of iNOS and COX-2
proteins (20). The present study
determined protein expression levels of iNOS and COX-2 by western
blotting. As shown in Fig. 3A,
RAW264.7 cells were stimulated by LPS for 24 h and the protein
expression levels of iNOS and COX-2 were enhanced compared with
untreated cells. Dysifragilone A downregulated the expression of
iNOS protein in a dose-dependent manner, which may account for the
reduced production of NO caused by dysifragilone A (Fig. 2). In addition, dysifragilone A also
significantly inhibited the expression of the COX-2 protein in a
dose-dependent manner. The density of the iNOS and COX-2 proteins
were normalized to β-actin and the data are presented in Fig. 3B and C, respectively.
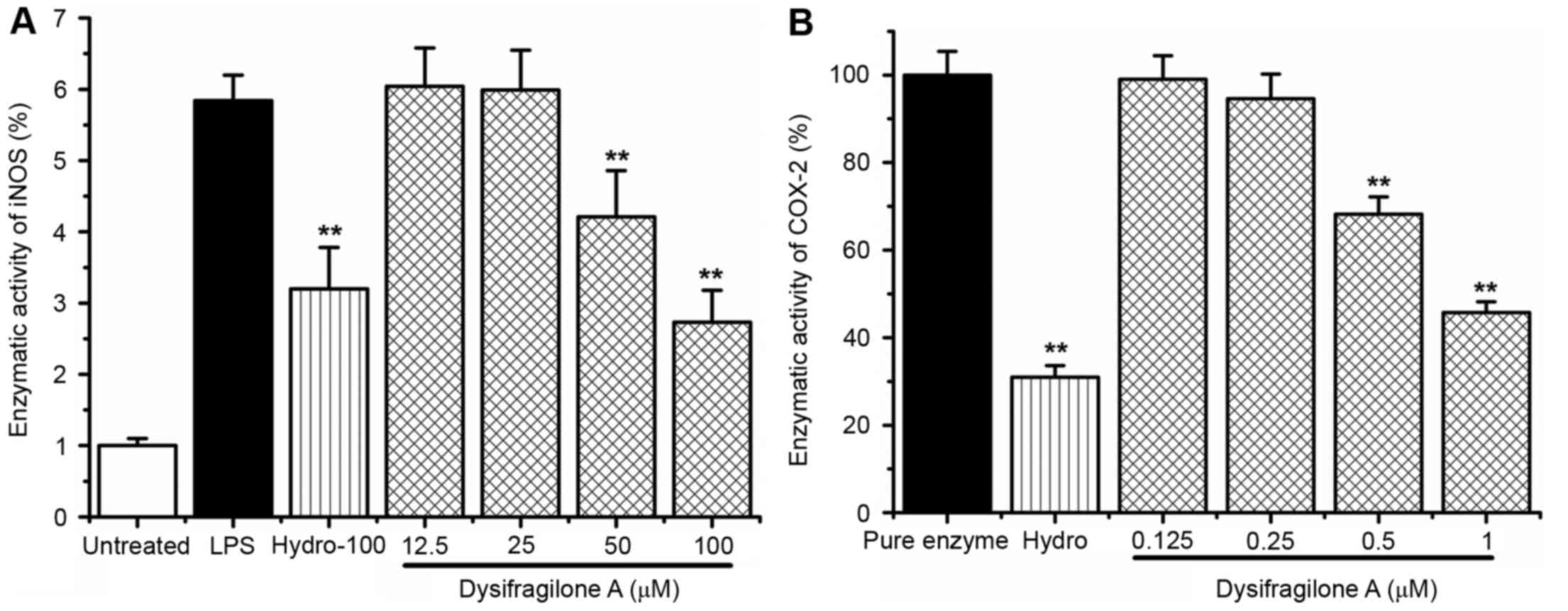
Effect of dysifragilone A on the iNOS
and COX-2 enzymatic activity
RAW264.7 cells were exposed to LPS (1 µg/ml),
various concentrations (12.5, 25, 50 and 100 µM) of dysifragilone A
with LPS (1 µg/ml) and hydrocortisone succinate (100 µM) with LPS
(1 µg/ml) for 24 h. The fluorimetric method was used to detect the
iNOS enzymatic activity. As shown in Fig. 4A, a 6-fold increase in iNOS
enzymatic activity was observed by LPS treatment within 120 min
compared with untreated cells. Dysifragilone A significantly
suppressed iNOS enzymatic activity in RAW264.7 cells at
concentrations of 50 and 100 µM. The cell-free colorimetric method
was used to detect the inhibitory effect of COX-2 enzymatic
activity induced by dysifragilone A. As demonstrated in Fig. 4B, dysifragilone A also inhibited
the COX-2 enzymatic activity at concentrations of 50 and 100
µM.
Effect of dysifragilone A on the
activation of the mitogen-activated protein kinase (MAPK) signaling
pathway
MAPKs are involved in regulating the inflammatory
reaction stimulated by LPS (15 min for JNK, 1 h for ERK and 10 min
for p38), and may also be treatment targets for inflammation
(21,22). In addition, the MAPK signaling
pathway, including ERK 1/2, JNK and p38 MAPK, was activated by LPS
(23). Therefore, in order to
research whether ERK 1/2, JNK and p38 MAPKs have inhibitory
activities on the inflammatory reaction by dysifragilone A, western
blot analysis was used to detect these three MAPKs. As shown in
Fig. 5A, LPS treatment
significantly induced high levels of the p-ERK 1/2, p-JNK and p-p38
proteins compared with untreated cells. Dysifragilone A blocked
LPS-induced p-p38 protein in a dose-dependent manner, whereas p-JNK
and p-ERK 1/2 were not inhibited by dysifragilone A. The results
suggested that dysifragilone A may exhibit an anti-inflammatory
potential via suppression of the p38/MAPK signaling pathway
following treatment with dysifragilone A for 24 h and treated with
LPS for 10 min. However, total p38 was not detected, and this was a
potential limitation of the present study. Densitometric data of
p-ERK 1/2, p-JNK and p-p38 proteins are displayed in Fig. 5B-D, respectively.
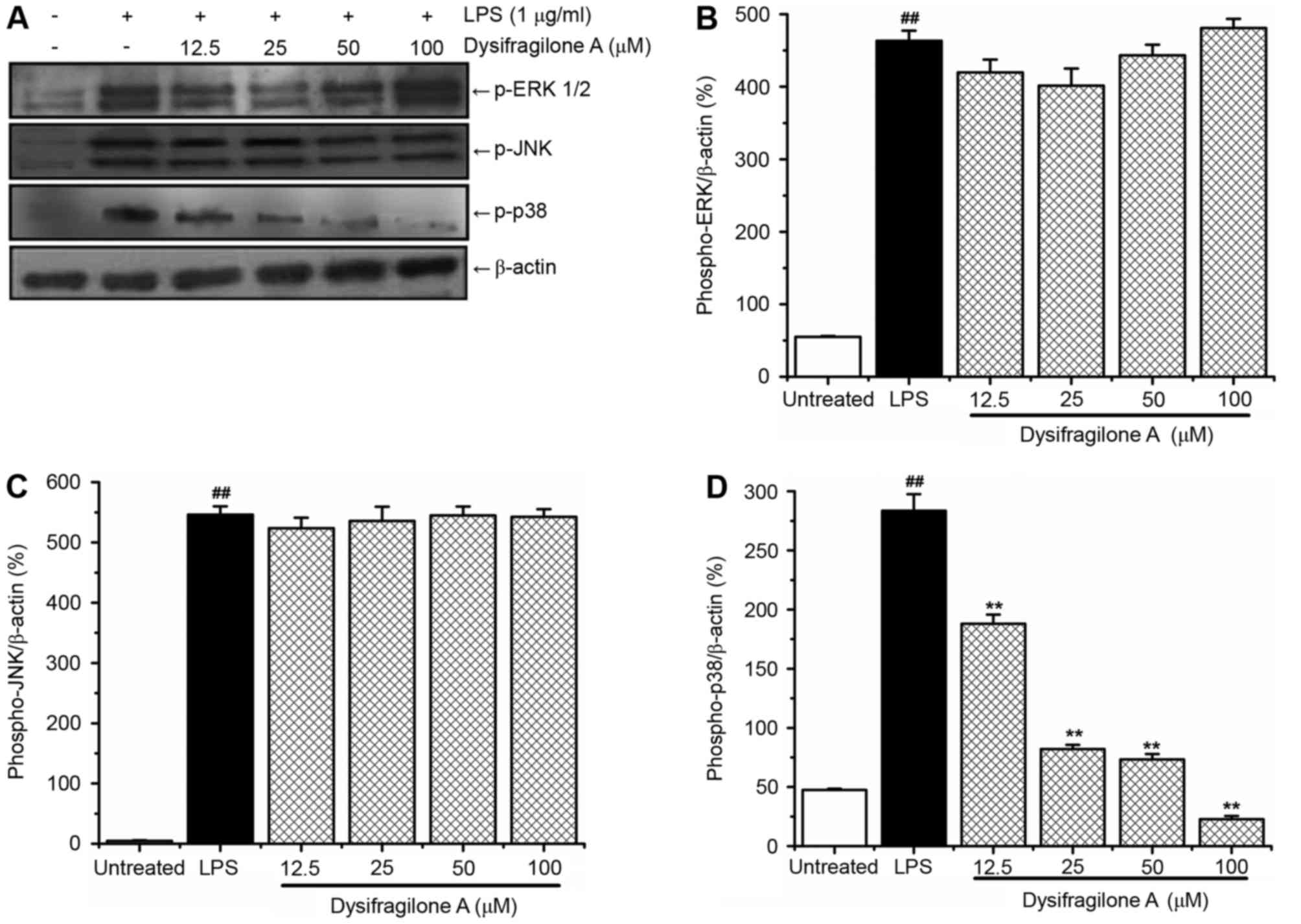 | Figure 5.Effect of dysifragilone A on p-ERK
1/2, p-JNK and p-p38 proteins induced by LPS. RAW264.7 cells were
treated with LPS (1 µg/ml), treated with various concentrations
(12.5, 25, 50 and 100 µM) of dysifragilone A with LPS (1 µg/ml) and
hydrocortisone succinate (100 µM) with LPS (1 µg/ml) for different
times (15 min for JNK, 1 h for ERK and 10 min for p38), and the
expression of (A) p-ERK 1/2, p-JNK and p-p38 proteins determined by
western blot analysis. β-actin was internal reference to confirm
the equal loading of proteins. Densitometric analysis represents
the mean of three independent experiments of (B) p-ERK 1/2 protein,
(C) p-JNK protein and (D) p-p38 protein. ##P<0.01 vs.
untreated group; **P<0.01 vs. LPS treatment group. LPS,
lipopolysaccharide; p-ERK 1/2, phosphorylated-extracellular
signal-regulated kinase 1/2; p-JNK, phosphorylated-c-Jun N-terminal
kinase; p-p38, phosphorylated-p38. |
Discussion
Inflammation is a stress reaction of the body to
resist environmental stimuli including pathogens, damage or other
foreign invasion (24). At
inflammatory sites, a clinical symptom is painful heat. Excessive
and continuous inflammatory responses may result in diseases
associated with inflammation, such as rheumatoid arthritis
(25), cancer (26,27),
atherosclerosis (28), coronary
artery disease (29), chronic
bronchitis (30), pneumonia
(31), and many other diseases
(32). There are two types of
medicines used in the clinic to treat inflammation, including
non-steroidal anti-inflammatory drug (NSAID) and steroidal agents.
Long-term use of NSAID may produce a gastrointestinal reaction,
liver damage, other secondary effects on the nervous system, and
effects on the urinary, blood and cardiovascular systems (33). The disadvantages of the steroidal
agents are retention of water and sodium, risk of infection,
rarefaction of bone and obesity. It is therefore essential to
identify an effective anti-inflammatory drug with low toxicity.
Macrophages are acquired from monocytes of bone
marrow precursors, and regulate inflammation by generation of
pro-inflammatory cytokines (including TNF-α, IL-6 and IL-1β),
chemokines (monocyte chemoattractant protein-1) and inflammatory
mediators (iNOS, COX-2 and PGE2) at the site of
inflammation (34). In the present
study, RAW264.7 cells were chosen to investigate the
anti-inflammatory activity of dysifragilone A. LPS, the primary
component of the outer membranes of gram-negative bacteria, is the
primary start factor of the inflammatory response and is recognized
by toll-like receptor 4 on the surface of host cells (35).
The bioactivities of sponges and marine bacteria
associated with sponges have been extensively studied, such as
antibacterial (36), antitumor
(37), antifungal (38), antiviral (39), as well as cardiovascular disease
(39) resistance activity.
However, limited research regarding the anti-inflammatory molecular
mechanism of dysifragilone A from Dysidea fragilis or
related derivatives, has been available until now. In the current
study, the anti-inflammatory activities of dysifragilone A in
LPS-stimulated RAW264.7 cells were investigated, perhaps for the
first time, and the underlying molecular mechanism was
explored.
Previous experiments have verified that NO
participates in the inflammatory reaction through recruiting
leucocytes to the effected tissue (40). PGE2 is one of the
prostaglandins with the highest bio-distribution and mediates a
variety of physiological and pathological processes, such as the
symptoms of redness, swelling, heat and pain in inflammation
(41). In addition, a study
confirmed the pathological action of PGE2 in most
inflammatory diseases (42).
Therefore, inhibiting the synthesis and release of NO and
PGE2 may be important for anti-inflammation. The results
of this study revealed that dysifragilone A suppressed the
overproduction of NO. The pro-inflammatory mediator PGE2
also exhibited similar inhibitory activities to hydrocortisone
succinate. Based on above analysis, further research of
anti-inflammatory activity is essential.
NO and PGE2 are overproduced by the
enzymes iNOS and COX-2, respectively (43). Thus, inhibiting the expression of
enzymes of iNOS and COX-2 may reduce the synthesis and release of
NO and PGE2. Consistent with the above analysis,
dysifragilone A also downregulated the enzymatic activity of iNOS
and COX-2 in a dose-dependent fashion.
During inflammation, activated RAW264.7 cells
produce a large number of pro-inflammatory factors, which expand
inflammatory responses (44).
TNF-α and IL-6 are the primary cytokines that mediate inflammation.
TNF-α is produced by activated mononuclear macrophages and
activated by nuclear factor-κB (NF-κB) and MAPK pathways, which
exert regulatory effects. IL-6 is produced by multiple cells,
promotes liver synthetic plasma protein and serves a crucial role
in acute responses and chronic inflammation. Thus, cytokines and
chemokines may be measured to evaluate anti-inflammatory
activities. The results demonstrated that dysifragilone A exhibited
weakly inhibitory activities on cytokine IL-6 induced by LPS.
However, dysifragilone A did not reduce the release of TNF-α
stimulated by LPS.
Western blotting was used to determine the possible
underlying molecular mechanism of anti-inflammation. Nuclear
transcription factor NF-κB serves an important role in the
mediating the expression levels of iNOS, COX-2 and pro-inflammatory
cytokines (45). NF-κB is a dimer
formed by the polypeptide chain p50 and p65 subunits, whereas in
the quiescent condition, NF-κB is located in the cytoplasm combined
with an inhibitory protein IκB-α. Stimulated by LPS,
phosphorylation of IκB-α dissociates from NF-κB and releases p65
with p50, resulting in translocation of activated NF-κB into the
nucleus, which then regulates the transcription of target genes,
including iNOS, COX-2 and other pro-inflammatory cytokines
(46). Further western blotting
studies revealed that high concentrations of dysifragilone A
downregulated the expression levels of iNOS and COX-2 proteins.
MAPK signaling pathways are known to influence the
modulation of the inflammatory response and cellular processes
(47). Three MAPK family members
have been identified in mammalian cells, including ERK1/2, JNK and
p38/MAPK (48). Signal
transduction pathways of JNK, ERK1/2 and p38/MAPK and other
pathways in cells are regulated by each other, and determine the
final biological effect of cells by external stimuli. An indicator
of activation of the three pathways is an increase in the
expression of phosphorylated protein involved in the pathways.
These three parallel MAPK signaling pathways promote the production
and release of pro-inflammatory cytokines (49), iNOS and COX-2 expression and
development of inflammation stimulated by LPS (50). Therefore, MAPK signaling pathways
have a profound involvement in inflammation, and are considered as
targets for screening compounds that have anti-inflammatory
activity at the molecular level. In the present study,
dysifragilone A prevented the activation of p38/MAPK signaling
pathway in LPS-induced RAW264.7 cells. However, dysifragilone A did
not exhibit inhibitory activities on p-ERK 1/2 and p-JNK. Based on
the above experimental results, it was speculated that
dysifragilone A is likely to selective in the p38/MAPK signaling
pathway.
In conclusion, the results of the present study
demonstrated that dysifragilone A may inhibit the enzymatic
activity of iNOS and COX-2 and the release of NO, PGE2
and IL-6 in LPS-stimulated RAW264.7 cells. Further investigations
demonstrated that dysifragilone A may also downregulate the
expression levels of iNOS and COX-2 proteins and block the p38/MAPK
signal pathway in RAW264.7 cells induced by LPS. These observations
suggested that dysifragilone A was a valuable candidate compound to
treat inflammatory diseases. However, animal experiments are
essential in revealing the potential of anti-inflammatories in a
more comprehensive way, and a limitation of the present study was a
lack of experiments in vivo. To support the findings of the
present study, samples of dysifragilone A are required for animal
experiments.
Acknowledgements
The research of the present study was supported by
the Taishan Scholar Project to Fenghua Fu, the Undergraduate
Scientific and Technological Innovation Project of Yantai
University, the Distinguished Young Scholars of China (grant no.
81225023), the Shandong Provincial Natural Science Foundation
(grant no. ZR2016HL54), the Shanghai Rising-Star Program (grant no.
14QA1402800) and the NSFC Programs (grant nos. 41576130, 41476121,
41476121 and U1605221).
References
|
1
|
Bell JJ: The functional roles of marine
sponges. Estuar Coast Shelf S. 79:341–353. 2008. View Article : Google Scholar
|
|
2
|
Braekman JC and Daloze D: Chemical and
biological aspects of sponge secondary metabolites. Phytochem Rev.
3:275–283. 2004. View Article : Google Scholar
|
|
3
|
Hooper JNA and Van Soest RWM: Systema
porifera: A guide to the classification of sponges. New York:
Kluwer Plenum; pp. 1–7. 2002, View Article : Google Scholar
|
|
4
|
Gerwick WH and Moore BS: Lessons from the
past and charting the future of marine natural products drug
discovery and chemical biology. Chem Biol. 19:85–98. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salomon CE, Williams DH and Faulkner DJ:
New azacyclopropene derivatives from Dysidea fragilis collected in
Pohnpei. J Nat Prod. 58:1463–1466. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su JY, Zhong YL, Zeng LM, Wei S, Wang QW,
Mak TCW and Zhou ZY: Three new diketopiperazines from a marine
sponge Dysidea fragilis. J Nat Prod. 56:637–642. 1993. View Article : Google Scholar
|
|
7
|
Fu X, Zeng LM, Su JY and Pais M: A new
diketopiperazine derivative from the South China Sea sponge Dysidea
fragilis. J Nat Prod. 60:695–696. 1997. View Article : Google Scholar
|
|
8
|
Milkova TS, Mikhova BP, Nikolov NM, Popov
SS and Andreev SN: Two new polyhydroxylated sterols from the sponge
Dysidea fragilis. J Nat Prod. 55:974–978. 1992. View Article : Google Scholar
|
|
9
|
Guella G, Guerriero A and Pietra F:
Sesquiterpenoids of the sponge Dysidea fragilis of the
North-Brittany Sea. Helv Chim Acta. 68:39–48. 1985. View Article : Google Scholar
|
|
10
|
Utkina NK, Kazantseva MV and Denisenko V:
Brominated diphenyl ethers from the marine sponge Dysidea fragilis.
Chem Nat Compd. 23:508–509. 1987. View Article : Google Scholar
|
|
11
|
Jiao WH, Huang XJ, Yang JS, Yang F, Piao
SJ, Gao H, Li J, Ye WC, Yao XS, Chen WS and Lin HW: Dysidavarones
A-D, new sesquiterpene quinones from the marine sponge Dysidea
avara. Org Lett. 14:202–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiao WH, Xu TT, Yu HB, Chen GD, Huang XJ,
Yang F, Li YS, Han BN, Liu XY and Lin HW: Dysideanones A-C, unusual
sesquiterpene quinones from the South China Sea sponge Dysidea
avara. J Nat Prod. 77:346–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao WH, Xu TT, Yu HB, Mu FR, Li J, Li YS,
Yang F, Han BN and Lin HW: Dysidaminones A-M, cytotoxic and NF-κB
inhibitory sesquiterpene aminoquinones from the South China Sea
sponge Dysidea fragilis. RSC Adv. 4:9236–9246. 2014. View Article : Google Scholar
|
|
14
|
Jiao WH, Xu TT, Zhao F, Gao H, Shi GH,
Wang J, Hong LL, Yu HB, Li YS, Yang F and Lin HW: Dysifragilones
A-C, unusual sesquiterpene aminoquinones and inhibitors of NO
production from the South China sea sponge Dysidea fragilis. Eur J
Org Chem. 2015:960–966. 2015. View Article : Google Scholar
|
|
15
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival: Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishihara T, Kohno K, Ushio S, Iwaki K,
Ikeda M and Kurimoto M: Tryptanthrin inhibits nitric oxide and
prostaglandin E(2) synthesis by murine macrophages. Eur J
Pharmacol. 407:197–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wohlmuth H, Deseo MA, Brushett DJ,
Thompson DR, Macfarlane G, Stevenson LM and Leach DN:
Diarylheptanoid from Pleuranthodium racemigerum with in vitro
prostaglandin E(2) inhibitory and cytotoxic activity. J Nat Prod.
73:743–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao F, Xu H, He EQ, Jiang YT and Liu K:
Inhibitory effects of sesquiterpenes from Saussurea lappa on the
overproduction of nitric oxide and TNF-alpha release in
LPS-activated macrophages. J Asian Nat Prod Res. 10:1045–1053.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of bata-carboline
alkaloids, isolated from Picrasma quassioiders, through inhibition
of the iNOS pathway. Planta Med. 78:1906–1911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akram M, Kim KA, Kim ES, Shin YJ, Noh D,
Kim E, Kim JH, Majid A, Chang SY, Kim JK and Bae ON: Selective
inhibition of JAK2/STAT1 signaling and iNOS expression mediates the
anti-inflammatory effects of coniferyl aldehyde. Chem Biol
Interact. 256:102–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YS, Ahn CB and Je JY:
Anti-inflammatory action of high molecular weight Mytilus edulis
hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB
and MAPK pathways. Food Chem. 202:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SH, Kim J and Sharma RP: Inhibition of
p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide
production and differentially modulates cytokine expression.
Pharmacol Res. 49:433–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaminska B: MAPK signaling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao KM: MAP kinase activation in
macrophages. J Leukoc Biol. 69:3–10. 2001.PubMed/NCBI
|
|
25
|
Valledor AF, Comalada M, Santamaría-Babi
LF, Lloberas J and Celada A: Macrophage proinflammatory activation
and deactivation: A question of balance. Adv Immunol. 108:1–20.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ignatova GL, Volchegorskii IA, Volkova EG
and Kolesnikov OL: Intensity of lipid peroxidation: Indicator of
inflammation severity in chronic bronchitis. B Exp Biol Med.
124:800–801. 1997. View Article : Google Scholar
|
|
31
|
Hoogendijk AJ, Roelofs J, van Lieshout M,
Blok DC, Poll TVD and Wieland CW: Cyclin-dependent kinase inhibitor
r-roscovitine reduces lipoteichoic acid lung inflammation and
improves the resolution of antibiotic-treated Streptococcus
Pneuumoniae pneumonia. Crit Care. 13 Suppl 4:S392009. View Article : Google Scholar
|
|
32
|
Wellen KE and Hotamisligil GS:
Inflammation, stress and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Tang J, Zuo Y, Yu Y, Luo P, Yao X,
Dong Y, Wang P, Liu L and Zhou H: Stauntoside B inhibits macrophage
activation by inhibiting NF-κB and ERK MAPK signaling. Pharmacol
Res. 111:303–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Butterfield TA, Best TM and Merrick MA:
The dual roles of neutrophils and macrophages in inflammation: A
critical balance between tissue damage and repair. J Athl Train.
41:457–465. 2006.PubMed/NCBI
|
|
35
|
Fitzpatrick JK and Downer EJ: Toll-like
receptor signaling as a cannabinoid target in Multiple Sclerosis.
Neuropharmacology. 113:618–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Melander RJ, Liu HB, Stephens MD, Bewley
CA and Melander C: Marine sponge alkaloids as a source of
anti-bacterial adjuvants. Bioorg Med Chem Lett. 26:5863–5866. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishimura S, Matsunaga S, Yoshida M,
Hirota H, Yokoyama S and Fusetani N: 13-Deoxytedanolide, a marine
sponge-derived antitumor macrolide, binds to the 60S large
ribosomal subunit. Bioorg Med Chem. 13:449–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
EI-Hossary EM, Cheng C, Hamed MM, El-Sayed
Hamed AN, Ohisen K, Hentschel U and Abdelmohsen UR: Antifungal
potential of marine natural products. Eur J Med Chem. 126:631–651.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mayer AM and Hamann MT: Marine
pharmacology in 2000: Marine compounds with antibacterial,
anticoagulant, antifungal, anti-inflammatory, antimalarial,
antiplatelet, antituberculosis, and antiviral activities; affecting
the cardiovascular, immune, and nervous systems and other
miscellaneous mechanisms of action. Mar Biotechnol (NY). 6:37–52.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Laroux FS, Pavlick KP, Hines IN, Kawachi
S, Harada H, Bharwani S, Hoffman JM and Grisham MB: Role of nitric
oxide in inflammation. Acta Physiol Scand. 173:113–118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bos CL, Richel DJ, Ritsema T,
Peppelenbosch MP and Versteeg HH: Prostanoids and prostanoid
receptors in signal transduction. Int J Biochem Cell Biol.
36:1187–1205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang CH, Han SH, Koo JR and So JS:
Chrysanthemum zawadskii var. latilobum extract inhibits the
production of nitric oxide and PGE2 through inducible
nitric oxide synthase (iNOS) and cyclooxygenase-2(COX-2) in RAW
264.7 cells. Biotechnol Bioproc Eng. 18:501–506. 2013. View Article : Google Scholar
|
|
43
|
Lee JK, Sayer BC, Chun KS, Lao HC,
Shipley-phillips JK, Bonner JC and Langenbach R: Multi-walled
carbon nanotubes induce COX-2 and iNOS expression via MAP
kinase-dependent and -independent mechanisms in mouse RAW264.7
macrophages. Part Fibre Toxicol. 9:142012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ham YM, Ko YJ, Song SM, Kim J, Kim KN, Yun
JH, Cho JH, Ahn G and Yoon WJ: Anti-inflammatory effect of
litsenolide B2 isolated from Litsea japonica fruit via suppressing
NF-κB and MAPK pathways in LPS-induced RAW264.7 cells. J Funct
Foods. 13:80–88. 2015. View Article : Google Scholar
|
|
47
|
Wu J, Xue X, Wu Z, Zhao HL, Cao HM, Sun
DQ, Wang RM, Sun J, Liu Y and Guo RC: Anti-tumor effect of paeonol
via regulating NF-κB, AKT and MAPKs activation: A quick review.
Biomed Pre Nutr. 4:9–14. 2014. View Article : Google Scholar
|
|
48
|
Liu Y, Shepherd EG and Nelin LD: MAPK
phosphatases-regulating the immune response. Nat Rev Immunol.
7:202–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu SJ, Shi Y, Liu C, Zhang M, Zuo ZC,
Zeng CJ, Zhou GB, Xian H and Song TZ: The upregulation of
pro-inflammatory cytokines in the rabbit uterus under the
lipopolysaccaride-induced reversible immunoresponse state. Anim
Reprod Sci. 176:70–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|