Introduction
Subfertility refers to the failure of conception
following unprotected intercourse for at least one year. Many
couples suffer from subfertility problems worldwide, with estimates
ranging from 10–20%. Of the total infertility cases, ~50% are
attributed to male infertility (1). Dysfunctional spermatogenesis is among
the leading causes of male subfertility, and assessment of the
quality of spermatogenesis is crucial for the evaluation and
treatment of subfertility in men. A previous study reported that
the Sertoli cell promotes sperm production through various
functions, including nurturing seminiferous epithelial cells,
transporting spermatids and secreting androgen-binding protein
(ABP) (2). In addition, studies
have also identified that cholesterol metabolism-associated genes,
including ATP-binding cassette (ABC) subfamily A member 1
(ABCA1)/G5 (3,4), apolipoprotein B (5), cystic fibrosis transmembrane
conductance regulator (6) and
cAMP-responsive element modulator (7), are essential for normal male
fertility. Sertoli cells exert phagocytic functions similar to
macrophages and possess the ability to efflux excess lipids and
cholesterols after engulfing membrane-rich structures. By means of
the basement membrane, Sertoli cells are isolated from interstitial
capillaries, which blocks the passage of low density lipoprotein
(LDL) but permits the entry of high density lipoprotein (HDL) to
the seminiferous tubules (8). The
molecular pathways responsible for the regulation of lipid exchange
between the periphery and testes remain unclear, but are of great
interest due to the critical role of cholesterol in spermatogenesis
and steroidogenesis. Furthermore, Leydig and Sertoli cells produce
reproductive steroid hormones. Leydig cells secrete a number of
different types of androgens, including dihydrotestosterone and
testosterone, which modulate the development and maturation of
spermatozoa (9). The uptake of
testosterone by Sertoli cells leads to its conversion into
dihydrotestosterone and estradiol. Leydig cells obtain cholesterol
either via de novo synthesis pathways or through the uptake
of cholesterol from HDL (10). In
addition, although Sertoli cells use acetate to synthesize
cholesterol, their primary source of cholesterol is from HDL entry
from the plasma or androgens that are transported from Leydig cells
(8,11,12).
The following review is a concise overview of the progress of
research concerning cholesterol metabolism in Sertoli cells in
addition to its role in spermatogenesis.
The role of Sertoli cells in
spermatogenesis
The Sertoli cell, which was initially identified by
Enrico Sertoli in 1865, performs a critical role during the process
of spermatogenesis. Sertoli cells are recognized as ‘nurse cells’
that are responsible for extending nutritional and energy support
to the development of germ cells. It has been widely demonstrated
that germ cells require a sufficient level of energy resources,
otherwise they decay and enter apoptosis (13,14).
The development of germ cells requires specific metabolic
substrates, such as lactate, which is used as a substrate for ATP
production (15). Through the
secretion of metabolic intermediates or nutrients, including
carbohydrates, lipids, vitamins, metal ions and amino acids,
Sertoli cells provide the nutritional requirements of germ cells
(16,17).
Additionally, through characteristic zones of
cellular membrane tight junctions, Sertoli cells form connections
with one another and divide the germinal epithelium into an
adluminal and a basal compartment; the blood-testis barrier of the
testis is also formed by these tight junctions. After passing this
barrier, the germ cells are protected from extraneous substance
diffusion in the adluminal compartment during spermatogenesis
(2). Therefore, the blood-testis
barrier acts as a multifunctional boundary between haploid and
diploid germ cells, in addition to separating testicular tissue
from blood. Furthermore, a functional Sertoli cell provides energy,
differentiation factors and adequate mitogens to the developing
germ cells, and prevents them from harm that may result from the
host's own immune system (18).
Sertoli cells also produce various types of enzymes, growth factors
and hormones, including plasminogen activator, ABP, ceruloplasmin,
insulin-like growth factor, transforming growth factors α and β,
anti-Müllerian hormone (AMH) and inhibin B (2,18).
Sertoli cells express the sex-determining region Y
gene, and thereby decide the male sex of the gonad. By producing
AMH, Sertoli cells also suppress the development of internal female
genitalia. Together with the peritubular cells, these cells are
critical for the formation of the testis cords. The immature
Sertoli cell varies greatly from the mature one in terms of
biochemical activity and morphology. During puberty, the Sertoli
cells are stretched and begin to form connections through tight
junctions. Furthermore, they secrete seminiferous fluid,
transforming testis cords into seminiferous tubules. The mature
differentiated Sertoli cell alters its protein expression pattern,
producing various factors, including the inflammatory cytokine
interleukin-1α and transferrin. As puberty develops, despite the
consecutive division of mature Sertoli cells, their proliferative
activity is reduced. After the formation of tight junctions,
Sertoli cells exhibit no proliferative capacity (18). Follicle-stimulating hormone (FSH)
serves as the predominant endocrine factor responsible for the
regulation of Sertoli cell function. In the testes, the Sertoli
cell exclusively expresses FSH receptors, which is required for the
appropriate proliferation of Sertoli cells. Spermatogenesis relies
on an appropriate intratesticular level of testosterone.
Furthermore, Sertoli cells, but not germ cells, express the
androgen receptor, indicating that Sertoli cells mediate the
effects of androgen on the seminiferous epithelium. The androgen
receptor is necessary for the correct functioning of the
blood-testis barrier, as well as for the normal development of germ
cells, and its expression on Sertoli cells was reported to be
amplified throughout the maturation process of germ cells (18). Therefore, Sertoli cells have
crucial roles in the autocrine and/or paracrine regulation of
spermatogenesis (2).
The role of cholesterol in Sertoli cells
during spermatogenesis
Lipids and cholesterols are essential for
spermatogenesis as they serve as ‘fuel’ for Sertoli cells and are
crucial for the membrane remodeling of developing germ cells. The
biosynthesis pathway of cholesterol comprises a succession of
enzymatic reactions implicating diverse intermediates. Among them,
meiosis-activating sterols were reported to have strong
meiosis-activating potency through screening of naturally occurring
compounds. In addition, various factors are responsible for the
regulation of cholesterol content in Sertoli cells, including ABCA1
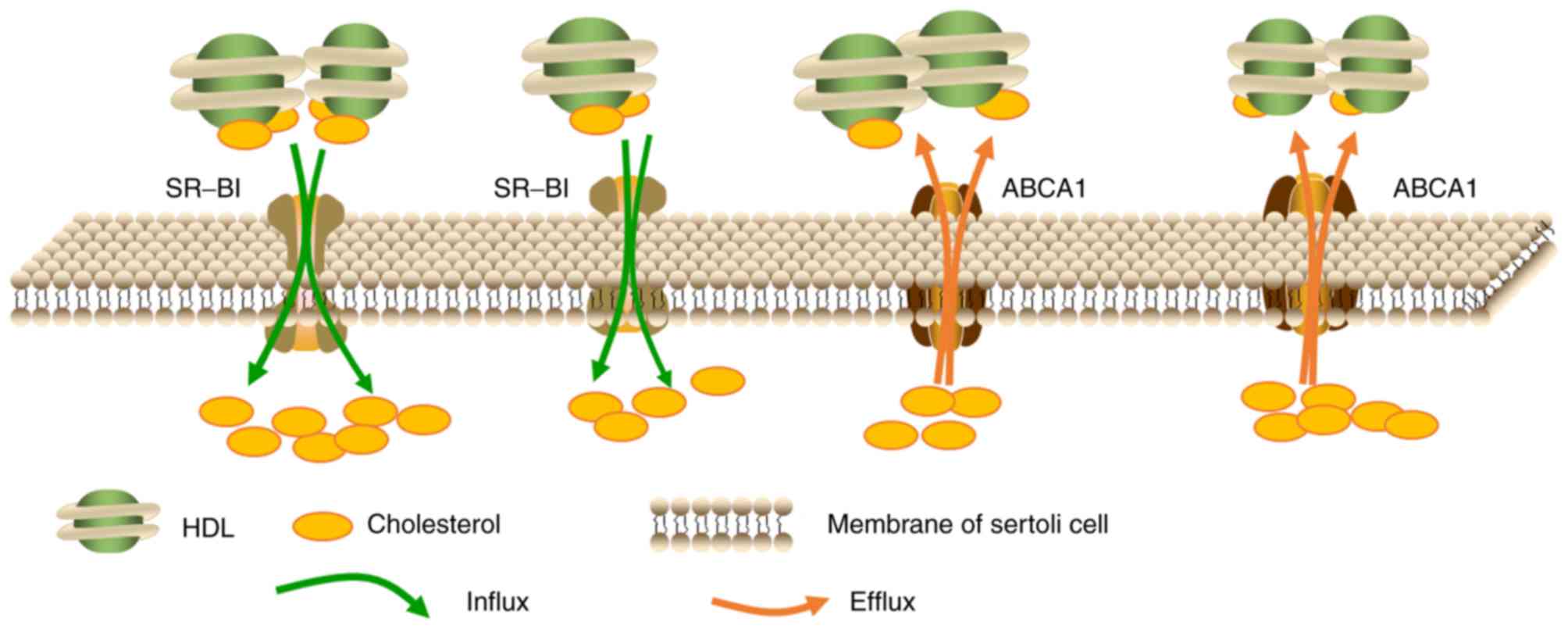
and scavenger receptor class B member 1 (SR-BI) (8,9,18,19).
The transport of cholesterol in
Sertoli cells
A large proportion of nutrients, such as lipids,
used for spermatogenesis are provided by supporting Sertoli cells.
Sertoli cells have the ability to synthesize cholesterol using
acetate in vitro (19),
however, it has been demonstrated that this source of cholesterol
is insufficient for steroidogenesis in vivo. As the amount
of cholesterol required for spermatogenesis far exceeds the
capacity of Sertoli cells biosynthesis, specialized cholesterol
transporters such as SR-BI facilitate the uptake of cholesterol
from the blood (20). Research has
revealed that, at least in rodents, HDL serves as the primary
source of cholesterol for Sertoli cells (21), which may be further supported by
the observations that the basal membrane segregating seminiferous
tubules from blood capillaries blocks the entry of LDL but permits
HDL entry (8,21). In rats, Sertoli cells obtain
cholesterol from HDL primarily through apolipoprotein E-dependent
pathways (22). In addition,
Sertoli cells may also obtain cholesterol from phagocytosed
apoptotic germ cells and lipid-rich remnant recycling (22). To eliminate toxicity, surplus
cholesterol undergoes esterification and storage in lipid droplets,
which is instrumental in the maintenance of cholesterol
equilibrium. Lipid droplets are highly mobile and dynamic
structures, and are reported to interact with cell junctions and
organelles via transient or stable surface exchange, ensuring the
effective synthesis, uptake, usage and elimination of cholesterol
(23). Furthermore, Sertoli cells
also maintain cholesterol homeostasis through reverse cholesterol
transport (RCT). RCT involves various procedures that enable the
efflux of excess cholesterol to HDL and promote the transport of
cholesterol from non-hepatic peripheral tissue back to the liver
via the plasma to maintain cholesterol homeostasis, which primarily
relies on the ABCA1 cholesterol transporters (3). The excessive accumulation of
cholesterol esters (CE) in Sertoli cells leads to disruption of
cholesterol homeostasis, ultimately resulting in complete
subfertility or infertility. This phenomenon was demonstrated in
various knockout mouse models that are deficient in functional
nuclear receptors, such as retinoid X receptor (RXR) (24,25),
and also in ABCA1 knockout mice (3). As ABCA1 is an established liver X
receptor (LXR) target gene, in double knockout mice (LXR
α/β−/−), the deficiency of ABCA1 decreases the
cholesterol efflux from Sertoli cells and results in the
accumulation of CE (3,24). Therefore, Sertoli cells modulate
every step of cholesterol metabolism, including cholesterol uptake,
efflux, storage and recycling, which indicates that Sertoli cells
should be a focus in the interpretation of spermatogenesis.
Fig. 1 is a graphic illustration
of the transport of cholesterol in Sertoli cells.
The role of cholesterol metabolism in
spermatogenesis
The role of cholesterol in sperm production and male
fertility is well established. The process of spermatogenesis
consists of a sequence of differentiation and proliferative phases,
which are further divided into spermatogenic, meiotic and mitotic
stages. Each stage involves different cell types, including
spermatids, spermatocytes and spermatogonia (26,27).
Additionally, increases in lipid droplets are observed throughout
spermatogenesis (28),
demonstrating an intimate association between lipid metabolism
alterations and fertility during spermatogenesis. Steroidogenesis
also requires large amounts of cholesterol (29), while in the seminiferous tubules,
cholesterol is required for the differentiation of germinal cells
to spermatozoids (gametogenesis/spermatogenesis) (30). Considerable evidence also indicates
that, in males and females, cholesterol is required for the
development of fertility and gametes. In male mice, the absence of
the 24-dehydrocholesterol reductase gene, which encodes a
cholesterol biosynthetic enzyme, induces infertility (31). Therefore, cholesterol is required
for the mass production of germ cells during spermatogenesis. The
plasma membranes of sperm are also heavily loaded with cholesterol
when sperm are released from the seminiferous epithelium.
Previously, experiments that determined the role of cholesterol
de novo synthesis identified that the incorporation rate of
14C acetate into cholesterol was increased during the
development of pachytene, leptotene and zygotene stages (32). This indicates that de novo
synthesis of cholesterol is increased in these germ cells, which
was associated with increased diameter and surface area of germ
cells. In late pachynema, the rate of cholesterol synthesis tends
to decrease and remains low throughout the following phases of
spermatogenesis, including sperm maturation. In contrast to
cholesterol, the incorporation capacity of acetate into dolichol
was enhanced in round spermatids and late pachytene spermatocytes,
and later diminished and remained low in mature sperm cells
(32). These observations indicate
that the preliminary phases of cholesterol synthesis in round
spermatids and pachytene spermatocytes may precede dolichol
synthesis, which acts as a critical constituent in the production
of membrane glycoproteins. However, the detailed role of
cholesterol metabolism within germ cells has not been identified
clearly, which is primarily due to the complex connections between
germ cells and the supporting Sertoli cells.
Proteins responsible for the
regulation of cholesterol metabolism in Sertoli cells
ABCA1
ABCA1, a member of the ABC transporter superfamily,
transfers phospholipids and cholesterol out of the peripheral cells
to lipid-free apolipoprotein A1, which results in the formation of
pre-βHDL and is the rate-limiting step in the process of HDL
biosynthesis (33,34). Genetic mutations in ABCA1 lead to
the absence of plasma HDL, the accumulation of CE in various body
tissues and an increased incidence of atherosclerosis (AS), which
are collectively termed Tangier disease (35). By contrast, ABCA1 overexpression in
mice enhances plasma HDL levels and prevents the development AS
(36,37). However, research has demonstrated
that, although ABCA1 in macrophages has an important role in
preventing atherosclerosis, it has limited influence on HDL levels
in the plasma. In addition, in mice, liver ABCA1 serves as a
predominant factor to determine plasma HDL levels (38,39).
ABCA1 is highly expressed in the testes (40,41),
indicating that ABCA1 may also be involved in the regulation of
testicular lipid transport, which is largely segregated from the
peripheral circulation (42).
Selva et al (3) observed
that, in the seminiferous tubule, Sertoli cells account for the
majority of the ABCA1 expression. Consistent with the high ABCA1
expression levels, Sertoli cells that express ABCA1 exhibit
substantial cholesterol efflux to lipid-free apolipoprotein A1.
Conversely, ABCA1-deficient Sertoli cells exhibited decreased
cholesterol efflux and accumulation of oil red O-positive lipid
droplets, effects that were reversed by ABCA1 restoration,
demonstrating that the cholesterol efflux from cultured Sertoli
cells is mediated by ABCA1. Fig. 2
is a schematic representation of the regulation of cholesterol
content in Sertoli cells. Importantly, in apolipoprotein
A1−/− mice, Sertoli cells do not exhibit excess lipids,
indicating that deficiency of plasma HDL is insufficient to lead to
lipid accumulation in Sertoli cells and that ABCA1 has a direct
role in modulating Sertoli cell lipid efflux (43). Furthermore, ABCA1−/−
mice exhibit reduced testosterone levels and fewer spermatozoa
compared with wild-type (WT) mice, indicating that ABCA1 deficiency
compromises the Sertoli cell functions and male fertility, but does
not lead to complete sterility (3). A deeper understanding of the roles of
ABCA1 in lipid transport in spermatogenesis requires further
investigation.
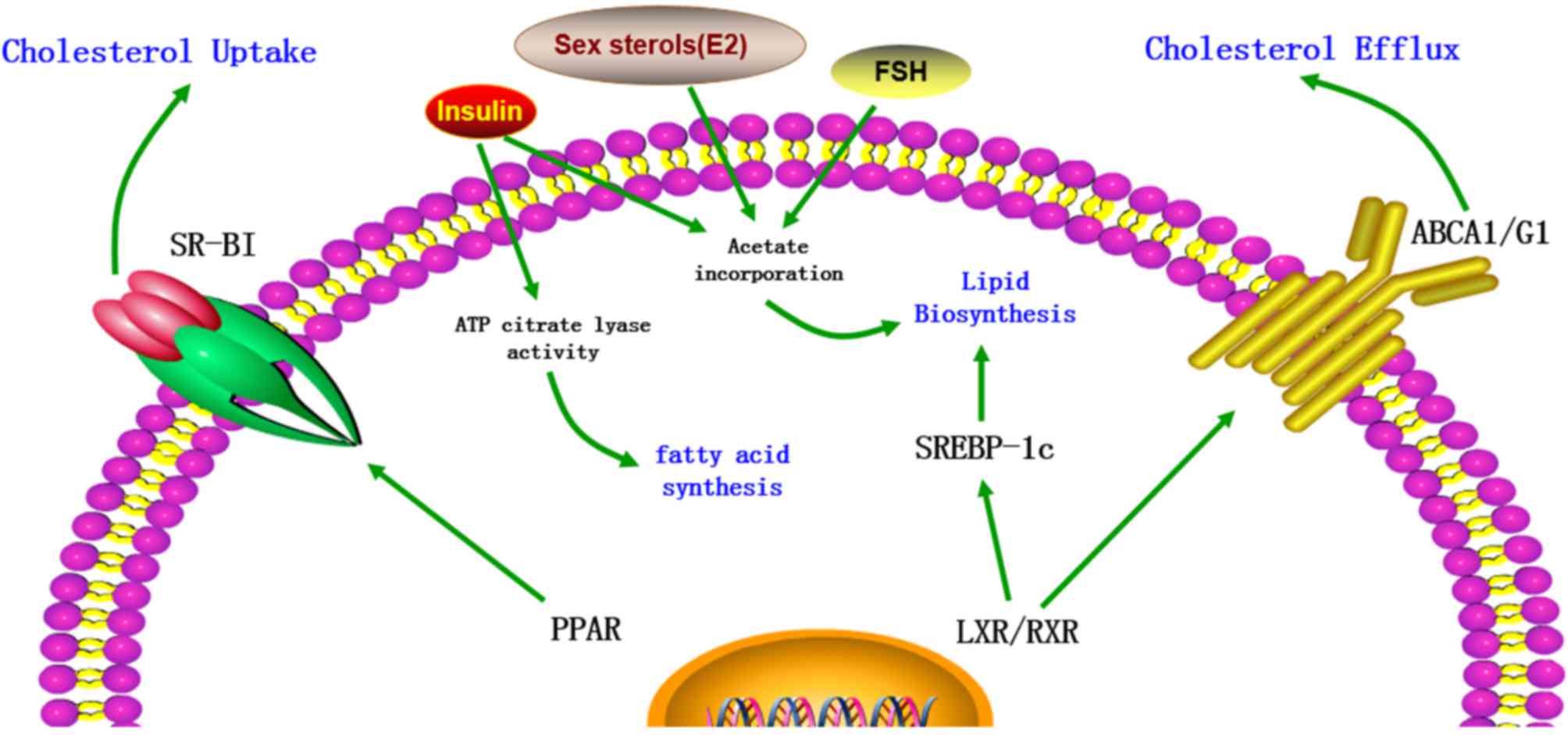 | Figure 2.The regulation of cholesterol content
in Sertoli cells. The cholesterol metabolism in Sertoli cells is
regulated by various factors, including transporters, transcription
factors and hormones. LXR/RXR heterodimers increase the cellular
cholesterol content by enhancing the levels of SREBP-1c and
decrease cholesterol levels by increasing ABCA1 expression. In
addition, cholesterol levels are increased by PPAR via increased
expression of SR-BI. Furthermore, FSH, insulin and certain sex
sterols, including 17β-estradiol (E2), increase the cholesterol
content in Sertoli cells by increasing acetate incorporation.
Insulin also increases the levels of cholesterol and fatty acids by
stimulating the activity of ATP citrate lyase. LXR, liver X
receptor; RXR, retinoid X receptor; SREBP-1c, sterol regulatory
element-binding protein-1c; ABCA1, ATP-binding cassette subfamily A
member 1; PPAR, peroxisome proliferator-activated receptor; SR-BI,
scavenger receptor class B member I; FSH, follicle-stimulating
hormone; ATP, adenosine triphosphate; E2, 17β-estradiol (E2). |
SR-BI
SR-BI, which belongs to the SR superfamily, is an
important integral membrane glycoprotein on the cell membrane.
Structurally, SR-BI and CD36 share 30% homology in amino acid
sequence. Functionally, it is a physiological receptor for HDL that
facilitates the uptake of HDL-CE, a process that is termed RCT
(44). Unlike LDL receptors, which
internalize whole lipoprotein particles, SR-BI selectively uptakes
cholesterol from HDL into cells. In the testes, the highest
expression of SR-BI is observed in steroidogenic Leydig cells
(45,46), and the remaining expression occurs
in Sertoli cells (46).
Previously, Shiratsuchi et al (47) demonstrated that the activity of
phosphatidylserine (PS)-mediated phagocytosis of spermatogenic
cells by Sertoli cells was enhanced by SR-BI upregulation and
inhibited by an anti-SR-BI antibody, indicating that SR-BI acts as
a PS receptor, enabling Sertoli cells to recognize and phagocytose
spermatogenic cells. Furthermore, Rigotti et al (45) demonstrated that in the testes, a
large proportion of lipoprotein-derived cholesterol used for
steroidogenesis is acquired through SR-BI (45). Akpovi et al (20) observed that, in mink testes, the
overexpression of SR-BI is associated with increased esterified
cholesterol levels in Sertoli cells. In addition, Casado et
al (48) reported that
hormone-sensitive lipase knockout mice exhibited upregulated SR-BI
expression, increased phosphorylated (p)-extracellular
signal-regulated kinase, p-Akt and p-SRC proto-oncogene levels, and
lipid accumulation in Sertoli cells, indicating that SR-BI may be
involved in the uptake of CE for spermatogenesis and
steroidogenesis in the testes.
LXRα/β
LXRα/β have important roles in the maintenance of
cellular cholesterol homeostasis. The tissue distribution of these
two LXR isoforms is different. LXRβ is expressed ubiquitously
throughout the body, while LXRα is predominantly distributed in the
kidney, liver and intestine (49,50).
Oxysterols, which are the oxidized derivatives of cholesterol, are
the endogenous ligands for LXRs. In addition, T0901317 and GW3965
are synthetic specific ligands. Upon activation, LXRs form
heterodimers with RXRs, which are capable of activating the
transcription of genes involved in cholesterol efflux. Previously
established LXR target genes include ABCA1/G1/G5/G8 (51–53),
apolipoprotein A1, apolipoprotein E (54,55)
and SR-BI (56). Robertson et
al (25) demonstrated that
from as early as 2.5 months, cholesterol began to accumulate in the
Sertoli cells of LXRβ−/− mice. At around 10 months,
although Sertoli cells structure remained comparatively intact and
spermatocytes and spermatogonia were still detectable, large and
numerous droplets, in addition to the absence of mature germ cells,
were observed. By 20 months, lipid accumulation was highest and few
cell types were observed. Genetic data from whole testis indicated
that the LXRβ was the predominant transcript in the testis
(25). Furthermore, Annicotte
et al (57) reported that
LXRβ was present specifically in the Sertoli cells and seminiferous
tubules as early as 16.5 days post-conception in embryos, which
further highlights the importance of LXRβ in these cells.
Furthermore, results also indicated that LXRα cannot be detected
with in situ hybridization (57), and that LXRα−/− mice did
not exhibit lipid accumulation and exhibited almost undisturbed
spermatogenesis, indicating that the presence of LXRβ is enough to
maintain cholesterol homeostasis in the testis. More importantly,
in the LXRα−/−/β−/− mouse, the testicular
phenotype is more destructive compared with that observed in the
LXRβ−/− mouse. Furthermore, it was also demonstrated
that WT animals that were fed a diet mixed with the synthetic LXR
agonist T0901317 exhibited increased levels of the LXR target genes
sterol regulatory element-binding protein-1c (SREBP-1c) and ABCG1
in their testes (53,58), which was supported by results in
MSC-1 cells treated with T0901317 where ABCA1 levels were increased
(51). LXR deficiency may also
cause dysfunction in these regulatory elements in Sertoli cells.
However, the accurate molecular mechanisms underlying the
regulation of cholesterol homeostasis by LXR in Sertoli cells and
male fertility require further investigation.
RXR α/β/γ
RXRα/β/γ belongs to the vertebrate nuclear receptor
superfamily (59). In rats, RXRα/β
transcripts are distributed widely throughout the body, whereas
RXRγ is only expressed in certain types of tissues. It has been
demonstrated that RXRβ is necessary for normal spermatogenesis in
rats, and RXRβ−/− males exhibit abnormalities in
spermiogenesis and spermiation. The biological functions of Sertoli
cells may be severely affected by abnormal RXRβ expression, which
is supported by various observations. In the testes of WT mice,
only Sertoli cells express RXRβ. In addition, in
RXRβ−/−mice, Sertoli cell abnormalities precede the
presence of abnormal spermatids by at least a week. In 29-day-old
mutants, prior to the completion of the first spermatogenic cycle,
lipid droplets were observed in Sertoli cells (49). Therefore, the earliest lipid
accumulation detected in Sertoli cells cannot due to the
phagocytosis of spermatids remnants. Furthermore, in the testes of
retinoic acid receptor (RAR)α−/− mice and not
RARβ−/−mice, although sperm release was blocked during
phagocytosis of retained spermatids, lipid droplets in Sertoli
cells were not observed (60),
indicating that lipid accumulation cannot be attributed to the
impaired spermiation. Combined, the results indicate that the lipid
deposition observed in the Sertoli cells of RXRβ−/− mice
reflects a complex metabolic disorder. As Sertoli cells are
critical for spermatid maturation, the obstacles of spermiation and
spermiogenesis in RXRβ−/− mutant testes may due to
compromised function of Sertoli cells. Furthermore, as RXRβ may
serve as a heterodimeric partner for peroxisome
proliferator-activated receptor (PPAR)β, which is expressed
abundantly in Sertoli cells (61),
compromised PPAR function may also contribute to the lipid
deposition in RXRβ−/− mutant Sertoli cells (62). However, the role of RXR in the
lipid metabolism of Sertoli cells requires further
investigation.
FSH and insulin
FSH, which is produced and secreted by the anterior
pituitary gland, is a crucial reproduction factor in mammals. In
females, FSH facilitates the maturation of follicles and the
production of estrogen, while in males it enhances Sertoli cell
proliferation in the immature testis and mature spermatogenesis
(63,64). The levels of FSH are regulated by
hormones released by gonadotropin through estrogen feedback and the
hypothalamic pituitary gonadal axis (65). Guma et al (66) demonstrated that FSH and insulin
promoted the lipid metabolism of Sertoli cells by augmenting
acetate incorporation into Sertoli cell lipids. These stimulatory
activities of FSH and insulin do not involve protein synthesis,
indicating a potential effect via modulation of enzyme activity or
glucose transport in Sertoli cells. In addition, treatment with
insulin, but not FSH, also enhanced the ATP citrate lyase activity,
indicating the biological relevance of insulin on the fatty acid
synthesis of Sertoli cells. Oliveira et al (67) demonstrated that insulin signaling
is indispensable for Sertoli cell lipid metabolism, as the absence
of insulin transforms Sertoli cell metabolism from glycolysis to
the Krebs cycle, which inhibits the development of germ cells.
Sex sterols and PPAR α/β/δ/γ
Sex steroids are also involved in the modulation of
cholesterol metabolism in Sertoli cells. The effects of
5α-dihydrotestosterone on Sertoli cell cholesterol metabolism
resembled those of insulin, but were less obvious. By contrast,
17β-estradiol (E2) promotes increases in acetate concentration by
amplifying the transcript levels of acetyl-CoA hydrolase, therefore
contributing to the production of by-products that are essential
for the maintenance of cholesterol synthesis in Sertoli cells
(68). In mature Sertoli cells,
18-carbon polyunsaturated fatty acids (PUFAs) are efficiently
converted into 22- and 24-carbon PUFAs with the assistance of
certain metabolic enzymes, including fatty acid elongases, and Δ5
and Δ6 desaturases (69). However,
hormonal dysregulation may disrupt PUFA synthesis, as Sertoli cells
treated with testosterone exhibited reduced activities of the Δ5
and Δ6 desaturases, therefore repressing the steps involving Δ5 and
Δ6 desaturases (70). These
effects are important as inhibition of Δ5 and Δ6 desaturases
activity is likely to restrict the incorporative process of long
chain PUFAs into sperm membranes, which may ultimately decrease
sperm membrane flexibility and fluidity. PUFAs possess several
double bonds in their backbone, which, with an increased number of
lipids, improves the fluidity and flexibility of sperm membranes
(71).
In addition, lipid oxidation in Sertoli cells is
also regulated by PPARα/β/δ/γ, which serve as sensors and
derivatives of fatty acids and thus influence the lipid and
cholesterol metabolic pathways. Regueira et al (72) demonstrated that the activation of
PPARα and PPARβ/δ augmented the expression of the cholesterol
transporter SR-BI in Sertoli cells, therefore promoting the
selective uptake of CE. Furthermore, PPAR activation enhanced
acetyl-CoA carboxylase phosphorylation, resulting in reduced enzyme
activity, which promotes the incorporation of fatty acyl-CoA into
the mitochondria for further oxidation, and increased long and
medium chain dehydrogenase enzyme and L-carnitine palmitoyl
transferase 1 mRNA levels (72).
These results indicate that PPARα and PPARβ/δ are indispensable for
cholesterol metabolism and lipid oxidation in Sertoli cells, while
the upstream factors regulated by the PPAR system remain
uncertain.
Conclusions and perspectives
Sertoli cells are essential for spermatogenesis as
they provide developing germ cells with physical support and a
source of growth factors, hormones, nutrients and energy. The
regulation of Sertoli cell metabolism has received attention from
numerous reproductive biologists as it may be involved in the fate
of germ cells. In fact, the maintenance of spermatogenesis
primarily relies on the metabolic collaboration between Sertoli and
germ cells, which is shown as a schematic representation in
Fig. 3. It involves various
metabolic pathways and a network of signal transduction. Various
factors regulate metabolic activity in Sertoli cells, which are
primary targets for hormonal signaling. Any alterations in the
metabolic behavior of Sertoli cells may cause abnormalities in
spermatogenesis and ultimately lead to male fertility. Thus,
Sertoli cells metabolism has a key role in the regulation of
spermatogenesis.
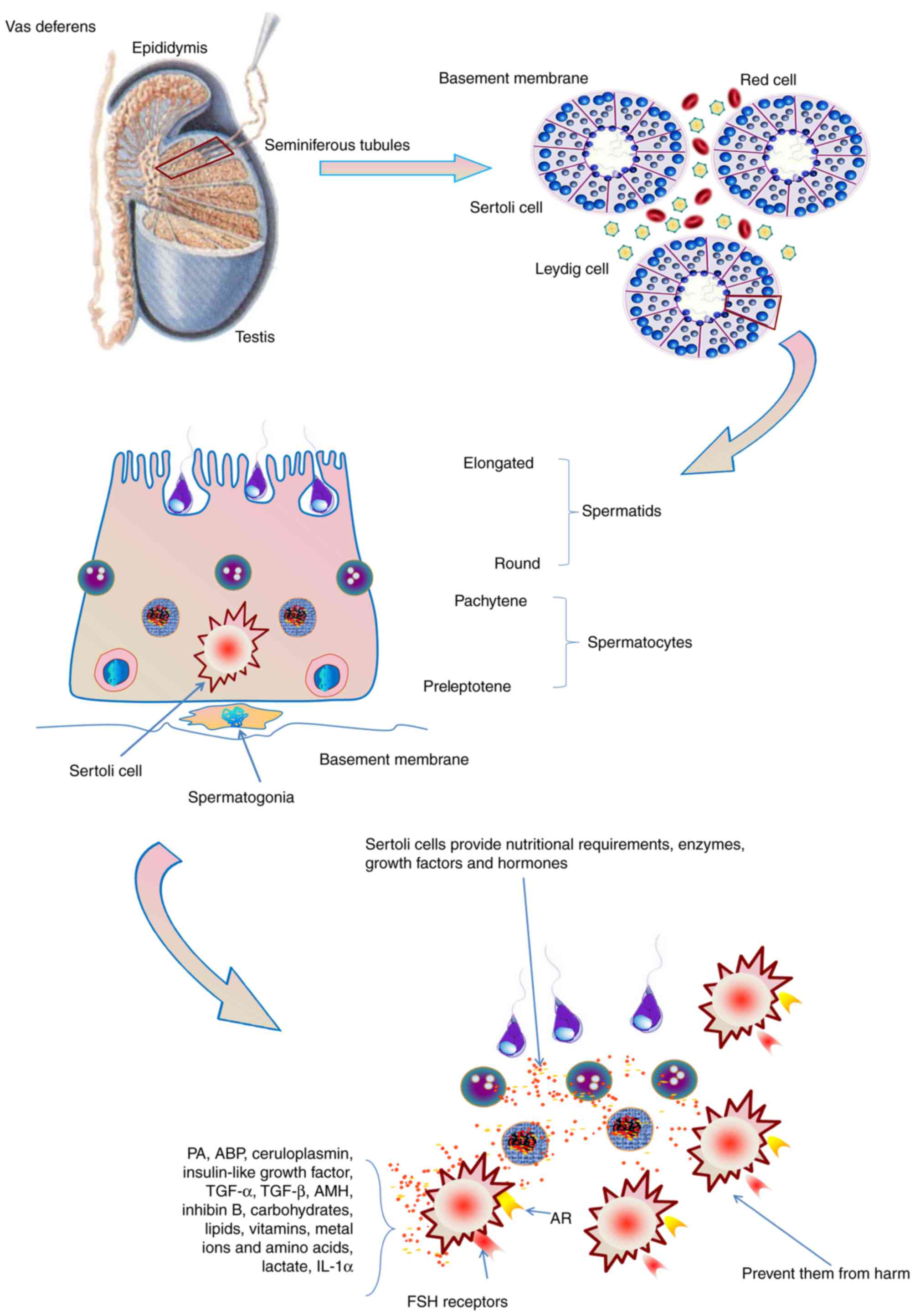 | Figure 3.The role of Sertoli cells in
spermatogenesis. Sertoli cells provide the nutritional requirements
of germ cells, including carbohydrates, lipids, vitamins, metal
ions and amino acids in spermatogenesis. Furthermore, a functional
Sertoli cell produces various types of enzymes, growth factors and
hormones, and prevent germ cells from harm. Sertoli cells also
express the AR and receptors for FSH. AR, androgen receptor; FSH,
follicle-stimulating hormone; PA, plasminogen activator; ABP,
androgen-binding protein; TGF, transforming growth factor; AMH,
anti-Müllerian hormone; IL, interleukin. |
Currently, the source of cholesterol utilized for
Sertoli cell metabolism and spermatogenesis is not
well-established, which is largely because the process is complex
and suitable in vitro systems are scarce. Sertoli cells
obtain a small portion of cholesterol from the de novo
synthesis pathway, whereas the majority of cholesterol is obtained
from lipoprotein particles in the interstitial compartment, as
described above. The uncertainty may be resolved by thoroughly
checking the phenotypes of conditional cell knockouts of genes that
are implicated in cholesterol synthesis and transport in various
types of testicular cells. In this respect, such a model has not
previously been developed. Certain studies have examined mouse
models with large-scale inactivation of genes responsible for
cholesterol transport, with the results indicating that cholesterol
transport is essential for normal spermatogenesis. For example,
mice with overall knockout of type 2 apolipoprotein E receptor
(73) and mice with partial
knockout of apolipoprotein B (74)
exhibited severely abnormal fertility. In addition, further
cholesterol-associated genes and proteins are also implicated in
male fertility, as demonstrated by different mouse models (51–56).
Furthermore, negative effects of overload of dietary cholesterol on
fertility in mice further demonstrated the importance of
cholesterol transport in sperm development (75,76).
The examination of conditional knockouts of cytochrome P450 family
51 in the testis indicated that de novo cholesterol
synthesis has an important role in spermatogenesis. Detecting the
phenotypes of the testis and fertility in these knockout models
provides important insights into the importance of cholesterol
synthesis and transport in germ and Sertoli cells, as well as in
male fertility.
Additionally, certain developed methods, including
high-resolution lipid imaging in vivo or in vitro,
may be instrumental in understanding the process of cholesterol
metabolism in Sertoli cells (77).
For example, Gimpl and Gehrig-Burger (78) reported that fluorescent and
photoreactive sterol probes reflect the movement and location of
these lipids, in addition to the cross-talk with proteins. Although
these novel methods have not been largely applied to the
investigation of Sertoli cell metabolism, they hold huge promise
for understanding cholesterol and lipid metabolism in Sertoli cells
during spermatogenesis, and may also help to explain previously
unexplained male infertility cases, which may be caused by
perturbations of cholesterol metabolism in the testes due to
various environmental or genetic factors.
Acknowledgements
The present review was supported by grants from the
Natural Sciences Foundation of Hunan Province (grant nos. 14JJ2084
and 2015JJ2128), the Zhengxiang Scholar (Xiangyang Tang) Program of
the University of South China, The Construct Program of the Key
Discipline in Hunan Province (Basic Medicine Sciences in University
of South China) and Hunan Province Cooperative Innovation Center
for Molecular Target New Drug Study (2015–351).
Glossary
Abbreviations
Abbreviations:
|
ABP
|
androgen-binding protein
|
|
ABCA1
|
ATP-binding cassette subfamily A
member 1
|
|
LDL
|
low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
AMH
|
anti-Müllerian hormone
|
|
FSH
|
follicle-stimulating hormone
|
|
SR-BI
|
scavenger receptor class B member
I
|
|
RCT
|
reverse cholesterol transport
|
|
CE
|
cholesterol esters
|
|
LXR
|
liver X receptor
|
|
AS
|
atherosclerosis
|
|
RXR
|
retinoid X receptor
|
|
SREBP-1c
|
sterol regulatory element-binding
protein-1c
|
|
PS
|
phosphatidylserine
|
References
|
1
|
Bhasin S: Approach to the infertile man. J
Clin Endocrinol Metab. 92:1995–2004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holstein AF, Schulze W and Davidoff M:
Understanding spermatogenesis is a prerequisite for treatment.
Reprod Biol Endocrinol. 1:1072003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selva DM, Hirsch-Reinshagen V, Burgess B,
Zhou S, Chan J, McIsaac S, Hayden MR, Hammond GL, Vogl AW and
Wellington CL: The ATP-binding cassette transporter 1 mediates
lipid efflux from Sertoli cells and influences male fertility. J
Lipid Res. 45:1040–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chase TH, Lyons BL, Bronson RT, Foreman O,
Donahue LR, Burzenski LM, Gott B, Lane P, Harris B, Ceglarek U, et
al: The mouse mutation ‘thrombocytopenia and cardiomyopathy’ (trac)
disrupts Abcg5: A spontaneous single gene model for human
hereditary phytosterolemia/sitosterolemia. Blood. 115:1267–1276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang LS, Voyiaziakis E, Markenson DF,
Sokol KA, Hayek T and Breslow JL: apo B gene knockout in mice
results in embryonic lethality in homozygotes and neural tube
defects, male infertility, and reduced HDL cholesterol ester and
apo A-I transport rates in heterozygotes. The Journal of clinical
investigation. 96:2152–2161. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snouwaert JN, Brigman KK, Latour AM,
Malouf NN, Boucher RC, Smithies O and Koller BH: An animal model
for cystic fibrosis made by gene targeting. Science. 257:1083–1088.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blendy JA, Kaestner KH, Weinbauer GF,
Nieschlag E and Schütz G: Severe impairment of spermatogenesis in
mice lacking the CREM gene. Nature. 380:162–165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fofana M, Maboundou JC, Bocquet J and Le
Goff D: Transfer of cholesterol between high density lipoproteins
and cultured rat Sertoli cells. Biochem Cell Biol. 74:681–686.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umehara T, Kawashima I, Kawai T, Hoshino
Y, Morohashi KI, Shima Y, Zeng W, Richards JS and Shimada M:
Neuregulin 1 regulates proliferation of Leydig cells to support
spermatogenesis and sexual behavior in adult mice. Endocrinology.
157:4899–4913. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen JM and Dietschy JM: Relative
importance of high and low density lipoproteins in the regulation
of cholesterol synthesis in the adrenal gland, ovary, and testis of
the rat. J Biol Chem. 253:9024–9032. 1978.PubMed/NCBI
|
|
11
|
Steinberger E, Root A, Ficher M and Smith
KD: The role of androgens in the initiation of spermatogenesis in
man. J Clin Endocrinol Metab. 37:746–751. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLachlan RI, Wreford NG, O'Donnell L, de
Kretser DM and Robertson DM: The endocrine regulation of
spermatogenesis: Independent roles for testosterone and FSH. J
Endocrinol. 148:1–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boussouar F and Benahmed M: Lactate and
energy metabolism in male germ cells. Trends Endocrinol Metab.
15:345–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grass J and Hauser ER: The influence of
early age mastectomy and unilateral ovariectomy on reproductive
performance of the bovine. J Anim Sci. 53:171–176. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grootegoed JA, Oonk RB, Jansen R and van
der Molen HJ: Metabolism of radiolabelled energy-yielding
substrates by rat Sertoli cells. J Reprod Fertil. 77:109–118. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson R and Fritz IB: Metabolism of
glucose by Sertoli cells in culture. Biol Reprod. 24:1032–1041.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mruk DD and Cheng CY: Sertoli-Sertoli and
Sertoli-germ cell interactions and their significance in germ cell
movement in the seminiferous epithelium during spermatogenesis.
Endocr Rev. 25:747–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petersen C and Soder O: The sertoli cell-a
hormonal target and ‘super’ nurse for germ cells that determines
testicular size. Horm Res. 66:153–161. 2006.PubMed/NCBI
|
|
19
|
Wiebe JP and Tilbe KS: De novo synthesis
of steroids (from acetate) by isolated rat Sertoli cells. Biochem
Biophys Res Commun. 89:1107–1113. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akpovi CD, Yoon SR, Vitale ML and
Pelletier RM: The predominance of one of the SR-BI isoforms is
associated with increased esterified cholesterol levels not
apoptosis in mink testis. J Lipid Res. 47:2233–2247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fofana M, Travert C, Carreau S and Le Goff
D: Evaluation of cholesteryl ester transfer in the seminiferous
tubule cells of immature rats in vivo and in vitro. J Reprod
Fertil. 118:79–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakanishi Y and Shiratsuchi A: Phagocytic
removal of apoptotic spermatogenic cells by Sertoli cells:
Mechanisms and consequences. Biol Pharm Bull. 27:13–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pelletier RM: The blood-testis barrier:
The junctional permeability, the proteins and the lipids. Prog
Histochem Cytochem. 46:49–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nebel A, Flachsbart F, Till A, Caliebe A,
Blanché H, Arlt A, Häsler R, Jacobs G, Kleindorp R, Franke A, et
al: A functional EXO1 promoter variant is associated with prolonged
life expectancy in centenarians. Mech Ageing Dev. 130:691–699.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robertson KM, Schuster GU, Steffensen KR,
Hovatta O, Meaney S, Hultenby K, Johansson LC, Svechnikov K, Söder
O and Gustafsson JA: The liver X receptor-{beta} is essential for
maintaining cholesterol homeostasis in the testis. Endocrinology.
146:2519–2530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eddy EM: Regulation of gene expression
during spermatogenesis. Semin Cell Dev Biol. 9:451–457. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodgson YM, Irby DC, Kerr JB and de
Kretser DM: Studies of the structure and function of the Sertoli
cell in a seasonally breeding rodent. Biol Reprod. 21:1091–1098.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osuga J, Ishibashi S, Oka T, Yagyu H,
Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O
and Yamada N: Targeted disruption of hormone-sensitive lipase
results in male sterility and adipocyte hypertrophy, but not in
obesity. Proc Natl Acad Sci USA. 97:787–792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gwynne JT and Strauss JF III: The role of
lipoproteins in steroidogenesis and cholesterol metabolism in
steroidogenic glands. Endocr Rev. 3:299–329. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kabbaj O, Yoon SR, Holm C, Rose J, Vitale
ML and Pelletier RM: Relationship of the hormone-sensitive
lipase-mediated modulation of cholesterol metabolism in individual
compartments of the testis to serum pituitary hormone and
testosterone concentrations in a seasonal breeder, the mink
(Mustela vison). Biol Reprod. 68:722–734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wechsler A, Brafman A, Shafir M, Heverin
M, Gottlieb H, Damari G, Gozlan-Kelner S, Spivak I, Moshkin O,
Fridman E, et al: Generation of viable cholesterol-free mice.
Science. 302:20872003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Potter JE, Millette CF, James MJ and
Kandutsch AA: Elevated cholesterol and dolichol synthesis in mouse
pachytene spermatocytes. J Biol Chem. 256:7150–7154.
1981.PubMed/NCBI
|
|
33
|
Brewer HB Jr and Santamarina-Fojo S:
Clinical significance of high-density lipoproteins and the
development of atherosclerosis: Focus on the role of the adenosine
triphosphate-binding cassette protein A1 transporter. Am J Cardiol.
92:10K–6K. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayden MR, Clee SM, Brooks-Wilson A,
Genest J Jr, Attie A and Kastelein JJ: Cholesterol efflux
regulatory protein, Tangier disease and familial high-density
lipoprotein deficiency. Curr Opin Lipidol. 11:117–122. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singaraja RR, Brunham LR, Visscher H,
Kastelein JJ and Hayden MR: Efflux and atherosclerosis: The
clinical and biochemical impact of variations in the ABCA1 gene.
Arterioscler Thromb Vasc Biol. 23:1322–1332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singaraja RR, Bocher V, James ER, Clee SM,
Zhang LH, Leavitt BR, Tan B, Brooks-Wilson A, Kwok A, Bissada N, et
al: Human ABCA1 BAC transgenic mice show increased high density
lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an
internal promoter containing liver X receptor response elements in
intron 1. J Biol Chem. 276:33969–33979. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singaraja RR, Fievet C, Castro G, James
ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus
BM, et al: Increased ABCA1 activity protects against
atherosclerosis. J Clin Invest. 110:35–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haghpassand M, Bourassa PA, Francone OL
and Aiello RJ: Monocyte/macrophage expression of ABCA1 has minimal
contribution to plasma HDL levels. J Clin Invest. 108:1315–1320.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aiello RJ, Brees D, Bourassa PA, Royer L,
Lindsey S, Coskran T, Haghpassand M and Francone OL: Increased
atherosclerosis in hyperlipidemic mice with inactivation of ABCA1
in macrophages. Arterioscler Thromb Vasc Biol. 22:630–637. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wellington CL, Walker EK, Suarez A, Kwok
A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE,
et al: ABCA1 mRNA and protein distribution patterns predict
multiple different roles and levels of regulation. Lab Invest.
82:273–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lawn RM, Wade DP, Couse TL and Wilcox JN:
Localization of human ATP-binding cassette transporter 1 (ABC1) in
normal and atherosclerotic tissues. Arterioscler Thromb Vasc Biol.
21:378–385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pelletier RM and Byers SW: The
blood-testis barrier and Sertoli cell junctions: Structural
considerations. Microsc Res Tech. 20:3–33. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Plump AS, Erickson SK, Weng W, Partin JS,
Breslow JL and Williams DL: Apolipoprotein A-I is required for
cholesteryl ester accumulation in steroidogenic cells and for
normal adrenal steroid production. J Clin Invest. 97:2660–2671.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Pietro N, Formoso G and Pandolfi A:
Physiology and pathophysiology of oxLDL uptake by vascular wall
cells in atherosclerosis. Vascul Pharmacol. 84:1–7. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rigotti A, Miettinen HE and Krieger M: The
role of the high-density lipoprotein receptor SR-BI in the lipid
metabolism of endocrine and other tissues. Endocr Rev. 24:357–387.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Landschulz KT, Pathak RK, Rigotti A,
Krieger M and Hobbs HH: Regulation of scavenger receptor, class B,
type I, a high density lipoprotein receptor, in liver and
steroidogenic tissues of the rat. J Clin Invest. 98:984–995. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shiratsuchi A, Kawasaki Y, Ikemoto M, Arai
H and Nakanishi Y: Role of class B scavenger receptor type I in
phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells.
J Biol Chem. 274:5901–5908. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Casado ME, Huerta L, Ortiz AI,
Pérez-Crespo M, Gutiérrez-Adán A, Kraemer FB, Lasunción MÁ, Busto R
and Martín-Hidalgo A: HSL-knockout mouse testis exhibits class B
scavenger receptor upregulation and disrupted lipid raft
microdomains. J Lipid Res. 53:2586–2597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Seol W, Choi HS and Moore DD: Isolation of
proteins that interact specifically with the retinoid X receptor:
Two novel orphan receptors. Mol Endocrinol. 9:72–85. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Willy PJ, Umesono K, Ong ES, Evans RM,
Heyman RA and Mangelsdorf DJ: LXR, a nuclear receptor that defines
a distinct retinoid response pathway. Genes Dev. 9:1033–1045. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Costet P, Luo Y, Wang N and Tall AR:
Sterol-dependent transactivation of the ABC1 promoter by the liver
X receptor/retinoid X receptor. J Biol Chem. 275:28240–28245.
2000.PubMed/NCBI
|
|
52
|
Repa JJ, Turley SD, Lobaccaro JA, Medina
J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM and Mangelsdorf
DJ: Regulation of absorption and ABC1-mediated efflux of
cholesterol by RXR heterodimers. Science. 289:1524–1529. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kennedy MA, Venkateswaran A, Tarr PT,
Xenarios I, Kudoh J, Shimizu N and Edwards PA: Characterization of
the human ABCG1 gene: Liver X receptor activates an internal
promoter that produces a novel transcript encoding an alternative
form of the protein. J Biol Chem. 276:39438–39447. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fayard E, Schoonjans K and Auwerx J: Xol
INXS: Role of the liver X and the farnesol X receptors. Curr Opin
Lipidol. 12:113–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oram JF and Vaughan AM: ABCA1-mediated
transport of cellular cholesterol and phospholipids to HDL
apolipoproteins. Curr Opin Lipidol. 11:253–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Malerød L, Juvet LK, Hanssen-Bauer A,
Eskild W and Berg T: Oxysterol-activated LXRalpha/RXR induces
hSR-BI-promoter activity in hepatoma cells and preadipocytes.
Biochem Biophys Res Commun. 299:916–923. 2000. View Article : Google Scholar
|
|
57
|
Annicotte JS, Schoonjans K and Auwerx J:
Expression of the liver X receptor alpha and beta in embryonic and
adult mice. Anat Rec A Discov Mol Cell Evol Biol. 277:312–316.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Repa JJ, Liang G, Ou J, Bashmakov Y,
Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL and
Mangelsdorf DJ: Regulation of mouse sterol regulatory
element-binding protein-1c gene (SREBP-1c) by oxysterol receptors,
LXRalpha and LXRbeta. Genes Dev. 14:2819–2830. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Evans RM and Mangelsdorf DJ: Nuclear
Receptors, RXR, and the Big Bang. Cell. 157:255–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lohnes D, Mark M, Mendelsohn C, Dollé P,
Dierich A, Gorry P, Gansmuller A and Chambon P: Function of the
retinoic acid receptors (RARs) during development (I). Craniofacial
and skeletal abnormalities in RAR double mutants. Development.
120:2723–2748. 1994.PubMed/NCBI
|
|
61
|
Braissant O, Foufelle F, Scotto C, Dauca M
and Wahli W: Differential expression of peroxisome
proliferator-activated receptors (PPARs): Tissue distribution of
PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology.
137:354–366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kastner P, Mark M, Leid M, Gansmuller A,
Chin W, Grondona JM, Décimo D, Krezel W, Dierich A and Chambon P:
Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev.
10:80–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Macklon NS and Fauser BC: Follicle
development during the normal menstrual cycle. Maturitas.
30:181–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Plant TM and Marshall GR: The functional
significance of FSH in spermatogenesis and the control of its
secretion in male primates. Endocr Rev. 22:764–786. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Midgley Ar J and Jaffe RB: Regulation of
human gonadotropins: 4. Correlation of serum concentration s of
follicle stimulating and luteinizing hormones during the menstrual
cycle. J Clin Endocrinol. 28:1699–1703. 1999. View Article : Google Scholar
|
|
66
|
Guma FC, Wagner M, Martini LH and Bernard
EA: Effect of FSH and insulin on lipogenesis in cultures of Sertoli
cells from immature rats. Braz J Med Biol Res. 30:591–597. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Oliveira PF, Alves MG, Rato L, Laurentino
S, Silva J, Sá R, Barros A, Sousa M, Carvalho RA, Cavaco JE and
Socorro S: Effect of insulin deprivation on metabolism and
metabolism-associated gene transcript levels of in vitro cultured
human Sertoli cells. Biochim Biophys Acta. 1820:84–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Oliveira PF, Alves MG, Rato L, Silva J, Sá
R, Barros A, Sousa M, Carvalho RA, Cavaco JE and Socorro S:
Influence of 5α-dihydrotestosterone and 17β-estradiol on human
Sertoli cells metabolism. Int J Androl. 34:e612–e620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Saether T, Tran TN, Rootwelt H,
Christophersen BO and Haugen TB: Expression and regulation of
delta5-desaturase, delta6-desaturase, stearoyl-coenzyme A (CoA)
desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol
Reprod. 69:117–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Carosa E, Radico C, Giansante N, Rossi S,
D'Adamo F, Di Stasi SM, Lenzi A and Jannini EA: Ontogenetic profile
and thyroid hormone regulation of type-1 and type-8 glucose
transporters in rat Sertoli cells. Int J Androl. 28:99–106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Israelachvili JN, Marcelja S and Horn RG:
Physical principles of membrane organization. Q Rev Biophys.
13:121–200. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Regueira M, Riera MF, Galardo MN,
Pellizzari EH, Cigorraga SB and Meroni SB: Activation of PPAR α and
PPAR β/δ regulates Sertoli cell metabolism. Mol Cell Endocrinol.
382:271–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Manova K and Bachvarova RF: Expression of
c-kit encoded at the W locus of mice in developing embryonic germ
cells and presumptive melanoblasts. Dev Biol. 146:312–324. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Orth JM, Jester WF Jr and Qiu J: Gonocytes
in testes of neonatal rats express the c-kit gene. Mol Reprod Dev.
45:123–131. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rossi P, Albanesi C, Grimaldi P and
Geremia R: Expression of the mRNA for the ligand of c-kit in mouse
Sertoli cells. Biochem Biophys Res Commun. 176:910–914. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mauduit C, Hamamah S and Benahmed M: Stem
cell factor/c-kit system in spermatogenesis. Hum Reprod Update.
5:535–545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dias TR, Rato L, Martins AD, Simões VL,
Jesus TT, Alves MG and Oliveira PF: Insulin deprivation decreases
caspase-dependent apoptotic signaling in cultured rat sertoli
cells. ISRN Urol. 2013:9703702013.PubMed/NCBI
|
|
78
|
Gimpl G and Gehrig BK: Probes for studying
cholesterol binding and cell biology. Steroids. 76:216–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|