Introduction
Spinal cord injury (SCI) is a severe condition of
the central nervous system, which represents a severe health
problem that is associated with life-long disabilities and high
mortality (1–3). The WHO reported in November 2013 that
worldwide, 250,000–500,000 people annually suffer a spinal cord
injury (4). It is well known that
in addition to white matter axon damage, oligodendrocyte cell death
(5) and demyelination in spared
axons (6–9) may lead to the initial deficits
associated with human SCC and impair functional recovery, which
represents a challenge for the effective treatment of SCI.
Oligodendrocytes are cells that produce the myelin sheath, which
have been observed to die hours to weeks after SCI, near and
distant to the epicenter, leading to demyelination (10–16).
Therefore, therapeutic measures are required to reduce
oligodendrocyte death and/or to enhance remyelination. Replacing
lost oligodendrocytes, preventing the progression of demyelination
or promoting remyelination is critical for functional recovery
following SCC. In previous years, the advancement of cell therapy
for SCI has been encouraging (17,18).
Cell transplantation has been reported to promote neuroprotection
by releasing trophic factors, providing a growth-promoting matrix
for regeneration of axons or replacing lost cells (19). However, mature oligodendrocytes are
unable to remyelinate naked axons in SCI, and also inhibit axonal
regeneration by releasing myelin-associated inhibitors, including
Nogo, myelin-associated glycoprotein and oligodendrocyte myelin
glycoprotein (20,21). Therefore, to the best of our
knowledge, no studies regarding the treatment of SCI with
oligodendrocyte transplantation have been conducted. However, with
the progress made regarding neural development, more studies have
reported the possibility of using oligodendrocyte precursor cell
(OPC) transplantation for the treatment of demyelinating diseases,
including SCC (22,23). Therefore, OPC transplantation may
be considered a potential therapy for the treatment of SCI
(24).
It has been reported that human embryonic stem cell
(hESC)-derived OPC transplantation may attenuate lesion
pathogenesis and improve recovery of forelimb function in rats with
cervical SCC (25). In addition,
hESC-derived OPC transplantation into SCI sites in adult rats
resulted in remyelination and functional repair (22). Combined treatment with OPC grafts
expressing ciliary neurotrophic factor (CNTF) can also enhance
remyelination and facilitate functional recovery following SCC
(26). However, the molecular
mechanism underlying the effects of OPC transplantation on SCI
treatment, particularly regarding microRNA (miRNA) expression, is
not fully understood.
miRNAs are ~23 nucleotide long endogenous
ribonucleic acid molecules that specifically pair with the 3′
untranslated region of target mRNAs post-transcription, in order to
direct mRNA degradation and suppress protein translation (27–29).
Accumulating evidence has indicated that miRNAs serve crucial roles
in various human diseases, including cancer, metabolic diseases,
cardiovascular diseases, viral infections and contusive
neurological injuries (30,31).
miRNAs are also involved in numerous cellular processes, including
development, proliferation and differentiation (28,32).
Furthermore, numerous miRNAs have been detected in the mammalian
central nervous system, including the brain and spinal cord, where
they have key roles in neurodevelopment and are likely to be
important mediators of plasticity (33–36).
In addition, altered miRNA expression has been revealed in the
spinal cord following SCC through microarray analysis (37,38).
However, whether miRNAs are involved in the regulatory effects of
OPC transplantation on the spinal cord of rats with SCC remains
unclear.
In the present study, following OPC transplantation
into the spinal cord of rats with SCC, the Basso, Beattie and
Bresnahan (BBB) score and pain value were determined, in order to
assess functional recovery of rats. Subsequently, a miRNA assay was
performed to detect the differentially expressed miRNAs in the
spinal cord of rats with SCC and OPC transplantation compared with
in rats with SCC and medium transplantation. Furthermore,
quantitative polymerase chain reaction (qPCR) was used to verify
the significantly altered miRNA expression. These results may
identify the regulatory effects of miRNA in rats with SCC following
OPC transplantation, which may provide a novel potential molecular
target for the clinical treatment of SCI in the future.
Materials and methods
Induced pluripotent stem cell (iPSC)
preparation
The present study was approved by the ethics
committee of Kunming Medical University (Kunming, China;
KMMU2015013). Two pregnant mice were purchased from the
Experimental Animal Center of Kunming Medical University. Mice were
housed in cages at 22±2°C with 55±5% humidity under a 12-h
light/dark cycle with free access to food and water. The skin of
green fluorescent protein (GFP)-transgenic fetuses, obtained from
13.5-day-pregnant mice, was cut into small pieces and the skin
samples were digested using 0.25% trypsin. Subsequently, cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. On the same day, fetal skin cells were
co-transfected with 100 µl Moloney-based retroviral vectors
(provided by Su Liu, Johns Hopkins University, Baltimore, MD, USA)
that expressed octamer-binding transcription factor 3/4 (Oct3/4),
sex determining region Y box-2 Sox2), c-Myc and Kruppel-like factor
4 (Klf4) (Oct3/4: Sox2: c-Myc: Klf4=1:1:1:1) which were transfected
into PlatE clles using FuGENE 6 transfection reagent (Roche
Diagnostics, Basel, Switzerland), twice within 2 days. On day 5
post-transfection, the transfected cells (1×105) were
inoculated into mouse embryonic fibroblasts (MEFs; MTI-GlobalStem,
Gaithersburg, MD, USA) that were pre-processed with 10 µg/ml
mitomycin (cat. no. 01035, BBI Solutions, Cardiff, UK) for 1 day.
Subsequently, the medium was replaced with embryonic stem cell
growth medium (Invitrogen; Thermo Fisher Scientific, Inc.) every
day. On day 16, iPSC clones were picked, digested using trypsin and
inoculated into radiated MEFs that served as a feeder layer to
obtain amplification.
iPSC differentiation
The iPSCs were cultured as aforementioned. iPSCs
were separated by 0.25% trypsin every 4 days until passage 3 and
were incubated with collagenase IV (1% in DMEM/F12; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 15 min in order to
separate them from MEFs. After 4 days, iPSCs cultured in stem cell
differentiation medium became embryonic bodies. Subsequently, the
embryonic bodies were incubated with tretinoin (all-trans-retinoic
acid, 500 nM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
purmorphamin (1 µm; Calbiochem; EMD Millipore, Billerica, MA, USA)
for 4 days at 37°C. The stem cell differentiation medium consisted
of 50% DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.), 50%
Neurobasal medium (Gibco; Thermo Fisher Scientific, Inc.), 1% N2
(Gibco; Thermo Fisher Scientific, Inc.), 1% B27 (Gibco; Thermo
Fisher Scientific, Inc.), 10% serum fluid replacement, 0.1 mM
mercaptoethanol (Sigma-Aldrich; Merck KGaA), 2 mM Glutamax (Gibco;
Thermo Fisher Scientific, Inc.) and 2 µg/ml heparin (Sigma-Aldrich;
Merck KGaA). A total of 24 days were required for iPSC preparation
and differentiation.
Culture of iPSC-induced OPCs
At the 8th day of differentiation stage, the medium
was replaced with OPC+ medium supplemented with
platelet-derived growth factor (PDGF) and the cells were
continually cultured for 8–10 days. Subsequently, cells were
inoculated into plates that were coated with poly-D-lysine/laminin
(BioCoat; BD Biosciences, Franklin Lakes, NJ, USA) at 37°C to
proliferate for 2–3 days. The OPC+ medium contained 48%
neurobasal medium (Gibco; Thermo Fisher Scientific, Inc.), 48% ml
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.), 50X OPC medium
(Johns Hopkins University, Baltimore, MD, USA) 1/50 ml, 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
0.5% Glutamax (Invitrogen; Thermo Fisher Scientific, Inc.), 1%
non-essential amino acids and 0.02% fibroblast growth factor.
Animal care and grouping
A total of 32 male Sprague-Dawley rats (7 weeks-old,
weight, 200–250 g) were purchased from the Experimental Animal
Center of Kunming Medical University and were randomly divided into
four groups: Sham, SCC, SCC + medium (Medium) and SCC + OPC
transplantation (OPC) groups (Table
I). All animal care, breeding and testing procedures conformed
to the principles of guidance suggested in the National Institutes
of Health Guide for the Care and Use of Laboratory Animals and
supported by the Use Committee of Kunming Medical University. Rats
were housed in cages at 22±2°C with 55±5% humidity under a 12-h
light/dark cycle with free access to food and water.
 | Table I.Animals and grouping. |
Table I.
Animals and grouping.
| Group | BBB score (no. of
rats) | miRNA chip (no. of
rats) | qPCR (no. of
rats) |
|---|
| Sham | 5 | 0 | 0 |
| SCC | 5 | 0 | 0 |
| Medium | 5 | 3 | 3 |
| OPC | 5 | 3 | 3 |
Animal model
SCC was performed to induce SCI in the present
study. The rats were anesthetized by intraperitoneal injection with
3.6% chloral hydrate (380 mg/kg) and were placed in the prone
position. A surgical incision was made in the thoracic region
(T9-T11), and the paravertebral muscles and supraspinal ligaments
were separated. Subsequently, the rats underwent a T9-T11
laminectomy, and were subjected to SCC at T10 level using a 10 g
weight that was dropped from a 30 mm height. In addition, rats in
the Sham group underwent the laminectomy only. Finally, the
surgical wounds were sutured with a 3–0 silk suture. The rats were
then injected with 5 ml saline, and received 0.5 ml cefotaxime
sodium for 7 days and 0.05 ml Ciclosporin A injection (Novartis
International AG, Basel, Switzerland) for 28 days. Their bladders
were manually compressed three times a day until recovery of the
micturition reflex.
Immunofluorescence staining
Immunofluorescence staining of the differentiated
cells was performed as follows. Briefly, after OPCs were fixed with
4% paraformaldehyde for 10 min, they were washed three times (5 min
each) with PBS (0.01 mol/l). OPCs were subsequently incubated with
0.3% Triton X-100 for 30 min at 37°C and 5% goat serum for 30 min
at 37°C, respectively, and incubated with the primary antibody
against PDGF (1:100; Neomarkers, Inc., Fremont, CA) to identify
OPCs (39), at 4°C for 24 h.
Finally, OPCs were washed using 0.01 mol/l PBS three times and
incubated with Cy3 (1:100; Abcam, Cambridge, UK) for 60 min at
37°C. The nuclei were stained using DAPI and images were captured
under a Leica DMI6000B (LAS AF system; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA).
OPC transplantation
Rats in each group were placed in the prone position
and their vertebral laminae were reopened 7 days after SCC surgery.
Subsequently, 5 µl cell suspensions (2×105 cells/µl)
were transplanted into each rat at two different sites at the
rostral and tail end of the injury with a constant speed of 2.5
µl/min using a micropipette. The microinjector was maintained in
the injection site for 5 min prior to removal. The Medium group was
treated with OPC+ culture medium using the same
procedure, while the vertebral laminae of rats in the sham and SCC
control groups were re-opened without transplanting OPC or
OPC+ culture medium.
Assessment of locomotion recovery
Locomotion recovery of rats was evaluated using the
BBB Locomotion Rating Scale (40).
The BBB scores range between 0 and 21. Score 0 indicates flaccid
paralysis, whereas score 21 indicates normal gait. Three
investigators, blinded to the experimental groups, performed
behavioral experiments for rats in the Sham, SCC, Medium and OPC
groups, in order to reduce the error of subjectivity. The
assessments were performed on days 1, 3, 7, 14, 21 and 28 days
following the transplantation operation in an open field (41). The average score from the three
investigators was considered the final score.
Detection of mechanical pain
threshold
Mechanical pain threshold assessments were performed
on days 1, 3, 7, 14, 21 and 28 following the operation. Briefly,
prior to the paw withdrawal threshold test, the value of the
instrument was adjusted to zero. As the weight increased, the
pressure that the rat felt was gradually increased. When the
maximum pressure was reached it was recorded. This test was
repeated three times and interval time was maintained at ≥10
min.
Tissues collection
After the rats were anesthetized by intraperitoneal
injection with 3.6% chloral hydrate (380 mg/kg), spinal cord
tissues were harvested from Medium and OPC group rats 7 days
following the OPC/medium transplantation operation. The samples
were stored at −80°C for miRNA microarray analysis and qPCR.
miRNA microarray analysis
Samples were transported to Genminix Informatics
Co., Ltd. (Shanghai, China) for miRNA microarray analysis. Briefly,
total RNA was extracted from the spinal cord samples using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), quantified
and assessed for quality using a Nanodrop ND-1000 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA), and reverse transcribed
into cDNA using SuperScript II Reverse Transcriptase (cat. no.
18064014, Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, the cDNA samples were hybridized to the Affymetrix
Gene 2.0 Array (Affymetrix; Thermo Fisher Scientific, Inc.) to
detect differentially expressed miRNAs. Subsequently, the positive
signal was recorded using an Axon GenePix 4000B scanner (Molecular
Devices, LLC, Sunnyvale, CA, UAS). The scanned images were imported
into GenePix Pro 6.0 software (Axon Instruments; Molecular Devices,
LLC) for grid alignment and data extraction. All microarray signals
were analyzed by subtracting the background and were normalized
using median normalization. Following normalization, differentially
expressed miRNAs were identified according to fold change. In all
samples, only miRNAs with a fold change >2 and P<0.05 were
defined as significant.
Bioinformatics analysis of
differentially expressed miRNAs
Initially, the target genes of the miRNAs were
identified using the following prediction databases: miRecords
(c1.accurascience.com/miRecords/), TargetScan
(www.targetscan.org/vert_71/),
MicroCosm (www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/),
miRanda (www.microrna.org/microrna/home.do) and PicTar
(pictar.mdc-berlin.de/), and genes that
were identified across two or more databases were selected for
further analysis. Subsequently, a Gene Ontology (GO; www.geneontology.org/) enrichment analysis was
conducted to determine the significantly enriched biological
processes associated with the target genes of the differentially
expressed miRNAs. Furthermore, predicted target genes of the
differentially expressed miRNAs were analyzed using a functional
method mapping genes to Kyoto Encyclopedia of Genes and Genomes
(KEGG; www.genome.jp/kegg/) pathways.
qPCR
Total RNA was extracted from the spinal cord samples
of rats in the Medium and SCC groups using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), after which RNA was
reverse transcribed to produce cDNA using an reverse transcribed
reagent kit (Takara Bio, Inc.). Subsequently, the expression levels
of miR-375-3p, miR-1-3p, miR-363-3p, miR-449a-5p and miR-3074 were
analyzed using an SYBR Green RT-PCR Master Mix Kit (Takara Bio,
Inc.). The primers were provided by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China) and the catalogue numbers are provided in
Table II. β-actin was used as an
internal control; the primer sequences and annealing temperature
for β-actin were as follows: Forward 5′-GCCAACACAGTGCTGTCT-3′ and
reverse 5′-GGAGCAATGATCTTGATCTT-3′; annealing temperature, 53°C.
The reaction was performed at 95°C for 2 min and 40 cycles of 95°C
for 20 sec, 53°C for 30 sec and 60°C for 40 sec. Data were analyzed
using the quantification cycle (Cq) method (42), and target gene expression was
normalized to internal control expression.
 | Table II.Primer sequence catalogue numbers for
miRNAs. |
Table II.
Primer sequence catalogue numbers for
miRNAs.
| miRNA | Number |
|---|
| rno-miR-375-5p | RmiRQP3367 |
| rno-miR-1-3p | RmiRQP1188 |
| rno-miR-363-3p | RmiRQP3254 |
|
rno-miR-449a-5p | RmiRQP0504 |
| rno-miR-3074 | RmiRQP1482 |
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used to
analyze the data. Differences between two groups were assessed
using a t-test and among multiple groups using one-way analysis of
variance followed by least significant difference. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of GFP-OPCs induced
from iPSCs derived from MEFs
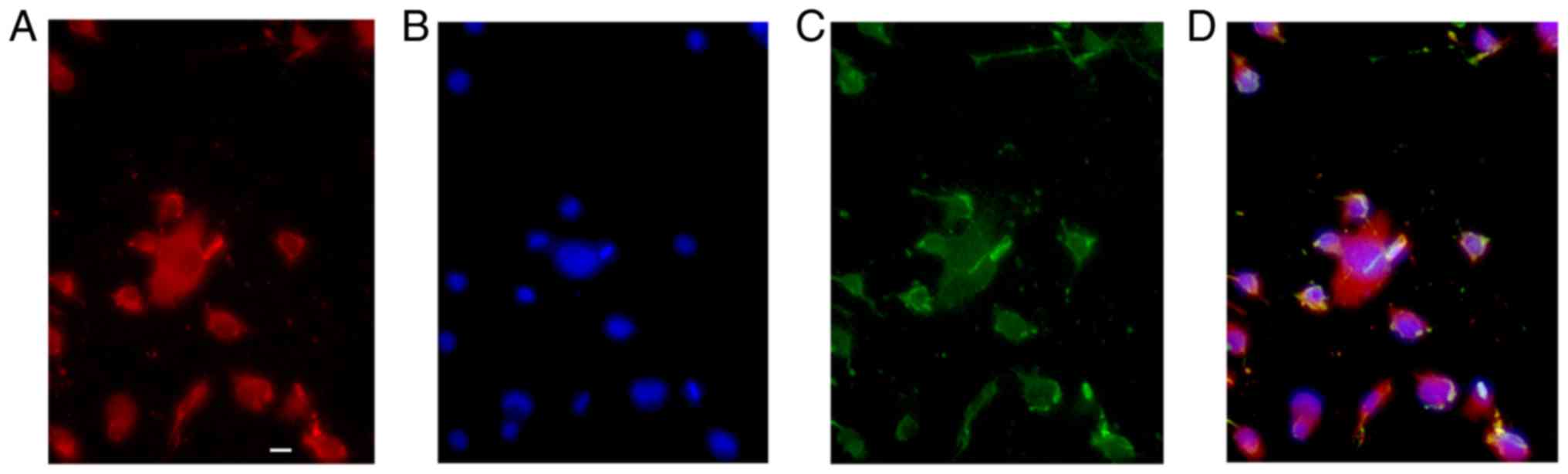
To identify the characteristics of GFP-OPCs induced
from iPSCs derived from MEFs, they were cultured for 24 days in
differentiation medium (Fig. 1).
When GFP-OPCs were cultured in basal-OPC-medium supplemented with
PDGF, almost all of the cells displayed the typical morphology of
OPCs (Fig. 1C), and >90% of
cells expressed PDGF (Fig. 1A and
D).
Transplantation of OPCs promotes motor
and sensory recovery of rats subjected to SCC
To investigate the effects of OPC transplantation on
the motor and sensory function of rats subjected to SCC, BBB and
paw withdrawal threshold analyses were conducted on rats from the
Sham, SCC, Medium and OPC groups. As presented in Fig. 2A, with the exception of day 1 and
3, post-transplantation, the BBB score demonstrated that rats in
the OPC group exhibited significant motor improvement compared with
those in the Medium group. Results of the paw withdrawal threshold
analysis indicated that OPCs attenuated SCC-induced mechanical
allodynia. Days 1 and 3 post-transplantation, there were no
significant differences between the average paw withdrawal
threshold for rats in the Medium and OPC groups. However, 7, 14, 21
and 28 days following the transplantation, the OPC group exhibited
a marked attenuation of mechanical allodynia (i.e. an increase in
paw-withdrawal threshold) compared with in the Medium group
(Fig. 2B).
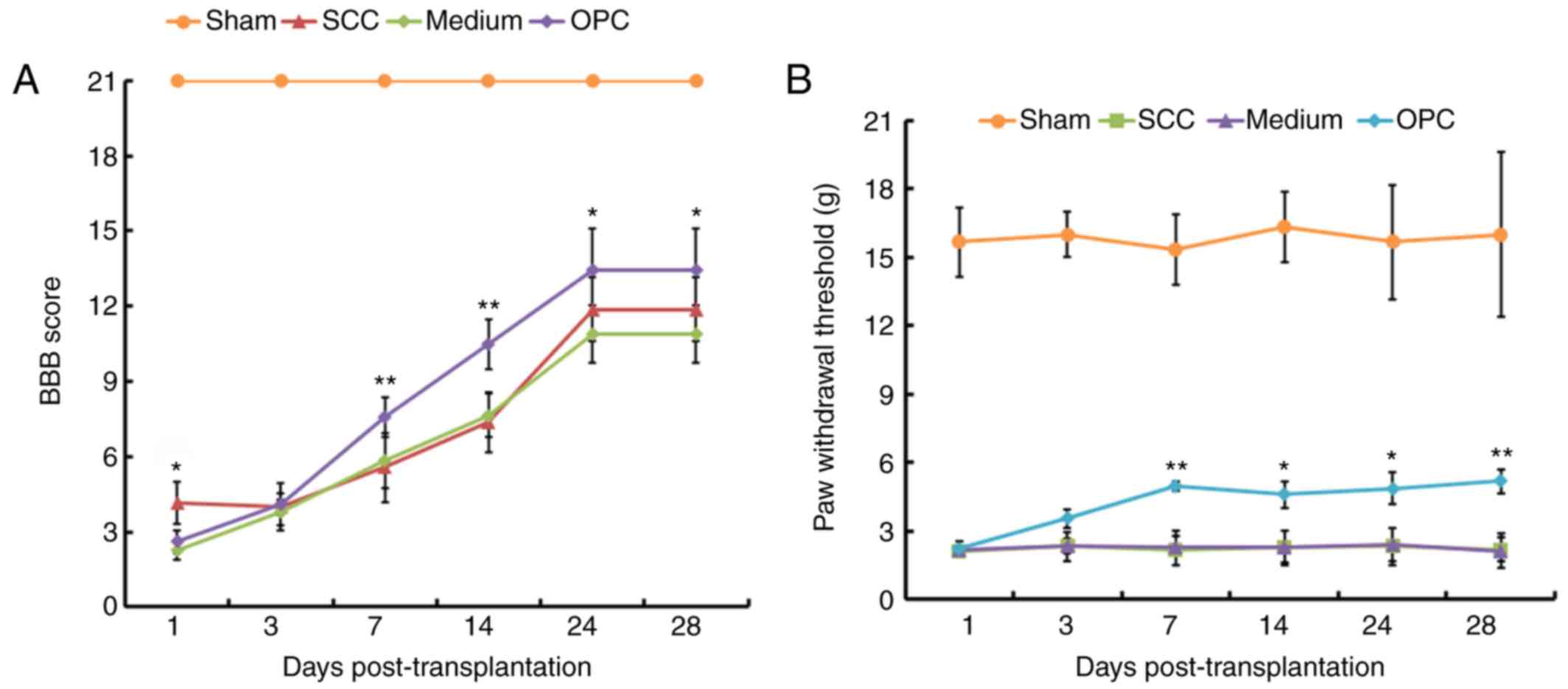 | Figure 2.Results of behavioral analyses. (A)
With the exception of day 1 and 3, treatment with OPCs resulted in
significantly higher BBB scores compared with in the Medium group.
(B) Mechanical allodynia data from the paw withdrawal threshold
analysis. On days 1 and 3 post-transplantation, the OPC, Medium and
SCC groups exhibited a decreased paw withdrawal threshold,
indicating mechanical allodynia, compared with in the Sham group.
Rats in the OPC group exhibited significant attenuation of paw
withdrawal threshold compared with in the Medium group. *P<0.05,
**P<0.01 vs. the Medium group. BBB, Basso, Beattie and
Bresnahan; OPC, oligodendrocyte precursor cell; SCC, contusive
spinal cord injury. |
miRNA expression profile in the spinal
cord of rats in the OPC group compared with the Medium group
A miRNA assay was conducted on spinal cords obtained
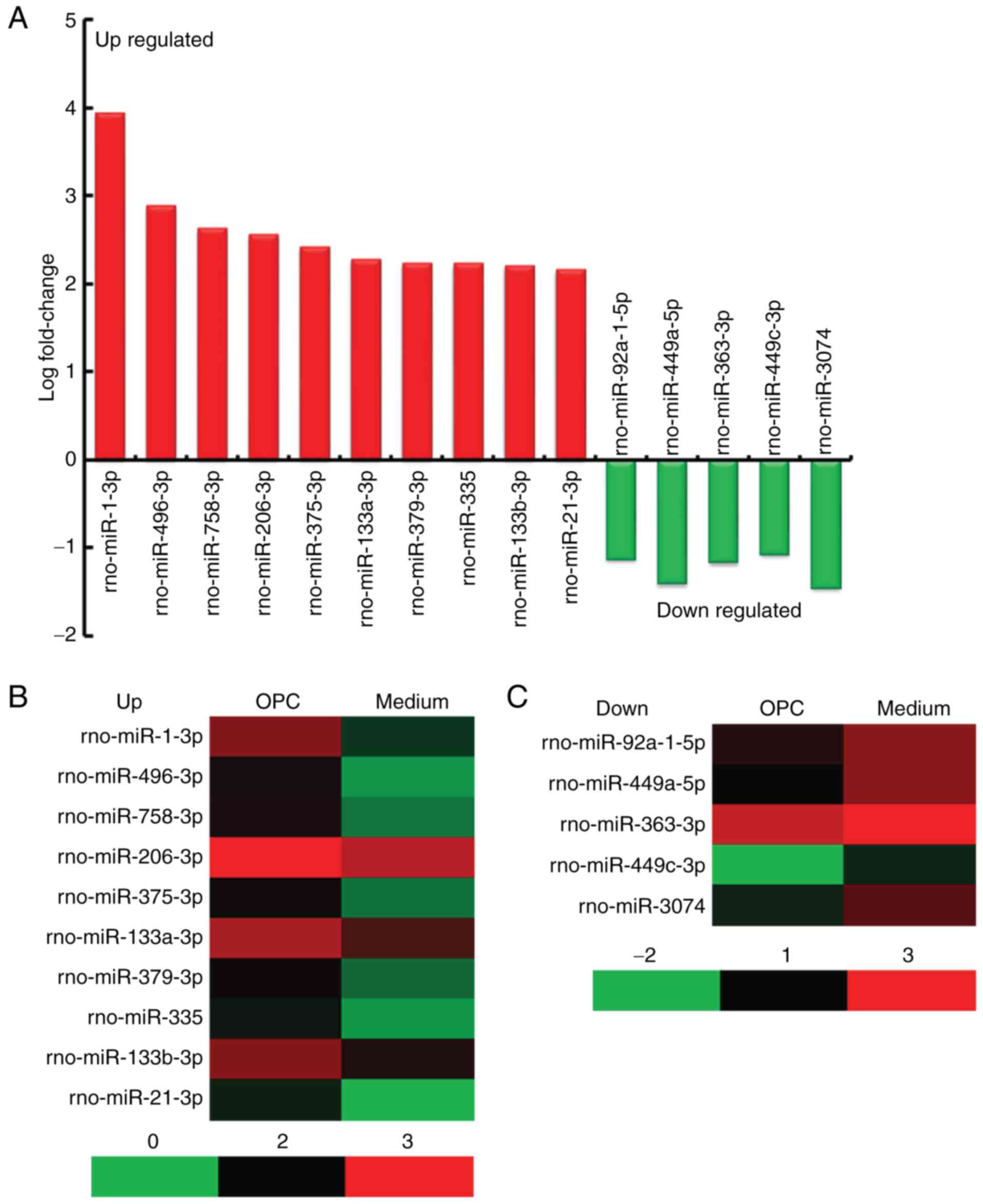
from rats in the OPC and Medium groups. The results indicated that
there were 45 differentially expressed miRNAs (40 upregulated and 5
downregulated) in the spinal cord of rats from the OPC group
compared with in the Medium group, according to the following
criteria: Fold change >2 and P<0.05 (Table III). The top 10 upregulated
miRNAs were rno-miR-1-3p, rno-miR-496-3p, rno-miR-758-3p,
rno-miR-206-3p, rno-miR-375-3p, rno-miR-133a-3p, rno-miR-379-3p,
rno-miR-335, rno-miR-133b-3p and rno-miR-21-3p, the 5 downregulated
miRNAs were rno-miR-92a-1-5p, rno-miR-449a-5p, rno-miR-363-3p,
rno-miR-449c-3p and rno-miR-3074 (Fig.
3A). A heat map of the miRNAs is presented in Fig. 3B.
 | Table III.Differentially expressed miRNAs in
the spinal cord of rats from the oligodendrocyte precursor cell
transplantation group compared with the Medium group. |
Table III.
Differentially expressed miRNAs in
the spinal cord of rats from the oligodendrocyte precursor cell
transplantation group compared with the Medium group.
| miRNA | log2 (fold
change) | Expression | Species | Sequence length
(bp) | Sequence
(5′-3′) |
|---|
| rno-miR-1-3p | 3.932485 | Up | Rattus
norvegicus | 22 |
UGGAAUGUAAAGAAGUGUGUAU |
| rno-miR-496-3p | 2.874668 | Up | Rattus
norvegicus | 21 |
AGUAUUACAUGGCCAAUCUCC |
| rno-miR-758-3p | 2.632376 | Up | Rattus
norvegicus | 22 |
UUUGUGACCUGGUCCACUAACC |
| rno-miR-206-3p | 2.552146 | Up | Rattus
norvegicus | 22 |
UGGAAUGUAAGGAAGUGUGUGG |
| rno-miR-375-3p | 2.413651 | Up | Rattus
norvegicus | 22 |
UUUGUUCGUUCGGCUCGCGUGA |
|
rno-miR-133a-3p | 2.264416 | Up | Rattus
norvegicus | 22 |
UUUGGUCCCCUUCAACCAGCUG |
| rno-miR-379-3p | 2.227997 | Up | Rattus
norvegicus | 22 |
CUAUGUAACAUGGUCCACUAAC |
| rno-miR-335 | 2.224635 | Up | Rattus
norvegicus | 23 |
UCAAGAGCAAUAACGAAAAAUGU |
|
rno-miR-133b-3p | 2.198147 | Up | Rattus
norvegicus | 22 |
UUUGGUCCCCUUCAACCAGCUA |
| rno-miR-21-3p | 2.15513 | Up | Rattus
norvegicus | 22 |
CAACAGCAGUCGAUGGGCUGUC |
| rno-miR-134-3p | 1.966194 | Up | Rattus
norvegicus | 22 |
CUGUGGGCCACCUAGUCACCAA |
|
rno-let-7a-2-3p | 1.875046 | Up | Rattus
norvegicus | 22 |
CUGUACAGCCUCCUAGCUUUCC |
| rno-miR-764-5p | 1.757712 | Up | Rattus
norvegicus | 22 |
GGUGCUCACAUGUCCUCCUCCA |
| rno-miR-369-5p | 1.688398 | Up | Rattus
norvegicus | 22 |
AGAUCGACCGUGUUAUAUUCGC |
| rno-miR-485-3p | 1.6806526 | Up | Rattus
norvegicus | 22 |
CAUACACGGCUCUCCUCUCUUC |
|
rno-miR-376a-3p | 1.67571 | Up | Rattus
norvegicus | 21 |
AUCGUAGAGGAAAAUCCACGU |
|
rno-miR-133a-5p | 1.5515371 | Up | Rattus
norvegicus | 22 |
AGCUGGUAAAAUGGAACCAAAU |
| rno-miR-124-5p | 1.505714 | Up | Rattus
norvegicus | 22 |
CGUGUUCACAGCGGACCUUGAU |
|
rno-miR-218a-5p | 1.499168 | Up | Rattus
norvegicus | 21 |
UUGUGCUUGAUCUAACCAUGU |
| rno-miR-323-3p | 1.491767 | Up | Rattus
norvegicus | 21 |
CACAUUACACGGUCGACCUCU |
| rno-miR-361-3p | 1.343701 | Up | Rattus
norvegicus | 24 |
CCCCCAGGUGUGAUUCUGAUUCGU |
| rno-miR-500-5p | 1.336195 | Up | Rattus
norvegicus | 24 |
AAUCCUUGCUAUCUGGGUGCUUAG |
| rno-miR-20a-3p | 1.3328006 | Up | Rattus
norvegicus | 21 |
ACUGCAUUACGAGCACUUACA |
| rno-miR-493-3p | 1.332116 | Up | Rattus
norvegicus | 21 |
UGAAGGUCUACUGUGUGCCAG |
|
rno-miR-551b-3p | 1.316522 | Up | Rattus
norvegicus | 23 |
GGCGACCCAUACUUGGUUUCAGU |
| rno-miR-122-5p | 1.3071 | Up | Rattus
norvegicus | 22 |
UGGAGUGUGACAAUGGUGUUUG |
| rno-miR-764-3p | 1.268782 | Up | Rattus
norvegicus | 23 |
GAGGAGGCCAUAGUGGCAACUGU |
| rno-miR-433-5p | 1.248092 | Up | Rattus
norvegicus | 22 |
UACGGUGAGCCUGUCAUUAUUC |
| rno-miR-411-5p | 1.203803 | Up | Rattus
norvegicus | 21 |
UAGUAGACCGUAUAGCGUACG |
|
rno-miR-200b-5p | 1.201743 | Up | Rattus
norvegicus | 22 |
CAUCUUACUGGGCAGCAUUGGA |
| rno-miR-511-3p | 1.200015 | Up | Rattus
norvegicus | 21 |
AAUGUGUAGCAAAAGACAGGA |
| rno-miR-380-3p | 1.17496 | Up | Rattus
norvegicus | 21 |
UAUGUAGUAUGGUCCACAUCU |
| rno-miR-137-5p | 1.142088 | Up | Rattus
norvegicus | 22 |
ACGGGUAUUCUUGGGUGGAUAA |
| rno-miR-292-3p | 1.134055 | Up | Rattus
norvegicus | 23 |
AAGUGCCGCCAGGUUUUGAGUGU |
| rno-miR-381-5p | 1.117327 | Up | Rattus
norvegicus | 22 |
AGCGAGGUUGCCCUUUGUAUAU |
| rno-miR-873-3p | 1.064982 | Up | Rattus
norvegicus | 21 |
GAGACUGACAAGUUCCCGGGA |
| rno-miR-10b-3p | 1.063514 | Up | Rattus
norvegicus | 21 |
ACAGAUUCGAUUCUAGGGGAA |
| rno-miR-434-5p | 1.02408 | Up | Rattus
norvegicus | 23 |
AGCUCGACUCAUGGUUUGAACCA |
| rno-miR-205 | 1.015991 | Up | Rattus
norvegicus | 23 |
UCCUUCAUUCCACCGGAGUCUGU |
| rno-miR-154-5p | 1.003104 | Up | Rattus
norvegicus | 22 |
UAGGUUAUCCGUGUUGCCUUCG |
|
rno-miR-92a-1-5p | −1.138072 | Down | Rattus
norvegicus | 23 |
AGGUUGGGAUUUGUCGCAAUGCU |
|
rno-miR-449a-5p | −1.414279 | Down | Rattus
norvegicus | 22 |
UGGCAGUGUAUUGUUAGCUGGU |
| rno-miR-363-3p | −1.172769 | Down | Rattus
norvegicus | 21 |
AAUUGCACGGUAUCCAUCUGU |
|
rno-miR-449c-3p | −1.0767871 | Down | Rattus
norvegicus | 17 |
UAGAACUUCGUCCCAAC |
| rno-miR-3074 | −1.463293 | Down | Rattus
norvegicus | 22 |
GAUAUCAGCUCAGUAGGCACCG |
GO analysis of the target genes of
altered miRNAs
It has previously been reported that miRNAs regulate
gene expression by controlling protein translation or destabilizing
mRNA transcripts (29,43). Therefore, using prediction
databases (miRecords, TargetScan, MicroCosm, Miranda and Pictar),
the target genes of all of the altered miRNAs were screened.
Subsequently, the main functional categories associated with the
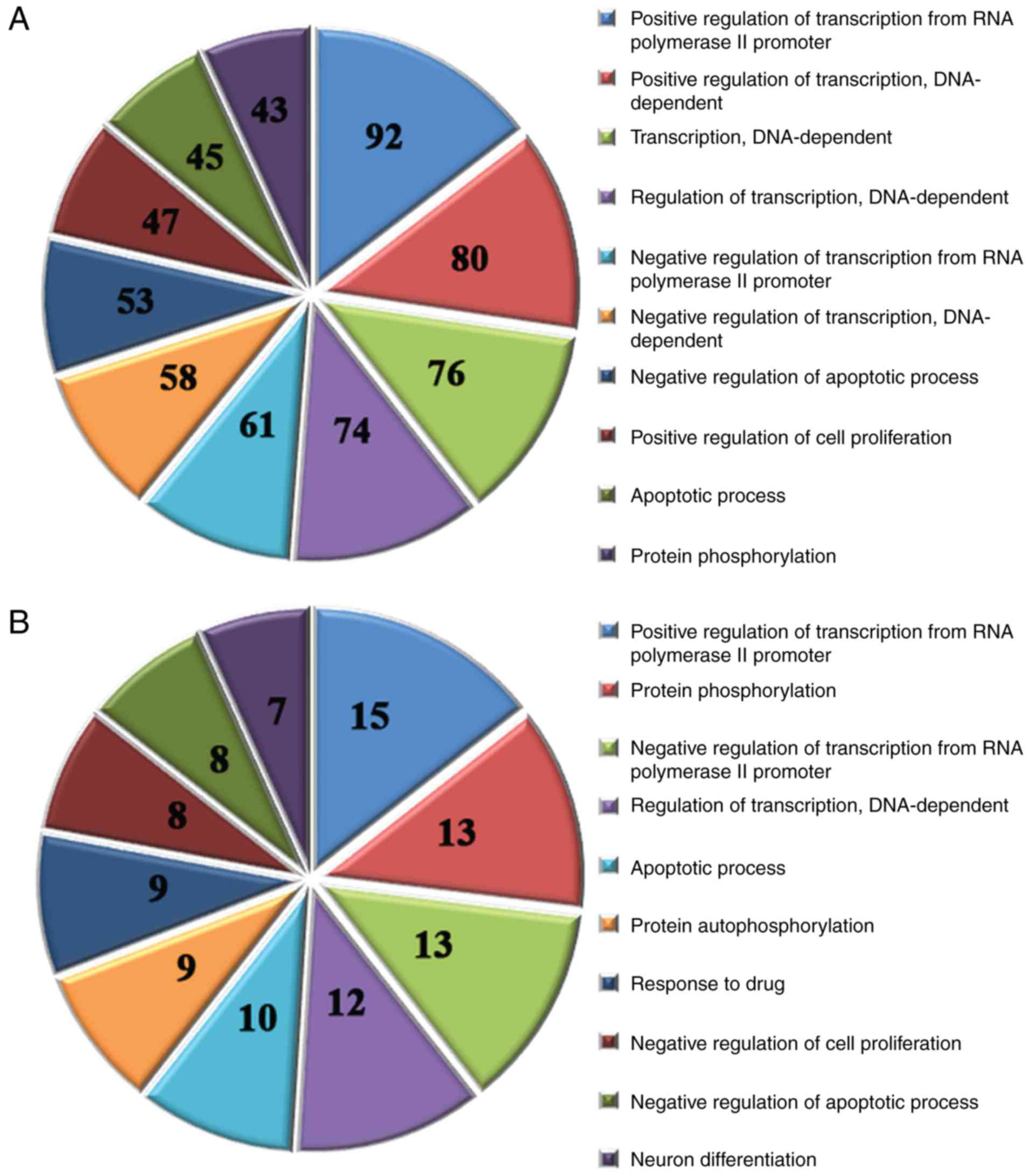
target genes were identified by GO analysis. The results identified
578 significantly enriched GO terms by analyzing the target genes
of the 40 upregulated miRNAs (P<0.05); the top 10 GO terms are
presented in Fig. 4A. In addition,
185 significantly enriched GO terms were identified by analyzing
the target genes of the 5 downregulated miRNAs (P<0.05); the top
10 GO terms are shown in Fig.
4B.
KEGG pathways associated with the
target genes of altered miRNAs
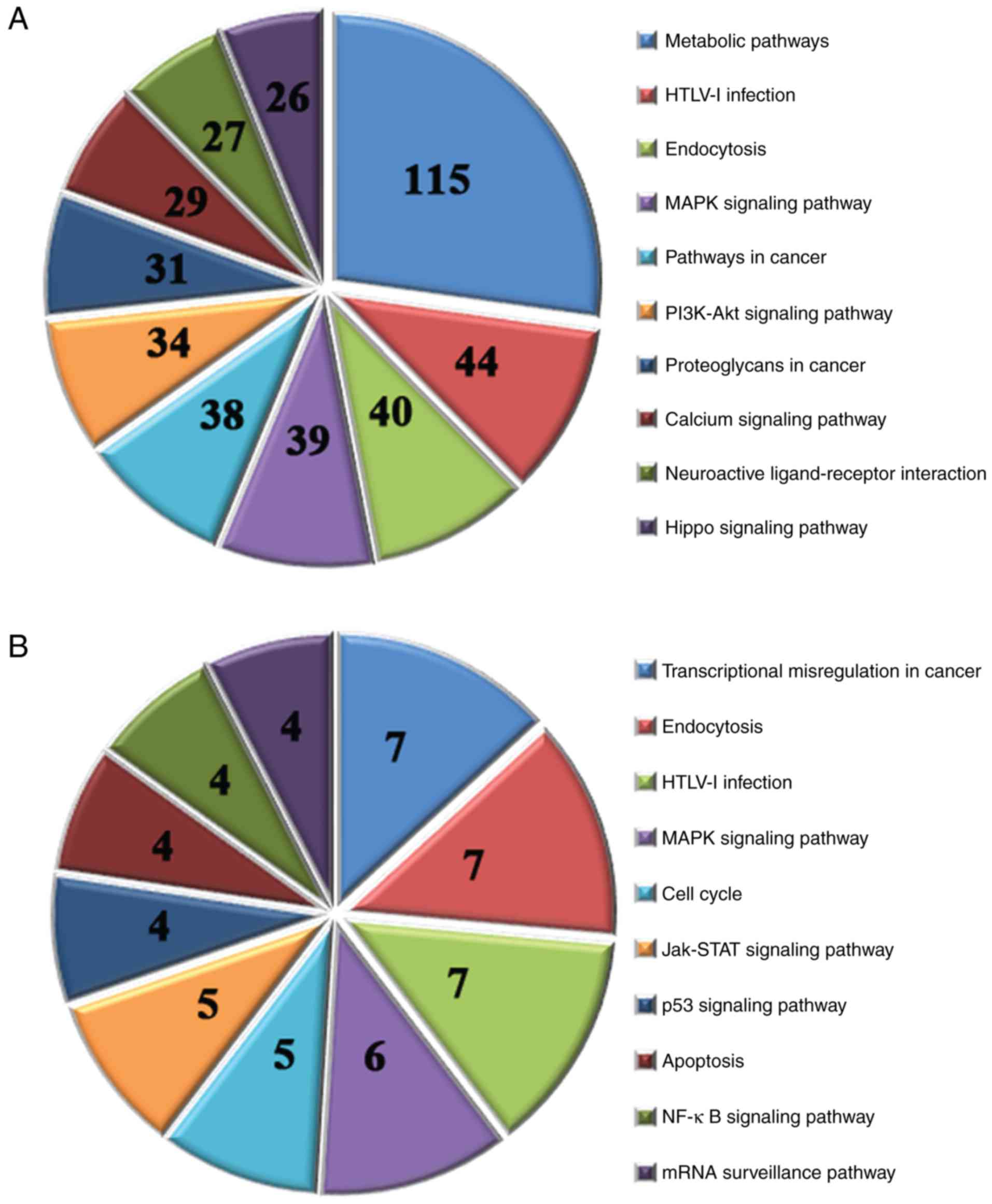
A KEGG pathway analysis was performed for the target
genes of all of the differentially expressed miRNAs. The results
identified 107 associated pathways by analyzing the target genes of
the 40 upregulated miRNAs (P<0.05); the top 10 pathways are
presented in Fig. 5A. In addition,
14 associated pathways were identified by analyzing the target
genes of the 5 downregulated miRNAs (P<0.05); the top 10
pathways are presented in Fig.
5B.
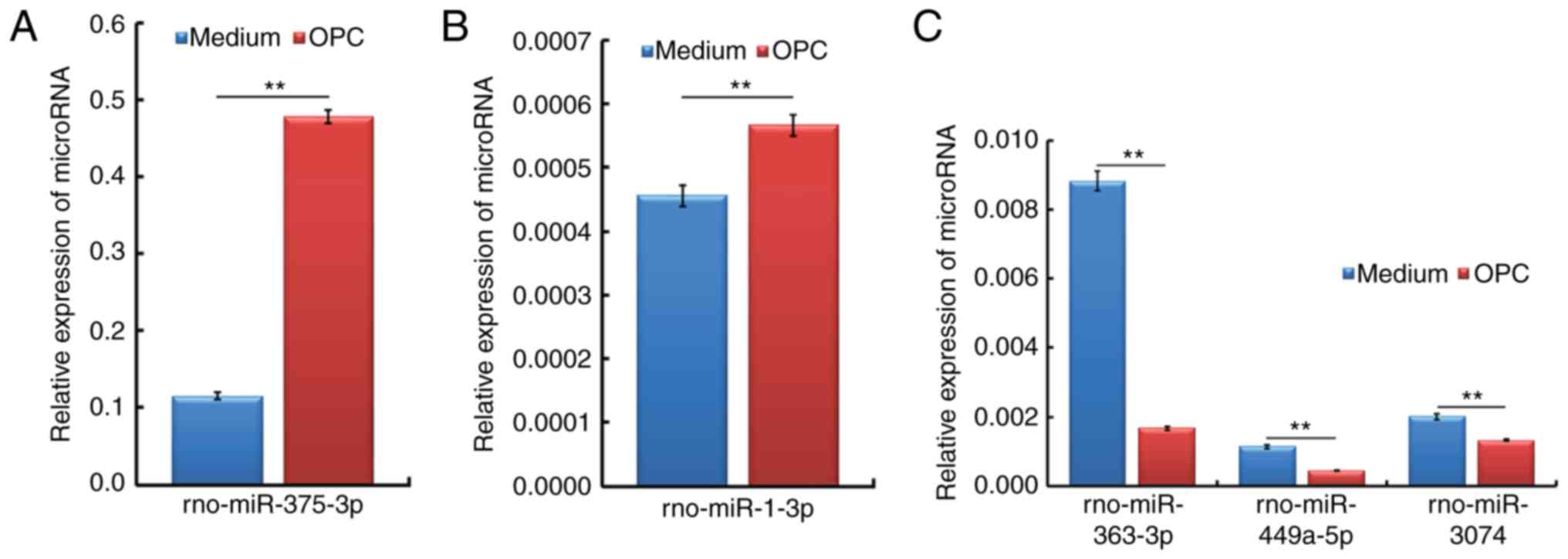
qPCR verification of OPC
transplantation-responsive spinal cord miRNAs from rats subjected
to SCC
In order to validate the expression profiles
generated from the miRNA assay, a qPCR analysis was performed to
identify the association between the two datasets. As presented in
Fig. 6, the most highly
upregulated (miR-375-3p and miR-1-3p) and downregulated
(miR-363-3p, miR-449a-5p and miR-3074) spinal cord miRNAs
identified in the miRNA assay were further verified, and showed
consistent alterations, in a qPCR analysis.
Bioinformatics analysis of the
important and verified miRNAs
The results of the qPCR analysis demonstrated that
miR-375 and miR-1 were upregulated in the OPC group compared with
in the Medium group, and a bioinformatics analysis indicated that
they targeted similar types of genes; therefore, miR-375 and miR-1
were grouped together for functional analysis. The results
suggested that miR-375 and miR-1 may act primarily to inhibit cell
proliferation and apoptosis through the regulation of transcription
and translation. Furthermore, due to their association within the
same annotation cluster (such as cell proliferation), it is
possible that the primary method by which these two miRNAs regulate
cell proliferation is via the inhibition of GO terms, including the
mitogen-activated protein kinases signaling pathway (Table IV). miR-363, miR-449a and miR-3074
were downregulated in the OPC group compared with in the Medium
group, as confirmed by qPCR, and a bioinformatics analysis
demonstrated that they targeted similar types of genes; therefore,
miR-363, miR-449a and miR-3074 were grouped together for functional
analysis. The results indicated that miR-363, miR-449a and miR-3074
may primarily inhibit cell proliferation and neuronal
differentiation through the regulation of transcription (Table IV).
 | Table IV.Statistical annotation of miRNA
function. |
Table IV.
Statistical annotation of miRNA
function.
| GO ID/pathway
function term | Gene count | P-value |
|---|
| miR-375 and miR-1
common targets |
|
|
|
GO:0045893-positive regulation
of transcription, DNA-dependent | 2 |
1.96792×10−24 |
|
GO:0045944-positive regulation
of transcription from RNA polymerase II promoter | 2 |
9.15325×10−21 |
|
GO:0006351-transcription,
DNA-dependent | 1 |
2.48966×10−15 |
|
GO:0000122-negative regulation
of transcription from RNA polymerase II promoter | 1 |
5.12873×10−13 |
|
GO:0006355-regulation of
transcription, DNA-dependent | 1 |
5.9179×10−13 |
|
GO:0006417-regulation of
translation | 1 | 0.000151831 |
|
GO:0006486-protein
glycosylation | 1 |
1.21081×10−10 |
|
GO:0006915-apoptotic
process | 3 |
3.19378×10−10 |
|
GO:0043066-negative regulation
of apoptotic process | 3 |
7.94504×10−10 |
|
GO:0043065-positive regulation
of apoptotic process | 1 |
2.34005×10−6 |
|
GO:0051402-neuron apoptotic
process | 1 |
1.43174×10−5 |
|
GO:0043524-negative regulation
of neuron apoptotic process | 2 |
7.17984×10−6 |
|
GO:0008283-cell
proliferation | 3 | 0.001320952 |
|
GO:0008285-negative regulation
of cell proliferation | 1 |
3.71268×10−10 |
|
GO:0008284-positive regulation
of cell proliferation | 4 |
4.37506×10−10 |
|
GO:0042552-myelination | 2 | 0.0003695 |
|
GO:0035556-intracellular
signal transduction | 3 |
1.05697×10−10 |
|
GO:0000165-MAPK cascade | 1 | 0.000318552 |
|
Path_id:1100-Metabolic
pathways | 4 |
7.89723×10−14 |
|
Path_id:4144-Endocytosis | 1 |
2.5492×10−13 |
|
Path_id:4010-MAPK signaling
pathway | 1 |
4.94224×10−11 |
|
Path_id:4020-Calcium signaling
pathway | 2 |
1.24247×10−8 |
|
Path_id:4070-Phosphatidylinositol
signaling system | 1 |
1.86855×10−7 |
|
Path_id:4310-Wnt signaling
pathway | 1 |
2.71935×10−7 |
|
Path_id:4151-PI3K-Akt
signaling pathway | 5 |
1.92934×10−5 |
|
Path_id:3015-mRNA surveillance
pathway | 1 | 0.000876288 |
|
Path_id:4064-NF-κB signaling
pathway | 2 | 0.008087159 |
|
Path_id:4514-Cell adhesion
molecules (CAMs) | 1 | 0.048139487 |
| miR-363, miR-449a
and miR-3074 common targets |
|
|
|
GO:0000122-negative regulation
of transcription from RNA polymerase II promoter | 2 |
9.32468×10−5 |
|
GO:0045944-positive regulation
of transcription from RNA polymerase II promoter | 3 | 0.000267853 |
|
GO:0006355-regulation of
transcription, DNA-dependent | 1 | 0.006384786 |
|
GO:0006468-protein
phosphorylation | 1 |
2.21252×10−6 |
|
GO:0015031-protein
transport | 1 | 0.032924475 |
|
GO:0030182-neuron
differentiation | 2 |
6.76381×10−5 |
|
GO:0008285-negative regulation
of cell proliferation | 1 | 0.003549981 |
|
GO:0007264-small GTPase
mediated signal transduction | 1 | 0.014162188 |
|
GO:0007165-signal
transduction | 2 | 0.044607167 |
|
Path_id:4630-Jak-STAT
signaling pathway | 2 | 0.009890945 |
Discussion
Human SCC can cause severe and long-term disability
(3). At present, there is no
effective clinical treatment for functional recovery following SCI.
However, it has been reported that OPC transplantation may have
potential for the treatment of SCI (44,45),
and abnormal miRNA expression may contribute to the pathogenesis of
SCI (37). However, to the best of
our knowledge, there are no reports regarding the expression of
miRNAs in the spinal cord of rats following SCC and OPC
transplantation. The present study aimed to investigate the effects
of OPC transplantation on rats with SCC; initially, BBB score and
paw withdrawal threshold were tested following OPC transplantation
into the spinal cord of rats with SCC. Subsequently, in order to
detect the differentially expressed miRNAs in the spinal cord of
rats in the OPC group compared with in the Medium group, a miRNA
assay was conducted. Finally, qPCR was performed to verify the
significantly altered miRNAs.
Firstly, following identification of OPCs, the cells
were transplanted into rats that were subjected to SCC for 7 days.
The BBB score demonstrated that OPC transplantation was able to
improve the motor recovery of rats with SCC. A previous study
demonstrated that OPC transplantation improved forelimb motor
function following cervical SCI (25). In addition, animals that were
subjected to SCC and hESC-derived OPCs transplantation at 7 days
performed significantly better on the BBB locomotor test compared
with those transplanted with human fibroblasts or DMEM (22). Furthermore, CNTF-OPC-grafted
animals exhibited significantly increased BBB locomotor scores
compared with those receiving DMEM, enhanced green fluorescent
protein (EGFP)-fibroblasts or CNTF-fibroblasts at 3–7 weeks
following injury; the BBB scores of the EGFP-OPC-grafted animals
were also significantly higher than the other three groups at 5–7
weeks following injury (26). In
the present study, paw withdrawal testing indicated that OPC
transplantation may relieve SCC-induced mechanical allodynia. It
has previously been reported that transplanted hESC-derived OPCs
promoted repair of the sensory pathways of rats with SCC (46). The results of the present study
were concordant with those of the previous aforementioned
studies.
The results of a miRNA assay detected 40 upregulated
miRNAs and 5 downregulated miRNAs in the spinal cord of rats from
the OPC group compared with in the Medium group, according to the
following criteria: Fold change >2 and P<0.05. Bioinformatics
analysis indicated that positive regulation of transcription from
RNA polymerase II promoter was the most significantly enriched GO
term, and metabolic pathways was the most significantly enriched
KEGG pathway, according to the number of target genes of
differentially expressed miRNAs enriched in them. A previous study
detected 97 differentially expressed miRNAs in the adult rat spinal
cord following traumatic SCI through miRNA analysis, and the
potential targets for these SCI-altered miRNAs included genes
associated with inflammation, oxidation and apoptosis, which are
known to serve important roles in the pathogenesis of SCI (37). Furthermore, microarray comparisons
of the injury sites of spinal cords from contused and sham rats,
harvested 4 and 14 days following SCC, demonstrated that 32 miRNAs,
including miR-124, miR-129, and miR-1, were significantly
downregulated. In addition, a bioinformatics analysis of validated
miRNA-targeted genes indicated that miRNA dysregulation may explain
the apoptotic susceptibility and aberrant cell cycle associated
with a loss of neuronal identity, which underlies the pathogenesis
of secondary SCC (38).
Furthermore, microarray data has previously revealed that the
induction of a specific miRNA expression pattern following moderate
SCC is characterized by a marked increase in the number of
downregulated miRNAs, particularly at 7 days after injury, and a
bioinformatics analysis indicated that alterations in miRNA
expression may affect key processes in SCI physiopathology,
including inflammation and apoptosis (31). To the best of our knowledge, the
present study is the first to report that dysregulated miRNA
expression is present in the spinal cord of SCC rats transplanted
with OPCs compared with in SCC rats treated with medium.
The present study also demonstrated that the most
highly upregulated (miR-375-3p and miR-1-3p) and downregulated
(miR-363-3p, miR-449a-5p and miR-3074) spinal cord miRNAs
identified in the miRNA assay were consistently altered in a qPCR
analysis. Furthermore, functional analysis demonstrated that
miR-375 and miR-1 may primarily act to inhibit cell proliferation
and apoptosis through the regulation of transcription and
translation, whereas miR-363, miR-449a and miR-3074 may primarily
act to inhibit cell proliferation and neuronal differentiation
through the regulation of transcription. A previous study
demonstrated that overexpression of miR-375 significantly inhibited
gastric cancer cell proliferation by targeting Janus kinase 2 in
vitro and in vivo (47). In addition, by regulating the
expression of B-cell lymphoma (Bcl)-2, Bcl-2-associated X protein
and miR-375 in vitro and in vivo, formononetin was
able to induce apoptosis of U2OS human osteosarcoma cells (48). miR-375 could also affect the
proliferation and apoptosis of human papilloma virus 16-positive
human cervical cancer cells by targeting insulin-like growth factor
1 receptor (49). In addition, it
has been reported that miR-1 may control cell proliferation and
cell cycle in human osteosarcoma through modulation of MET protein
expression (50). miR-1 may also
inhibit gastric cancer cell proliferation by targeting MET
(51). Furthermore, miR-1 has been
reported to regulate heat shock protein 60 expression, thus
contributing to glucose-mediated apoptosis in cardiomyocytes
(52). Overexpression of miR-1 may
also induce apoptosis in mesothelioma cell lines (53). miR-1 has also been revealed to be
involved in the protective effects of hydrogen sulfide against
cardiomyocyte apoptosis via ischemia/reperfusion (54). Introduction of miR-363 mimics has
been reported to result in inhibition of cell proliferation in
renal cell carcinoma (55).
Conversely, miR-363 may promote proliferation of human gastric
cancer cells via targeting FBW7 ubiquitin ligase expression
(56). With regards to miR-449a,
it has been reported to regulate proliferation in response to
cisplatin by targeting cyclin D1 and Bcl-2 in SGC7901 cells
(57). In addition, overexpression
of miR-449a significantly inhibits the proliferation of HEC-1B
endometrial cancer cells (58).
miR-449a acts as a tumor suppressor by reducing the proliferation
of human glioblastoma cell lines and glioblastoma stem cells
(59). miR-449a may also inhibit
liver cancer cell proliferation by suppressing POU class 2 homeobox
1 and calpain 6 (60). miRNAs have
various target genes and serve roles in several diseases by
regulating the associated target genes, thus, some miRNAs have
exhibited opposing effects in different studies. OPC
transplantation may alter miRNA expression following SCC in rats,
and may be useful for treating SCC. To the best of our knowledge,
the present study is the first to identify OPC
transplantation-responsive spinal cord miRNAs in rats subjected to
SCC, which may suggest potential functional roles for these miRNAs
in transplantation of OPCs in rats with SCC.
In conclusion, the present study demonstrated that
OPC transplantation could improve motor recovery and relieve
mechanical allodynia of rats with SCC. After verifying the results
of a miRNA assay, the most highly upregulated (miR-375-3p and
miR-1-3p) and downregulated (miR-363-3p, miR-449a-5p and miR-3074)
spinal cord miRNAs were detected in SCC rats transplanted with
OPCs. These results suggested that OPC transplantation may promote
functional recovery of rats with SCC, which may be associated with
various miRNAs in the spinal cord, particularly miR-375-3p,
miR-1-3p, miR-363-3p, miR-449a-5p and miR-3074.
Acknowledgements
The authors of the present study would like to thank
Professor Qing-Jie Xia (West China Hospital, Sichuan University,
China) for suggestions on the paper and Professor Su Liu (Johns
Hopkins University, Baltimore, MD, USA) for providing the
viruses.
References
|
1
|
Hulsebosch CE: Recent advances in
pathophysiology and treatment of spinal cord injury. Adv Physiol
Educ. 26:238–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Penas C, Verdu E, Asensio-Pinilla E,
Guzmán-Lenis MS, Herrando-Grabulosa M, Navarro X and Casas C:
Valproate reduces CHOP levels and preserves oligodendrocytes and
axons after spinal cord injury. Neuroscience. 178:33–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beaumont E, Whitaker CM, Burke DA, Hetman
M and Onifer SM: Effects of rolipram on adult rat oligodendrocytes
and functional recovery after contusive cervical spinal cord
injury. Neuroscience. 163:985–990. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee BB, Cripps RA, Fitzharris M and Wing
PC: The global map for traumatic spinal cord injury epidemiology:
Update 2011, global incidence rate. Spinal Cord. 52:110–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emery E, Aldana P, Bunge MB, Puckett W,
Srinivasan A, Keane RW, Bethea J and Levi AD: Apoptosis after
traumatic human spinal cord injury. J Neurosurg. 89:911–920. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bunge RP, Puckett WR, Becerra JL, Marcillo
A and Quencer RM: Observations on the pathology of human spinal
cord injury. A review and classification of 22 new cases with
details from a case of chronic cord compression with extensive
focal demyelination. Adv Neurol. 59:75–89. 1993.PubMed/NCBI
|
|
7
|
Kakulas BA: A review of the neuropathology
of human spinal cord injury with emphasis on special features. J
Spinal Cord Med. 22:119–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buss A, Brook GA, Kakulas B, Martin D,
Franzen R, Schoenen J, Noth J and Schmitt AB: Gradual loss of
myelin and formation of an astrocytic scar during Wallerian
degeneration in the human spinal cord. Brain. 127:34–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guest JD, Hiester ED and Bunge RP:
Demyelination and Schwann cell responses adjacent to injury
epicenter cavities following chronic human spinal cord injury. Exp
Neurol. 192:384–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crowe MJ, Bresnahan JC, Shuman SL, Masters
JN and Beattie MS: Apoptosis and delayed degeneration after spinal
cord injury in rats and monkeys. Nat Med. 3:73–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li GL, Farooque M, Holtz A and Olsson Y:
Apoptosis of oligodendrocytes occurs for long distances away from
the primary injury after compression trauma to rat spinal cord.
Acta Neuropathol. 98:473–480. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shuman SL, Bresnahan JC and Beattie MS:
Apoptosis of microglia and oligodendrocytes after spinal cord
contusion in rats. J Neurosci Res. 50:798–808. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McTigue DM, Wei P and Stokes BT:
Proliferation of NG2-positive cells and altered oligodendrocyte
numbers in the contused rat spinal cord. J Neurosci. 21:3392–3400.
2001.PubMed/NCBI
|
|
14
|
McEwen ML and Springer JE: A mapping study
of caspase-3 activation following acute spinal cord contusion in
rats. J Histochem Cytochem. 53:809–819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casha S, Yu WR and Fehlings MG:
Oligodendroglial apoptosis occurs along degenerating axons and is
associated with FAS and p75 expression following spinal cord injury
in the rat. Neuroscience. 103:203–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossman SD, Rosenberg LJ and Wrathall JR:
Temporal-spatial pattern of acute neuronal and glial loss after
spinal cord contusion. Exp Neurol. 168:273–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hernández J, Torres-Espin A and Navarro X:
Adult stem cell transplants for spinal cord injury repair: Current
state in preclinical research. Curr Stem Cell Res Ther. 6:273–287.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grabel L: Prospects for pluripotent stem
cell therapies: Into the clinic and back to the bench. J Cell
Biochem. 113:381–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Führmann T, Tam RY, Ballarin B, Coles B,
Donaghue Elliott I, van der Kooy D, Nagy A, Tator CH, Morshead CM
and Shoichet MS: Injectable hydrogel promotes early survival of
induced pluripotent stem cell-derived oligodendrocytes and
attenuates longterm teratoma formation in a spinal cord injury
model. Biomaterials. 83:23–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKerracher L and Winton MJ: Nogo on the
go. Neuron. 36:345–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domeniconi M and Filbin MT: Overcoming
inhibitors in myelin to promote axonal regeneration. J Neurol Sci.
233:43–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faulkner J and Keirstead HS: Human
embryonic stem cell-derived oligodendrocyte progenitors for the
treatment of spinal cord injury. Transpl Immunol. 15:131–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuypers NJ, Bankston AN, Howard RM, Beare
JE and Whittemore SR: Remyelinating oligodendrocyte precursor cell
miRNAs from the Sfmbt2 cluster promote cell cycle arrest and
differentiation. J Neurosci. 36:1698–1710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang HL, Jiang ZS and Wang FW: Analysis
of gene expression profiles associated with functional recovery
after spinal cord injury caused by sema4D knockdown in
oligodendrocytes. Cell Biochem Biophys. 69:655–661. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharp J, Frame J, Siegenthaler M, Nistor G
and Keirstead HS: Human embryonic stem cell-derived oligodendrocyte
progenitor cell transplants improve recovery after cervical spinal
cord injury. Stem Cells. 28:152–163. 2010.PubMed/NCBI
|
|
26
|
Cao Q, He Q, Wang Y, Cheng X, Howard RM,
Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, et al:
Transplantation of ciliary neurotrophic factor-expressing adult
oligodendrocyte precursor cells promotes remyelination and
functional recovery after spinal cord injury. J Neurosci.
30:2989–3001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Sall A and Yang D: MicroRNA: An
emerging therapeutic target and intervention tool. Int J Mol Sci.
9:978–999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yunta M, Nieto-Diaz M, Esteban FJ,
Caballero-López M, Navarro-Ruiz R, Reigada D, Pita-Thomas DW, del
Águila A, Muñoz-Galdeano T and Maza RM: MicroRNA dysregulation in
the spinal cord following traumatic injury. PLoS One. 7:e345342012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krichevsky AM: MicroRNA profiling: From
dark matter to white matter, or identifying new players in
neurobiology. ScientificWorldJournal. 7:155–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kosik KS: The neuronal microRNA system.
Nat Rev Neurosci. 7:911–920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bak M, Silahtaroglu A, Møller M,
Christensen M, Rath MF, Skryabin B, Tommerup N and Kauppinen S:
MicroRNA expression in the adult mouse central nervous system. RNA.
14:432–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miska EA, Alvarez-Saavedra E, Townsend M,
Yoshii A, Sestan N, Rakic P, Constantine-Paton M and Horvitz HR:
Microarray analysis of microRNA expression in the developing
mammalian brain. Genome Biol. 5:R682004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu NK, Wang XF, Lu QB and Xu XM: Altered
microRNA expression following traumatic spinal cord injury. Exp
Neurol. 219:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Strickland ER, Hook MA, Balaraman S, Huie
JR, Grau JW and Miranda RC: MicroRNA dysregulation following spinal
cord contusion: Implications for neural plasticity and repair.
Neuroscience. 186:146–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lü HZ, Wang YX, Li Y, Fu SL, Hang Q and Lu
PH: Proliferation and differentiation of oligodendrocyte progenitor
cells induced from rat embryonic neural precursor cells followed by
flow cytometry. Cytometry A. 73:754–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu R, Zhao W, Zhao Q, Liu SJ, Liu J, He
M, Xu Y, Wang W, Liu W, Xia QJ, et al: Endoplasmic reticulum
protein 29 protects cortical neurons from apoptosis and promoting
corticospinal tract regeneration to improve neural behavior via
caspase and Erk signal in rats with spinal cord transection. Mol
Neurobiol. 50:1035–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ozdemir M, Attar A and Kuzu I:
Regenerative treatment in spinal cord injury. Curr Stem Cell Res
Ther. 7:364–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang HL, Wang J and Tang L: Sema4D
knockdown in oligodendrocytes promotes functional recovery after
spinal cord injury. Cell Biochem Biophys. 68:489–496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
All AH, Bazley FA, Gupta S, Pashai N, Hu
C, Pourmorteza A and Kerr C: Human embryonic stem cell-derived
oligodendrocyte progenitors aid in functional recovery of sensory
pathways following contusive spinal cord injury. PLoS One.
7:e476452012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y,
Yao H, Liu X, Ke Y, Si J and Zhou T: MiR-375 frequently
downregulated in gastric cancer inhibits cell proliferation by
targeting JAK2. Cell Res. 20:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu W and Xiao Z: Formononetin induces
apoptosis of human osteosarcoma cell line U2OS by regulating the
expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell
Physiol Biochem. 37:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu X, Zhao W, Yang X, Wang Z and Hao M:
miR-375 affects the proliferation, invasion, and apoptosis of
HPV16-positive human cervical cancer cells by targeting IGF-1R. Int
J Gynecol Cancer. 26:851–858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Novello C, Pazzaglia L, Cingolani C, Conti
A, Quattrini I, Manara MC, Tognon M, Picci P and Benassi MS: miRNA
expression profile in human osteosarcoma: Role of miR-1 and
miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han C, Zhou Y, An Q, Li F, Li D, Zhang X,
Yu Z, Zheng L, Duan Z and Kan Q: MicroRNA-1 (miR-1) inhibits
gastric cancer cell proliferation and migration by targeting MET.
Tumour Biol. 36:6715–6723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP,
Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, et al: miR-1/miR-206
regulate Hsp60 expression contributing to glucose-mediated
apoptosis in cardiomyocytes. FEBS Lett. 584:3592–3600. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu Y, Zheng M, Merritt RE, Shrager JB,
Wakelee H, Kratzke RA and Hoang CD: miR-1 induces growth arrest and
apoptosis in malignant mesothelioma. Chest. 144:1632–1643. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang
Y, He B and Wang Z: Involvement of miR-1 in the protective effect
of hydrogen sulfide against cardiomyocyte apoptosis induced by
ischemia/reperfusion. Mol Biol Rep. 41:6845–6853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y, Chen D, Li Y, Jin L, Liu J, Su Z, Qi
Z, Shi M, Jiang Z, Ni L, et al: Oncogenic cAMP responsive element
binding protein 1 is overexpressed upon loss of tumor suppressive
miR-10b-5p and miR-363-3p in renal cancer. Oncol Rep. 35:1967–1978.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang PF, Sheng LL, Wang G, Tian M, Zhu
LY, Zhang R, Zhang J and Zhu JS: miR-363 promotes proliferation and
chemo-resistance of human gastric cancer via targeting of FBW7
ubiquitin ligase expression. Oncotarget. 7:35284–35292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu J, Fang Y, Cao Y, Qin R and Chen Q:
miR-449a Regulates proliferation and chemosensitivity to cisplatin
by targeting cyclin D1 and BCL2 in SGC7901 cells. Dig Dis Sci.
59:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ye W, Xue J, Zhang Q, Li F, Zhang W, Chen
H, Huang Y and Zheng F: MiR-449a functions as a tumor suppressor in
endometrial cancer by targeting CDC25A. Oncol Rep. 32:1193–1199.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Li Z, Hu
Y, Shang X and Liu Y: MiR-449a exerts tumor-suppressive functions
in human glioblastoma by targeting Myc-associated zinc-finger
protein. Mol Oncol. 9:640–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Wang Y, Sun X, Mei C, Wang L, Li Z
and Zha X: miR-449a promotes liver cancer cell apoptosis by
downregulation of Calpain 6 and POU2F1. Oncotarget. 7:13491–13501.
2016. View Article : Google Scholar : PubMed/NCBI
|